Abstract
In 1984, there was considerable evidence that the hippocampus was important for spatial learning and some evidence that it was also involved in duration discrimination. The article Hippocampus, Time, and Memory (Meck, Church, and Olton, 1984), however, was the first to isolate the effects of hippocampal damage on specific stages of temporal processing. In this review, to celebrate the 30th anniversary of Behavioral Neuroscience, we look back on factors that contributed to the long-lasting influence of this article. The major results were that a fimbria-fornix lesion (a) interferes with the ability to retain information in temporal working memory and (b) distorts the content of temporal reference memory, but (c) it did not decrease sensitivity to signal duration. This was the first lesion experiment in which the results were interpreted by a well-developed theory of behavior (scalar timing theory). It has led to extensive research on the role of the hippocampus in temporal processing by many investigators. The most important ones are the development of computational models with plausible neural mechanisms (such as the Striatal Beat-Frequency model of interval timing), the use of multiple behavioral measures of timing, and empirical research on the neural mechanisms of timing and temporal memory using ensemble recording of neurons in prefrontal-striatal-hippocampal circuits.
There are several reasons that a scientific article might attain the status of ‘classic,’ innovation and significance chief among them. To the extent that the paper by Meck, Church, and Olton (1984) has attained that status, it’s worth noting that it had a very good title: “Hippocampus, Time, and Memory.” In 1984 each of these topics was “hot,” but the inclusion of all three in a single title was unusual, if not unprecedented. So the title helped. Our question now, however, is whether or not the article was innovative or significant. The article was focused on the behavioral effects of fimbria-fornix (FFx) lesions (which disconnect the hippocampus from subcortical innervation), and the cognitive interpretations of these results. The cognitive interpretations were based on scalar timing theory, which involved an information-processing (IP) model of interval timing that was developed in the early 1980’s by John Gibbon, Russell Church, Warren Meck, and others (Church, 1984, 2003; Gibbon, Church, & Meck, 1984; Meck, 2003). This IP model was expressed as a flow diagram with cognitive features of clock, memory, and decision stages, and as a computational model with closed-form equations as well as simulations. The model was consistent with the principles of scalar expectancy theory (Gibbon, 1977) and was being successfully fit to new experiments on timing and time perception (e.g., Church & Gibbon, 1982; Meck and Church, 1984). Meck, Church, and Olton (1984) explained the qualitative results with the IP model, but attempted to explain only a few of the quantitative results (e.g., Figure 8 on the effect of timing a stimulus with gaps, and Figure 10 on the probability that a choice was determined by previous choices).
The instructions to authors from Behavioral Neuroscience clearly required that “all manuscripts must include an abstract of 100–150 words.” The 407-word abstract by Meck, Church, and Olton (1984) described the overall problem, the primary method and results of each of the five experiments, and the major conclusions. The abstract ended with three main conclusions about the effects of FFx lesions: (a) they interfere with temporal, as well as spatial, working memory, (b) they reduce the remembered time of reinforcement stored in reference memory, and (c) they have no effect on the rat’s sensitivity to stimulus duration. Consequently, the extended abstract probably helped readers to appreciate interrelationships between hippocampus, time, and memory before reading the article.
Authors’ background
In 1984, papers authored by single investigators or small groups of investigators with similar skills were typical in the field of behavioral neuroscience, but papers authored by teams of investigators with different skills were still relatively rare. The team that contributed to Hippocampus, Time, and Memory was ideal for this topic at that time. David Olton was a well-established physiological psychologist at The Johns Hopkins University, who now would be recognized as a behavioral neuroscientist. He was an expert in the function of the hippocampus, and in the use of the radial-arm maze. Most researchers then referred to it as the “Olton maze;” David Olton did not, but he occasionally referred to it as his “Tenure maze” and believed that investigators should be known for their discoveries and not the pieces of apparatus that they build. When he came to Brown University to do the brain surgeries, he was asked how many he had done previously, and he said “about 1000;” to the follow-up question about how many he had done recently, he said “two—I practiced before coming.” Russell Church was an experimental psychologist at Brown University with considerable research experience on timing and animal learning. Warren Meck was a 1982 Ph.D. from Brown University who was focusing on the neural basis of interval timing. The Brown-Hopkins collaboration continued with four additional publications – Meck, Church, and Olton (1984), Meck, Church, and Wenk (1986), Olton, Meck, and Church (1987), and Olton, Wenk, Church, and Meck (1988).
