Abstract
A novel method for the simultaneous determination of 3-nitrotyrosine (NT) and 3-chlorotyrosine (CT) in human plasma has been developed based on direct analysis in real time–tandem mass spectrometry (DART–MS/MS). Analysis was performed in the positive ionization mode using multiple reaction monitoring (MRM) of the ion transitions at m/z 216.2/170.1 for CT, m/z 227.2/181.1 for NT and m/z 230.2/184.2 for the internal standard, d3-NT. The assay was linear in the ranges 0.5–100 μg/mL for CT and 4–100 μg/mL for NT with corresponding limits of detection of 0.2 and 2 μg/mL. Intra- and inter-day precisions and accuracies were respectively <15% and ±15%. Matrix effects were also evaluated. The method is potentially useful for high throughput analysis although sensitivity needs to be improved before it can be applied in clinical research.
KEY WORDS: 3-Nitrotyrosine, 3-Chlorotyrosine, Determintion, DART–MS/MS, Human plasma
Graphical abstract
A novel method for the simultaneous determination of 3-nitrotyrosine (NT) and 3-chlorotyrosine (CT) in human plasma has been developed based on direct analysis in real time–tandem mass spectrometry (DART–MS/MS). The method is potentially useful for high throughput analysis.
1. Introduction
3-Nitrotyrosine (NT) and 3-chlorotyrosin (CT) are oxidation products of reactive oxygen species and other radicals formed under inflammatory conditions. CT is formed when neutrophil- and monocyte-derived myeloperoxidase catalyzes the formation of hypochlorous acid which then chlorinates tyrosine residues in proteins1, 2, 3, 4. NT is formed when the superoxide anion reacts with nitric oxide (NO•) to produce the powerful oxidant peroxynitrite (ONOO−), which nitrates tyrosine residues3, 5, 6, 7. Many researchers have reported that NT and CT are associated with many disorders such as lung and cardiovascular pathologies, atherosclerosis, autoimmune diseases, type 2 diabetes mellitus, and other inflammatory conditions7, 8, 9, 10, 11, 12, 13, 14, 15, 16. Because of this, the determination of NT and/or CT is of great importance in understanding the etiology of these disorders.
To date, a number of analytical methods have been developed to determine plasma NT and CT including ELISA17, surface plasmon resonance immunoassay18, high performance liquid chromatography (HPLC) after derivatization19, HPLC with electrochemical20, 21 and fluorescence22 detection, gas chromatography (GC) with electrochemical (GC–ECD) detection23, GC with mass spectrometric (GC–MS) detection with24 and without25 derivatization, GC tandem mass spectrometry (GC–MS/MS)26, liquid chromatography mass spectrometry (LC–MS)27 and LC–MS/MS 25, 28. The application of MS to the determination of NT has been recently reviewed29. However, since these methods require sample preparation and, in the case of the chromatographic methods, retention and separation, they are limited for high throughput bioanalysis. Accordingly we have investigated the application of direct analysis in real time-tandem mass spectrometry (DART–MS/MS), a technique which requires minimal or no sample preparation.
Direct analysis in real time (DART)30, 32 is a novel ionization technique which relies on the fundamental principles of atmospheric pressure chemical ionization (APCI). The DART ion source consists of a tube containing a chamber through which helium or nitrogen flows at atmospheric pressure. A glow discharge is initiated by applying a kilovolt potential between a needle electrode and a grounded counter electrode. The gas exiting the chamber then passes through a tube containing a perforated intermediate electrode, an optional gas heater, and a grid electrode positioned at the exit behind an insulating cap. Ionization occurs when the gas makes contact with a sample in the open air gap between the DART outlet and the mass spectrometer sampling orifice30. The technique has been successfully employed for the analysis of human tissues and body fluids without sample preparation31, 32. This paper reports the application of DART–MS/MS to the determination of NT and CT in human plasma.
