Abstract
Since the discovery that non-small cell lung cancer (NSCLC) is driven by epidermal growth factor receptor (EGFR) mutations, the EGFR tyrosine kinase inhibitors (EGFR-TKIs, e.g., gefitinib and elrotinib) have been effectively used for clinical treatment. However, patients eventually develop drug resistance. Resistance to EGFR-TKIs is inevitable due to various mechanisms, such as the secondary mutation (T790M), activation of alternative pathways (c-Met, HGF, AXL), aberrance of the downstream pathways (K-RAS mutations, loss of PTEN), impairment of the EGFR-TKIs-mediated apoptosis pathway (BCL2-like 11/BIM deletion polymorphism), histologic transformation, ATP binding cassette (ABC) transporter effusion, etc. Here we review and summarize the known resistant mechanisms to EGFR-TKIs and provide potential targets for development of new therapeutic strategies.
Abbreviations: ABC, ATP binding cassette; ABCB1, ATP binding cassette, sub-family B, member 1; ABCC1, ATP binding cassette, sub-family C, member 1; ABCC10, ATP binding cassette, sub-family C, member 10; ABCG2, ATP binding cassette, sub-family G, member 2; AKT, protein kinase B; ALK, anaplastic lymphoma kinase; AXL, Anexelekto; BCL-2, B-cell CLL/lymphoma-2; BCL2L11/BIM, BCL2-like 11; BH3, BCL2-homology domain 3; BRAF, v-RAF murine sarcoma viral oncogene homolog B1; CML, chronic myelogenous leukemia; CRKL, Crk-like protein; EGFR, epidermal growth factor receptor; EGFR-TKIs, epidermal growth factor receptor tyrosine kinase inhibitors; EGFRvIII, EGFR variant III; EML4, echinoderm microtubule-associated protein-like 4; EMT, epithelial mesenchymal transition; ERK1/2, extracellular signal-regulated kinases; FGFs, fibroblast growth factors; FGFRs, fibroblast growth factor receptors; GAS6, growth-arrest-specific protein 6; HER, human epidermal receptor; HGF, hepatocyte growth factor; IGF, insulin growth factor; IGFBPs, IGF-binding proteins; IGF-1R, IGF-1 receptor; IL, interleukin; IL-6R, IL-6 receptor; JAK, janus kinase; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase; NSCLC, non-small cell lung cancer; PDGFs, platelet-derived growth factors; PDGFRs, platelet-derived growth factor receptors; PI3K, phosphatidylinositol-3-kinase; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase,catalytic subunit alpha; PTEN, phosphatase and tensin homolog; RAF, rapidly accelerated fibrosarcoma; RAS, rat sarcoma; RTK, tyrosine kinase receptor; SF, scatter factor; SOCS3, suppressor of cytokine signaling 3; STAT, signal transducers and activators of transcription; TKs, tyrosine kinases; TKIs, tyrosine kinase inhibitors; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor
KEY WORDS: EGFR, TKIs, Resistance, Mechanisms
Graphical abstract
We review and summarize the known resistant mechanisms to EGFR-TKIs and provide potential targets for development of new therapeutic strategies.
1. EGFR signal pathway and cancers
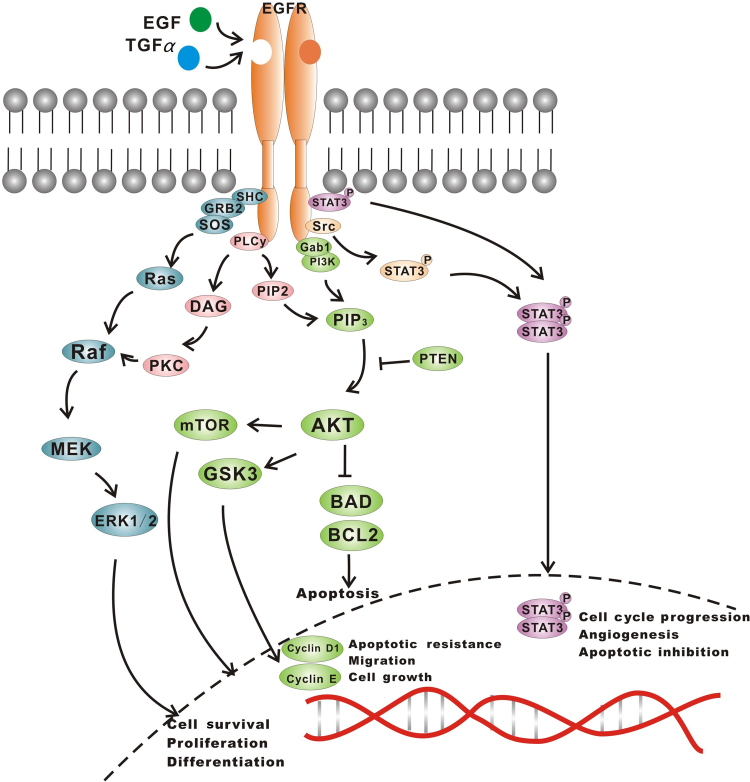
EGFR, also known as ERBB1 and HER1, is a transmembrane tyrosine kinase receptor (RTK). EGFR is a member of the human epidermal receptor (HER) family and a crucial component of cell signal pathways. Binding with ligands (EGF and TGF-α) leads to conformational changes in EGFR and homodimerization or heterodimerization with other HER family members. There is subsequent autophosphorylation of the cytoplasmic tyrosine kinase (TK) domain with the help of adapter proteins (e.g., SHC and GRB-2), which triggers downstream signaling. There are three main downstream pathways: (1) rat sarcoma (RAS)/rapidly accelerated fibrosarcoma (RAF)/mitogen-activated protein kinase (MAPK) pathway; (2) phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT) pathway and (3) janus kinase (JAK)/signal transducers and activators of transcription (STAT) pathway, which stimulates mitosis, leading to cell proliferation and inhibition of apoptosis1. These pathways are crucial in normal cell growth (Fig. 1).
Figure 1.
EGFR and its signal pathway. There is subsequent autophosphorylation of the cytoplasmic tyrosine kinase domain, which, with the aid of adapter proteins (e.g., SHC and GRB-2), triggers downstream signaling. The principal pathways included: (1) RAS/RAF/MEK, (2) PI3K/AKT and (3) JAK/STAT pathways.
EGFR also serves as a stimulus for cancer growth. EGFR gene mutations and protein overexpression, both of which activate downstream pathways, are associated with cancers, especially lung cancer. The importance of EGFR to lung cancers supports the concept of ‘oncogene addiction’. Tyrosine kinase inhibitors (TKIs) have been used to treat the cancer harboring EGFR mutations or aberrant activation of EGFR. TKIs can inhibit the EGFR TK domain reversibly through competitive binding with ATP2. TKIs also lead to tumor cell death through BCL2-like 11 (BIM)-mediated apoptosis. However, patients with EGFR-activating mutations benefit from treatment with EGFR-TKIs (e.g., gefitinb and erlotinib) for less than approximately 1 year, after which drug resistance develops.
The etiology of EGFR-TKI resistance is complex. According to the cell signal transduction pathway, the etiology of EGFR-TKI resistance can be divided into subsequent categories.
2. EGFR mutations induce drug resistance, especially the secondary mutation T790M
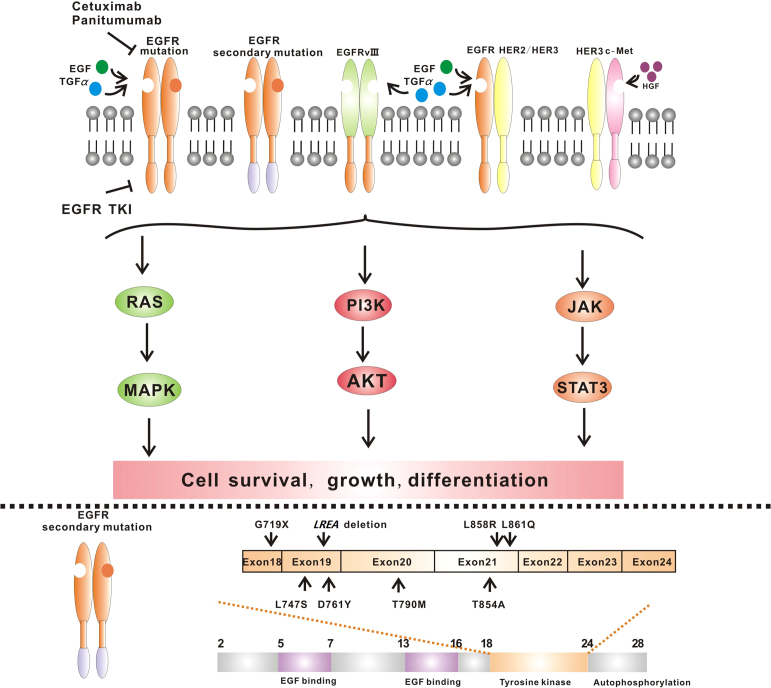
The EGFR gene, located in the 7p12−14 region in the short arm of chromosome 7, consists of 28 exons. The tyrosine kinase function is encoded by exons 18−24. Currently, more than 90% of the known EGFR mutations reside in exons 19−21 (Fig. 2). The rate of mutation in exon 19 is the highest, accounting for more than 60% of overall mutations3.
