Abstract
Cardiac side population (CSP) cells, defined by their ability to efflux the vital dye Hoechst 33342, have been identified as putative cardiac stem cells based on their potential to give rise to both cardiomyocytes and endothelial cells. The CSP phenotype relies on an active metabolic pathway and cell viability to identify a rare population of cells and therefore technical differences in the CSP staining protocol can lead to inconsistent results and discrepancies between studies. Here we describe an established protocol for CSP identification and have optimised a protocol for CSP analysis utilizing an automated cardiac digestion procedure using gentleMACs dissociation and Hoechst 33342 staining followed by dual wavelength flow cytometric analysis.
Keywords: Cardiac, Side population, Abcg2, Fumitremorgin C, Stem cell
Introduction
In recent years there has been an accumulation of evidence challenging the supposition that the heart is a terminally differentiated, post-mitotic organ. Initial studies focussed on the evidence of cardiomyocyte replication in the diseased human heart [1–3], and although the frequency was low, evidence for cardiomyocyte cell division was observed raising the possibility that an un-differentiated cardiac stem cell might also be present. These and other data prompted investigators to identify and characterise putative cardiac stem cells. One approach that has proven useful in the study of stem cells in other organ systems has been to identify candidates by virtue of the cell surface antigenic markers they express. Cardiac cells possessing markers characteristic of stem cell populations, such as Sca1 and c-kit [4–8] have been directly identified in adult hearts, and have also been shown to be enriched in cardiosphere culture [9–12]. Another common approach has been to use dye exclusion to isolate the cardiac ‘side population’ (SP) which express the ATP-binding cassette (ABC) transporters [13].
SP cells were originally identified in the haematopoietic lineage as a candidate stem cell population, and have also been used to isolate cell populations from tissues including lung, mammary gland, testes, kidney, skin, and skeletal muscle where they have been shown to overlap with stem cell activity and regenerative potential [14–23]. Within the heart, the CSP has been reported to comprise 0.03 %–3.5 %, of the total mononuclear cell fraction [3, 6, 13, 24–26]. CSP cells have been demonstrated to be capable of self-renewal, and in vitro can differentiate to form functionally mature cardiomyocytes. Furthermore, following transplantation CSP cells contribute to the formation of multiple cardiac cell types including cardiomyocytes [3, 6, 13, 27]. The combination of mechanical dissociation and enzymatic digestion is required to obtain single cell preparations for CSP analysis. To date, depending on the study, digestion has been achieved using enzymes including collagenase, trypsin, pronase and dispase. As with all SP populations, the CSP phenotype relies on functional biological processes and cell metabolism to efflux vital dyes, variations in the CSP percentage between different studies may reflect these different digestion protocols [3, 6, 13, 24–26]. In addition the concentration of Hoechst 33342 dye and ABC-transporter inhibitor used also affects the ability to discriminate CSP cells [28] increasing variability between the data obtained by independent laboratories. Standardised methods for SP analysis have been described and optimised for bone marrow but there remains a lack of standardisation in the techniques used for cardiac tissue. Here we describe in detail protocols we have used previously to identify and isolate CSP cells [13, 29]. In order to establish a more efficient method for CSP analysis we have also compared this with an automated mechanical dissociation system. Further, we have compared the use of collagenase II/DNase I to pronase, optimised the protocols for Hoechst 33342 staining and established the most appropriate ABC-transporter inhibitor to allow the robust identification and quantification of the CSP. This has allowed us to establish the reproducible digestion protocol for CSP analysis that we describe here.
Materials
Analysis of the Cardiac Side Population
C57BL/10 and 129S1/SvIMJ mice have been studied using this technique and gave comparable results1.
PBS: Phosphate-Buffered Saline (Cat No. 10010–015 Gibco, Invitrogen).
HBSS+: Hanks balanced Salt solution (HBSS, Cat No. 14025050, Gibco Invitrogen), Supplemented with Penicillin/streptomycin (Cat No. 10378–016, Gibco Invitrogen), and 2 % FBS (Cat No. 12003C, Sigma).
GentleMACS Dissociator (Cat No. 130-093-235, Miltenyi Biotec), gentleMACS C Tubes (Cat No. 130-093-237, Miltenyi Biotec), MACSmix™ Tube Rotator spinning at 12 rpm (Cat No. 130-090-753, Miltenyi Biotec) or alternative rotator with an rpm of approximately 12 rpm2.
Pronase: Dissolved in HBSS+ to a concentration of 100 mg/ml for 10X stock (Cat No. 537088-100KU, Merck-Millipore).
ACK Red Blood cell lysing buffer (Ammonium-Chloride-Potassium, Cat No. A10492-01 Gibco, Invitrogen).
Hoechst 33342 powder: (Cat. No. B2261, Sigma) is dissolved in H20 to 1 mg/ml.
Fumitremorgin C: (FTC) (Cat. No. alx-350-127-c250 Axxora).
Dissection tools, scissors and forceps.
Syringes (Cat No. 300188 BD Plastipak) and 23-gauge needles (Cat No. MD300700, BD)
70 μM Cell strainers (Cat No 352350, BD Falcon).
10 cm Petri dishes.
Tissue culture incubator at 37 °C.
