Abstract
The isoflavone calycosin-7-O-β-d-glucopyranoside (CG) is a principal constituent of Astragalus membranaceus (AR) and has been reported to inhibit osteoclast development in vitro and bone loss in vivo. The aim of this study was to investigate the osteogenic effects of CG and its underlying mechanism in ST2 cells. The results show that exposure of cells to CG in osteogenic differentiation medium increases ALP activity, osteocalcin (Ocal) mRNA expression and the osteoblastic mineralization process. Mechanistically, CG treatment increased the expression of bone morphogenetic protein 2 (BMP-2), p-Smad 1/5/8, β-catenin and Runx2, all of which are regulators of the BMP- or wingless-type MMTV integration site family (WNT)/β-catenin-signaling pathways. Moreover, the osteogenic effects of CG were inhibited by Noggin and DKK-1 which are classical inhibitors of the BMP and WNT/β-catenin-signaling pathways, respectively. Taken together, the results indicate that CG promotes the osteoblastic differentiation of ST2 cells through regulating the BMP/WNT signaling pathways. On this basis, CG may be a useful lead compound for improving the treatment of bone-decreasing diseases and enhancing bone regeneration.
Abbreviations: ALP, alkaline phosphatase; AR, Astragalus membranaceus; BMP, bone morphogenetic protein; CG, calycosin-7-O-β-d-glucopyranoside; DKK-1, dickkopf-1; ECL, enhanced chemiluminescence; FGF, fibroblast growth factor; HAase, hyaluronidase; IGF1, insulin-like growth factor 1; MAPK, mitogen-activated protein kinase; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; OBM, osteogenic differentiation medium; Ocal, osteocalcin; OPN, osteopontin; OVX, ovariectomized; PVDF, polyvinylidine fluoride; TGF-β, transforming growth factor β; WNT, wingless-type MMTV integration site family
KEY WORDS: BMP signaling pathway, WNT/β-catenin signaling pathway, Osteoblastic differentiation, Calycosin-7-O-β-d-glucopyranoside, ST2 cells
Graphical abstract
The results indicate that calycosin-7-O-β-d-glucopyranoside (CG) promotes the osteoblastic differentiation of ST2 cells through regulating the BMP/WNT signaling pathways. On this basis, CG may be a useful lead compound for improving the treatment of bone-decreasing diseases and enhancing bone regeneration.
1. Introduction
Regulation of bone mass is controlled by continuous bone remodeling through osteoblastic bone formation and resorption. Disorders of bone remodeling are implicated in a variety of diseases such as osteoporosis, hypercalcemia and rheumatoid arthritis as well as tumor metastasis into bone1. Understanding osteoblastic differentiation is therefore crucial to improving the treatment of such disorders.
Osteoblasts are the main bone-forming cells arising from mesenchymal stem cells. They produce alkaline phosphatase (ALP) and bone matrix proteins such as osteocalcin (Ocal) and osteopontin (OPN) which act to induce osteoblastic mineralization2. Osteoblast differentiation is regulated by various signaling pathways involving bone morphogenetic proteins (BMPs), wingless-type MMTV integration site family (WNT)/β-catenin proteins, transforming growth factor-β (TGF-β), insulin-like growth factor-1 (IGF-1), fibroblast growth factor (FGF), and mitogen-activated protein kinase (MAPK)3, 4, 5. Of these pathways, the one involving BMPs is key in skeletal development, maintaining adult bone homeostasis and stimulating bone formation in fracture healing6. Activation of the WNT/β-catenin signaling pathway is essential for proper bone development7 and, in cooperation with the BMP signaling pathway, regulates osteoblast differentiation and bone formation8.
Astragalus membranaceus (AR) is one of the most important medicinal plants in traditional Chinese medicine. In recent years, it has received considerable attention because of its immunostimulant effects9, antibacterial and antiviral properties, hepatoprotective and antiinflammatory activity and beneficial cardiovascular effects10. Of particular interest is the observation that AR inhibits osteoclast development in vitro and bone loss in vivo in ovariectomized (OVX) rats11. Moreover, AR combined with calcium has been shown to significantly improve bone mineral density, biomechanical strength, and ash weight of the femur and tibia of OVX rats12. However, the main osteogenically active components of AR remain to be identified.
The isoflavone calycosin-7-O-β-d-glucopyranoside (CG, Fig. 1) is a principal constituent of AR. It is a strong inhibitor of hyaluronidase (HAase)13 and of matrix degradation caused by IL-1β or HAase in human articular cartilage explant and chondrocytes14. Since it is known that isoflavones are active in preventing osteoporosis15, 16, it is reasonable to hypothesize that CG may exhibit osteogenic effects. Accordingly this study aimed to investigate the osteogenic effects of CG and its role in the osteogenic differentiation of bone marrow stromal cell.