2. Materials and methods
2.1. Materials
CT and NT were purchased from Sigma-Aldrich. Deuterium-labeled NT (d3-NT) for use as internal standard (IS) was purchased from CDN Isotopes, Inc. Acetonitrile was HPLC grade. Ultrapure water was obtained using a Milli-Q RG unit (Millipore, Bedford, USA). Dip-it samplers were purchased from Aspec Technologies Ltd. (Beijing). Plasma samples for analysis were prepared from blood samples immediately after collection for diagnostic tests by centrifugation at 3000 rpm for 10 min at 4 °C and kept frozen at −20 °C until required. All procedures were performed in accordance with the local Ethics Committee guidelines.
2.2. Instrumentation and experimental conditions
A DART 100 source (IonSense Inc, Saugus, USA) with Control Software (Version 2.03) was coupled to a 5500 triple quadrupole tandem mass spectrometer (Applied Biosystems, AB Sciex, Toronto Canada) using Analyst 1.5 software (AB Sciex). The DART orifice, the ceramic tube (4 mm i.d.×7.3 cm length) and the mass spectrometer orifice were aligned so that the stream of helium from the DART source was introduced into the mass spectrometer orifice. Introduction of samples into the DART gas stream was controlled by an acquiring module with dip-it samplers inserted into the DART source.
Analysis was performed in the positive ionization mode with multiple reaction monitoring (MRM) of the ion transitions at m/z 227.2/181.1 for NT, m/z 216.2/170.1 for CT and m/z 230.2/184.1 for the IS. Curtain gas was nitrogen (purity≥99.999%) set at 20 psi, declustering potential (DP) +80 V and collision energies (CEs) +16, +18 V and +16 V for NT, CT and IS, respectively. DART parameters were as follows: ionizing gas helium (purity≥99.999%) at 2.8 L/min and 350 °C; grid voltage +150 V; discharge needle voltage +350 V; distance between the DART orifice and the ceramic tube 4.5 cm; and sliding speed of the sample acquiring module 0.4 mm/s.
2.3. Sample preparation
Mixtures of 50 μL of plasma, 50 μL of the d3-NT solution, and 100 μL of the standard solutions were vortexed and injected directly into the DART–MS system. Concentration of analytes was calculated using calibration curves prepared using calibration standards prepared freshly on each assay day.
2.4. Assay validation
2.4.1. Preparation of calibration standards and quality control (QC) samples
Stock solutions (1 mg/mL) of CT, NT and IS were prepared with 5% acetonitrile. Standard solutions of analytes were prepared at concentrations of 0.5, 5, 10, 20, 40, 80, 100 μg/mL for CT and 4, 8, 10, 20, 40, 80, 100 μg/mL for NT. Calibration standards were prepared from plasma samples thawed at room temperature by mixing 50 μL plasma with 50 μL IS solution (200 μg/mL) and 100 μL CT and NT standard solutions. Low, medium and high QC samples were prepared in the same way at concentrations of 7.8, 25 and 78 μg/mL respectively.
2.4.2. Specificity
A number of blank plasma samples were vortexed and injected directly into the DART–MS system.
2.4.3. Linearity and sensitivity
Linearity of calibration curves based on ratios of peak areas of analyte to IS was assessed by linear regression. The limit of detection (LOD) and lower limit of quantitation (LLOQ) were calculated as 3.3×SD/slope and 10×SD/slope, respectively, where SD is the standard deviation of the analyte response at a concentration close to its LLOQ and slope is the slope of the corresponding calibration curve.
2.4.4. Accuracy, precision and matrix effects
Intra- and inter-day precision and accuracy were determined by assay of 5 replicates of low, medium and high QC samples on the same day and on three consecutive days. Precision was expressed as relative standard deviation (RSD%) and accuracy as relative error (RE%). Matrix effects (ME) were evaluated by assay of 5 replicates of QC samples and comparing the results with those obtained by assay of the corresponding standard solutions (non-matrix solutions). Matrix effects were calculated as percent of nominal concentrations (100×AQC/Astd).