Propidum Iodide (PI) (Cat No. P4170, Sigma) dissolved in PBS to 1 μg/ml 200X stock kept at 4 °C in the dark.
Flow/sorting equipment with UV laser capable of excitation at 350 nm and detection with 450/20 and 675LP optical filters.
Methods
Cardiac Cell Harvesting
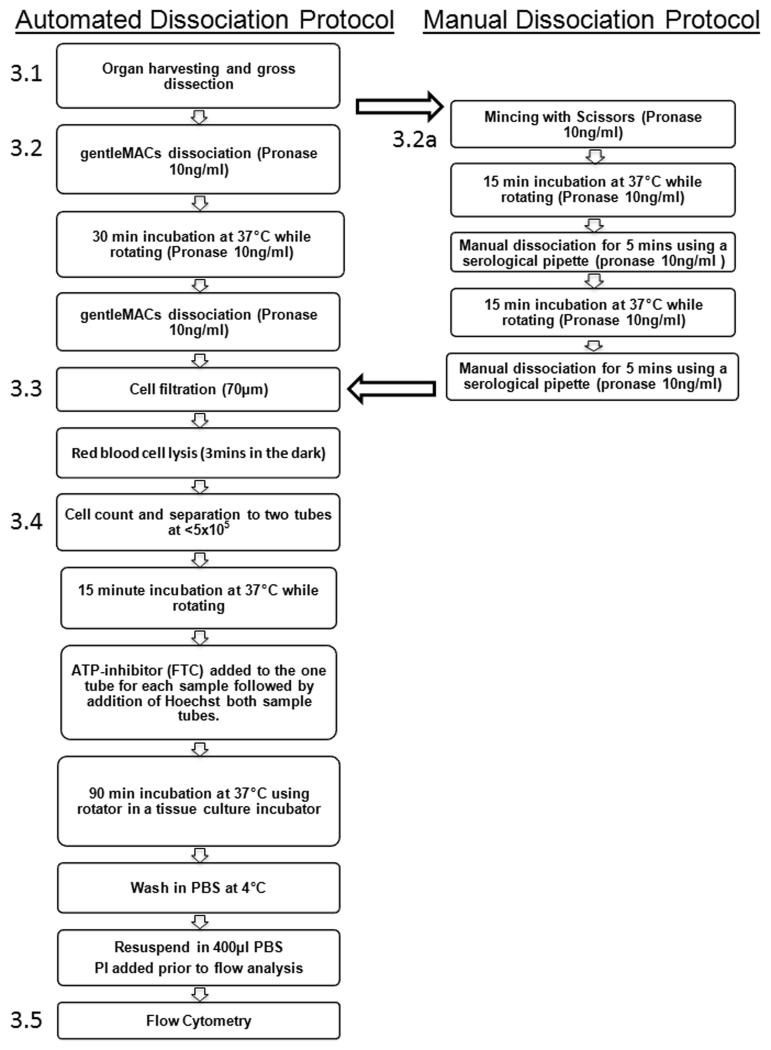
An important step in the analysis of SP cells from solid organs cells is the preparation of viable single cell suspension. CSP analysis has previously been performed following dissociation with both pronase and collagenase II/DNase I in association with various mechanical dissociation techniques [29–31]. To establish a standardised protocol for CSP analysis, and to reduce the variability that maybe associated with manual tissue dissociation, we utilised the MACS dissociation system and compared two combinations of enzymes3. We have established that Pronase digestion resulted in a more efficient dissociation, a more discrete CSP profile and fewer false positives than collagenase II/DNase I. We therefore describe this protocol in this section. As the use of the MACS dissociation system may be unavailable to some groups we have also described previously established manual dissociation techniques in detail. A flow diagram of both procedures is shown in Fig. 1.
Before starting dilute pronase to working concentration (10 mg/ml) with HBSS+ and warm HBSS+ and working concentration pronase to 37 °C in water bath.
Euthanize animals using Home Office approved methods. Open the rib cage to reveal the heart and using fine forceps and fine scissors make a small incision in the right atrium. Using a syringe and bent 23-gauge needle inject 5 ml of PBS (4 °C) into the left atrium. Immediately remove hearts and place in 5 ml PBS 4 °C.
Place hearts in separate 10 cm Petri dishes and dissected into quarters using dissection scissors. Wash three times in 10 ml of PBS (4 °C).
Fig. 1.
Flow diagram showing the key steps for both the automated and manual dissociation protocol for CSP analysis
Automated Dissociation
-
4.
Place 5 ml of pre-warmed pronase (10 mg/ml) into a gentleMACs C tube and use forceps to move the dissected hearts from the Petri dish to the gentleMACs C tube.
-
5.
Place the gentleMACs C tube into the gentleMACS dissociator and dissociate using the preloaded program m_heart_01.
-
6.
Place gentleMACs C tube into a MACSmix™ Tube Rotator or alternative rotation system2 (spinning at approximately 12 rpm) and place in an incubator at 37 °C for 30 min.
-
7.
Following incubation place gentleMACs C tubes into the gentleMACS dissociator and process using preloaded program m_heart_02.
-
8.
Immediately add 10 ml of PBS to the gentleMACs C tubes and spin in a centrifuge at 400 × g (4 °C) for 10 min. Discard the supernatant and resuspend cells in 10 mls of PBS (4 °C).
Manual Dissociation4
-
4a.