Figure 2.
Aberration of HER families. Members of HER families get involved in the resistance to EGFR-TKIs. The secondary mutations of EGFR, EGFR-vIII, the overexpression of HER2 or mutations of HER2 contribute to the resistance in the presence of EGFR-TKIs. Compared to the other HER proteins, there are currently no mutational alterations known to confer oncogenic activities to HER3. In most cases, HER3 phosphorylation is driven by one of HER family kinase partners, like HER1 and HER2. What׳s more, resistance can also occur through amplification of the proto-oncogene c-Met and the c-Met-mediated phosphorylation of HER3. HER3 serves as a key activator of downstream PI3K/AKT and MEK/MAPK signal pathways through dimerization with other HER family proteins or other molecules.
2.1. Secondary mutation—T790M
A secondary mutation of the EGFR gene reported in 2005 conferred acquired resistance to EGFR-TKIs4. This mutation (located in exon 20) results in the substitution of methionine for threonine at position 790 (T790M) in the kinase domain. Threonine 790 has been designated as a “gatekeeper” residue, important for regulating inhibitor specificity in the ATP binding pocket. The T790M mutation enhances affinity of the ATP binding pocket for ATP, thus successfully competing with the TKIs, thereby conferring resistance5. Currently, two theories can explain the production of the second mutations: subcloning and induced mutation/acquisition6. Although the second mutation rarely occurs prior to treatment, it is found in approximately half of EGFR TKIs-treated patients. Experiments have identified a proportion of TKI-naive tumors that carry T790M, and these resistant clones may be selected after exposure to TKIs7, 8. The T790M mutation can coexist with other mutations, like L858R and D761Y. The T790M mutation also possesses enhanced phosphorylating activity, especially in combination with the L858R mutation. The combination leads to lung cancer cell survival, indicating that the T790M mutant is actually an oncogene9. Furthermore, cyclin D1 and Hsp90 may contribute to resistance in cancer cells harboring the T790M mutant by inhibiting the degradation of EGFR and maintaining the conformation of mutant EGFR10. Recently, the Hsp90 inhibitor ganetespib has been shown to enhance the anti-tumor effect of TKIs11.
2.2. Other secondary resistance mutations: L747S, D761Y and T854A
The non-T790M secondary mutations mainly include D761Y, L747S and T854A12, 13, 14. They reduce the sensitivity of mutant EGFR to EGFR-TKIs, but the resistance mechanism remains unknown. A possible explanation may be that these secondary resistance mutations modify the conformation of EGFR and the combination between EGFR and TKIs. In addition, they may affect gefitinib-induced apoptosis and inhibit BIM up-regulation. Recently, another new insertion mutation on exon 20 of EGFR has been reported (Pro772_His773insGlnCysPro)15, 16. It was found in an individual who never smoked. The patient had previously been treated with cisplatin and gemcitabine, followed by carboplatin and pemetrexed. Finally, the patient developed resistance to erlotinib. Additional mutations still remain to be discovered. However, according to the reports of EGFR-mutated TKI-resistant patients, the frequency of non-T790M secondary mutations is low.
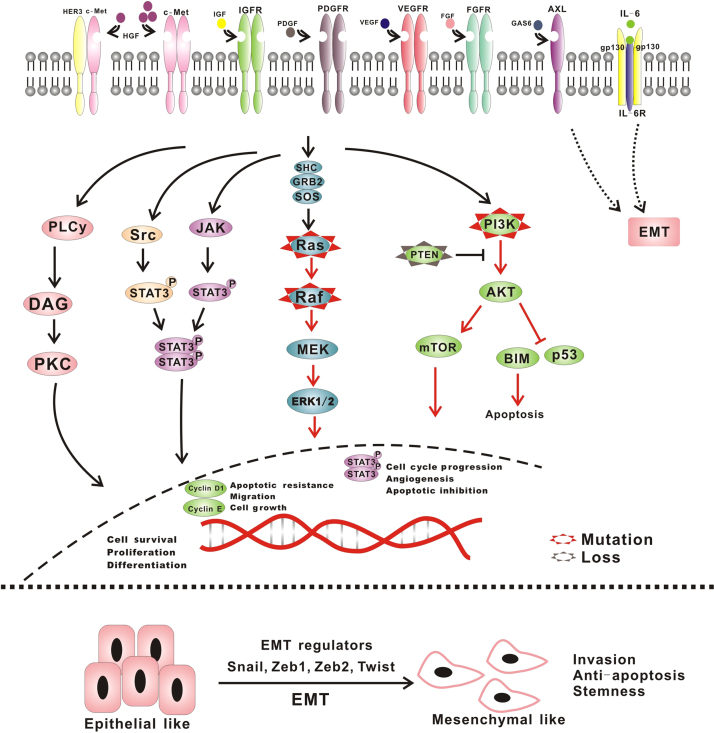
3. The aberrated activation of the bypass pathways induce resistance
The synchronous activation of redundant kinases also can induce resistance via activation of bypass pathways (Figure 2, Figure 3). EGFR-TKI treatment of patients harboring such a change is not effective.
Figure 3.
Synchronous activation of redundant kinases and abnormality of the downstream pathway.
3.1. The aberrance of other members of HER family
The HER family is comprised of EGFR, HER2, HER3 and HER4. These receptors have a cytoplasmic TK domain which can be activated by ligand binding, followed by dimerization. Although HER2 appears to have no intrinsic ligand-binding capability, it can interact reversibly with ligand-activated EGFR or HER3 to form active heterodimers which activate downstream signals to govern cell proliferation and migration. Overexpression of HER2 and mutations of HER2 are involved in resistance of EGFR-TKIs17. Therefore, HER2 is a useful target for treatment. A covalent, irreversible inhibitor of HER2, afatinib, can overcome the resistance of the patient harboring HER2 overexpression or HER2 gene mutations18. Compared to the other HER proteins, there are currently no mutational alterations known to confer oncogenic activities to HER3, but HER3 also takes part in resistance of EGFR-TKIs. HER3 phosphorylation is driven by one of the other HER family kinase partners, like HER1 and HER2, or through amplification of the proto-oncogene c-Met19. HER3 serves as a key activator of downstream PI3K/AKT and MEK/mitogen-activated protein kinase (MAPK) pathways and contributes to survival of the most tumor cells. However, researchers have found that a heregulin–EGFR–HER3 autocrine signaling axis mediated acquired lapatinib resistance in HER2+ breast cancer models which no longer depended on HER2–HER3–PI3K signal pathway20. What׳s more, overexpression of HER3 in COLM-5 cells can lead to significant resistance to gefitinib in vitro and in vivo21. All of these studies highlight the central role of HER3 in cancers. Targeting of HER3 receptor with a monoclonal antibody, such as MM-121 or MM-111 is an effective strategy currently under preclinical study and clinical evaluation22.
3.2. Amplification of c-Met
c-Met is a transmembrane RTK. Binding with its ligand, hepatocyte growth factor (HGF) triggers receptor dimerization and phosphorylation, leading to conformational changes of c-Met that activates the TK domain and activates a wide range of different cellular signal pathways, including those involved in proliferation, motility, migration and invasion. Although c-Met is important in the control of tissue homeostasis under normal physiological conditions, it has also been found to be aberrantly activated in human cancers via gene mutation, amplification or protein overexpression. In 2007, the second most common primary resistance to EGFR-TKIs involved amplification of the c-Met oncogene in HCC827 NSCLC cells after exposure to gefitinib. In c-Met-resistant patients, c-Met amplified clones existed prior to EGFR-TKIs therapy and were selected out by treatment23. The resistant mechanism of EGFR-TKIs may involve HER3-PI3K/AKT signaling by maintaining HER3 phosphorylation in the presence of gefitinib, which is independent of EGFR kinase activity19. Besides, a high MET gene copy number leads to shorter survival in patients with NSCLC. Clinical trials have demonstrated that concurrent inhibition of EGFR and c-Met can overcome resistance of EGFR-TKIs and improve patient outcomes.
3.3. Overexpression of HGF
HGF is the ligand for c-Met. HGF acts as a pleiotropic factor and cytokine, promoting cell proliferation, survival, motility, scattering, differentiation and morphogenesis.
Overexpression of HGF is another EGFR-TKIs resistance mechanism and it may be more common among patients with mutations who had no response24. One study showed that HGF induces resistance by activating c-Met which restores phosphorylation of downstream MAPK/extracellular signal-regulated kinases (ERK1/2) and PI3K/AKT pathways25. Interestingly, although amplification of c-Met activates downstream pathways by activating ERBB3, HGF induces downstream pathways through c-Met; this activation is independent of ERBB3 or EGFR. The resistance induced by HGF not only appears in the NSCLS, but also exists in breast cancers. Blockade of EGFR and the downstream pathways can overcome HGF-mediated resistance.