Results and cognitive explanations
In 1984, knowledge of the role of the hippocampus in spatial learning was much more advanced than in temporal learning. The primary purpose of the radial-arm maze experiment (Experiment 5) was to determine that the FFx-lesions were sufficient to replicate the well-established findings that they impair spatial working memory (Olton, Becker, & Handelmann, 1979; Olton & Papas, 1979; Olton & Samuelson, 1976).
The article formulated some basic questions about the effects of a FFx lesion on temporal learning, and provided the basis for the three strong conclusions in the abstract. The conclusions were that a FFx lesion had strong effects of temporal memory (both on temporal working and reference memory), but it did not decrease the sensitivity to stimulus duration (and sometimes increased the subject’s sensitivity to time). A basic finding was that a FFx lesion had no general impairment on a rat’s ability to perceive time intervals. The sensitivity to stimulus duration or rate was unimpaired by this lesion in psychophysical choice procedures (Experiment 1), and the sensitivity to stimulus duration was unimpaired by this lesion in a standard peak-interval (PI) reproduction procedure (Experiment 4). Lesions that do not have general debilitating effects make it possible to identify specific effects.
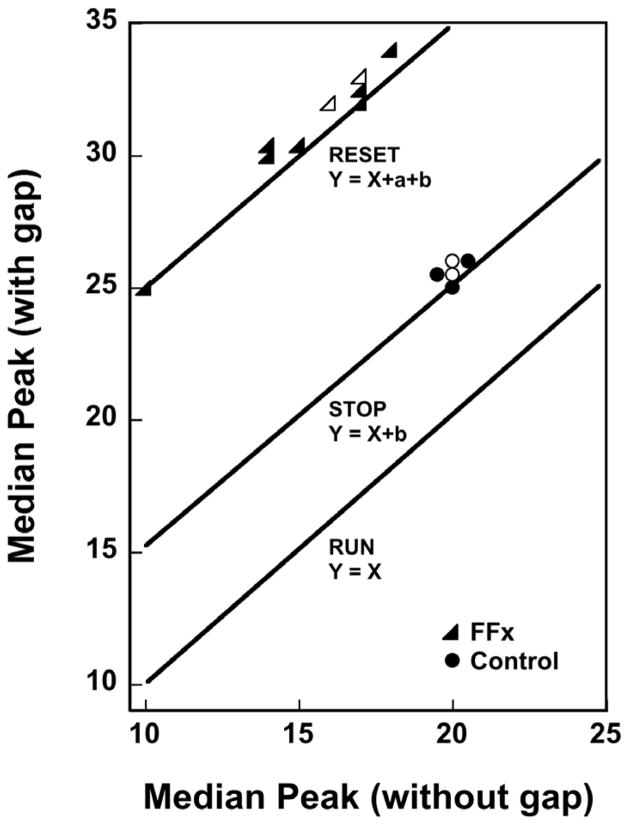
FFx lesions also produced a sustained leftward shift in the psychometric functions for both duration and rate of a stimulus indicative of a systematic decrease in the clock readings stored in temporal memory. This timing distortion was interpreted in terms of a biased encoding/retrieval process whereby clock readings are transferred to and stored in reference memory (Experiment 1) and the same interpretation was made of the leftward shift in the PI procedure experiment (Experiment 4). However, the most dramatic effect of the FFx lesions is shown in Figure 1 (Figure 8 in the original article) in which rats in the control and lesion groups were trained in a 20-s PI procedure and tested with a 5-s gap in some of the probe-trial presentations. The peak times during unreinforced probe trials with and without gaps are shown for each rat in both the control and FFx-lesion groups. The amount of increase in peak time between the ‘gap’ and ‘no gap’ conditions indicated whether rats continued timing during the gap (run mode), paused their clocks during the gap (stop mode), or forgot the signal duration that occurred prior to the gap (reset mode) – see Allman, Pelphrey, and Meck, 2012; Buhusi and Meck, 2009b; Cheng, Williams, and Meck (2006), Matell and Meck (1999), Meck and Church (1983) and Roberts and Church (1978) for a description of how subjects can operate their internal clocks in these different modes. The major finding was that the control rats ‘stopped’ timing during the gap, but that rats with FFx lesions ‘reset’ their clocks sometime during the gap, i.e., the working memory of the control rats was normal, but the working memory of the FFx-lesioned rats was non-operational in this respect. In other words, normal rats estimate the total interval by adding together the time periods before and after the gap. In contrast, rats with hippocampal damage ignore the estimated time period before the gap and initiate timing anew after the interruption is removed as if the time before the gap never occurred (see Table 1 for the results of other PI-gap experiments as a function of the type of hippocampal lesion).