3. Results and discussion
3.1. Optimizing DART–MS/MS parameters
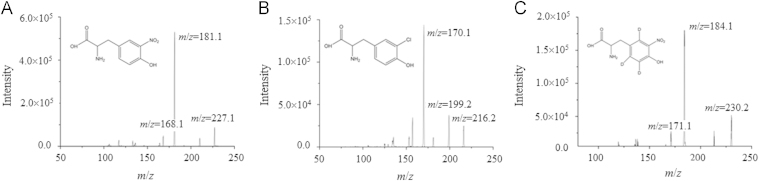
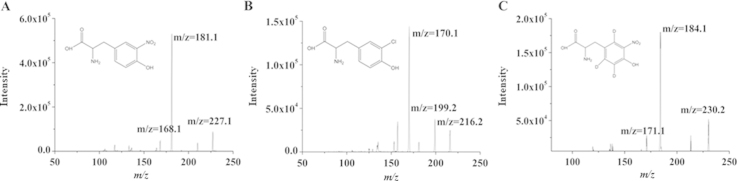
The structural formulae and product ion (MS2) spectra of the analytes and IS in the positive ionization mode are shown in Fig. 1. The precursor-product ion transitions giving the highest responses, namely m/z 227.2/181.1 for NT, m/z 216.2/170.1 for CT, and 230.2/184.1 for CT, were selected as quantifiers in the MRM mode and transitions giving the next highest response, namely m/z 227.2/168.1 for NT, m/z 216.2/199.2 for CT, and m/z 230.2/171.1 for IS, were selected as qualifiers. This assures the specificity of analysis in the absence of chromatographic separation.
Figure 1.
Structural formulae and product ion (MS2) spectra of NT, CT and IS, showing the ions selected for quantifier and qualifier transitions using the collision energies given: (A) NT m/z 227.2/181.1, 227.1/168.1 and +16 V; (B) CT m/z 216.2/170.1, 216.2/199.2 and +18 V; (C) IS m/z 230.2/184.1, 230.2/171.1, and +16 V.
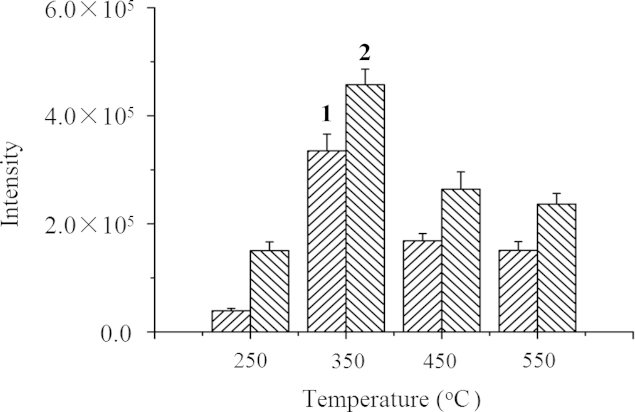
Both MS parameters (DP and CE) and DART parameters (choice of nitrogen or helium as ionizing gas, ionizing gas temperature (250, 350, 450 or 550 °C), grid voltage (+100, +150, +250 or +350 V), distance between the orifice of the DART source and the ceramic tube (45, 75 or 95 mm), and sliding speed of the sample acquiring module (0.2, 0.4, 0.6 or 0.8 mm/s) were optimized. MRM transitions and optimal parameters are as given in Section 2.2. Notably among the DART parameters, helium gave greater ionization and ion transmission than nitrogen but its temperature was a critical factor as shown in Fig. 2. CT gave a greater response than NT at all temperatures and both analytes gave the greatest response at 350 °C. The latter can be seen as the result of a balance between a temperature high enough to accelerate thermal desorption of analyte and allow more to enter the mass spectrometer and increase response and a temperature that is not too high as to cause too rapid thermal desorption or irreversible degradation resulting in loss of analyte and lower sensitivity.
Figure 2.
Effect of helium ionizing gas temperature on DART-MS/MS sensitivity for detection of NT (1) and CT (2).