Figure 1.
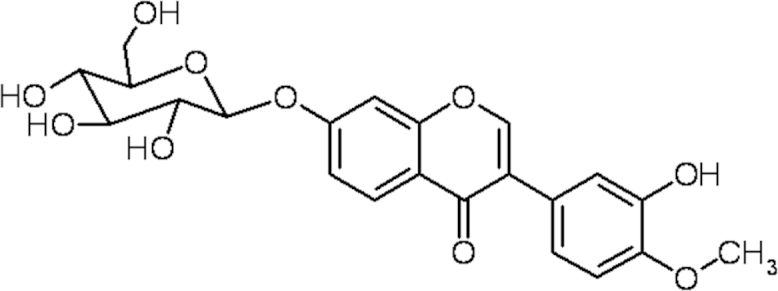
Chemical structure of calycosin-7-O-β-d-glucopyranoside (CG).
2. Materials and methods
2.1. Cell culture
Bone marrow stromal ST2 cells were seeded at a density of 1×105 cells/mL and cultured in regular growth culture medium containing α-minimum essential medium supplemented with 15% fetal bovine serum (Biochrom, Australia), 100 units/mL penicillin (Gibco, Australia) and 100 mg/L streptomycin (Gibco) in a humidified atmosphere of 5% CO2 at 37 °C. At 80% confluence, the cells were cultured in osteogenic differentiation medium (OBM) which consisted of the above culture medium containing 10 nmol/L dexamethasone (Sigma-Aldrich, USA), 10 mmol/L β-glycerophosphate (Sigma-Aldrich), 50 μg/mL ascorbic acid (Sinopharm Chemical Reagent, China) and various concentrations of CG (98.3%, National Institutes for Food and Drug Control, CAS 20633-67-4, China) added as a solution in dimethyl sulfoxide (Sigma-Aldrich, final concentration 0.1%).
2.2. Cell viability assay
Cell viability was assessed using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, Sigma-Aldrich) assay. Briefly, cells (1×104 cells/well in 96-well plates) were maintained in OBM at 37 °C for 24 h. Cells were then treated with CG (4, 8, 16 and 32 μmol/L) in OBM for 1, 3 and 6 days at which times 20 μL MTT (5 mg/mL) was added to each well and samples incubated in the dark at 37 °C. After 4 h, medium was discarded and the precipitated formazan dissolved in DMSO (150 μL/well). Absorbance was measured with a microplate reader (iMARKtm, BIO-RAD) at 570 nm. Cell viability in OBM (without CG) was used as control and designated as 100%.
2.3. ALP activity assay
After incubation with CG (4, 8, 16 and 32 μmol/L) in OBM for 3, 7 and 9 days, ST2 cells were collected and lysed with 0.1% Triton X-100. p-Nitrophenyl phosphate (pNPP, Sigma-Aldrich) was used as the substrate to measure the intracellular ALP activity. Briefly, 100 μL lysate supernatant was incubated with 100 μL substrate solution containing 3 mmol/L pNPP, 1 mol/L diethanolamine buffer and 0.5 mmol/L MgCl2 for 30 min at 37 °C. The reaction was stopped by adding 0.2 mol/L NaOH solution and absorbance determined using a microplate reader at 405 nm. Relative ALP activity was normalized to the protein concentration of each sample assayed using the BCA method and then to the control.
2.4. Alizarin red-S staining
After incubation of cells with CG (4, 8, 16 and 32 μmol/L) in OBM for 28 days, mineralized nodule formation was determined using Alizarin red-S (ARS)17. Briefly, cells were washed twice with PBS, fixed in ice-cold 70% ethanol for 1 h at room temperature and then stained with 40 mmol/L ARS solution (pH 4.2) at room temperature for 30 min. Images of the stained matrix were acquired using a digital camera. To quantify mineralization, stained cells were dissolved by adding 10% cetylpyridinium chloride for 1 h and transferred to a 96-well plate for measuring absorbance at 570 nm with a microplate reader.