Following the gross dissection of the heart place the heart into a 35 ml dish containing 500 μl of the pre-warmed pronase solution.
-
5a.
Using fine scissors mince the sample for 5mins. Following the mincing step add the remaining pronase (4.5 ml) and transfer to a 50 ml falcon tube.
-
6a.
Place falcon tubes into a MACSmix™ Tube Rotator (12 rpm) or alternative rotation system2 place in an incubator at 37 °C for 15 min
-
7a.
Remove tube from the rotator and using a 10 ml serological pipette, pipette up and down to dissociate the tissue for 30 s. Place the tube back into the rotator and incubate at 37 °C for a further 15 min
-
8a.
Using a 5 ml serological pipette, pipette up and down for 5 mins. A P1000 pipette can be used if required to dissociate the remaining tissue.
Both Protocols Red Blood Cell Lysis
-
9.
Filter cells through a 70 μm cell filter and spin in a centrifuge at 400 × g at 4 °C for 10 min and discard the supernatant.
-
10.
To prevent erythrocyte induced changes in Hoechst equilibration, red blood cells must be removed using osmotic lysis with ACK buffer. The cells are therefore resuspended in 5 ml of ACK buffer and placed on ice in the dark for 3 min.
-
11.
Following red blood cells lysis add 10 ml of pre warmed HBSS+ to dilute ACK buffer and centrifugation 400 × g for 10 min.
-
12.
Resuspend cells in 1 ml of pre-warmed HBSS+ (37 °C).
Hoechst 33342 Staining and ABC-Transporter Inhibition
To accurately quantify and compare SP populations control experiments are required in which the ABC-transporters responsible for Hoechst efflux are inhibited. This procedure is used to set the SP gate and therefore allows discrimination of the SP from the main population. To ascertain the most suitable ABC-transporter inhibitor for CSP analysis in adult hearts we compared the effect of Verapamil (VP) and FTC5. Additionally an optimal concentration of Hoechst 33342 needs to be established for CSP analysis. We have therefore titred the Hoechst dye concentration required for CSP analysis at a concentration of 5×105 cells per ml6. We have established that a concentration of 5ug/ml of Hoechst 33342 and the use of the Abcg2 inhibitor FTC (10 μm) are optimal for CSP analysis and therefore describe these in this protocol.
Using a haemocytometer perform a cell count and place cells into two separated eppendorfs. Using HBSS+ (37 °C) adjust the cell concentration ≤5×105 cells/ml per tube in a final volume of 1 ml. Label one of the paired tubes with “inhibitor” and one tube “SP”.
Seal the eppendorfs into a 50 ml falcon tube (each tube holds around 6 tubes) and place falcon tubes onto the MACSmix™ Tube Rotator. Start the rotator and place in incubator for 15 min7. Alternatively any rotator with an rpm of approximately 12 rpm can be used2.
Remove Falcon tubes from the MACSmix™ Tube Rotator and remove the eppendorfs from the tubes. To each tube labelled “inhibitor” add 5ul of stock FTC (final conc. 10 μM).
Once FTC has been added to all the inhibitor tubes, add 5ul of stock Hoechst 33342 dye (finial conc. 5 μg/ml) first to each SP labelled tube and then to the tubes to which FTC has been added8.
Place eppendorfs back into the 50 ml falcon tubes, place falcon tubes back into the Rotator and incubate in at 37 °C for precisely 90 min9.
For each sample place 10 ml of PBS (4 °C) into two 15 ml falcon tubes label one with “inhibitor” and one “SP” and place on ice.
Following incubation immediately place the entire sample (1 ml) into a 15 ml falcon tube prepared in step 610. Centrifuge tubes at 400 × g for 10 min discard the supernatant and re-suspended cells in 400 μl of PBS (4 °C), place on ice and cover with foil until flow cytometric analysis.
Cardiac Side Population Analysis
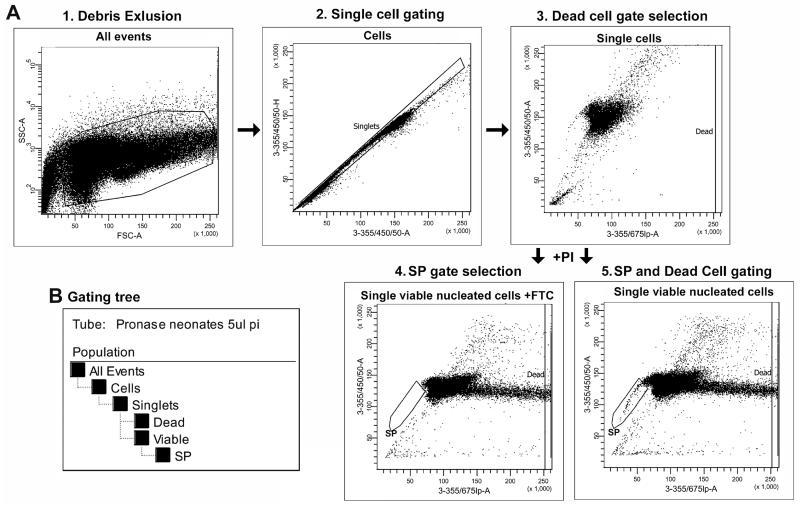
For analysis cytometers equipped with 355 nm UV to detect Hoechst red/Hoechst blue and 488 nm argon lasers to detect PI and 670 LP nm and 450/50 nm filters are required. In this study a LSRII flow cytometer (BD) was used. The gating strategies used for CSP analysis are detailed in Fig. 2.