Figure 1.
The effect of a 5-s gap inserted during the white-noise signal for fimbria-fornix (FFx) lesioned and control rats performing in a 20-s PI procedure. The gap affected the control and FFx rats differently. The control rats typically responded about 5 s later on trials with a 5-s gap than without a gap (i.e., they stopped timing during the gap); the FFx rats responded about 15 s later on trials with a 5-s gap than without a gap (i.e., they reset timing during the gap). Open triangles represent multiple FFx rats (n=12) and open circles represent multiple control rats (n=8). The peak time on trials with gaps for rats with FFx lesions is approximated by a line Y = X + a + b, where X is the peak time without a gap, a is the duration of the signal before the gap, and b is the duration of the gap. In contrast, the peak time on trials with gaps for control rats is approximated by a line Y = X + b. The three diagonal lines show what would occur if subjects operated their clocks using the run, stop, or reset modes during the gap. Adapted from Meck, Church, and Olton (1984).
Table 1.
Studies Investigating Hippocampal Involvement in Interval Timing.
| Species | Manipulation | Procedure(s) | Major Findings | Reference |
|---|---|---|---|---|
| Rat | electrolytic lesions of ffx | 2 vs. 8-s & 2 vs. 16-cps bisection; 20-s PI with gaps | leftward shifts of all functions (underestimation of target duration), sharpening of peak functions, reset following a 5-s gap | Meck et al. (1984) |
| Rat | ibotenic acid lesions of MSA | 40-s PI with gaps | leftward shift (underestimation of target durations), reset following 5 & 10-s gaps initiated 10 or 20s into the signal | Meck et al. (1987) |
| Rat | electrolytic lesions of ffx | 10-s & 20-s PI | proportional leftward shifts (underestimation of target durations); impaired regulation of peak time on sequential trials; normal transition from 20s to 10s | Meck (1988) |
| Rat | electrolytic lesions of ffx ibotenic acid lesions of MSA | 10-s & 20-s PI | proportional leftward shifts (underestimation of target durations), reset following 10-s gap, normal STP | Olton et al. (1988) |
| Rat | aspiration lesion of hpx | 12-s differential reinforcement of low-rate schedule | leftward shift of inter-response time distributions (underestimation of target durations) | Jaldow & Oakley (1990) |
| Rat | aspiration lesion of hpx | 40-s PI with gaps | no effects - likely due to procedural limitations* | Dietrich & Allen (1998) |
| Rat | ibotenic acid lesions of dhpx | 15-s and 30-s Pavlovian delay and trace conditioning with non-reinforced peak trials & gaps | proportional leftward shifts (underestimation of target durations) in both delay and trace conditioning; partial resetting in gap trials | Tam & Bonardi (2012a) |
| Rat | ibotenic acid lesions of dhpx | 40-s Pavlovian delay conditioning with non-reinforced peak trials abd gaps | leftward shift (underestimation of target duration); resetting in gap trials, but no group differences | Tam & Bonardi (2012b) |
| Rat | ibotenic acid lesions of dhpx | 15-s Pavlovian delay conditioning with non-reinforced peak trials and gaps | leftward shift (underestimation of target duration); partial resetting in gap trials | Tam et al. (2013) |
| Rat | ibotenic acid lesions along septotemporal extent of hpx | 20-s & 40-s PI with gaps | proportional leftward shifts (underestimation of target durations) & sharpening of response functions, resetting with gaps | Buhusi & Meck (2013); Buhusi et al. (2004) |
| Mouse | ibotenic acid lesions of hpx | 35-s PI | leftward shift of peak function (underestimation of target duration), including ‘start’ & ‘stop’ times | Balci et al. (2009) |
| Mouse | Close homolog to L1 (CHL1−/−) gene deletion | 20-s PI with 5 or 10-s gaps | leftward shift of peak function (underestimation of target duration), decreased effect of gaps | Buhusi et al. (2013) |