2.5. RNA isolation and real-time PCR (RT-PCR)
After incubation of cells with CG (4, 8, 16 and 32 μmol/L) in OBM for 9 days, the cells were collected and total RNA extracted using TRIzol reagent (Invitrogen, USA). RNA of each sample was reverse transcribed to cDNA using SuperScript™ III Reverse Transcriptase (Invitrogen). cDNA was then amplified using GoTaq® DNA Polymerase (Promega, USA) and SYBR Green PCR Master Mix (Applied Biosystems, USA). The expression levels were quantified using a CFX Connect™ Real-Time System (BIO-RAD). The primers used for real-time PCR were as follows: Runx2: 5′-TGCTTCATTCGCCTCACAAA-3′ (sense) and 5′-TTGCAGTCTTCCTGGAGAAAGTT-3′ (antisense); Ocal: 5′-TGCTTGTGACGAGCTATCAG-3′ (sense) and 5′-TGAACTAGGAGGGACAGGAG-3′ (antisense); BMP2: 5′-TGAGGATTAGCAGGTCTTTG-3′ (sense) and 5′-CACAACCATGTCCTGATAAT-3′ (antisense); β-catenin: 5′-CCGTTCGCCTTCATTATGGA-3′ (sense) and 5′-CCTAACTAAGCTTTGGAACGG-3′ (antisense); GAPDH: 5′-CCGTTCGCCTTCATTATGGA-3′ (sense) and 5′-CCTAACTAAGCTTTGGAACGG-3′ (antisense). Values were normalized to that of GAPDH using the 2−ΔΔCT method.
2.6. Protein isolation and Western blotting
After incubation of cells with CG (8, 16 and 32 μmol/L) in OBM for 9 days, the cells were collected and lysed in RIPA buffer (20 mmol/L Tris-HCl, 200 mmol/L NaCl, 1% Triton X-100, 1 mmol/L dithiothreitol) containing 1% protease inhibitor (Roche). The concentration of protein was measured using a Protein Assay Kit (BIO-RAD). Total protein from each sample was separated by SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidine fluoride (PVDF) membrane. The blotting membrane was then incubated with primary antibodies of anti-Runx2, anti-β-catenin and anti-p-Smad1/5/8 (Santa Cruz Biotechnology). Subsequently, the blots were washed with TBST (10 mmol/L Tris-HCl, 50 mmol/L NaCl, 0.25% Tween 20) and incubated with HRP-conjugated secondary antibody. The blots were visualized with enhanced chemiluminescence (ECL) and exposed to photographic film. β-Actin was used as a loading control.
2.7. Osteogenetic analysis after co-treatment with CG and Noggin or Dickkopf (DKK-1)
After incubation of cells with 16 μmol/L CG and 0.5 μg/mL Noggin (Sigma-Aldrich) or 0.1 μg/mL DKK-1 (Peprotech, USA) in OBM for 9 days, cells were collected, lysed with 0.1% Triton X-100 and subjected to determination of ALP activity as mentioned above. In addition, cells were collected, lysed in RIPA buffer and protein expression of p-Smad1/5/8 and β-catenin determined by Western blotting.
2.8. Statistical analyses
Data were analyzed using SPSS 13.0 software. Values are expressed as mean±S.E.M. unless otherwise indicated. Data analysis was performed by one way analysis of variance (ANOVA) followed by Tukey׳s post hoc test. Differences for which P<0.05 were considered to be statistically significant.
3. Results and discussion
3.1. Effect of CG on ST2 cell viability
ST2 cells are a type of bone marrow stromal cell which can be differentiated into osteoblast-like cells in OBM18 by inducing the formation of a matrix of type I collagen and, through subsequently activating the BMP signaling pathway19, be stimulated to further differentiate into mature osteoblasts. Activation of WNT/β-catenin signaling induces differentiation of pluripotent mesenchymal cells into osteoblast progenitors. When these osteoprogenitors become osteoblasts, both the BMP and WNT/β-catenin pathways can promote further differentiation as evidenced by increased ALP activity and mineralization20, 21. On this basis, ST2 cells were used in the present study to investigate the osteogenic effect of CG. After incubation of ST2 cells in OBM with up to 32 μmol/L CG for up to 6 days, there was no evidence of cytotoxicity to the cells (Fig. 2). In addition, DMSO was not cytotoxic to ST2 cells at the final concentration of 0.1% (data not shown).
Figure 2.