3.4. The abnormality of insulin growth factor receptor (IGFR)
As early as 2002, it had been suggested that IGF-1 receptor (IGF-1R) signaling through PI3K may represent a novel and potentially important mechanism of resistance to anti-EGFR therapy. In 2008, researchers found that the loss of expression of IGF-binding proteins (IGFBPs) in tumor cells treated with EGFR-TKIs increased the activation of IGF-IR signaling which, in turn, mediates resistance of EGFR-TKIs26, 27. Inhibition of IGF-IR signaling disrupted the association of IRS-1 with PI3K and restored the ability of gefitinib to down-regulate PI3K/AKT signaling and to inhibit cell growth. Concomitant inhibition of both EGFR and IGFIR was required to abort PI3K signaling, and treatment of the resistant cells with an IGFIR inhibitor restored their sensitivity to EGFR TKIs. Another study found that activation of IGF-1R can alter phosphorylation state and subcellular localization of p27, which can promote cell proliferation and cell motility28. Thus, IGF-1R and p27 can be used to be a biomarker of cell cycle arrest and response to therapy.
3.5. The abnormality molecules of multiple angiogenic pathways
Angiogenesis is an essential step in tumor growth and metastasis. Impaired vascularity and hypoxia can lead to increased metastasis and treatment resistance. Thus, targeting multiple angiogenic pathways may not only improve antitumor activity but also reduce the risk of resistance29. Important targets for the development of novel antiangiogenic therapies include vascular endothelial growth factors (VEGFs), fibroblast growth factors (FGFs), platelet-derived growth factors (PDGFs), and their receptors.
3.5.1. The VEGFs and their receptors
The VEGF family and receptors are important regulators of angiogenesis and vascular permeability. Overexpression of vascular endothelial growth factor receptor (VEGFR) 1 in tumor cells leads to cell survival and invasion. It also reduces the inhibition by EGFR inhibitors and reduces sensitivity to gefitinib. Activation of the EGFR signal can increase the expression of VEGF. Researchers have demonstrated that a VEGF/VEGFR2 feed-forward loop in NSCLC cells expressing VEGFR2, which leads to a signal amplification and a boost in VEGF secretion, is required for establishment of fully angiogenic tumors in vivo. This VEGF/VEGFR2 signaling cascade via VEGFR2/PI3K/mTOR induces an mTOR-dependent regulation of VEGF secretion30. And VEGF secretion is induced by the upregulation of HIF-1α. Thus, the VEGF/VEGFR system is associated with resistance of anti-EGFR drugs through activation of downstream signal pathways via EGFR-independent mechanisms31. The therapeutic use of agents able to inhibit both EGFR and VEGFR may help to efficiently inhibit the activation of bypass pathways and overcome EGFR inhibitor resistance.
3.5.2. The fibroblast growth factors and their receptors
The FGFs and the receptors (FGFRs) are involved in multiple cellular functions. During embryonic development, FGFs play a part in morphogenesis. In adults, FGFs are involved in wound healing and tissue repair as well as regulating the nervous system. FGFs also participate in tumor angiogenesis. FGFR autocrine signaling has been implicated in NSCLC cell lines. In 2010, researchers found EGFR-TKIs (gefitinib) increased the expression of FGFR2 and FGFR3. Importantly, FGFR2 and FGFR3 are capable of mediating FGF2 and FGF7 stimulated ERK activation, leading to cell survival and invasion and reducing the sensitivity to EGFR-TKIs32. FGFR activation is an escape mechanism in human lung cancer cells resistance to afatiniab, that may compensate for the loss of EGFR-driven signaling pathway33. Treatment of NSCLC patients with combinations of EGFR and FGFR specific inhibitors (e.g., PD173074) or FGF antibodies may be potential strategy to enhance efficacy of single EGFR-TKIs.
3.5.3. The platelet-derived growth factors and their receptors (PDGFRs)
PDGFs are isolated from human platelets and promote angiogenesis. As angiogenesis factors, PDGFs/PDGFRs are closely related to tumor development. PDGFs/PDGFRs can induce tumor cell proliferation and migration, and inhibit apoptosis. In 2013, Akhavan et al.34 found that a physiologic RTK switch to the PDGFRβ was required to maintain the growth of EGFR variant III (EGFRvIII)/EGFR-activated in response to EGFR-TKIs. Combination of EGFR and PDGFR inhibitors may overcome resistance of EGFR-TKIs.
3.6. EGFRvIII
EGFRvIII is a tumor-specific mutation that results from in-frame deletion of 801 base pairs spanning exons 2–7 of the coding sequence. This deletion removes 267 amino acids from the extracellular domain, creating a junction site between exons 1 and 8 and a new glycine residue. EGERvIII has expressed in many kinds of tumors.
EGFRvIII confers enhanced tumorigenicity through multiple mechanisms and pathways. EGFRvIII expression is associated with the activation of downstream PI3K/AKT/mTOR pathway and increases proliferation and cell cycle progression mediated by a decrease in the level of p27KIP1. EGFRvIII also has been shown to activate the NF-κB pathway and regulate expression of IL-835. Furthermore, EGFRvIII induces angiopoietin-like 4 expression through the ERK/c-Myc pathway and promotes tumor angiogenesis in malignant gliomas36. Cells harboring EGFRvIII have an enhanced capacity for dysregulated growth, survival, invasion and angiogenesis. EGFRvIII is an attractive target and predictor in cancer immunotherapy because it is not expressed in normal tissue.
3.7. Overexpression of or overactivated Anexelekto (AXL)
AXL belongs to the Tyro/Axl/Mer (TAM) family of RTK. Growth-arrest-specific protein 6 (GAS6) is a ligand for AXL. Activation of AXL increases cell migration, aggregation and growth through multiple downstream pathways. AXL was overexpressed in EGFR-mutant NSCLC tumor xenografts with acquired resistance to erlotinib37. The researchers suggested that the AXL upregulation may activate AKT, MAPK or NF-κB signaling to promote resistance to erlotinib, perhaps in association with epithelial mesenchymal transition (EMT) process via inducing Slug expression38. Inhibitors of AXL (MP-470 or XL-880) enhance erlotinib sensitivity particularly in the context of NSCLC with acquired resistance to EGFR-targeting TKIs. Therefore, the inhibitors of AXL or GAS6 may overcome the AXL-mediated resistance of EGFR-TKIs.
3.8. Excess secretion of interleukin-6 (IL-6)
IL-6 is a cytokine which plays an important role in many chronic inflammatory diseases. Binding to the membrane-bound IL-6 receptor (IL-6R) causes the recruitment of two gp130 co-receptor molecules and the activation of intracellular signaling cascades via gp130. However, some reports suggest that IL-6 expression is involved in the regulation of tumor growth and metastatic spread, including lung cancers. One study demonstrated that activation of the IL-6R/JAK1/STAT3 pathway induced de novo resistance to irreversible EGFR-TKIs in NSCLC harboring T790M39. Afatinib activated IL-6R/JAK1/STAT3 signaling via autocrine IL-6 production40. Inhibition of the IL-6R/JAK1/STAT3 signal pathway can reverse the resistance. JAK1 activates STAT3 activity which is stopped rapidly by the major negative regulator suppressor of cytokine signaling 3 (SOCS3) in normal cells. However, association of the IL-6 receptor with the EGFR can activate STAT3 in presence of the inhibition of SOCS3 to JAK1 and JAK241. Besides, paracrine or autocrine stimulation of the TGF-β axis also increases the secretion of IL-6 and promotes resistance42.
3.9. Amplification of Crk-like protein (CRKL)
CRKL is a member of adapter proteins that participates in signal transduction in response to both extracellular and intracellular stimuli, such as growth factors, cytokines and the oncogenic BCR-ABL fusion protein. Oncogenic CRKL activates the SOS1/RAS/RAF/ERK and SRC/C3G/RAP1 pathways. Amplification of the CRKL gene was observed in 1/11 lung cancer patients with EGFR mutations who acquired resistance to EGFR-TKIs43. Amplification of CRKL in EGFR-mutant cells induces resistance to gefitinib by activating ERK and AKT signaling44. What׳s more, overexpression of CRKL promotes cell invasion via upregulating MMP9 expression and activating ERK pathway45. Therefore, CRKL can be a potential target in the treatment of NSCLC.