| Mouse | NMDA lesions of dhpx or vhpx δ opioid receptor (Oprd1−/−) gene deletion |
15-s & 45-s Bi-PI | leftward shift of peak functions (underestimation of target durations) for dorsal hpx lesions & Oprd1−/− mice; disruption of motivational effects for ventral hpx lesions | Yin & Meck (2012, 2013) |
| Rabbit | excitotoxic lesions of dhpx + vhpx | 300-ms & 500-ms trace eye-blink conditioning | disruption of extinction for 300-ms & learning impairment for 500-ms condition | Moyer et al. (1990) |
| Rabbit | single-unit recording in CA1 area of hpx | 10-s & 20-s trace eye-blink conditioning with non-reinforced peak trials | CA1 pyramidal neurons showed encoding of target duration on peak trials | McEchron et al. (2003) |
| Human | intracarotid AMO assessment following left or right temporal lobe resection in epilepsy patients | retrospective estimation of how much time had passed since AMO administration (multiple minutes) | underestimation of time for LTR & RTR, increased variability for RTR | Drane et al. (1999) |
| Human | left or right medial-temporal lobe resection in epilepsy patients | 1 vs. 2-s visual bisection | leftward shift (underestimation of anchor durations) for LTR; increased WF for RTR | Vidalaki et al. (1999) |
| Human | left or right medial-temporal lobe resection in epilepsy patients | 5, 14, or 38 s reproduction and production tasks under conditions of silence, counting, or articulatory suppression | underestimation of duration in the production task for RTR | Perbal et al. (2001) |
| Human | left or right medial-temporal lobe resection in epilepsy patients | 2 vs. 8-s auditory and visual bisection; 50 vs. 200-ms auditory bisection | decreased WF for LTR & increased WF for RTR in all conditions | Melgire et al. (2005) |
| Human | Left or right medial-temporal lobe resection in epilepsy patients | 1 – 8 s verbal estimation and duration reproduction tasks | overestimation and underproduction for both LTR and RTR (effect larger for LTR) | Noulhiane et al. (2007) |
AMO = amobarbital; ffx = fimbria-fornix; hpx = hippocampus; medial septal area = MSA; RTR = right temporal lobe resection; LTR = left temporal lobe resection; NMDA = N-Methyl-D-aspartic acid; PI = peak-interval procedure; Bi-PI = bi-peak-interval procedure in which both target durations are timed beginning at trial onset with no external cue provided to indicate which, if any, lever/target duration will be selected for reinforcement on any trial; STP = simultaneous temporal processing of an auditory and visual signal paired with different target durations presented in compound with asynchronous signal onsets; WF = Weber fraction used to measure sensitivity to signal duration, lower values indicate better sensitivity;
study based differences in peak time on the number of sessions required to reach a criterion of 10% (± 4s) from the scheduled time of reinforcement (40 s), thus precluding observation of the nature of the deviations in peak time prior to this (typically more than 80 sessions). Moreover, 5-s gaps were contained in both fixed-interval and unreinforced probe trials used in the PI procedure, thus precluding their usefulness in evaluating working memory.
Note: Trace conditioning, a form of Pavlovian conditioning in which the presentation of the conditioned stimulus (CS) and the unconditioned stimulus (US) is separated in time by an interstimulus interval (ISI), requires an intact hippocampus (Moyer, Deyo, & Disterhoft, 1990). In contrast, Pavlovian conditioning procedures in which the CS and US are not separated by an ISI (i.e., delay conditioning procedures) typically do not (Solomon, Vander Schaaf, Thompson, & Weisz, 1986; Woodruff-Pak & Disterhoft, 2008). However, why trace conditioning is dependent on the hippocampus is unknown, but may be related to the absence of temporal contiguity (Bangasser, Waxler, Santollo, & Shors, 2006; Haritha, Wood, Ver Hoef, & Knight, 2013).