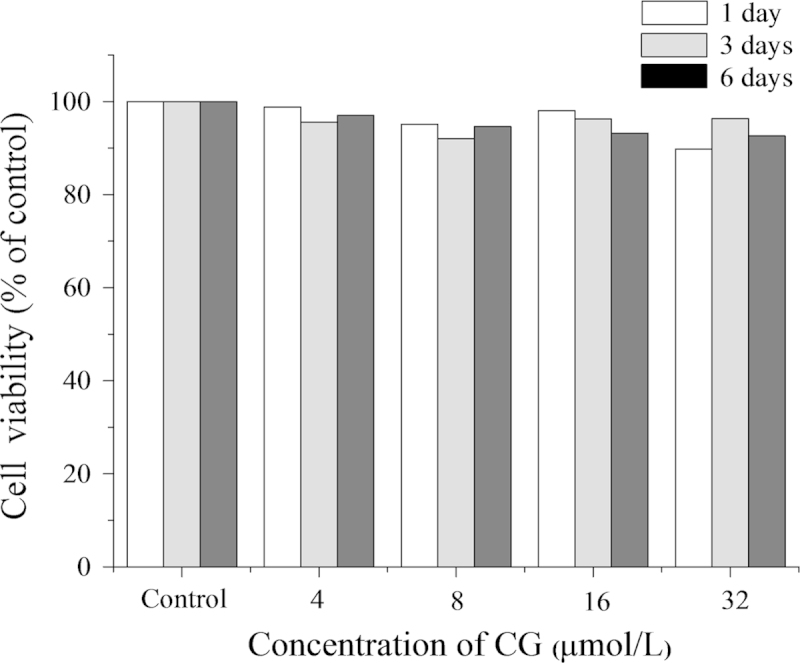
Effect of CG on the proliferation of ST2 cells. Cells were seeded in 96-well plates for 24 h and then treated with different concentrations of CG for 1, 3 and 6 days. Cellular proliferation was determined using the MTT assay. Results are expressed as means±SD (n=3).
3.2. CG promotes osteoblastic differentiation of ST2 cells
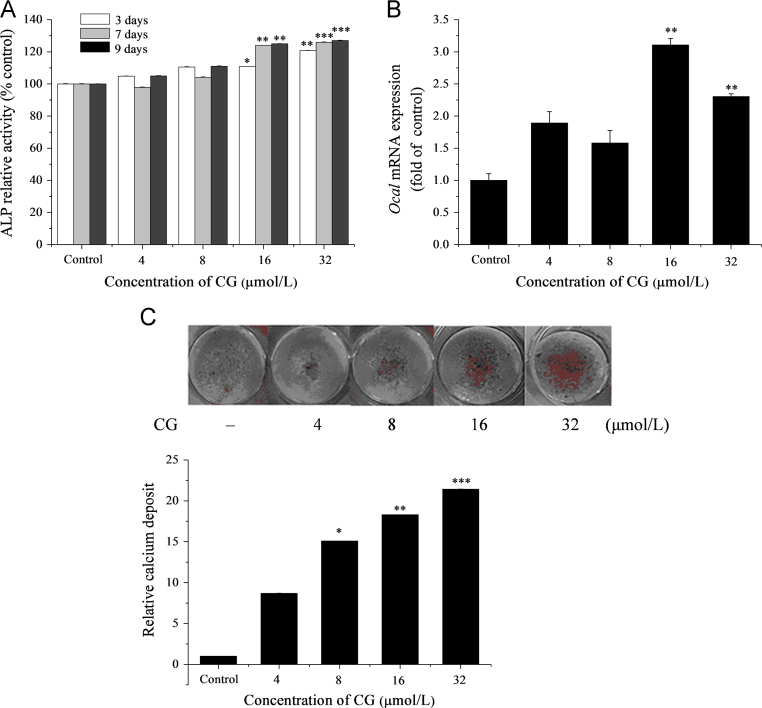
The process of bone formation is reported to first involve osteoblast proliferation followed by increased ALP activity. The latter is a well-recognized early marker of osteoblast differentiation, development and maturation of extracellular matrix leading ultimately to mineralization22. Our in vitro data show that exposure to increasing concentrations of CG for 3, 7 and 9 days caused increases in ALP activity which was significant at 16 μmol/L and more significant at 32 μmol/L (Fig. 3A). Expression of mRNA by the osteogenic marker gene Ocal was also increased by treatment with 16 and 32 μmol/L CG for 9 days (Fig. 3B). In addition, exposure to CG treatment for 28 days caused a significant increase in mineralized nodule formation (a well-recognized late marker of osteoblast differentiation) at concentrations >8 μmol/L (Fig. 3C). These indicators of bone-formation appear to have little correlation with stimulation of cellular proliferation as the growth rate of ST2 cells slowed only slightly with exposure to increasing CG concentrations (Fig. 2).
Figure 3.
Effect of CG on osteoblast differentiation in ST2 cells as indicated by: (A) ALP activity where cells were cultured in OBM as described in Section 2 and treated with different concentrations of CG for 3, 7 and 9 days prior to determination of ALP activity; (B) expression of the osteoblast marker gene Ocal after exposure to CG at the indicated concentrations for 9 days. mRNA was determined by real-time PCR analysis; and (C) osteoblastic mineralization. Cells were cultured in OBM and treated with CG for 28 days after which mineralization deposits were identified by Alizarin red S staining. *P<0.05, **P<0.01, ***P<0.001 versus control.