3.2. Sample preparation
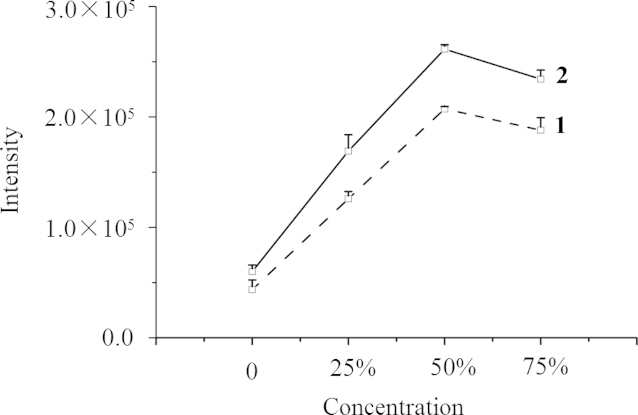
Although plasma samples were analyzed essentially without sample preparation, we optimized the preparation method to decrease the extent of the matrix effect and to obtain the maximum signal intensity. Because the organic solvent-to-water ratio (v/v) might affect the ionization efficiency of the target analyte, the standard solution was diluted with four different mixtures (water, 25%, 50%, and 75% acetonitrile). The effect is shown in Fig. 3 where it is clear that 50% acetonitrile gave a higher response for CT than for NT and the highest response for both analytes. As regards the effect of the plasma: solvent ratio, the results in Fig. 4 show that the responses of the analytes were similar at ratios of 1:1 and 1:3, although the concentrations of analytes in the latter case (method B) were one-half of those in the former case (method A). This indicates that decreasing the plasma volume in samples increases the assay sensitivity. After systematic consideration of the effects of acetonitrile concentration, plasma volume, and solubility of analytes, sample preparation involved addition of 50 μL IS solution (200 μg/mL containing 10% acetonitrile) and 100 μL 80% acetonitrile to 50 µL plasma was used. This ensured complete dissolution of the analytes while maintaining the final acetonitrile concentration as close to 50% as possible.
Figure 3.
Effect of concentration of acetonitrile on sensitivity for detection of NT (1) and CT (2).
Figure 4.
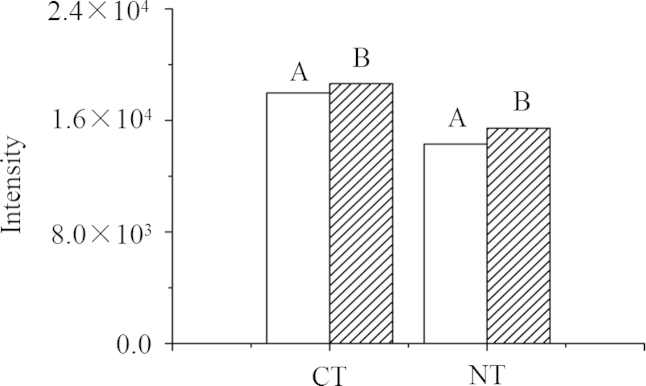
Effect of plasma:solvent ratio (v/v) on sensitivity. Method (A): 100 μL plasma+100 μL 50% acetonitrile; plasma:solvent (v/v) 1:1 (analyte concentration 20 μg/mL); method (B): 50 μL plasma+50 μL 50% acetonitrile+100 μL water, plasma:solvent (v/v) 1:3 (analyte concentration 10 μg/mL).
3.3. Assay validation
In terms of specificity, the assay was found to be free of interference from other components in plasma. Results of linearity and sensitivity assessment together with equations of calibration curves are given in Table 1. Intra- and inter-day precisions (RSD) were 2.3%–7.9% and 1.1%–5.5% for NT and 4.0%–8.4% and 2.5%–5.3% for CT with accuracies (RE) of −3.7% to 0.2% for NT and −1.7% to 7.5% for CT (Table 2). Compared with previous methods17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, DART–MS/MS was found to be as reproducible and more rapid (2–3 s), if somewhat less sensitive than some techniques. Moreover, the method is economical and not harmful to the environment as a result of no mobile phase being used.
Table 1.
Linearity and sensitivity assessment for DART–MS/MS analysis of CT and NT in human plasma.
| Analyte | Linearity | Correlation coefficient (R2) | LOD (μg/mL) | LLOQ (μg/mL) |
|---|---|---|---|---|
| CT | Y=0.0923X+1.7316 (0.5–100 μg/mL) | 0.9958 | 0.1 | 0.3 |
| NT | Y=0.1053X+0.0384 (4–100 μg/mL) | 0.9994 | 0.2 | 0.6 |
Table 2.