Combined with previous work reviewed by Church (1984), these findings demonstrated that subjects process temporal information as if they were using an internal stopwatch that can be run, stopped, and reset on command, and whose speed is adjustable (cf., Swearingen & Buhusi, 2010). Previous data suggest that dopaminergic drugs affect the speed of this internal clock (Maricq & Church, 1983; Maricq, Roberts, & Church, 1981; Meck, 1983, 1996 – see Coull, Cheng, & Meck, 2011 and Williamson, Cheng, Etchegaray, & Meck, 2008 for reviews). Using a paradigm in which rats have to filter out the gaps that (sometimes) interrupted timing, Buhusi and Meck (2002) found that methamphetamine (dopamine agonist) and haloperidol (dopamine antagonist) also affect the ‘stop’ and ‘reset’ mechanism of the internal clock, possibly by modulating attentional components that are dependent on the content and salience of the timed events. This article was published in Behavioral Neuroscience and is the first report of both clock and attentional effects of dopaminergic drugs on interval timing in the same experimental setting. The behavioral methods relied on the use of the standard PI procedure with gaps as applied by Meck, Church, and Olton (1984) as well as the ‘reversed gap’ procedure as developed by Buhusi and Meck (2000) in order to isolate effects on attention, clock speed, and memory (e.g., Buhusi & Meck, 2006a, b, 2009b; Buhusi, Mocanu, & Meck, 2004; Buhusi, Perera, & Meck, 2005; Buhusi, Sasaki, & Meck, 2002; Buhusi, Scripa, Williams, & Buhusi, 2013). The basic discovery is that changes in attention can be observed in unreinforced peak trials with gaps where lesions and/or drugs have a more pronounced effect on the perceived salience of the gap relative to the ‘to-be-timed’ signal and the intertrial interval. In contrast, changes in clock speed are more easily detected in unreinforced peak trials without gaps using the same manipulations (drugs or lesions) presumably because the absence of a gap doesn’t engage the same attentional processing (Buhusi & Meck, 2007, 2009a). Moreover, the proportional underestimation of time following neurotoxic lesions along the septotemporal extent of the hippocampus is reversed by the dopamine D2 receptor antagonist raclopride, suggesting a role for dopaminergic supersensitivity in the striatum following hippocampal damage (Buhusi & Meck, 2013; Yin & Meck, 2013).
From our current perspective, this work has been influential in multiple respects. First, the findings themselves have inspired numerous empirical reports investigating the role of the hippocampus in temporal processing (e.g., Abela & Chudasama, 2013; Abela, Dougherty, Fagen, Hill, & Chudasama, 2013; Brasted, Bussey, Murray, & Wise, 2003; MacDonald, Lepage, Eden, & Eichenbaum, 2011 – see Table 1 for a list of studies using prospective and retrospective timing procedures to investigate the effects of hippocampal lesions) and a variety of reviews (e.g., Allman, Teki, Griffiths, & Meck, in press; Balci, Meck, Moore, & Brunner, 2009; Kesner, 2002; Meck, 2002b, 2005; Squire, 1992; Wallenstein, Hasselmo, & Eichenbaum, 1998; Yin & Troger, 2011). Second, in addition to demonstrating that hippocampal damage contributes to distortions in long-term (reference) memory and impairments of short-term (working) memory as described above, it also revealed, by way of negation, that this type of short-term memory isn’t necessary for the duration discrimination in basic timing tasks such as the standard PI and temporal bisection procedures in which the signal is present for the entire interval (see Church, 1984; Church & Deluty, 1977; Church, Miller, Meck, & Gibbon, 1991; Paule et al., 1999). This distinction is similar to the difference between trace and delay conditioning in terms of the temporal contiguity of the conditioned stimulus with the unconditioned stimulus (Bangasser, Waxler, Santollo, & Shors, 2006; Haritha, Wood, Ver Hoef, & Knight, 2013; Kehoe, Ludvig, and Sutton, 2009; Moyer, Deyo, & Disterhoft, 1990; Solomon, Vander Schaaf, Thompson, & Weisz, 1986).