3.3. Mechanism of CG-induced osteogenesis
The BMP pathway is one of the main signaling cascades that stimulate bone formation. The mechanism of receptor activation involves BMP-induced phosphorylation of two sequentially activated kinases, with the type I receptor acting as a substrate for the type II receptor kinase. The activated type I receptor relays the signal to the cytoplasm by phosphorylating its downstream target, Smad1/5/8 protein, which then interacts with Smad4 and translocates into the nucleus23. A number of compounds have been found to affect this pathway by increasing the expression of BMPs and/or activating the downstream signaling pathway24, 25, 26, 27.
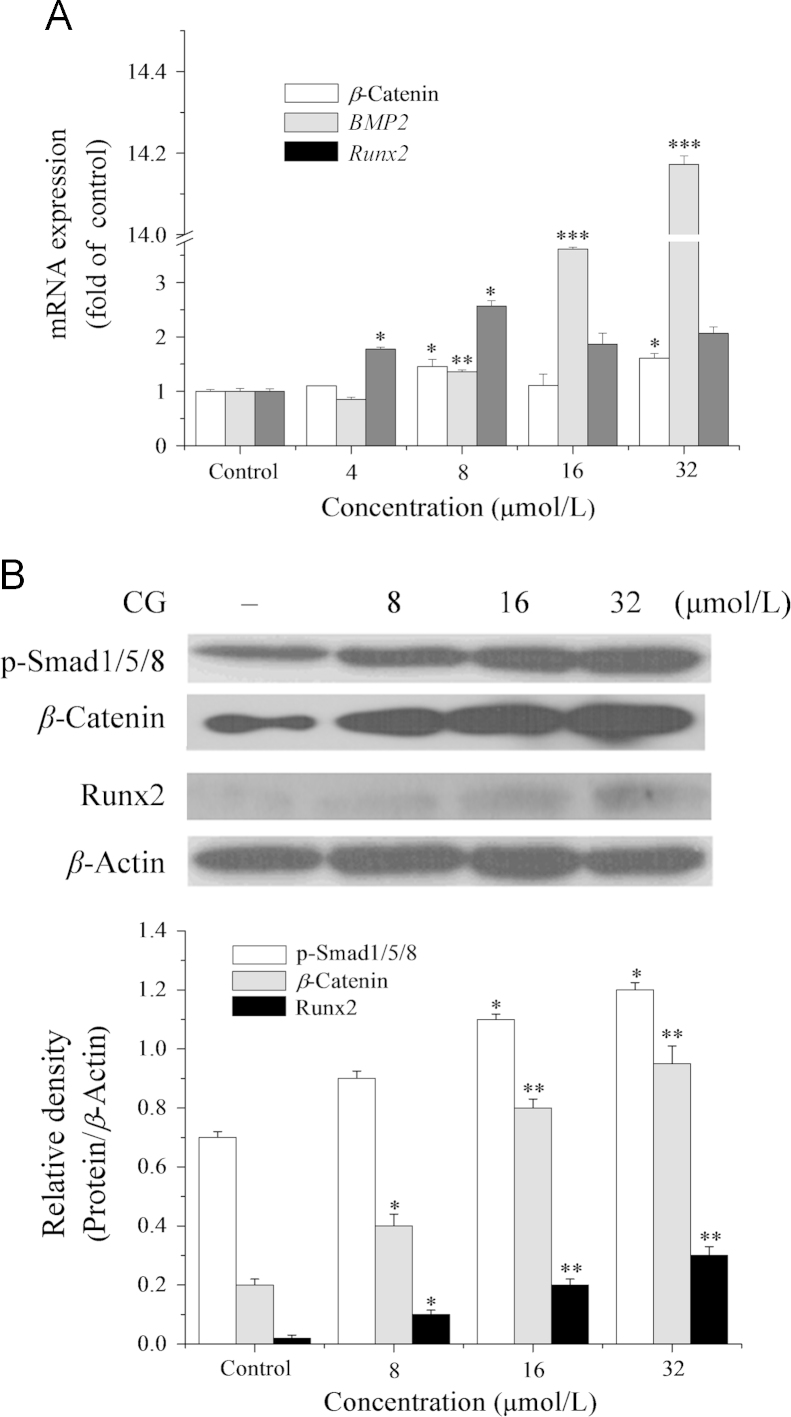
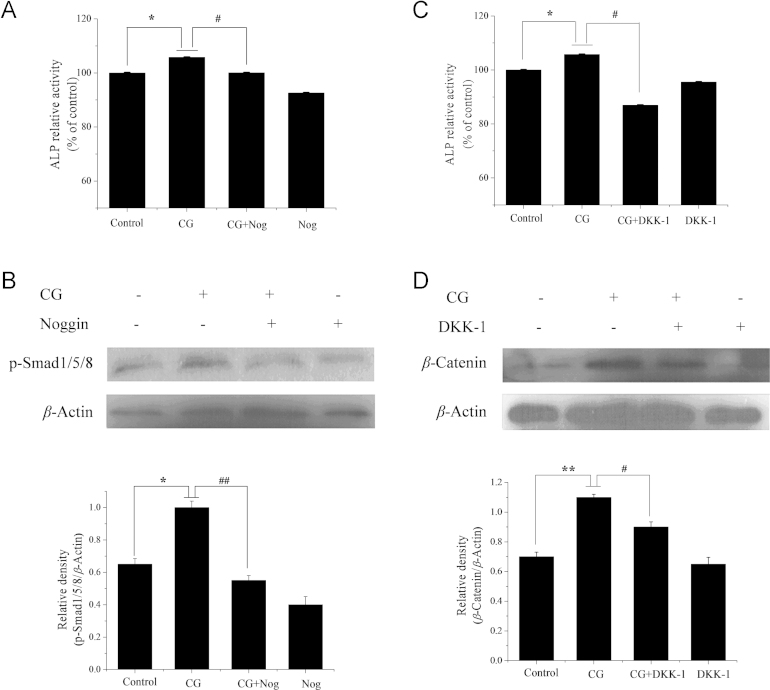
The results of this study indicate that not only BMP-2 mRNA expression is dose-dependently increased by CG treatment (4−32 μmol/L) for 9 days (Fig. 4A), but also the translational level of phosphorylated Smad1/5/8 (Fig. 4B). We also showed that Noggin (a specific inhibitor of the BMP pathway28) significantly inhibited the increase in CG-induced ALP activity and Smad1/5/8 phosphorylation in ST2 cells (Fig. 5A and B). These two findings are the first evidence that BMP signaling is involved in bone metabolism regulated by CG. Also the fact that Noggin significantly inhibited the CG-induced increase in ALP activity and Smad1/5/8 phosphorylation further confirms that CG-induced osteogenic regulation is involved in the BMP pathway.
Figure 4.