First a plot describing Forward Scatter (FSC, related to cell size) and Side scatter (SSC, related to cell granularity) is created to allow the discrimination of cell from debris (Fig. 2A1).
A plot allowing the discrimination of single cells from cell aggregates and doublets is then created (Fig. 2A2). This is achieved by plotting Hoechst blue area (3-355/405/50-A) versus height (3-355/405/50-H) dot plot11.
Finally a plot that allows both analysis of the CSP and the discrimination of dead cells is created using Hoechst blue area (3-355/405/50-A) vs Hoechst red area (3-355/675lp-A). In the first series of experiments a small amount of samples should be analysed before the addition of PI (Fig. 2A3). This allows a gate for the exclusion of dead cell to be established. Dead cells are recognized by their strong positivity for PI on the (3-355/675lp-A) axis.
Immediately prior to analysis PI (5 ng/ml) is added to the cell suspension and the sample analysed. Between 100,000 and 500,000 cells should be collected for analysis. Following data acquisition the procedures detailed in Fig. 2 should be used to discard cells not in the singlet gate and dead cells from the analysis. The appropriate gating tree is shown in Fig. 2B.
The FTC inhibited sample should be used to establish the SP gate (Fig. 2A4).
The SP cells are recognized as a dim tail extending first on the left side of G0/G1 cells toward the lower “Hoechst Blue” signal as a proportion of live cells established (Fig. 2A5). The CSP at 18 weeks in the CJ57/Bl10 strain is approximately 0.9 % of viable single cells (Fig. 3 and 4).
Fig. 2.
Gating Strategy for SP Data Analysis. Example of a step-by-step gating strategy, example shows pronase digestion, 5 mg/ml Hoechst labelling and 10 μM FTC inhibition. a Gating strategy. 1. Based on Forward Scatter (FSC, related to the cell size) and Side Scatter (SSC, related to cell granularity) cells are distinguished from debris. 2. A gate is used to assure that only single cells are included in subsequent analysis. Single cells are distinguished from cell doublets and aggregates based on Hoechst labelling and their properties displayed on the Hoechst blue area (3-355/405/50-A) versus height (3-355/405/50-H) dot plot. 3. To set the gating for dead cell exclusion samples are analysed a Hoechst blue area (3-355/405/50-A) vs Hoechst red area (3-355/675lp-A) dot plot before the addition of PI. 4 and 5. Following the addition of Propidium Iodide samples with and without the addition inhibitor (FTC or VP) are analysed for Hoechst blue area (3-355/405/50-A) vs Hoechst red area (3-355/675lp-A) dot plot. Dead cells are recognized by their strong positivity for Propidium Iodide on the (3-355/675lp-A) axis. SP cells are recognized as a dim tail extending first on the left side of G0/G1 cells toward the lower “Hoechst Blue” signal. 4. b Gating tree. The gating tree indicates the sequential procedure applied to select out the final population for CSP
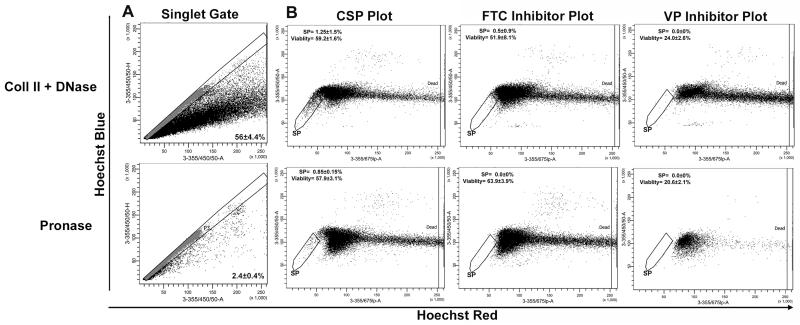
Fig. 3.
Comparison of collagenase II/DNase I and pronase enzymatic digestion and assessment of CSP inhibition by the ABC-transporter inhibitors FTC and Verapamil. a Following either the collagenase II/DNase I or pronase digestion protocols the efficiency of the digestion was assessed by establishing a “singlet” gate and the number of cell doublets and aggregates quantified. b For CSP analysis, following digestion and Hoechst labelling (5ug/ml), single cells were gated based on their properties displayed on the Hoechst blue area (3-355/405/50-A) versus height (3-355/405/50-H) dot plot. CSP cells are recognized as a dim tail extending first on the left side of the main population of cells toward the lower “Hoechst Blue” signal (SP gate). The Hoechst Blue vs Hoechst red plots were also used to assess the ability of FTC (10 μM) or VP (50 mM) to inhibit the CSP (as identified by an absence of events in the SP gate) and their effects on cell viability. Viable cells are defined by exclusion of dead cells as recognized by their strong positivity for Propidium Iodide on the 3-355/675lp-A axis. Data represents the mean±SD of a least three individual experiments
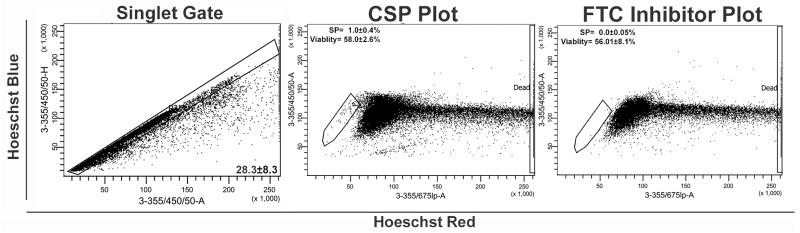
Fig. 4.