The true importance of this work, however, extends beyond these specific findings, in that it began a theoretically driven systems neuroscience approach to the investigation of timing and time perception (MacDonald & Meck, 2004). While there were previous investigations of the impact of lesions on temporally controlled behavior using differential reinforcement of low rate and fixed-interval schedules (e.g., Ellen & Powell, 1963; Glickstein, Quigley, & Stebbins, 1964; Rawlins, Winocur, & Gray, 1983; Schmaltz & Isaacson, 1968; Spiegel, Wycis, Orchinik, & Freed, 1955), this paper was the first lesion experiment in which the results were interpreted within a well-developed theory of behavior, i.e., scalar timing theory (Church, 2003; Church, Meck, & Gibbon, 1994; Gibbon, 1977; Gibbon & Church, 1984; Gibbon, Church, & Meck, 1984). Briefly, scalar timing theory proposes that time perception is achieved via a linear pacemaker-accumulator process (clock stage), a distributed reference memory store of previously reinforced clock times (memory stage), and a ratio-based comparison process (decision stage). Moreover, the standard deviation observed in timing behavior increases proportionally with the mean of the target duration – referred to as the ‘scalar property’ of interval timing – see Buhusi et al., 2009; Cheng & Meck, 2007; Gibbon, Church, & Meck, 1984. As the authors not only interpreted the data with respect to theory, but also designed this collection of experiments based on the information processing stages subsumed by the theory, this paper, as an anonymous reviewer stated, “still stands as an instructional example of how to mesh the psychological, biological and mathematical levels of explanation”.
Search for neural mechanisms
As indicted above, the Hippocampus, Time, and Memory paper was quickly followed by a number of other neuroanatomical investigations of temporal processing by various members of the team (e.g., Meck, 1985, 1988, 2002a, b; Meck, Church, Wenk, & Olton, 1987; Olton, 1989; Olton, Meck, & Church, 1987; Olton, Wenk, Church, & Meck, 1988), leading to systematic growth in the field. Integrating the results from these and other studies investigating the roles of various temporal lobe structures (e.g., amygdala and hippocampus) in timing and temporal memory (Drane, Lee, Loring, & Meador, 1999; Droit-Volet & Meck, 2007; Lustig & Meck, 2009; Meck & MacDonald, 2007; Melgire et al., 2005; Olton, Meck, & Church, 1987; Vidalaki, Ho, Bradshaw, & Szabadi, 1999).
While these investigations have shown that the hippocampus organizes experiences in time and plays an important modulatory role in the translation (i.e., encoding/retrieval) of temporal sequences, this structure does not appear to be as critical for prospective timing and the detection of specific event durations as the striatum (Dalla Barba & La Corte, 2013; Eichenbaum, 2013; Meck, 2006b; Pastalkova, Itskov, Amarasingham, & Buzsáki, 2008; Shapiro, 2011; Shapiro & Ferbinteanu, 2006). Moreover, it has recently been proposed that the hippocampus may be preferentially involved in retrospective timing, which is more dependent on incidental memory for the number and temporal sequence of events than prospective timing (e.g., MacDonald, 2013; Ogden, Wearden, Gallagher, & Montgomery, 2011; Zakay & Block, 2004). As such, the remainder of this commemorative retrospective will highlight subsequent work by members of the original team and/or their colleagues that investigate the role of cortico-thalamic-basal ganglia circuits in time perception and timed performance (e.g., Allman & Meck, 2012; Coull, Cheng, & Meck, 2011; Gibbon, Malapani, Dale, & Gallistel, 1997; Gu, Laubach, & Meck, 2013; Matell & Meck, 2004; Matell, 2013; Meck, 1996; Merchant, Harrington, & Meck, 2013; Yin & Meck, 2013).
Following from other anatomical, pharmacological, and imaging work (e.g., Agostino et al., 2013; Allman & Meck, 2012; Buhusi & Meck, 2005; Cheng, Ali, & Meck, 2007; Cheng, Etchegaray, and Meck, 2007; Cheng, Hakak, and Meck, 2007; Cheng, MacDonald, & Meck, 2006; Gu, Cheng, Yin, & Meck, 2011; Hata, 2011; Jaldow, Oakley, & Davey, 1990; Jones & Jahanshahi, 2011; Lake & Meck, 2012; MacDonald, Cheng, & Meck, 2012; Matell, Bateson, Meck, 2006; Matell, King, & Meck, 2004; Meck, 2006a, b, c; Meck, Cheng, MacDonald, Gainetdinov, Caron, & Çevik, 2012; Meck, Penney, & Pouthas, 2009; Miller, McAuley, & Pang, 2006), combined with sophisticated behavioral and analytic techniques (Cheng & Westwood, 1993; Church, Meck, & Gibbon, 1994; Gibbon & Church, 1990; MacDonald, Cheng, Williams, & Meck, 2007; Meck, 2001; Penney, Gibbon, & Meck, 2008; Rakitin et al., 1998), a number of investigators have proposed that interval timing capacities rely on interactions between cortico-thalamic-basal ganglia and hippocampal circuits (e.g., Cheng, Jesuthasan, & Penney, 2011, 2013; Gu, Laubach, & Meck, 2013; Onoda & Sakata, 2006; Onoda, Takahashi, & Sakata, 2003; Sakata, 2006; Sakata & Onoda, 2003; Yin & Meck, 2013).