Intra-day and inter-day accuracy, precision and matrix effect.
| Analyte | Spiked concentration (μg/mL) | Precision (RSD%) |
Accuracy (RE%) | Matrix effect (%) | |
|---|---|---|---|---|---|
| Intra-day | Inter-day | ||||
| NT | 7.8 | 7.9 | 5.5 | 0.2 | 3.3 |
| 25 | 7.0 | 5.1 | –3.7 | 5.8 | |
| 78 | 2.3 | 1.1 | –1.9 | 5.5 | |
| CT | 7.8 | 8.4 | 5.3 | 7.5 | 5.5 |
| 25 | 6.9 | 2.9 | 0.1 | 2.7 | |
| 78 | 4.0 | 2.5 | –1.7 | 1.8 | |
Precision (RSD%)=(Standard deviation/mean)×100; Accuracy (RE%)=(Mean obtained concentration–nominal concentration)/nominal concentration×100; ME (%)=AQC/Astd×100.
3.4. Matrix effects
Matrix effects are a potentially significant problem in tandem mass spectrometric detection using direct injection with minimal sample preparation. As shown in Fig. 4, the signal responses of both CT and NT did not increase much as their concentration doubled presumably due to ion suppression resulting from the relative increase in plasma volume. As shown in Table 2, the matrix effect in plasma was severe because analytes were introduced simultaneously into the mass spectrometer with sample matrix, without LC separation being performed.
4. Conclusions
A method based on DART–MS/MS has been developed for the simultaneous determination of CT and NT in human plasma. The method required neither sample preparation nor chromatographic separation and was therefore potentially useful for high-throughput analysis. However, the levels of CT and NT in human plasma were very low and only 0.5–17.5 nmol/L and 8.0±1.0 nmol/L for NT and CT17, 19, 25, respectively, which are much lower than their respective LODs, which indicates that the sensitivity is relatively low and needs to be improved in order to facilitate application in clinical research.
Acknowledgments
The authors acknowledge Professor Qinghe Zhang and Engineer Zong Yang for kindly providing support and assistance.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Talib J, Pattison DI, Harmer JA, Celermajer DS, Davies MJ. High plasma thiocyanate levels modulate protein damage induced by myeloperoxidase and perturb measurement of 3-chlorotyrosine. Free Radic Biol Med. 2012;53:20–29. doi: 10.1016/j.freeradbiomed.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Gujral JS, Hinson JA, Jaeschke H. Chlorotyrosine protein adducts are reliable biomarkers of neutrophil-induced cytotoxicity in vivo. Comp Hepatol. 2004;3 Suppl 1:S48. doi: 10.1186/1476-5926-2-S1-S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohiuddin I, Chai H, Lin PH, Lumsden AB, Yao QZ, Chen CY. Nitrotyrosine and chlorotyrosine: clinical significance and biological functions in the vascular system. J Surg Res. 2006;133:143–149. doi: 10.1016/j.jss.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Mani AR, Ippolito S, Moreno JC, Visser TJ, Moore KP. The metabolism and dechlorination of chlorotyrosine in vivo. J Biol Chem. 2007;282:29114–29121. doi: 10.1074/jbc.M704270200. [DOI] [PubMed] [Google Scholar]
- 5.Ulrich M, Petre A, Youhnovski N, Prömm F, Schirle M, Schumm M. Post-translational tyrosine nitration of eosinophil granule toxins mediated by eosinophil peroxidase. J Biol Chem. 2008;283:28629–28640. doi: 10.1074/jbc.M801196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahsan H. 3-Nitrotyrosine: a biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum Immunol. 2013;74:1392–1399. doi: 10.1016/j.humimm.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Paier A, Agewall S, Kublickiene K. Expression of heat shock proteins and nitrotyrosine in small arteries from patients with coronary heart disease. Heart Vessels. 2009;24:260–266. doi: 10.1007/s00380-008-1117-y. [DOI] [PubMed] [Google Scholar]