3.10. Overexpression and activation of integrin beta1
Beta1 subunit of integrin is an adhesion molecule, sharing common downstream signaling elements with EGFR, such as the PI3K/AKT and ERK1/2 pathways. Overexpression of integrin β1 induces gefitinib resistance, accompanied by increases in cell adhesion and migration46. Subsequently, researchers found that β1 integrin activated an alternative survival pathway in breast cancer cells harboring resistance to lafatinib, which led to activation of β1 integrin׳s downstream kinases, FAK and SRC. Inhibition of β1 integrin by AIIB2 can enhance the sensitivity to EGFR-TKIs47. Further study found that ligand-dependent activation of integrin β1 could induce EGFR-TKIs resistance through activating c-Met and downstream pathways48. Kanda et al.49 identified the integrin β1/Src/AKT signal pathway as a key mediator of acquired resistance to EGFR-targeted anticancer drugs. Researchers have also found that integrin mediates a stem-like phenotype and confers resistance to EGFR-targeted therapy through enhancing downstream coupling to a KRAS/RalB/NF-κB pathway50. Therefore, integrin β1 and the pathway molecules provide potential agents for overcoming the resistance mediated by integrin β1.
4. Abnormal downstream pathways induce drug resistance
The abnormal downstream pathways also can result in the resistance to EGFR-TKIs, even though the cells have not harbored any other mutations. The mutation of K-RAS, the loss of phosphatase and tensin homolog (PTEN), the mutations of PIK3CA and BRAF are the main points (Fig. 3).
4.1. K-RAS mutation
The RAS proteins include K-RAS, N-RAS and H-RAS. The RAS proteins are GTPases that are molecular switches for a variety of critical cellular activities and their function is tightly and temporally regulated in normal cells. Oncogenic mutations of RAS genes, which create constitutively-active RAS proteins, can result in uncontrolled proliferation or survival in tumor cells. In 2005, Pao et al.51 showed that mutations in K-RAS are associated with primary resistance to single-agent gefitinib or erlotinib. If the mutations occur at codons 12, 13, 59, 61, 63, 116, 117, 119 or 146, its structure is altered by binding sites for guanine, affecting normal function. The effects of these mutations can be translated either in a reduction of the activity of oncoprotein GTPases, blocking it into the active form bound to GTP, or in decreased binding affinity and increasing the change in GDP by GTP attachment52. In total, 80% of K-RAS mutations occur in codon 12, and other mutations are mainly located in codons 13 and 61. RAS gene alteration is poor prognostic factors for survival of patients with NSCLC. Additionally, several studies have clearly demonstrated that RAS uses additional effectors to promote tumorigenesis53. Very little is known about the relevant mechanisms.
4.2. Loss of PTEN
PTEN dephosphorylates PI-(3, 4, 5)-triphosphate, which mediates activation of AKT, thereby negatively regulating the PI3K/AKT/mTOR pathway and leading to G1 cell cycle arrest and apoptosis. In addition, PTEN inhibits cell migration and spreading through regulation of focal adhesion kinase as well as regulates p53 protein levels and activity.
PTEN deleted on chromosome 10 is a tumor suppressor gene on chromosome 10q23.3 and encodes a 403 amino acid dual-specificity lipid and protein phosphatase. The loss of PTEN has only been investigated in a small number of NSCLC cases. However, PTEN loss contributes to erlotinib resistance in EGFR mutant lung cancer by activation of AKT and EGFR54, 55. The absence of PTEN protein expression is an independent prognostic marker in early stage resected lung adenocarcinoma. Inhibition of the PI3K/AKT/mTOR signal pathway can be an effective strategy to NSCLC harboring the EGFR activating mutations that acquires resistance to both TKIs and radiotherapy due to PTEN loss56.
4.3. Mutations of BRAF (v-RAF murine sarcoma viral oncogene homolog B1)
BRAF, another component of the EGFR/RAS/RAF signal transduction pathway, encodes a RAS-regulated kinase that mediates cell growth, differentiation, apoptosis and malignant transformation. Mutations of BRAF (G469A, V600E and V599E) were found in several tumors, including malignant melanoma and colorectal cancer. Thus, BRAF mutations also induce drug resistance57. Activating BRAF mutations, especially the common mutant V599E, induces constitutive activation of the signal transduction pathway, providing a potent pro-mitogenic force that drives malignant transformation. The BRAF V599E mutant shows greatly increased activity in the RAF/MEK/ERK pathway both in vitro and in vivo. Here, BRAF provides a new target for the treatment to overcome the mutant BRAF-mediated resistance.
4.4. A downstream mutation in PIK3CA
The PIK3CA gene, encoding a catalytic subunit of the PI3K, is mutated or amplified in various neoplasias, including lung cancer. Infrequently, a downstream mutation in PIK3CA has been identified as a mechanism of resistance58. Recently, a study demonstrated that PIK3CA mutations frequently coexist with EGFR or K-RAS mutations59. Patients with single PIK3CA mutation in NSCLC have poor prognosis. The role of mutant PIK3CA in oncogenic signaling requires further investigation, including development of novel targets for therapeutic intervention in cancers harboring PIK3CA mutations.
4.5. The aberrant expression of NF1
Neurofibromin, the RAS GTPase-activating protein, is encoded by NF1 gene. A recent study demonstrated that reduced expression of NF1 was associated with erlotinib resistance due to a failure to fully inhibit RAS/RAF/MEK/ERK pathway60. Combination therapy with EGFR and MEK inhibitors may restore sensitivity to EGFR-TKIs.
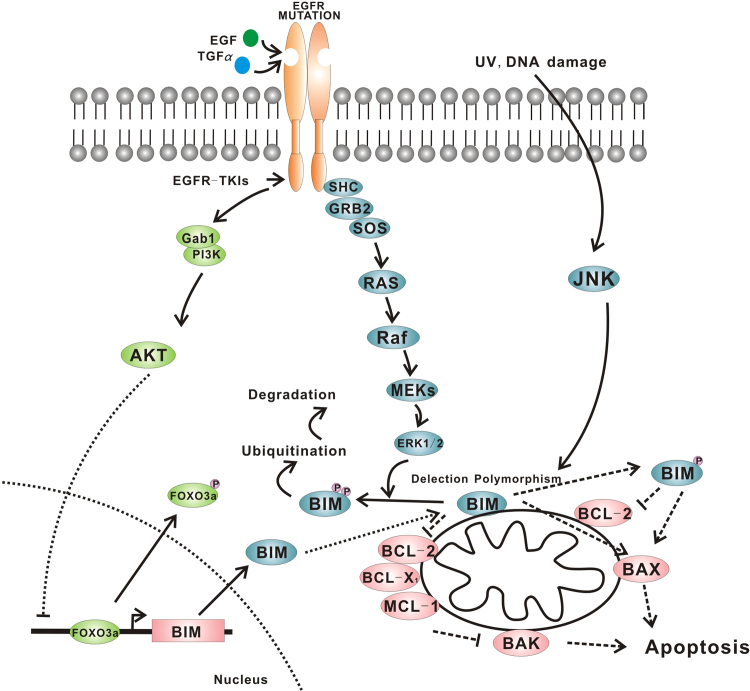
5. Impairment of a pathway that is essential for EGFR-TKIs-mediated apoptosis: a common intrinsic deletion polymorphism in the gene encoding BIM
BIM is a pro-apoptotic member of the B-cell CLL/lymphoma-2 (BCL-2) family, and plays a critical role in inducing cell apoptosis and tumor metastasis. Consequently, BIM has become the focus of intense interest as a potential target for cancer chemotherapy. Its upregulation is required for apoptosis induced by EGFR and EGFR-TKIs in tumors harboring EGFR mutations. The polymorphism switched BIM splicing from exon 4 to exon 3, which resulted in expression of BIM isoforms lacking the pro-apoptotic BCL2-homology domain 3 (BH3)61 (Fig. 4). Although the polymorphism was sufficient to confer intrinsic TKI resistance in chronic myelogenous leukemia (CML) and EGFR NSCLC cell lines, this resistance could be overcome with BH3-mimetic drugs. Recently, researchers have shown that the PP2A activator FTY720 could induce apoptosis of CML cells via dual activation of BIM and BID and overcome various types of resistance of TKIs62.
Figure 4.
Apoptosis pathway mediated by BIM.
6. Histologic transformation
6.1. The EMT
EMT is a physiological process during embryogenesis that appears to be reinstated in adult tissues undergoing wound healing and tissue regeneration, or under certain pathological conditions, such as fibrosis and cancer. EMT is characterized by the combined loss of epithelial cell junction proteins, such as E-cadherin, and the gain of mesenchymal markers, such as vimentin and N-cadherin63. In the EMT process, epithelial cells lose their features, gain properties of mesenchymal, and become motile and invasive (Fig. 3).