Analysis of digestion efficiency, cell viability and the CSP profile using manual dissociation techniques. Following manual dissociation protocols the efficiency of the digestion was assessed by establishing a “singlet” gate and the number of cell doublets and aggregates quantified. For CSP analysis, following digestion and Hoechst labelling (5ug/ml), single cells were gated based on their properties displayed on the Hoechst blue area (3-355/405/50-A) versus height (3-355/405/50-H) dot plot. CSP cells are recognized as a dim tail extending first on the left side of the main population of cells toward the lower “Hoechst Blue” signal (SP gate). The Hoechst Blue vs Hoechst red plots were also used to assess the ability of FTC (10 μM) to inhibit the CSP (as identified by an absence of events in the SP gate) and the effects on cell viability. Viable cells are defined by exclusion of dead cells as recognized by their strong positivity for Propidium Iodide on the 3-355/675lp-A axis. Data represents the mean±SD of a least three individual experiments
Discussion
Recently the CSP has gained increased attention as a key player in cardiac regeneration as reviewed in [32]. However small changes in tissue preparation including tissue digestion protocols, Hoechst staining and the ABC-transporter inhibitor used can lead to large variability in quantification of such a rare population [28, 33]. In this study we have provided detailed protocols for CSP analysis using an established manual dissociation protocol we have previously used to isolate CSP cells from both human and murine cardiac tissue [13, 29]. Further, we have adapted and optimised this protocol in order to establish an automated digestion protocol using the commercially available MAC dissociator. The automated dissociation protocol increases the speed at which cardiac tissue can be processed compared with manually mincing the tissue. This enables a reduction in the time to final analysis and also limits variability due to differences between the technical skills of those manually processing tissue. In laboratories where there is a high need for tissue dissociation the cost of the tissue dissociator can be offset by the cost of time taken by laboratory staff to manually dissociate tissues as the automated dissociation takes seconds per heart. In terms of other improvements our data suggests that in comparison to manual dissociation the tissue dissociator increases the yield of single cells while reducing the number of cell aggregates seen with manual dissociation. As other protocols for CSP cells isolation published to date have relied on manual dissociation and CSP cells are rare cells improvements even if relatively small in cell yields will be of value. Also a reduction in the number of cell aggregates reduces the risk of problems associated with FACS analysis such a clogging of the sort nozzle due to poorly disaggregated cell suspensions.
Previously studies have used a number of different digestion protocols to obtain the single cell preparations for CSP and regardless of the protocol used the CSP has demonstrated cardiogenic potential. However, it is well documented that different enzymes have differing levels of cytotoxicity [34, 35]. We have therefore used the automated dissociation protocol to compare two enzymatic digestions collagenase II/DNase I and pronase which have previously been used for CSP preparation [3, 6, 13, 24–26]. We have demonstrated the use of pronase together with tissue dissociation allows a reproducible digestion of murine hearts into a single cell suspension with constituent levels of cell viability. This system results in reduced cell death and the absence of false positive cells with a SP phenotype, which remain present following ABC-transporter inhibition.
In the adult murine heart there remains debate regarding the principle ABC-transporter that is responsible for Hoechst 33342 efflux. Pfister et al. [36] has demonstrated that Abcg2 is required for the CSP phenotype in the neonatal heart where as Mdr1 (abcg1) is responsible for at least a proportion of the CSP phenotype in the adult. Therefore which ABC-transporter inhibitor is most appropriate for the analysis of the CSP is brought into question. FTC has been reported to be a specific inhibitor of ABCG2 [37, 38], while VP is a calcium-channel blocker which has the potential to inhibit multiple members of the ABC family of transporters [39]. In the current study VP had the ability to inhibit the efflux ability of CSP of adult hearts, albeit with high levels of cytotoxicity whereas FTC was able to completely inhibit the efflux potential of the CSP from hearts of animals of the same age and gender as those in the VP treated group, while maintaining cell viability.
The ability to discriminate SP cells is also affected by the concentration of Hoechst 33342 dye employed [28]. Concentrations of Hoechst that are too low can result in cells which have not been saturated with Hoechst being mistaken for SP cells, in contrast concentrations too high can result in decreased viability or a reduced SP due to toxicity (Fig. 5) as described previously [33]. In the heart different studies have employed Hoechst dye concentrations ranging from 1.25 μg/ml to 6 μg/ml. Such studies did not describe the titration process for optimising dye concentration [3, 6, 13, 24–26]. Further, duration of incubation in these studies has been variable. Given that greater exposure to the Hoechst dye results in greater toxicity, identifying the shortest incubation time which results in optimal SP discrimination is valuable. This study demonstrates that the use of pronase and automated digestion together with Hoechst 33342 at a concentration of 5ug/ml for 90 min at 37 °C leads to optimal CSP FACS profile resolution whilst maintaining maximum cell viability. We also demonstrate that the use of ABCG2 transporter inhibitor FTC reliably confirms the CSP phenotype. This allows for a reproducible method for analyses of murine CSP, enabling more detailed and accurate comparisons of changes in this resident cardiac stem cell population under pathological conditions.
