Abstract
Collagen-induced arthritis (CIA) is a common autoimmune animal model used to study rheumatoid arthritis (RA). The development of CIA involves infiltration of macrophages and neutrophils into the joint, as well as T and B cell responses to type II collagen. In murine CIA, genetically susceptible mice (DBA/1J) are immunized with a type II bovine collagen emulsion in complete Freund’s adjuvant (CFA), and receive a boost of type II bovine collagen in incomplete Freund’s adjuvant (IFA) 21 days after the first injection. These mice typically develop disease 26 to 35 days after the initial injection. C57BL/6J mice are resistant to arthritis induced by type II bovine collagen, but can develop arthritis when immunized with type II chicken collagen in CFA, and receive a boost of type II chicken collagen in IFA 21 days after the first injection. The concentration of heat-killed Mycobacterium tuberculosis H37RA (MT) in CFA also differs for each strain. DBA/1J mice develop arthritis with 1 mg/ml MT, while C57BL/6J mice require and 3–4 mg/ml MT in order to develop arthritis. CIA develops slowly in C57BL/6J mice and cases of arthritis are mild when compared to DBA/1J mice. This protocol describes immunization of DBA/1J mice with type II bovine collagen and the immunization of C57BL/6J mice with type II chicken collagen.
Materials and Reagents
Bovine Collagen Type II (Chondrex, catalog number: 20021)
Chicken Collagen Type II (Chondrex, catalog number: 20011)
Complete Freund's Adjuvant (CFA) (Chondrex, catalog number: 1mg/ml 7008& 4mg/ml 7001)
Incomplete Freund's Adjuvant (IFA) (Sigma-Aldrich, catalog number: F5506)
Ketamine (KetaVed) (VEDCO, catalog number: 078908598)
Xylazine (AnaSed) (LLOYD Laboratories, catalog number: 078081939)
Glacial acetic acid(Fischer Scientific, catalog number: A38-212)
Mice
DBA/1J mice, male, 8 weeks old
C57BL/6, male, 8 weeks old
Equipment
Filtration System (Corning, catalog number: 431096)
Interchangeable Syringes (Micro-Mate, catalog number: 148251A)
Nylon 3-Way Stopcock (Kimble Chase, catalog number: 4201634503)
Needle (BD, catalog number: 305109)
Disposable syringe (BD, catalog number: 309623)
Mouse Restraint (Braintree Scientific, catalog number: TV-150)
Caliper (Kafer, catalog number: 217901)
Procedure
A. Collagen-induced arthritis: Immunization of DBA/1J mice with type II bovine collagen
Preparation of bovine collagen stock
-
1.
Prepare 0.01N acetic acid in ddH2O and filter through a 0.2 µM membrane to sterilize diluent. Store at 4 °C.
-
2.
Dilute 10 mg of bovine collagen with 2.5 ml of 0.01 N acetic acid for a final concentration of 4 mg/ml collagen.
-
3.
Secure lid and wrap the collagen bottle in aluminum foil to avoid light exposure.
-
4.
Rotate the bottle overnight at 4 °C or until collagen is completely dissolved.
-
5.
Aliquot collagen solution (500µl aliquots) and freeze at −20 °C.
Aliquots of collagen can be stored at −20°C for 6 months
Aliquots should not be thawed more than 2 times prior to injection.
Preparation of anesthetic
-
6.
Prepare a working solution of 25 mg/ml ketamine and 2.5 mg/ml xylazine in PBS. Store working solution for 2 weeks at room temperature, or 1–3 months at 4 °C.
For a 10 ml solution, combine 2.5 ml of ketamine stock, and 0.25 ml of xylazine stock, with 7.25 ml PBS.
Each mouse will be anesthetized through an intraperitoneal (i.p.) injection with 0.1 ml of stock solution for a final concentration of 2.5 mg ketamine and 0.25 mg xyalzine per mouse.
Preparation of bovine collagen emulsion with CFA for Day 0 immunization
*Important preform in sterile tissue culture hood
-
7.
Connect two autoclaved 2 ml glass luer lock syringes to the 3-way stopcock.
-
8.