In 2005, a transition to a mesenchymal phenotype was noted among patients treated with EGFR-TKIs. EMT may be induced by the activation of AXL via the PI3K/AKT pathway. One study also showed that loss of E-cadherin can activate EGFR–MEK/ERK/ZEB1/MMP2 axis, which is responsible for promoting invasion in NSCLC64. Tumor cells undergoing EMT are also known to increase the secretion of specific factors, including cytokines, chemokines and growth factors, which could play an important role in tumor progression. Well-established signals include those initiated by TGF-β, FGF, EGF and HGF, all of which have been shown to promote EMT in various tumor cell models. Other possible pathways or factors that have been reported to be associated with EMT include IL-8, IL-6, Notch-1, SOX9, FoxO4, SRC and CRIPTO-165, 66, 67, 68, 69. Co-targeting the relative molecules of these pathways and EGFR can reverse the resistance mediated by EMT70. However, the specific mechanism of EMT still remains unknown.
6.2. Small cell transformation
Conversion to small-cell carcinoma has been seen at the time of development of resistance. Tumor cells retained the original EGFR mutations but developed a histopathologic small-cell phenotype, which may benefit from a standard chemotherapy for small cell lung cancer71. Interestingly, after a period of conventional cytotoxic treatment, susceptibility to TKIs may redevelop. Research into this phenomenon is still insufficient.
7. ATP binding cassette (ABC) effusion
The ABC transporters are transmembrane proteins that involved in transporting biologically important substrates across the cellular membranes, such as amino acids, cholesterol, hydrophobic drugs and antibiotic. Overexpression of ABC transporters can reduce drug uptake, increase drug efflux and lead to low drug density in the cytoplasm, which will come to a lower drug efficacy and finally acquire drug resistance. When EGFR-TKIs (lapatinib) binds to the ATP binding cassette, sub-family B, member 1 (ABCB1) and ATP binding cassette, sub-family G, member 2 (ABCG2), substrate-binding sites with high affinity induced overexpression of ABC transporters72. Other ABC transporters, such as ATP binding cassette, sub-family C, member 1 (ABCC1) and ABCC10 also participate in drug resistance. Given the ABC transporter influence on TKI actions, ABC transporter inhibitors may reverse the resistance. In addition, researchers73 found that GW583340 and GW2974, EGFR and HER-2 inhibitors can reverse ABCG2- and ABCB1-mediated drug resistance.
8. Echinoderm microtubule-associated protein-like 4-the anaplastic lymphoma kinase (EML4-ALK) fusion gene and the ALK secondary mutation
The EML4-ALK fusion oncogene was identified as a novel genetic alteration in NSCLC74. Patients harboring ALK rearrangements tend to be non-smokers or light smokers, have a history of adenocarcinoma, and tend to be younger in age75. The EML4-ALK fusion gene was present at a high frequency in Chinese NSCLC patients, particularly in those with adenocarcinomas lacking EGFR/K-RAS mutations76. Recently, crizotinib was identified as a potent inhibitor of ALK and MET tyrosine kinases77. Crizotinib was well tolerated and resulted in important tumor shrinkage in NSCLC EML4-ALK positive patients. However, some patients have resistance to crizotinib and other EML4-ALK inhibitors are in development. Researchers78, 79 found that the naive NSCLC patients with ALK rearrangements also had concurrent EGFR activating mutations. The resistance mechanisms to ALK TKIs may be mediated by both ALK secondary mutation and a bypass signaling pathway such as EGFR. These mechanisms can occur independently, or in the same cancer. Combination therapy with EGFR-TKIs and ALK inhibitors can improve the anti-tumor effect.
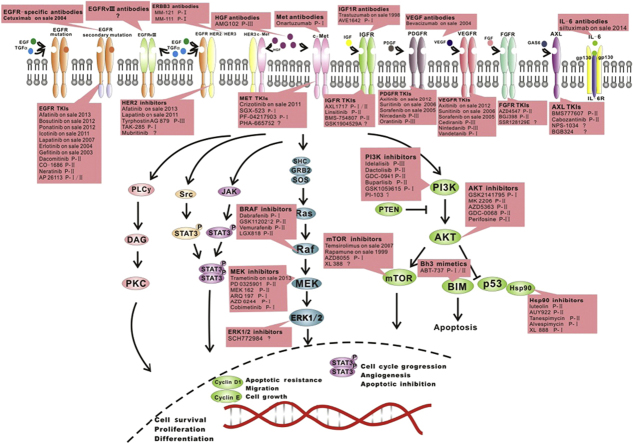
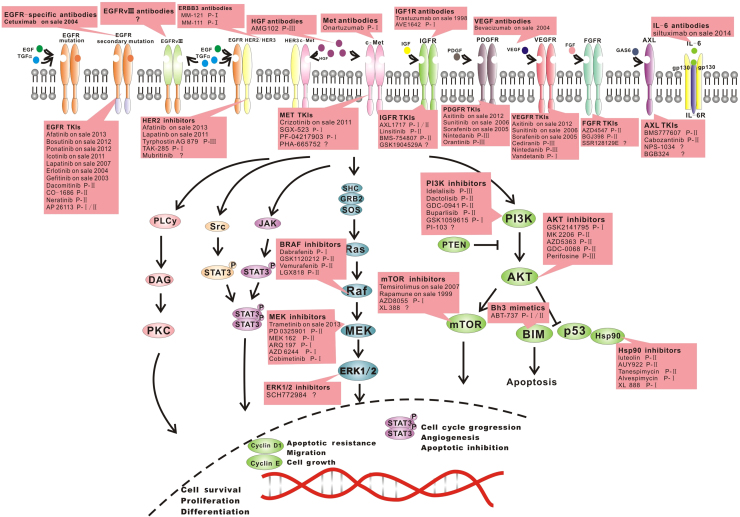
9. Therapeutic strategies to overcome resistance
The existence of various resistance mechanisms is clear, and it is wise to identify the specific mechanisms in a patient so that a suitable, effective strategy can be chosen. The next generation of EGFR-TKIs and specific antibodies of the relative molecules are under development. MicroRNAs will soon be employed in treating resistance to EGFR-TKIs in cancers. Clinical trials are engaged in examining the potential targets less susceptible to resistance. The relative clinical trials or therapeutic strategies are listed in the table (Table 111, 18, 30, 62, 73, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96 and Fig. 5). In practice, combined treatments or therapies with multiple targets may show more powerful efficacy, and the multi-targeting drugs may show superior efficacy.
Table 1.
Therapeutic strategies and clinical trials to overcome resistance of EGFR-TKIs, "–" stands for no drugs for therapy.
| Resistant mechanism | Strategy | Clinical research | Ref. |
|---|---|---|---|
| EGFRmutation | |||
| T790M | EGFR-TKIs combined/+antibodies | Afatinib+cexitumab | 80 |
| T790M-specific inhibitors | CO-1686/AZD9291 | 81, 82 | |
| c-Met inhibitors+PI3K inhibitors | GDC0973+GDC0941 | 83 | |
| HSP90 inhibitors | Luteolin/ganetespib | 11, 84 | |
| EGFR-TKIs+MEK inhibitors | Afatinib+ARQ 197 | 85 | |
| Glycolysis inhibition+EGFR-TKIs | Afatinib+AUY922 | 86 | |
| Bypass pathway | |||
| HER family abnormality | HER inhibitors+EGFR-TKIs | Afatinib/dacomitinib | 18, 87 |
| c-Met amplification | EGFR-TKIs+c-Met inhibitors | Erlotinib+crizotinib | 88 |
| Dacomitinib+crizotinib | |||
| HGF overexpression | EGFR-TKIs+PI3K inhibitors | Gefitinib+PI-103 | 89 |
| Triple inhibition of EGFR/Met/VEGF | – | 90 | |
| IGFR abnormality | IGFR inhibitors+EGFR-TKIs | AG1024+gefitinib | – |
| EGFRvIII | EGFRvIII antibodies | – | – |
| VEGF/VEGFR abnormality | EGFR-TKIs+VEGF inhibitors | ZD6474 | 91 |
| MEK inhibitors+VEGF inhibitors | ZD6474+PD0325901 | 30 | |
| PDGF/PDGFR abnormality | EGFR-TKIs+PDGF inhibitors | – | – |
| FGF/FGFR abnormality | EGFR-TKIs+FGF inhibitors | – | – |
| IL-6 abnormality | IL-6 antibodies | Siltuximab | 92 |
| AXL abnormality | AXL inhibitors | NPS-1034 | 93 |
| CRKL amplification | Unknown | Unknown | – |
| Integrin beta1 overexpression | Unknown | Unknown | – |
| Downstream pathway | |||
| K-RAS mutations | PI3K inhibitors+MEK inhibitors | GDC-0941+AZD6244 | 94 |
| BRAF mutations | BRAF inhibitors+MEK inhibitors | Dabrafenib+trametinib | 95 |
| Loss of PTEN | mTOR inhibitors/AKT inhibitors | – | – |
| PIK3CA mutation | EGFR-TKIs+PI3K inhibitors | Gefitinib+BKM120 | 96 |
| Low expression of NF1 | Unknown | Unknown | – |
| Apoptosis pathway | |||
| BIM BH3 deletion | EGFR-TKIs+PP2A activator | Erlotinib+FTY720 gefitinib+FTY720 | 62 |
| Histologic transformation | |||
| EMT | EGFR-TKIs+MEK1/2 inhibitors | – | – |
| SCLC transformation | Platinum+VP16/EGFR-TKIs | – | – |
| ABC effusion | EGFR-TKIs+HER-2 inhibitors | GW583340/GW2974 | 73 |
| Unknown mechanism | EGFR-TKIs combined | Afatinib+cexitumab | 80 |
| EGFR-TKIs+glycolysis inhibitors | Erlotinib+AUY922 | ||
Figure 5.