- 8.Tanabe K, Kawai Y, Kitayama M, Akao H, Ishida R, Motoyama A. Increased levels of the oxidative stress marker, nitrotyrosine in patients with provocation test-induced coronary vasospasm. J Cardiol. 2014;64:86–90. doi: 10.1016/j.jjcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Mu H, Wang XW, Lin PH, Yao QZ, Chen CY. Chlorotyrosine promotes human aortic smooth muscle cell migration through increasing superoxide anion production and ERK1/2 activation. Atherosclerosis. 2008;201:67–75. doi: 10.1016/j.atherosclerosis.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng ML, Chen CM, Gu PW, Ho HY, Chiu DTY. Elevated levels of myeloperoxidase, white blood cell count and 3-chlorotyrosine in Taiwanese patients with acute myocardial infarction. Clin Biochem. 2008;41:554–560. doi: 10.1016/j.clinbiochem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Bo S, Gambino R, Guidi S, Silli B, Gentile L, Gassader M. Plasma nitrotyrosine levels, antioxidant vitamins and hyperglycaemia. Diabetic Med. 2005;22:1185–1189. doi: 10.1111/j.1464-5491.2005.01588.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang BS, Liu YH, Xu Q, Wang J, Yu XM, Guo CJ. Investigation to the levels of 3-nitrotyrosine and their correlation factors in type 2 diabetic patients. J Hygiene Res. 2009;38:433–436. [PubMed] [Google Scholar]
- 13.Lee JS, Shin JH, Hwang JH, Baek JE, Choi BS. Malondialdehyde and 3-nitrotyrosine in exhaled breath condensate in retired elderly coal miners with chronic obstructive pulmonary disease. Safety Health Work. 2014;5:91–96. doi: 10.1016/j.shaw.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricciardolo FLM, Caramori G, Ito K, Capelli A, Brun P, Abatangelo G. Nitrosative stress in the bronchial mucosa of severe chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2005;116:1028–1035. doi: 10.1016/j.jaci.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 15.Ricciardolo FLM, Di Stefano A, Sabatini F, Folkerts G. Reactive nitrogen species in the respiratory tract. Eur J Pharmacol. 2006;533:240–252. doi: 10.1016/j.ejphar.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 16.Whiteman M, Spencer JPE. Loss of 3-chlorotyrosine by inflammatory oxidants: implications for the use of 3-chlorotyrosine as a bio-marker in vivo. Biochem Biophys Res Comm. 2008;371:50–53. doi: 10.1016/j.bbrc.2008.03.153. [DOI] [PubMed] [Google Scholar]
- 17.Steege JCAT, Koster-kamphuis L, Van Straaten EA, Forget PP, Buurman WA. Nitrotyrosine in plasma of celiac disease patients as detected by a new sandwich ELISA. Free Rad Biol Med. 1998;25:953–963. doi: 10.1016/s0891-5849(98)00184-1. [DOI] [PubMed] [Google Scholar]
- 18.Jin J, Wang CY, Tao Y, Tan YJ, Yang DC, Gu Y. Determination of 3-nitrotyrosine in human urine samples by surface plasmon resonance immunoassay. Sensor Actuat B. 2011;153:164–469. [Google Scholar]
- 19.Zhang WZ, Lang C, Kaye DM. Determination of plasma free 3-nitrotyrosine and tyrosine by reversed-phase liquid chromatography with 4-fluoro-7-nitrobenzofurazan derivatization. Biomed Chromatogr. 2007;21:273–278. doi: 10.1002/bmc.750. [DOI] [PubMed] [Google Scholar]
- 20.Lu YW, Li HM, Xin J, Zhu QY, Ding F, Gu Y. Determination of 3-chlorotyrosine concentration in human plasma by HPLC with electrochemical detection. Chin J Clin Pharm. 2009;18:213–216. [Google Scholar]