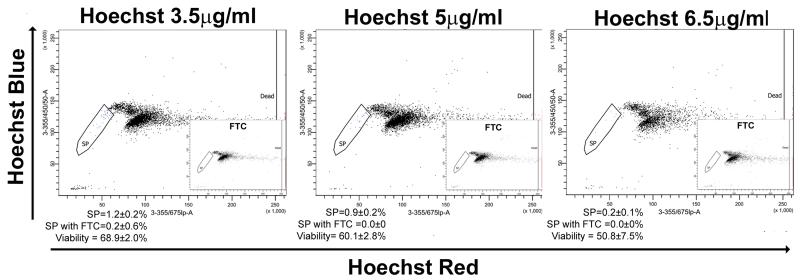
Fig. 5.
Hoechst titration to establish the optimal labelling protocol for CSP analysis. Following singlet gating the Hoechst blue vs Hoechst red plots were used to analyse CSP phenotype following labelling with different concentrations of Hoechst. FTC was used to confirm the SP cell phenotype. To quantify viability, dead cells are recognized by their strong positivity for the dead cell discrimination marker Propidium Iodide on the 3-355/675lp-A axis. Data represents the mean±SD of a least three individual experiments
Acknowledgments
Sources of Funding This work was supported by Newcastle University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- FACS
Fluorescence-activated cell sorting
- SP
Side population
- CSP
Cardiac side population
- FTC
Fumitremorgin C
Footnotes
Disclosures None.
Conflict of Interest The authors indicate no potential conflicts of interest.
Adult (18 week) C57BL/10 mice were used for comparison of digestion techniques, Abcg2 inhibitors comparison and Hoechst 33342 titration in this study.
We have previously used a pre-warmed (37 °C) hybridisation oven spinning at approximately 12 rpm.
We compared two alternate enzyme blends which have previously been described for CSP analysis; Collagenase II/DNase I was chosen specifically as it has been previously reported to be less traumatic to cells than trypsin[40]. Pronase was investigated as it was previously reported to be less cytotoxic than collagenase II[41]. No significant difference in cell viability was observed between the two digestion protocols (57.9±3.1 vs. 59.2±1.6). However, the use of pronase resulted in a more efficient dissociation than collagenase II/DNase I (Fig. 3). Furthermore, following pronase digestion, the CSP appeared as a more discrete population with less deviation between individual samples (0.85±0.15 % vs 1.25±1.5 %) (Fig. 3). Additionally following pronase digestion no false positive CSP cells were identified, as determined by the absence of cells with a CSP phenotype in the FTC inhibited sample. This is in contrast to the analysis following collagenase II/DNase I digestion where 0.5±0.9 % of cells were present in the CSP gate following FTC inhibition.
Examples of flow cytometry plots demonstrating singlet gating, CSP identification and FTC inhibition of the CSP, using the manual dissociation protocol, are shown in Fig. 4. As expected no difference in relative CSP percentage was observed between the manual and automated dissociation protocols. Further, viability was not significantly different. When using the manual protocol our laboratory obtained an approximately 10 % reduction in cell yield and a 12-fold increase in the number of incompletely dissociated (doublet or cells aggregates) cells.
In the adult murine heart there remains debate regarding the principle ABC-transporter that is responsible for Hoechst 33342 efflux. Pfister et al.[36] have demonstrated that Abcg2 is required for the CSP phenotype in the neonatal heart whereas Mdr1 (abcg1) is responsible for at least a proportion of the CSP phenotype in the adult. Therefore which ABC-transporter inhibitor is most appropriate for the analysis of the CSP is brought into question. FTC has been reported to be a specific inhibitor of ABCG2[37, 38], while VP is a calcium-channel blocker which has the potential to inhibit multiple members of the ABC family of transporters[39]. To ascertain the most suitable ABC-transporter inhibitor for CSP analysis in adult hearts we compared the effect of VP (50 μm) and FTC (10 μM) using both pronase and collagenase II/DNase I protocols. Each sample preparation was separated into three tubes (at 5×105 cells per ml); two of the individual tubes were either incubated with FTC or VP prior to labelling with 5 μg/ml Hoechst. The remaining tube was labelled with 5 μg/ml Hoechst without the addition of an inhibitor. Both VP and FTC demonstrated the ability to inhibit the CSP phenotype (Fig. 3) when combined with pronase digestion. FTC demonstrated a complete inhibition and was less toxic, regardless of the digestion protocol used, compared to VP (Fig. 3). Reduced VP concentrations (40 μm) resulted in an incomplete CSP inhibition but remained more toxic than FTC (data not shown).
To establish the optimal concentration of Hoechst 33342 for CSP analysis we titrated the Hoechst dye concentration using cells at a concentration of 5×105 cells per ml. Samples were separated into individual tubes containing 5×105 cells per ml and incubated with 3.5, 5 or 6.5 μg/ml of Hoechst for precisely 90 min. A concentration of 5 μg/ml gave the optimal SP profile balancing resolution and viability (Fig. 5). These data gave a high reproducibility in CSP percentages in the C57Bl/6 mouse strain. However, optimization of the Hoechst 33342 concentrations may be required using other murine strains.