The potential targets for the relative treatment to overcome the resistance to EGFR-TKIs.
10. Conclusions
Increasing evidence shows that the primary or acquired resistance to first- or second-generation EGFR-TKIs explains why patients who initially benefited from these treatments later do not. Though some of the mechanisms of resistance have been identified, much additional information is needed to understand and overcome resistance to these agents.
Acknowledgment
The work was supported by the National Natural Science Foundation of China (No. 81473233).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Ciardiello F., Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 2.Bethune G., Bethune D., Ridgway N., Xu Z. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis. 2010;2:48–51. [PMC free article] [PubMed] [Google Scholar]
- 3.Yu H.A., Arcila M.E., Rekhtman N., Sima C.S., Zakowski M.F., Pao W. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi S., Boggon T.J., Dayaram T., Jänne P.A., Kocher O., Meyerson M. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 5.Yun C.H., Mengwasser K.E., Toms A.V., Woo M.S., Greulich H., Wong K.K. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma C., Wei S., Song Y. T790M and acquired resistance of EGFR TKI: a literature review of clinical reports. J Thorac Dis. 2011;3:10–18. doi: 10.3978/j.issn.2072-1439.2010.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita Y., Suda K., Kimura H., Matsumoto K., Arao T., Nagai T. Highly sensitive detection of EGFR T790M mutation using colony hybridization predicts favorable prognosis of patients with lung cancer harboring activating EGFR mutation. J Thorac Oncol. 2012;7:1640–1644. doi: 10.1097/JTO.0b013e3182653d7f. [DOI] [PubMed] [Google Scholar]
- 8.Su K.Y., Chen H.Y., Li K.C., Kuo M.L., Yang J.C., Chan W.K. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:433–440. doi: 10.1200/JCO.2011.38.3224. [DOI] [PubMed] [Google Scholar]
- 9.Suda K., Onozato R., Yatabe Y., Mitsudomi T. EGFR T790M mutation: a double role in lung cancer cell survival? J Thorac Oncol. 2009;4:1–4. doi: 10.1097/JTO.0b013e3181913c9f. [DOI] [PubMed] [Google Scholar]
- 10.Hong Y.S., Jang W.J., Chun K.S., Jeong C.H. Hsp90 inhibition by WK88-1 potently suppresses the growth of gefitinib-resistant H1975 cells harboring the T790M mutation in EGFR. Oncol Rep. 2014;31:2619–2624. doi: 10.3892/or.2014.3161. [DOI] [PubMed] [Google Scholar]
- 11.Smith D.L., Acquaviva J., Sequeira M., Jimenez J.P., Zhang C., Sang J. The HSP90 inhibitor ganetespib potentiates the antitumor activity of EGFR tyrosine kinase inhibition in mutant and wild-type non-small cell lung cancer. Target Oncol. 2015;10:235–245. doi: 10.1007/s11523-014-0329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa D.B., Halmos B., Kumar A., Schumer S.T., Huberman M.S., Boggon T.J. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007;4:1669–1679. doi: 10.1371/journal.pmed.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toyooka S., Date H., Uchida A., Kiura K., Takata M. The epidermal growth factor receptor D761Y mutation and effect of tyrosine kinase inhibitor. Clin Cancer Res. 2007;13:3431. doi: 10.1158/1078-0432.CCR-07-0070. [DOI] [PubMed] [Google Scholar]
- 14.Bean J., Riely G.J., Balak M., Marks J.L., Ladanyi M., Miller V.A. Acquired resistance to epidermal growth factor receptor kinase inhibitors associated with a novel T854A mutation in a patient with EGFR-mutant lung adenocarcinoma. Clin Cancer Res. 2008;14:7519–7525. doi: 10.1158/1078-0432.CCR-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo H.S., Ahn H.K., Lee H.Y., Park I., Kim Y.S., Hong J. Epidermal growth factor receptor (EGFR) exon 20 mutations in non-small-cell lung cancer and resistance to EGFR-tyrosine kinase inhibitors. Invest New Drugs. 2014;32:1311–1315. doi: 10.1007/s10637-014-0146-x. [DOI] [PubMed] [Google Scholar]
- 16.Khan N.A., Mirshahidi S., Mirshahidi H.R. A novel insertion mutation on exon 20 of epidermal growth factor receptor, conferring resistance to erlotinib. Case Rep Oncol. 2014;7:491–496. doi: 10.1159/000365325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landi L., Cappuzzo F. HER2 and lung cancer. Expert Rev Anticancer Ther. 2013;13:1219–1228. doi: 10.1586/14737140.2013.846830. [DOI] [PubMed] [Google Scholar]
- 18.Nishida Y., Kuwata T., Nitta H., Dennis E., Aizawa M., Kinoshita T. A novel gene–protein assay for evaluating HER2 status in gastric cancer: simultaneous analyses of HER2 protein overexpression and gene amplification reveal intratumoral heterogeneity. Gastric Cancer. 2014 Jun 11 doi: 10.1007/s10120-014-0394-7. [DOI] [PubMed] [Google Scholar]
- 19.Engelman J.A., Zejnullahu K., Mitsudomi T., Song Y., Hyland C., Park J.O. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 20.Xia W., Petricoin E.F., 3rd, Zhao S., Liu L., Osada T., Cheng Q. An heregulin–EGFR–HER3 autocrine signaling axis can mediate acquired lapatinib resistance in HER2+ breast cancer models. Breast Cancer Res. 2013;15:R85. doi: 10.1186/bcr3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakata S., Tanaka H., Ito Y., Hara M., Fujita M., Kondo E. Deficient HER3 expression in poorly-differentiated colorectal cancer cells enhances gefitinib sensitivity. Int J Oncol. 2014;45:1583–1593. doi: 10.3892/ijo.2014.2538. [DOI] [PubMed] [Google Scholar]
- 22.Schoeberl B., Faber A.C., Li D., Liang M.C., Crosby K., Onsum M. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res. 2010;70:2485–2494. doi: 10.1158/0008-5472.CAN-09-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubo T., Yamamoto H., Lockwood W.W., Valencia I., Soh J., Peyton M. MET gene amplification or EGFR mutation activate MET in lung cancers untreated with EGFR tyrosine kinase inhibitors. Int J Cancer. 2009;124:1778–1784. doi: 10.1002/ijc.24150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yano S., Wang W., Li Q., Matsumoto K., Sakurama H., Nakamura T. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–9487. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 25.Mueller K.L., Madden J.M., Zoratti G.L., Kuperwasser C., List K., Boerner J.L. Fibroblast-secreted hepatocyte growth factor mediates epidermal growth factor receptor tyrosine kinase inhibitor resistance in triple-negative breast cancers through paracrine activation of Met. Breast Cancer Res. 2012;14:R104. doi: 10.1186/bcr3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guix M., Faber A.C., Wang S.E., Olivares M.G., Song Y., Qu S. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–2619. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peled N., Wynes M.W., Ikeda N., Ohira T., Yoshida K., Qian J. Insulin-like growth factor-1 receptor (IGF-1R) as a biomarker for resistance to the tyrosine kinase inhibitor gefitinib in non-small cell lung cancer. Cell Oncol. 2013;36:277–288. doi: 10.1007/s13402-013-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jameson M.J., Taniguchi L.E., VanKoevering K.K., Stuart M.M., Francom C.R., Mendez R.E. Activation of the insulin-like growth factor-1 receptor alters p27 regulation by the epidermal growth factor receptor in oral squamous carcinoma cells. J Oral Pathol Med. 2013;42:332–338. doi: 10.1111/jop.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballas M.S., Chachoua A. Rationale for targeting VEGF, FGF, and PDGF for the treatment of NSCLC. Onco Targets Ther. 2011;4:43–58. doi: 10.2147/OTT.S18155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee S., Heukamp L.C., Siobal M., Schöttle J., Wieczorek C., Peifer M. Tumor VEGF:VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J Clin Invest. 2013;123:1732–1740. doi: 10.1172/JCI65385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bianco R., Rosa R., Damiano V., Daniele G., Gelardi T., Garofalo S. Vascular endothelial growth factor receptor-1 contributes to resistance to anti-epidermal growth factor receptor drugs in human cancer cells. Clin Cancer Res. 2008;14:5069–5080. doi: 10.1158/1078-0432.CCR-07-4905. [DOI] [PubMed] [Google Scholar]