Neurophysiological realism
Unfortunately, these proposals lacked detail regarding the precise mechanisms that could be utilized to achieve control of timing in the seconds-to-minutes range at the same level as timing systems in the millisecond range (e.g., Buonomano & Mauk, 1994), and concerns regarding the physiological realizability of scalar timing theory were becoming apparent (Hinton & Meck, 1997a, b). Therefore, following the example of a theoretically driven research program set by this classic paper, Matell and Meck (2000, 2004) developed the Striatal Beat-Frequency (SBF) model of interval timing which provided a ‘neurophysiologically plausible’ mechanism for the temporal control of behavior (cf., Humphries, Stewart, & Gurney, 2006). Briefly, they proposed that striatal medium spiny neurons (MSNs) could learn, through synaptic strength changes, to recognize in a ‘perceptron-like’ manner, patterns or states of cortical activity, thereby providing a mechanism for the IP components of scalar timing theory. Specifically, the SBF model proposed that MSNs could be trained to represent target durations by detecting the coincident firing of an array of cortical neurons oscillating at different periodicities. See Gibbon (1991), Morell (1996), Bhattacharjee (2006), and Treisman (2013) for a historical perspective on the origins of pacemaker/accumulator conceptualizations of the ‘internal clock’ and the subsequent evolution of the SBF model of interval timing.
To assess the SBF model, Matell and Meck began recording from ensembles of dorsal striatal and anterior cingulate cortical neurons in rats trained on a two-duration (10 & 40 s) version of the PI procedure used by Meck, Church, & Olton, 1984. By using different reinforcement probabilities for the two durations, the rats lever pressed at approximately the same rate at each target duration. In this manner, neural activity associated with lever pressing at each duration could be meaningfully compared without the inherent confound of differences in motor output. They found that MSNs fired at different rates when the rat responded at the 10-s target duration than when it responded in an equivalent manner at the 40-s target duration, thereby suggesting that the striatum could represent different durations independently of the motor control exerted by the striatum and basal ganglia circuits. They also found that cortical neurons fired at differed rates when the rats responded at the two target durations, although the differences in firing rate were less robust than that seen in the striatum. Taken together, these data were broadly consistent with the SBF model in that the striatum could represent specific target durations by integrating less-distinct cortical activity that co-varied with signal duration (Matell, Meck, & Nicolelis, 2003b.) While they found no evidence suggesting that neurons in the general area of the premotor and cingulate cortex fired in an oscillatory manner, the obvious possibility that other cortical areas provide this input has led to continued development of the SBF model (e.g., Buhusi & Oprisan, 2013; Oprisan & Buhusi, 2011, 2013; Lustig, Matell, & Meck, 2005; Meck & N’Diaye, 2005; Van Rijn, Gu, & Meck, 2013; Van Rijn, Kononowicz, Meck, Ng, and Penney, 2011). Of course, future theoretical work should examine the extent to which consistent, but non-oscillatory patterns of cortical activity could generate the same type of temporal control as specified by the SBF model (see Matell & Meck, 2004 for alternative descriptions of the time base for the coincidence-detection process specified by the SBF model).