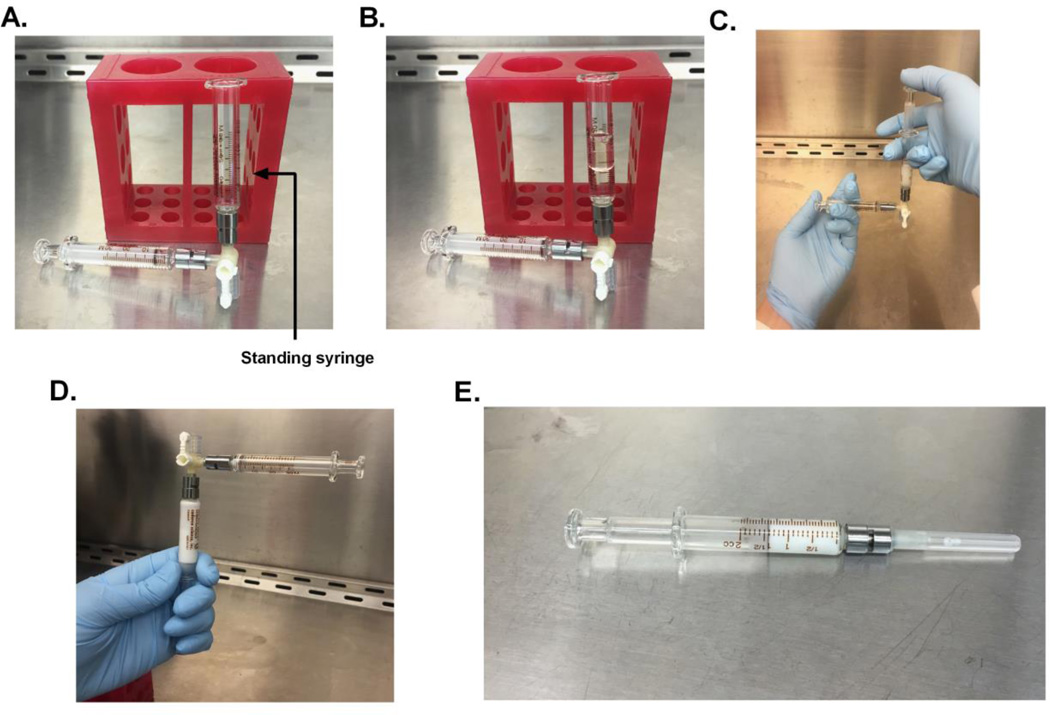
Place glass plunger into one of the syringes and stand the opposite syringe against a 50 ml tube rack. The syringe with the plunger should lay on the surface of the hood, while the second syringe stands at a 90° angle to the syringe with the plunger (Figure 1a).
-
9.
Create a working solution of collagen by adding 4 mg/ml collagen stock to 0.01 N acetic acid at a 1:1 ratio in the standing syringe (Figure 1b).
-
10.
Add CFA to the syringe at a 1:1 ratio of collagen working solution in the standing syringe.
Example: 500µl 4mg/ml collagen, 500µl 0.01N acetic acid, and 1mL CFA
Actual volumes of the working solution and CFA will be determined by number of mice to be immunized, where each DBA/1J mouse receives 0.1 ml of the final emulsion.
Mixing the emulsion will cause 0.4 ml of the emulsion to be lost in the stopcock, be sure to account for this when determining volumes of reagents.
-
11.
Turn the stop cock lever to close the valve opening not connected to a syringe.
-
12.
Place the plunger in the standing syringe.
-
13.
Mix the solution between the two syringes slowly, by plunging the entire solution into the opposite syringe 20 times. This will create a white emulsion (Figure 1c).
-
14.
Plunge all of the collagen into a single syringe. While holding the syringe containing the collagen stopcock end up (Figure 1d), remove the stopcock and connect a 27 gauge ½ inch luer lock needle to the end of syringe.
-
15.
Gently and slowly lower the plunger to remove air from syringe until the collagen emulsion fills the needle (Figure 1e).
-
16.
Place collagen emulsion on ice and away from light until immunization.
Figure 1. Syringe Assembly.