- 32.Ware K.E., Marshall M.E., Heasley L.R., Marek L., Hinz T.K., Hercule P. Rapidly acquired resistance to EGFR tyrosine kinase inhibitors in NSCLC cell lines through de-repression of FGFR2 and FGFR3 expression. PLoS One. 2010;5:e14117. doi: 10.1371/journal.pone.0014117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azuma K., Kawahara A., Sonoda K., Nakashima K., Tashiro K., Watari K. FGFR1 activation is an escape mechanism in human lung cancer cells resistant to afatinib, a pan-EGFR family kinase inhibitor. Oncotarget. 2014;5:5908–5919. doi: 10.18632/oncotarget.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akhavan D., Pourzia A.L., Nourian A.A., Williams K.J., Nathanson D., Babic I. De-repression of PDGFRβ transcription promotes acquired resistance to EGFR tyrosine kinase inhibitors in glioblastoma patients. Cancer Discov. 2013;3:534–547. doi: 10.1158/2159-8290.CD-12-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonavia R., Inda M.M., Vandenberg S., Cheng S.Y., Nagane M., Hadwiger P. EGFRvIII promotes glioma angiogenesis and growth through the NF-κB, interleukin-8 pathway. Oncogene. 2012;31:4054–4066. doi: 10.1038/onc.2011.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katanasaka Y., Kodera Y., Kitamura Y., Morimoto T., Tamura T., Koizumi F. Epidermal growth factor receptor variant type III markedly accelerates angiogenesis and tumor growth via inducing c-myc mediated angiopoietin-like 4 expression in malignant glioma. Mol Cancer. 2013;12:31. doi: 10.1186/1476-4598-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z., Lee J.C., Lin L., Olivas V., Au V., LaFramboise T. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee Y., Lee M., Kim S. GAS6 induces cancer cell migration and epithelial–mesenchymal transition through upregulation of MAPK and Slug. Biochem Biophys Res Commun. 2013;434:8–14. doi: 10.1016/j.bbrc.2013.03.082. [DOI] [PubMed] [Google Scholar]
- 39.Kim S.M., Kwon O.J., Hong Y.K., Kim J.H., Solca F., Ha S.J. Activation of IL-6R/JAK1/STAT3 signaling induces de novo resistance to irreversible EGFR inhibitors in non-small cell lung cancer with T790M resistance mutation. Mol Cancer Ther. 2012;11:2254–2264. doi: 10.1158/1535-7163.MCT-12-0311. [DOI] [PubMed] [Google Scholar]
- 40.Ishiguro Y., Ishiguro H., Miyamoto H. Epidermal growth factor receptor tyrosine kinase inhibition up-regulates interleukin-6 in cancer cells and induces subsequent development of interstitial pneumonia. Oncotarget. 2013;4:550–559. doi: 10.18632/oncotarget.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y., van Boxel-Dezaire A.H., Cheon H., Yang J., Stark G.R. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc Natl Acad Sci U S A. 2013;110:16975–16980. doi: 10.1073/pnas.1315862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao Z., Fenoglio S., Gao D.C., Camiolo M., Stiles B., Lindsted T. TGF-β IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci U S A. 2010;107:15535–15540. doi: 10.1073/pnas.1009472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suda K., Mizuuchi H., Murakami I., Uramoto H., Tanaka F., Sato K. CRKL amplification is rare as a mechanism for acquired resistance to kinase inhibitors in lung cancers with epidermal growth factor receptor mutation. Lung Cancer. 2014;85:147–151. doi: 10.1016/j.lungcan.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 44.Cheung H.W., Du J., Boehm J.S., He F., Weir B.A., Wang X. Amplification of CRKL induces transformation and epidermal growth factor receptor inhibitor resistance in human non-small cell lung cancers. Cancer Discov. 2011;1:608–625. doi: 10.1158/2159-8290.CD-11-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin F., Xie C.Y., Li Q.C., Dong Q.Z., Wang E.H., Wang Y. CRKL promotes lung cancer cell invasion through ERK–MMP9 pathway. Mol Carcinog. 2015;54 Suppl 1:E35–44. doi: 10.1002/mc.22148. [DOI] [PubMed] [Google Scholar]
- 46.Ju L., Zhou C., Li W., Yan L. Integrin beta1 over-expression associates with resistance to tyrosine kinase inhibitor gefitinib in non-small cell lung cancer. J Cell Biochem. 2010;111:1565–1574. doi: 10.1002/jcb.22888. [DOI] [PubMed] [Google Scholar]
- 47.Huang C., Park C.C., Hilsenbeck S.G., Ward R., Rimawi M.F., Wang Y.C. β1 Integrin mediates an alternative survival pathway in breast cancer cells resistant to lapatinib. Breast Cancer Res. 2011;13:R84. doi: 10.1186/bcr2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ju L., Zhou C. Association of integrin beta1 and c-MET in mediating EGFR TKI gefitinib resistance in non-small cell lung cancer. Cancer Cell Int. 2013;13:15. doi: 10.1186/1475-2867-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanda R., Kawahara A., Watari K., Murakami Y., Sonoda K., Maeda M. Erlotinib resistance in lung cancer cells mediated by integrin β1/Src/Akt-driven bypass signaling. Cancer Res. 2013;73:6243–6253. doi: 10.1158/0008-5472.CAN-12-4502. [DOI] [PubMed] [Google Scholar]
- 50.Brown W.S., Wendt M.K. Integrin-mediated resistance to epidermal growth factor receptor-targeted therapy: an inflammatory situation. Breast Cancer Res. 2014;16:448. doi: 10.1186/s13058-014-0448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pao W., Wang T.Y., Riely G.J., Miller V.A., Pan Q., Ladanyi M. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Mello R.A., Marques D.S., Medeiros R., Araújo A.M. Epidermal growth factor receptor and K-RAS in non-small cell lung cancer-molecular pathways involved and targeted therapies. World J Clin Oncol. 2011;2:367–376. doi: 10.5306/wjco.v2.i11.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Repasky G.A., Chenette E.J., Der C.J. Renewing the conspiracy theory debate: does RAF function alone to mediate RAS oncogenesis? Trends Cell Biol. 2004;14:639–647. doi: 10.1016/j.tcb.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Bidkhori G., Moeini A., Masoudi-Nejad A. Modeling of tumor progression in NSCLC and intrinsic resistance to TKI in loss of PTEN expression. PLoS One. 2012;7:e48004. doi: 10.1371/journal.pone.0048004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sos M.L., Koker M., Weir B.A., Heynck S., Rabinovsky R., Zander T. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009;69:3256–3261. doi: 10.1158/0008-5472.CAN-08-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim E.J., Jeong J.H., Bae S., Kang S., Kim C.H., Lim Y.B. mTOR inhibitors radiosensitize PTEN-deficient non-small-cell lung cancer cells harboring an EGFR activating mutation by inducing autophagy. J Cell Biochem. 2013;114:1248–1256. doi: 10.1002/jcb.24465. [DOI] [PubMed] [Google Scholar]
- 57.Ohashi K., Sequist L.V., Arcila M.E., Moran T., Chmielecki J., Lin Y.L. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A. 2012;109:E2127–E2133. doi: 10.1073/pnas.1203530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whyte D.B., Holbeck S.L. Correlation of PIK3CA mutations with gene expression and drug sensitivity in NCI-60 cell lines. Biochem Biophys Res Commun. 2006;340:469–475. doi: 10.1016/j.bbrc.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 59.Wang L., Hu H., Pan Y., Wang R., Li Y., Shen L. PIK3CA mutations frequently coexist with EGFR/KRAS mutations in non-small cell lung cancer and suggest poor prognosis in EGFR/KRAS wildtype subgroup. PLoS One. 2014;9:e88291. doi: 10.1371/journal.pone.0088291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruin E.C., Cowell C., Warne P.H., Jiang M., Saunders R.E., Melnick M.A. Reduced NF1 expression confers resistance to EGFR inhibition in lung cancer. Cancer Discov. 2014;4:606–619. doi: 10.1158/2159-8290.CD-13-0741. de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ng K.P., Hillmer A.M., Chuah C.T., Juan W.C., Ko T.K., Teo A.S. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012;18:521–528. doi: 10.1038/nm.2713. [DOI] [PubMed] [Google Scholar]
- 62.Kiyota M., Kuroda J., Yamamoto-Sugitani M., Shimura Y., Nakayama R., Nagoshi H. FTY720 induces apoptosis of chronic myelogenous leukemia cells via dual activation of BIM and BID and overcomes various types of resistance to tyrosine kinase inhibitors. Apoptosis. 2013;18:1437–1446. doi: 10.1007/s10495-013-0882-y. [DOI] [PubMed] [Google Scholar]