The temperature and time in contact with Hoechst 33342 is critical; therefore pre-incubation allows the samples time to equilibrate to the optimum temperature of 37 °C before Hoechst 33342 is added.
Adding the Hoechst 33342 to the “SP” tubes first (without Abcg2 inhibitor) allows time for the FTC to inhibit Abcg2 function in the “Inhibited” tubes before the addition of Hoechst 33342.
It is critical that the sample remains at a constant 37 °C throughout the staining incubation. The door the incubator should not be opened during this step of the protocol.
Following incubation the Hoechst 33342 efflux should be stopped immediately by quickly cooling samples to 4 °C. Therefore it is very important to use PBS at 4 °C at this step.
As only singlet cells should be analysed for CSP, it is important to eliminate doublet cells and aggregates from further analysis. Doublets, i.e. single cells that pass through the laser simultaneously, rather than one by one, are distinguished by the disproportionate laser generated area signal (i.e. the sum of total fluorescence analysed) to the height signal (i.e. the maximum fluorescence intensity of the cell). In the case of a single cell the area and height signal, after correct adjustment for digital area scaling factor, should give the same channel value when viewed on an Area versus height do plot, however in the case of a doublet the area signal becomes proportionally larger with reference to the degree of doublet detection as demonstrated in Figs. 2A2 and 4.
Contributor Information
Annette Meeson, Institute of Genetic Medicine, International Centre for Life, Newcastle University, Newcastle upon Tyne NE1 3BZ, UK.
Andrew Fuller, Institute of Genetic Medicine, International Centre for Life, Newcastle University, Newcastle upon Tyne NE1 3BZ, UK.
David T. Breault, Division of Endocrinology, Children’s Hospital Boston, Harvard Medical School, Boston, MA 02115, USA; Harvard Stem Cell Institute, Cambridge, MA 02138, USA
W. Andrew Owens, Institute of Genetic Medicine, International Centre for Life, Newcastle University, Newcastle upon Tyne NE1 3BZ, UK; Department of Cardiothoracic Surgery, South Tees Hospitals NHS Foundation Trust, Middlesbrough TS4 3BW, UK.
Gavin D. Richardson, Institute of Genetic Medicine, International Centre for Life, Newcastle University, Newcastle upon Tyne NE1 3BZ, UK
References
- 1.Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. The New England Journal of Medicine. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 2.Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proceedings of the National Academy of Science of the United States of America. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfister O, Mouquet F, Jain M, et al. CD31− but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circulation Research. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 4.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 5.Matsuura K, Nagai T, Nishigaki N, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. Journal of Biological Chemistry. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 6.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proceedings of the National Academy of Science of the United States of America. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tateishi K, Ashihara E, Takehara N, et al. Clonally amplified cardiac stem cells are regulated by Sca-1 signaling for efficient cardiovascular regeneration. Journal of Cell Science. 2007;120:1791–1800. doi: 10.1242/jcs.006122. [DOI] [PubMed] [Google Scholar]
- 8.Urbanek K, Torella D, Sheikh F, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proceedings of the National Academy of Science of the United States of America. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aghila Rani KG, Jayakumar K, Srinivas G, Nair RR, Kartha CC. Isolation of ckit-positive cardiosphere-forming cells from human atrial biopsy. Asian Cardiovascular & Thoracic Annals. 2008;16:50–56. doi: 10.1177/021849230801600113. [DOI] [PubMed] [Google Scholar]
- 10.Davis DR, Kizana E, Terrovitis J, et al. Isolation and expansion of functionally-competent cardiac progenitor cells directly from heart biopsies. Journal of Molecular and Cellular Cardiology. 2010;49(2):312–21. doi: 10.1016/j.yjmcc.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circulation Research. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto S, Kawaguchi N, Ellison GM, Matsuoka R, Shin’oka T, Kurosawa H. Characterization of long-term cultured c-kit+ cardiac stem cells derived from adult rat hearts. Stem Cells and Development. 2009;19(1):105–16. doi: 10.1089/scd.2009.0041. [DOI] [PubMed] [Google Scholar]
- 13.Martin CM, Meeson AP, Robertson SM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Developmental Biology. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Alvi AJ, Clayton H, Joshi C, et al. Functional and molecular characterisation of mammary side population cells. Breast Cancer Research. 2003;5:R1–R8. doi: 10.1186/bcr547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. The Journal of Cell Biology. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inowa T, Hishikawa K, Takeuchi T, Kitamura T, Fujita T. Isolation and potential existence of side population cells in adult human kidney. International Journal of Urology. 2008;15:272–274. doi: 10.1111/j.1442-2042.2007.01984.x. [DOI] [PubMed] [Google Scholar]