Effect of exposure to CG for 9 days on the BMP/WNT pathway in ST2 cells as indicated by: (A) expression of the osteoblast marker genes, BMP-2, Runx2 and β-catenin. mRNAs determined by real-time PCR analysis; (B) expression of the osteoblast markers, p-Smad1/5/8, β-catenin and Runx2. Protein expression was determined by Western blot analysis. *P<0.05, **P<0.01, ***P<0.001 versus control.
Figure 5.
Effect of BMP/WNT pathway inhibitors on the osteogenic effects of CG in ST2 cells after 9 days. (A) Effect of Noggin (Nog) on CG-induced ALP activity. Cells were cultured with Noggin (0.5 μg/mL) in the presence of CG (16 μmol/L) prior to determination of ALP. (B) Effect of Noggin on Smad1/5/8 phosphorylation. (C) Effect of DKK-1 on ALP activity. Cells were cultured with DKK-1 (0.1 μg/mL) in the presence of CG (16 μmol/L) prior to determination of ALP assay. (D) Effect of DKK-1 on β-catenin expression. Protein expression was determined by Western blot analysis. *P<0.05, **P<0.01 versus control group, #P<0.05, ##P<0.01 versus CG alone treated group.
WNT/β-catenin signaling is another key pathway in osteoblastic differentiation that contributes to regulating bone formation and remodeling29, 30. The results show that CG treatment (8−32 μmol/L) of ST2 cells increased the transcriptional and translational level of β-catenin, and that co-treatment with CG and DKK-1 (a specific inhibitor of the WNT/β-catenin signaling pathway29) significantly inhibited increases in ALP activity and β-catenin protein expression (Figure 4, Figure 5). Thus it is clear that WNT/β-catenin signaling is also involved in CG-induced osteogenesis in ST2 cells.