- 21.Hensley K, Williamson KS, Floyd RA. Measurement of 3-nitrotyrosine and 5-nitro-γ-tocopherol by high-performance liquid chromatography with electrochemical detection. Free Rad Biol Med. 2000;28:520–528. doi: 10.1016/s0891-5849(00)00155-6. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki Y, Mochizuki K, Nakano Y, Maruya N, Goto M, Maruyama Y. Comparison of fluorescence reagents for simultaneous determination of hydroxylated phenylalanine and nitrated tyrosine by high-performance liquid chromatography with flurescence detection. Biomed Chromatogr. 2012;26:41–50. doi: 10.1002/bmc.1623. [DOI] [PubMed] [Google Scholar]
- 23.Pavlovic R, Santaniello E, Chiesa LM, Biondi PA. New procedure for the determination of 3-nitrotyrosine in plasma by GC–ECD. Chromatographia. 2009;70:637–641. [Google Scholar]
- 24.Pietzsch J, Kopprasch S, Bergmann R. Analysis of 3-chlorotyrosine as a specific marker of protein oxidation: the use of N(O,S)-ethoxycarbonyl trifluoroethyl ester derivatives and gas chromatography/mass spectrometry. Rapid Commun Mass Sp. 2003;17:767–770. doi: 10.1002/rcm.977. [DOI] [PubMed] [Google Scholar]
- 25.Gaut JP, Byun J, Tran HD, Heinecke JW. Artifact-free quantification of free 3-chlorotyrosine, 3-bromotyrosine, and 3-nitrotyrosine in human plasma by electron capture-negative chemical ionization gas chromatography mass spectrometry and liquid chromatography-electrospray ionization tandem mass spectrometry. Anal Biochem. 2002;300:252–259. doi: 10.1006/abio.2001.5469. [DOI] [PubMed] [Google Scholar]
- 26.Tsikas D, Mitschke A, Gntzki FM. Measurement of 3-nitrotyrosine in human plasma and urine gas chromatography tendem mass spectrometry. Amino Acid Analysis. 2012;828:255–270. doi: 10.1007/978-1-61779-445-2_20. [DOI] [PubMed] [Google Scholar]
- 27.Nicholls SJ, Shen ZZ, Fu XM, Levison BS, Hazen SL. Quantification of 3-nitrotyrosine levels using a benchtop ion trap mass spectrometry method. Meth Enzymol. 2005;396:245–266. doi: 10.1016/S0076-6879(05)96022-9. [DOI] [PubMed] [Google Scholar]
- 28.Conventz A, Musiol A, Brodowsky C, M¨uller-Lux A, Dewes P, Kraus T. Simultaneous determination of 3-nitrotyrosine, tyrosine, hydroxyproline and proline in exhaled breath condensate by hydrophilic interaction liquid chromatography/electrospray ionization tandem mass spectrometry. J Chromatogr B. 2007;860:78–85. doi: 10.1016/j.jchromb.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 29.Tsikas D, Duncan MW. Mass spectrometry and 3-nitrotyrosine: strategies, controversies, and our current perspective. Mass Spec Rev. 2014;33:273–276. doi: 10.1002/mas.21396. [DOI] [PubMed] [Google Scholar]
- 30.Zhao YP, Lam M, Wu DL, Mak R. Quantification of small molecules in plasma with direct analysis in real time tandem mass spectrometry, without sample preparation and liquid chromatographic separation. Rapid Commun Mass Spectrom. 2008;22:3217–3224. doi: 10.1002/rcm.3726. [DOI] [PubMed] [Google Scholar]
- 31.Jones CM, Fernández FM. Transmission mode direct analysis in real time mass spectrometry for fast untargeted metabolic fingerprinting. Rapid Commun Mass Spectrom. 2013;27:1311–1318. doi: 10.1002/rcm.6566. [DOI] [PubMed] [Google Scholar]
- 32.Wang CY, Zhu HB, Cai ZW, Song FR, Liu ZQ, Liu SY. Newborn screening of phenylketonuria using direct analysis in real time (DART) mass spectrometry. Anal Bioanal Chem. 2013;405:3159–3164. doi: 10.1007/s00216-013-6713-8. [DOI] [PubMed] [Google Scholar]