- 17.Lapan AD, Rozkalne A, Gussoni E. Human fetal skeletal muscle contains a myogenic side population that expresses the melanoma cell-adhesion molecule. Human Molecular Genetics. 2012;21(16):3668–80. doi: 10.1093/hmg/dds196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majka SM, Beutz MA, Hagen M, Izzo AA, Voelkel N, Helm KM. Identification of novel resident pulmonary stem cells: form and function of the lung side population. Stem Cells. 2005;23:1073–1081. doi: 10.1634/stemcells.2005-0039. [DOI] [PubMed] [Google Scholar]
- 19.Motohashi N, Uezumi A, Yada E, et al. Muscle CD31(−) CD45(−) side population cells promote muscle regeneration by stimulating proliferation and migration of myoblasts. American Journal of Pathology. 2008;173:781–791. doi: 10.2353/ajpath.2008.070902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muskiewicz KR, Frank NY, Flint AF, Gussoni E. Myogenic potential of muscle side and main population cells after intravenous injection into sub-lethally irradiated mdx mice. Journal of Histochemistry and Cytochemistry. 2005;53:861–873. doi: 10.1369/jhc.4A6573.2005. [DOI] [PubMed] [Google Scholar]
- 21.Scaldaferri ML, Fera S, Grisanti L, et al. Identification of side population cells in mouse primordial germ cells and prenatal testis. Internatonal Journal of Development Biology. 2011;55(2):209–14. doi: 10.1387/ijdb.092977ms. [DOI] [PubMed] [Google Scholar]
- 22.Smalley MJ, Titley I, Ashworth A. An improved definition of mouse mammary epithelial side population cells. Cytotherapy. 2005;7:497–508. doi: 10.1080/14653240500361145. [DOI] [PubMed] [Google Scholar]
- 23.Yano S, Ito Y, Fujimoto M, Hamazaki TS, Tamaki K, Okochi H. Characterization and localization of side population cells in mouse skin. Stem Cells. 2005;23:834–841. doi: 10.1634/stemcells.2004-0226. [DOI] [PubMed] [Google Scholar]
- 24.Tomita Y, Matsumura K, Wakamatsu Y, et al. Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. The Journal of Cell Biology. 2005;170:1135–1146. doi: 10.1083/jcb.200504061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oyama T, Nagai T, Wada H, et al. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. The Journal of Cell Biology. 2007;176:329–341. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Letters. 2002;530:239–243. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 27.Pfister O, Oikonomopoulos A, Sereti KI, Liao R. Isolation of resident cardiac progenitor cells by Hoechst 33342 staining. Methods in Molecular Biology. 2010;660:53–63. doi: 10.1007/978-1-60761-705-1_4. [DOI] [PubMed] [Google Scholar]
- 28.Golebiewska A, Brons NH, Bjerkvig R, Niclou SP. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8(2):136–47. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Alfakir M, Dawe N, Eyre R, et al. The temporal and spatial expression patterns of ABCG2 in the developing human heart. International Journal of Cardiology. 2010;156(2):133–8. doi: 10.1016/j.ijcard.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hackett TL, Shaheen F, Johnson A, et al. Characterization of side population cells from human airway epithelium. Stem Cells. 2008;26:2576–2585. doi: 10.1634/stemcells.2008-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wulf GG, Luo KL, Jackson KA, Brenner MK, Goodell MA. Cells of the hepatic side population contribute to liver regeneration and can be replenished with bone marrow stem cells. Haematologica. 2003;88:368–378. [PubMed] [Google Scholar]
- 32.Unno K, Jain M, Liao R. Cardiac side population cells: moving toward the center stage in cardiac regeneration. Circulation Research. 2012;110(10):1355–63. doi: 10.1161/CIRCRESAHA.111.243014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montanaro F, Liadaki K, Schienda J, Flint A, Gussoni E, Kunkel LM. Demystifying SP cell purification: viability, yield, and phenotype are defined by isolation parameters. Experimental Cell Research. 2004;298:144–154. doi: 10.1016/j.yexcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Iglesias I, Valiente L, Shiang KD, Ichii H, Kandeel F, Al-Abdullah IH. The effects of digestion enzymes on islet viability and cellular composition. Cell Transplantation. 2012 doi: 10.3727/096368911X623826. doi: 10.3727/096368911X623826. [DOI] [PubMed] [Google Scholar]
- 35.Fletcher AL, Malhotra D, Acton SE, et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Frontiers in Immunology. 2011;2:35. doi: 10.3389/fimmu.2011.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfister O, Oikonomopoulos A, Sereti KI, et al. Role of the ATP-binding cassette transporter Abcg2 in the phenotype and function of cardiac side population cells. Circulation Research. 2008;103:825–835. doi: 10.1161/CIRCRESAHA.108.174615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen JD, van Loevezijn A, Lakhai JM, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Molecular Cancer Therapeutics. 2002;1:417–425. [PubMed] [Google Scholar]
- 38.Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Research. 2000;60:47–50. [PubMed] [Google Scholar]
- 39.Yusa K, Tsuruo T. Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Research. 1989;49:5002–5006. [PubMed] [Google Scholar]
- 40.Kirkpatrick CJ, Melzner I, Goller T. Comparative effects of trypsin, collagenase and mechanical harvesting on cell membrane lipids studied in monolayer-cultured endothelial cells and a green monkey kidney cell line. Biochimica et Biophysica Acta. 1985;846:120–126. doi: 10.1016/0167-4889(85)90117-x. [DOI] [PubMed] [Google Scholar]
- 41.Watson JV. Introduction to flow cytometry. Cambridge University Press; 2004. [Google Scholar]