- 63.Thiery J.P. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 64.Bae G.Y., Choi S.J., Lee J.S., Jo J., Lee J., Kim J. Loss of E-cadherin activates EGFR–MEK/ERK signaling, which promotes invasion via the ZEB1/MMP2 axis in non-small cell lung cancer. Oncotarget. 2013;4:2512–2522. doi: 10.18632/oncotarget.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palena C., Hamilton D.H., Fernando R.I. Influence of IL-8 on the epithelial–mesenchymal transition and the tumor microenvironment. Future Oncol. 2012;8:713–722. doi: 10.2217/fon.12.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie M., He C.S., Wei S.H., Zhang L. Notch-1 contributes to epidermal growth factor receptor tyrosine kinase inhibitor acquired resistance in non-small cell lung cancer in vitro and in vivo. Eur J Cancer. 2013;49:3559–3572. doi: 10.1016/j.ejca.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Capaccione K.M., Hong X., Morgan K.M., Liu W., Bishop J.M., Liu L. Sox9 mediates Notch1-induced mesenchymal features in lung adenocarcinoma. Oncotarget. 2014;5:3636–3650. doi: 10.18632/oncotarget.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu M.M., Mao G.X., Liu J., Li J.C., Huang H., Liu Y.F. Low expression of the FoxO4 gene may contribute to the phenomenon of EMT in non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15:4013–4018. doi: 10.7314/apjcp.2014.15.9.4013. [DOI] [PubMed] [Google Scholar]
- 69.Park K.S., Raffeld M., Moon Y.W., Xi L., Bianco C., Pham T. CRIPTO1 expression in EGFR-mutant NSCLC elicits intrinsic EGFR-inhibitor resistance. J Clin Invest. 2014;124:3003–3015. doi: 10.1172/JCI73048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson C., Nicholes K., Bustos D., Lin E., Song Q., Stephan J.P. Overcoming EMT-associated resistance to anti-cancer drugs via Src/FAK pathway inhibition. Oncotarget. 2014;5:7328–7341. doi: 10.18632/oncotarget.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alam N., Gustafson K.S., Ladanyi M., Zakowski M.F., Kapoor A., Truskinovsky A.M. Small-cell carcinoma with an epidermal growth factor receptor mutation in a never-smoker with gefitinib-responsive adenocarcinoma of the lung. Clin Lung Cancer. 2010;11:E1–E4. doi: 10.3816/CLC.2010.n.046. [DOI] [PubMed] [Google Scholar]
- 72.Shi Z., Tiwari A.K., Shukla S., Robey R.W., Singh S., Kim I.W. Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011;71:3029–3041. doi: 10.1158/0008-5472.CAN-10-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sodani K., Tiwari A.K., Singh S., Patel A., Xiao Z.J., Chen J.J. GW583340 and GW2974, human EGFR and HER-2 inhibitors, reverse ABCG2- and ABCB1-mediated drug resistance. Biochem Pharmacol. 2012;83:1613–1622. doi: 10.1016/j.bcp.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soda M., Choi Y.L., Enomoto M., Takada S., Yamashita Y., Ishikawa S. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 75.Kwak E.L., Bang Y.J., Camidge D.R., Shaw A.T., Solomon B., Maki R.G. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X., Zhang S., Yang X., Yang J., Zhou Q., Yin L. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer. 2010;9:188. doi: 10.1186/1476-4598-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pearson R., Kolesar J.M. Targeted therapy for NSCLC: ALK inhibition. J Oncol Pharm Pract. 2012;18:271–274. doi: 10.1177/1078155211417477. [DOI] [PubMed] [Google Scholar]
- 78.Yamada T., Takeuchi S., Nakade J., Kita K., Nakagawa T., Nanjo S. Paracrine receptor activation by microenvironment triggers bypass survival signals and ALK inhibitor resistance in EML4-ALK lung cancer cells. Clin Cancer Res. 2012;18:3592–3602. doi: 10.1158/1078-0432.CCR-11-2972. [DOI] [PubMed] [Google Scholar]
- 79.Sasaki T., Koivunen J., Ogino A., Yanagita M., Nikiforow S., Zheng W. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011;71:6051–6060. doi: 10.1158/0008-5472.CAN-11-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perez-Torres M., Guix M., Gonzalez A., Arteaga C.L. Epidermal growth factor receptor (EGFR) antibody down-regulates mutant receptors and inhibits tumors expressing EGFR mutations. J Biol Chem. 2006;281:40183–40192. doi: 10.1074/jbc.M607958200. [DOI] [PubMed] [Google Scholar]
- 81.Walter A.O., Sjin R.T., Haringsma H.J., Ohashi K., Sun J., Lee K. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov. 2013;3:1404–1415. doi: 10.1158/2159-8290.CD-13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cross D.A., Ashton S.E., Ghiorghiu S., Eberlein C., Nebhan C.A., Spitzler P.J. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoeflich K.P., Merchant M., Orr C., Chan J., Den Otter D., Berry L. Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res. 2012;72:210–219. doi: 10.1158/0008-5472.CAN-11-1515. [DOI] [PubMed] [Google Scholar]
- 84.Hong Z., Cao X., Li N., Zhang Y., Lan L., Zhou Y. Luteolin is effective in the non-small cell lung cancer model with L858R/T790M EGF receptor mutation and erlotinib resistance. Br J Pharmacol. 2014;171:2842–2853. doi: 10.1111/bph.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qu G., Liu C., Sun B., Zhou C., Zhang Z., Wang P. Combination of BIBW2992 and ARQ 197 is effective against erlotinib-resistant human lung cancer cells with the EGFR T790M mutation. Oncol Rep. 2014;32:341–347. doi: 10.3892/or.2014.3178. [DOI] [PubMed] [Google Scholar]
- 86.Kim S.M., Yun M.R., Hong Y.K., Solca F., Kim J.H., Kim H.J. Glycolysis inhibition sensitizes non-small cell lung cancer with T790M mutation to irreversible EGFR inhibitors via translational suppression of Mcl-1 by AMPK activation. Mol Cancer Ther. 2013;12:2145–2156. doi: 10.1158/1535-7163.MCT-12-1188. [DOI] [PubMed] [Google Scholar]
- 87.Engelman J.A., Zejnullahu K., Gale C.M., Lifshits E., Gonzales A.J., Shimamura T. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67:11924–11932. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]
- 88.Nanjo S., Yamada T., Nishihara H., Takeuchi S., Sano T., Nakagawa T. Ability of the Met kinase inhibitor crizotinib and new generation EGFR inhibitors to overcome resistance to EGFR inhibitors. PLoS One. 2013;8:e84700. doi: 10.1371/journal.pone.0084700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Graves E.E., Maity A., Le Q.T. The tumor microenvironment in non-small-cell lung cancer. Semin Radiat Oncol. 2010;20:156–163. doi: 10.1016/j.semradonc.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakade J., Takeuchi S., Nakagawa T., Ishikawa D., Sano T., Nanjo S. Triple inhibition of EGFR, Met, and VEGF suppresses regrowth of HGF-triggered, erlotinib-resistant lung cancer harboring an EGFR mutation. J Thorac Oncol. 2014;9:775–783. doi: 10.1097/JTO.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sarkar S., Rajput S., Tripathi A.K., Mandal M. Targeted therapy against EGFR and VEGFR using ZD6474 enhances the therapeutic potential of UV-B phototherapy in breast cancer cells. Mol Cancer. 2013;12:122. doi: 10.1186/1476-4598-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song L., Smith M.A., Doshi P., Sasser K., Fulp W., Altiok S. Antitumor efficacy of the anti-interleukin-6 (IL-6) antibody siltuximab in mouse xenograft models of lung cancer. J Thorac Oncol. 2014;9:974–982. doi: 10.1097/JTO.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rho J.K., Choi Y.J., Kim S.Y., Kim T.W., Choi E.K., Yoon S.J. MET and AXL inhibitor NPS-1034 exerts efficacy against lung cancer cells resistant to EGFR kinase inhibitors because of MET or AXL activation. Cancer Res. 2014;74:253–262. doi: 10.1158/0008-5472.CAN-13-1103. [DOI] [PubMed] [Google Scholar]
- 94.Hofmann I., Weiss A., Elain G., Schwaederle M., Sterker D., Romanet V. K-RAS mutant pancreatic tumors show higher sensitivity to MEK than to PI3K inhibition in vivo. PLoS One. 2012;7:e44146. doi: 10.1371/journal.pone.0044146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rutkowski P., Blank C. Dabrafenib for the treatment of BRAF V600-positive melanoma: a safety evaluation. Expert Opin Drug Saf. 2014;13:1249–1258. doi: 10.1517/14740338.2014.939954. [DOI] [PubMed] [Google Scholar]
- 96.Ren H., Zhao L., Li Y., Yue P., Deng X., Owonikoko T.K. The PI3 kinase inhibitor NVP-BKM120 induces GSK3/FBXW7-dependent Mcl-1 degradation, contributing to induction of apoptosis and enhancement of TRAIL-induced apoptosis. Cancer Lett. 2013;338:229–238. doi: 10.1016/j.canlet.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]