Attach 2, 2mL leur-lock syringes with a stop cock at a 90° angle. Insert the plunger to the syringe perpendicular to the standing syringe. Do no insert plunger into standing syringe (A). Fill the standing syringe with the collagen working solution and CFA (B). Insert the plunger into the standing syringe and exchange the solution to the opposite syringe 20 times, until a white emulsion forms (C). Turn the syringe so leur-lock faces upward and the entire emulsion falls against a single plunger(D). Remove the stop cock and syringe without the emulsion and assemble the syringe with the 27 ½’ gauge needle (E).
Immunization of DBA/1J mice with Bovine Collagen Emulsion in CFA: Day 0
-
17.
Anesthetize each mouse with a 0.1ml injection of the ketamine/xylazine working solution described in 6a
-
18.
Place the fully anesthetized mouse in the restraint with tail exposed.
-
19.
Turn the bevel of the needle upwards and inject 0.1 ml of the collagen emulsion subcutaneously in the tail, approximately 2 cm from the base of the tail. Be careful not to inject into tail veins, as this will result in death.
Preparation and injection of DBA/1J mice with Type II Bovine Collagen Emulsion in IFA for Day 21 Boost
-
20.
Prepare bovine collagen emulsion for 21-day boost in the same concentrations as the day 0 immunization, substituting CFA for IFA.
-
21.
Mix the collagen slowly, and assemble the needle with the same methods described for day 0 immunization.
-
22.
Anesthetize each mouse with an 0.1ml injection of the ketamine/xylazine working solution described in 6a
-
23.
Place the fully anesthetized mouse in the restraint with tail exposed.
-
24.
Inject 0.1 ml of collagen emulsion into the tail of the mouse closer to the base of the tail than the original injection site.
If the boost injection is administered further from the base of the tail than the original injection spot, arthritis may develop slowly, or to a lesser extent than boost injections given closer to the base of the tail.
B. Collagen-induced arthritis: Immunization of C57BL/6J mice with type II chicken collagen
The procedure to elicit CIA in C57BL/6J mice is modeled after the DBA/1J procedure, but contains two major changes. Immunization of C57BL/6J mice uses type II chicken collagen in place of type II bovine collagen. Additionally, the concentration of MT in CFA is increased to 3–4 mg/mL for C57BL/6J mice from than 1 mg/ml used for DBA/1J mice. All other details are identical to the DBA/1J procedure.
C. Paw measurement and scoring arthritis
-
1.
Anesthetize mice with the drop method of isoflurane anesthesia (add approximately 0.3 ml to a 500 µl container per IACUC guidelines).
-
2.
Weigh each mouse, score paws, and measure paw thickness on day 0 and once a week until day 21. After day 21, weigh, measure and score paws twice a week.
-
3.
Apply an arthritis score to each paw with the following criteria:
0: Normal, no inflammation or redness
1: Redness and swelling in one digit
2: Redness and swelling in more than one digit or redness and swelling in one digit and ankle and wrist joint.
3: Redness and swelling present in all digits and joints
-
4.
Record the severity of paw swelling by measuring paw thickness at wrist joint of the front paws and the ankle joints of the hind paws. Each measurement should be noted in mm.
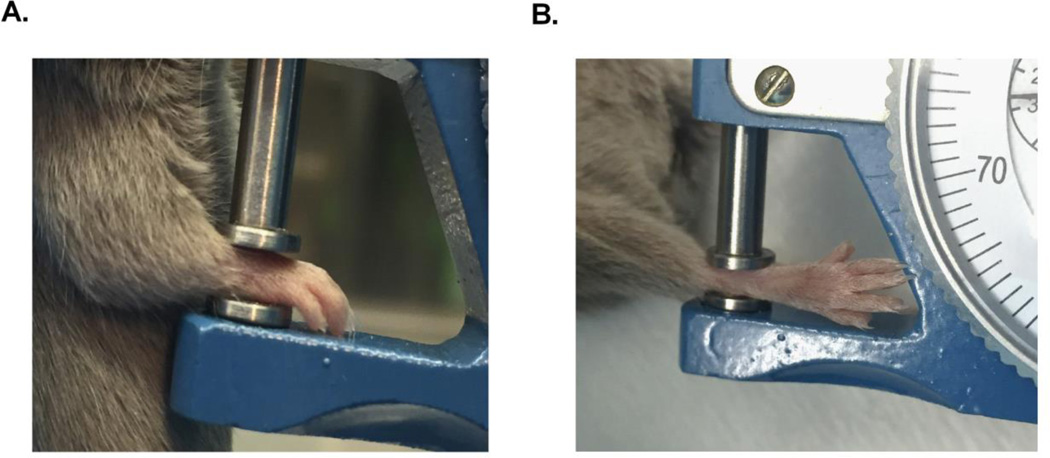
Figure 2. Paw Measurement.