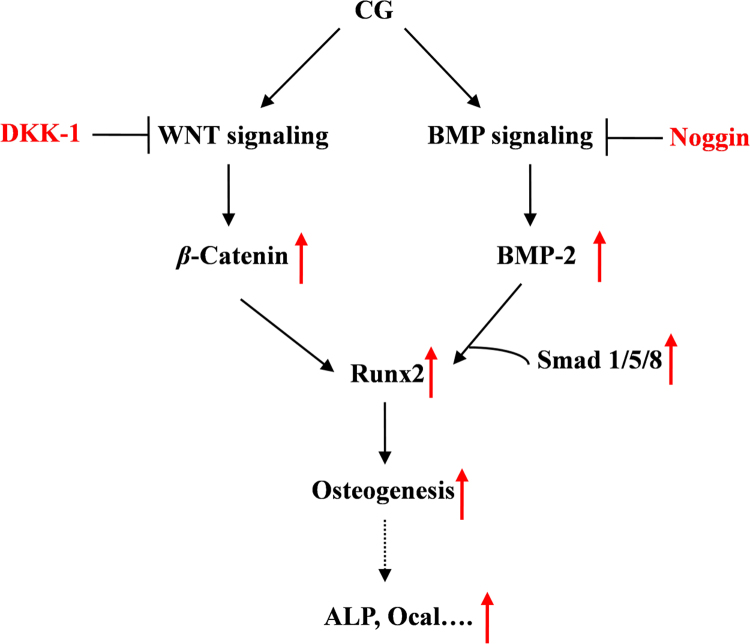
As the master osteogenic transcription factor, Runx2, is a downstream regulator of the WNT/BMP pathway and plays a critical role in the process of osteoblast maturation31 and Ocal expression32. The results of this study indicate that mRNA and protein expression of Runx2 in ST2 cells are increased by exposure to CG (8−32 μmol/L) for 9 days (Fig. 4). On this basis, we speculate that CG-induced Runx2 translocation occurs through regulating the WNT/BMP signaling pathway and subsequently increases ALP activity and Ocal expression to perform its osteogenic activity (Fig. 6).
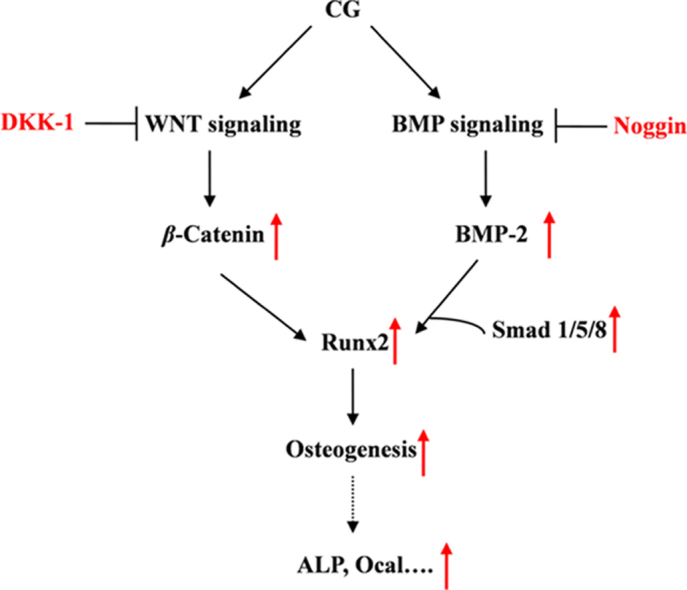
Figure 6.
The underlying mechanism of CG-induced osteogenesis in ST2 cells. The scheme indicates the effect of CG on the BMP- and WNT-signaling pathways leading to osteogenesis.
In conclusion, our findings demonstrate that CG can stimulate osteoblastic differentiation of ST2 cells by regulating the WNT/BMP signaling pathways. The underlying osteogenic mechanism of CG in ST2 cells is presented in Fig. 6. Considering safety and cost, CG appears to be an alternative therapeutic agent for osteoporosis and bone-related diseases that merits further investigations.
Acknowledgments
This work was supported by the National Natural Science Foundation of China, China (No. 31400304), the Natural Science Foundation of Hubei Province, China (No. 2012FFB00303), the Youth & Middle-aged Talent Project of Hubei Province (No. Q20111005), and the Science and Technology Program of Shandong Province (No. J12LL07).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Lijuan Sun, Email: lijuansun1212@163.com.
Yong Chen, Email: cy101610@qq.com.