Measure front paws by placing the front paw in the caliper so the palm and back of paw touch either side of the caliper (A). Read measurement in mm. Measure the hind paws by placing the ankle joint in the caliper so either side of the ankle touches the caliper (B). Read measurement in mm.
D. Data analysis
-
1.
Plot the arthritis score data by adding the score of each paw together, for a maximum score of 12.
-
2.
Display the data in an XY plot, where Y is arthritis score and X is days after immunization (Figure 3a).
-
3.
Plot the measurement data by subtract the thickness of the paw in mm from the original day 0 thickness.
-
4.
Display the data in an XY plot, where Y is swelling in mm and X is days after immunization (Figure 3b).
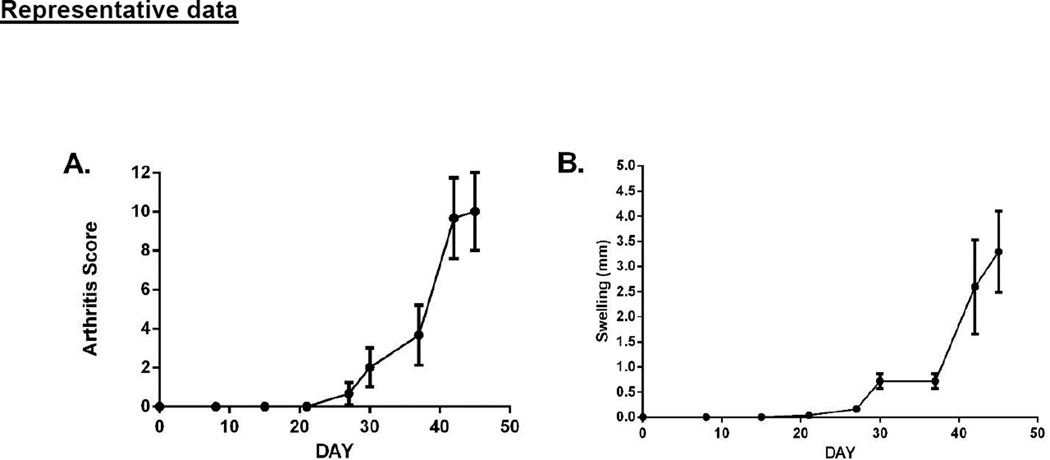
Figure 3. DBA/1J mice were immunized with type II bovine collagen in CFA on day 0, and boosted with type II bovine collagen in IFA on day 21.
Over the course of disease, an arthritis score was attributed to each paw of each mouse, based on the features described in section C. The score of each paw was summed for a score of 0–12 for each mouse (A). The thickness of each paw was also measured and the increase in paw thickness in mm was plotted (B). The displayed results represent three individual mice
Acknowledgments
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIAMS-NIH) under Award Number 1R01AR063132.
References
- 1.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2(5):1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 2.Crook KR, Jin M, Weeks MF, Rampersad RR, Baldi RM, Glekas AS, Shen Y, Esserman DA, Little P, Schwartz TA, Liu P. Myeloid-derived suppressor cells regulate T cell and B cell responses during autoimmune disease. J Leukoc Biol. 2015;97(3):573–582. doi: 10.1189/jlb.4A0314-139R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inglis JJ, Simelyte E, McCann FE, Criado G, Williams RO. Protocol for the induction of arthritis in C57BL/6 mice. Nat Protoc. 2008;3(4):612–618. doi: 10.1038/nprot.2008.19. [DOI] [PubMed] [Google Scholar]