References
- 1.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 2.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13:27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 3.Huang W, Yang S, Shao J, Li YP. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. 2007;12:3068–3092. doi: 10.2741/2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 5.Kalajzic I, Staal A, Yang WP, Wu YL, Johnson SE, Feyen JHM. Expression profile of osteoblast lineage at defined stages of differentiation. J Biol Chem. 2005;280:24618–24626. doi: 10.1074/jbc.M413834200. [DOI] [PubMed] [Google Scholar]
- 6.Lee SS, Sharma AR, Choi BS, Jung JS, Chang JD, Park S. The effect of TNFα secreted from macrophages activated by titanium particles on osteogenic activity regulated by WNT/BMP signaling in osteoprogenitor cells. Biomaterials. 2012;33:4251–4263. doi: 10.1016/j.biomaterials.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113:517–525. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JJ, Zhang NF, Mao GX, He XB, Zhan YC, Deng HB. Salidroside stimulates osteoblast differentiation through BMP signaling pathway. Food Chem Toxicol. 2013;62:499–505. doi: 10.1016/j.fct.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Yesilada E, Bedir E, Çalıs I, Takaishic Y, Ohmotod Y. Effects of triterpene saponins from Astragalus species on in vitro cytokine release. J Ethnopharmacol. 2005;96:71–77. doi: 10.1016/j.jep.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 10.Rios JL, Waterman PG. A review of the pharmacology and toxicology of Astragalus. Phytother Res. 1997;11:411–418. [Google Scholar]
- 11.Kim C, Ha H, Lee JH, Kim JS, Song K, Park SW. Herbal extract prevents bone loss in ovariectomized rats. Arch Pharm Res. 2003;26:917–924. doi: 10.1007/BF02980200. [DOI] [PubMed] [Google Scholar]
- 12.Kang SC, Kim HJ, Kim MH. Effects of Astragalus membranaceus with supplemental calcium on bone mineral density and bone metabolism in calcium-deficient ovariectomized rats. Biol Trace Elem Res. 2013;151:68–74. doi: 10.1007/s12011-012-9527-1. [DOI] [PubMed] [Google Scholar]
- 13.Choi SI, Lee YM, Heo TR. Screening of hyaluronidase inhibitory and free radical scavenging activity in vitro of traditional herbal medicine extracts. Korean J Bio-technol Bioeng. 2003;18:282–288. [Google Scholar]
- 14.Choi SI, Park SR, Heo TR. Inhibitory effect of Astragali radix on matrix degradation in human articular cartilage. J Microbiol Biotechnol. 2005;15:1258–1266. [Google Scholar]
- 15.Taku K, Melby MK, Nishi N, Omori T, Kurzer MS. Soy isoflavones for osteoporosis: an evidence-based approach. Maturitas. 2011;70:333–338. doi: 10.1016/j.maturitas.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Wei P, Liu M, Chen Y, Chen DC. Systematic review of soy isoflavone supplements on osteoporosis in women. Asian Pac J Trop Med. 2012;5:243–248. doi: 10.1016/S1995-7645(12)60033-9. [DOI] [PubMed] [Google Scholar]
- 17.Stanford CM, Jacobson PA, Eanes ED, Lembke LA, Midura RJ. Rapidly forming apatitic mineral in an osteoblastic cell-line. J Biol Chem. 1995;270:9420–9428. doi: 10.1074/jbc.270.16.9420. [DOI] [PubMed] [Google Scholar]
- 18.Otsuka E, Yamaguchi A, Hirose S, Hagiwara H. Characterization of osteoblastic differentiation of stromal cell line ST2 that is induced by ascorbic acid. Am J Physiol. 1999;277:C132–C138. doi: 10.1152/ajpcell.1999.277.1.C132. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi A, Ishizuya T, Kintou N, Wada Y, Katagiri T, Wozney JM. Effects of BMP-2, BMP-4, and BMP-6 on osteoblastic differentiation of bone marrow-derived stromal cell lines, ST2 and MC3T3-G2/PA6. Biochem Biophys Res Commun. 1996;220:366–371. doi: 10.1006/bbrc.1996.0411. [DOI] [PubMed] [Google Scholar]
- 20.Zhang R, Oyajobi BO, Harris SE, Chen D, Tsao C, Deng HW. Wnt/β-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone. 2013;52:145–156. doi: 10.1016/j.bone.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rawadi G, Vayssière B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18:1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 22.Aubin JE. Bone stem cells. J Cell Biochem Suppl. 1998;30−31:73–82. [PubMed] [Google Scholar]
- 23.Cao X, Chen D. The BMP signaling and in vivo bone formation. Gene. 2005;357:1–8. doi: 10.1016/j.gene.2005.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang JF, Li G, Chan CY, Meng CL, Lin MC, Chen YC. Flavonoids of Herba Epimedii regulate osteogenesis of human mesenchymal stem cells through BMP and Wnt/β-catenin signaling pathway. Mol Cell Endocrinol. 2010;314:70–74. doi: 10.1016/j.mce.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Yonezawa T, Lee JW, Hibino A, Asai M, Hojo H, Cha BY. Harmine promotes osteoblast differentiation through bone morphogenetic protein signaling. Biochem Biophys Res Commun. 2011;409:260–265. doi: 10.1016/j.bbrc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Yuan L, Wang X, Zhang TL, Wang K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 2007;81:832–840. doi: 10.1016/j.lfs.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Vali B, Rao LG, El-Sohemy A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J Nutr Biochem. 2007;18:341–347. doi: 10.1016/j.jnutbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AN, Kwiatkowski W. Structural basis of BMP signaling inhibition by the cystine knot protein Noggin. Nature. 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- 29.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML. Essential role of β-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 31.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 32.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]