Subsequent electrophysiological work by Matell and colleagues has followed up on these intriguing findings. In one study, they examined whether individual MSNs represented time in an ‘abstract’ manner, divorced from the motor behaviors necessary to obtain reinforcement (Meck & Church, 1982a, b; Portugal, Wilson, & Matell, 2011). Specifically, they trained rats that reinforcement could be earned for nose-pokes in one location at a specific time (i.e., 15 s), whereas nose-poking at a different location would be reinforced at random times. As a consequence, the rats responded at nearly constant levels during non-reinforced probe trials; they initially responded at a high rate on the random-interval nosepoke, abruptly switched to the fixed-interval nosepoke around 15s, and then abruptly switched back to the random-interval nosepoke. Critically, they found that the vast majority of MSNs had different firing rates for the same motor behavior as a function of the temporal phase in the task (e.g., initial responding on the random-interval nosepoke compared to responding on the fixed-interval nosepoke compared to terminal responding on the random-interval nosepoke), further supporting the notion that the striatum represents temporal information. On the other hand, this temporal modulation of firing rates was embedded within modulation related to the execution of overt motor behaviors (e.g., nose-poking, moving towards or away from the nose-poke location, etc.), thereby arguing against a purely ‘abstract’ representation of time – see Matell, Meck, & Nicolelis, 2003a. Again, these data were consistent with the coincidence-detection framework specified by the SBF model, which postulates that the temporally specific firing of MSNs serves as an instantiation of the ‘decision stage’ described by scalar timing theory (Gibbon et al., 1984).
Another follow-up study conducted by this research group (Matell, Shea-Brown, Gooch, Wilson, & Rinzel, 2011) examined neural activity in medial agranular cortex, a possible rodent homolog of primate pre-motor/supplementary-motor cortex (Reep & Corwin, 1999), which is one of the few cortical structures consistently activated in a variety of timing tasks (Wiener, Turkeltaub, & Coslett, 2010). As in the earlier study, they found that cortical neurons had firing rates that varied as a function of time, even though the rat’s behavior during the analysis period was stationary. Using extracellular recordings, they found no evidence of oscillatory firing in this cortical area, further suggesting that the time base proposed by the SBF model may be less dependent upon oscillatory firing of cortical neurons than on other patterns of neural activity in the cortex or thalamus. However, they did find a wide variety of systematic firing patterns (i.e., positive and negative ramp patterns, as well as peak and dip patterns) that could be used by an ‘ideal observer’ to distinguish the subject’s ‘location’ in time. Subsequent computer simulations demonstrated that this variety of firing patterns provided an improved estimate of time (i.e., with less noise) than a single firing pattern. Notably, this article by Matell, Shea-Brown, Gooch, Wilson, and Rinzel (2011) received the D.G. Marquis award for the best paper in Behavioral Neuroscience, in 2011.
Given the massive anatomical convergence of cortical neurons to individual MSNs (i.e., 30,000 to 1), these findings suggest that investigation of the extent to which both oscillatory and non-oscillatory patterns of cortical activity can generate temporally structured behavior should prove fruitful for continued revision and/or expansion of the model (Allman & Meck, 2012; Coull, Cheng, & Meck, 2011; Gu, Laubach, & Meck, 2013; Gu & Meck, 2012; Lewis & Meck, 2012; Merchant, Harrington, & Meck, 2013). In this regard, current work is taking into account that the hippocampus normally provides tonic inhibition to the striatum, such that firing is delayed in some proportion of MSNs – perhaps those corresponding with the ‘representation’ of the previous trial’s sequence of timed responding and reward outcome in order to provide feedback regulation of successive peak times (Lustig & Meck, 2005; Meck, 1988; Meck et al. 1987; Shi, Church, & Meck, 2013). Following hippocampal lesions, these MSNs may become sensitized or ‘overexcited’ in the absence of this tonic inhibition. It has been shown, for example, that hippocampal lesions can increase dopamine sensitivity in the striatum (Fidalgo, Conejo, González-Pardo, & Arias, 2012; Seeman et al., 2005) possibly altering the synaptic weights of the coincidence-detection processes predicted by the SBF model as illustrated in Figure 2 (Allman & Meck, 2012; Matell & Meck, 2004; Yin & Troger, 2011; Yin & Meck, 2012, 2013) and thereby providing an account of the memory-associated leftward shifts in PI functions first reported by Meck, Church, and Olton (1984). Such compensatory responses between the hippocampus and dorsal striatum, in terms of mutual inhibition and excitation, have become a focus of investigation for understanding complementary interactions among memory systems (e.g., Fouquet et al., 2013; Lee, Duman, & Pittenger, 2008; Packard & White, 1991; Poldrack & Packard, 2003).
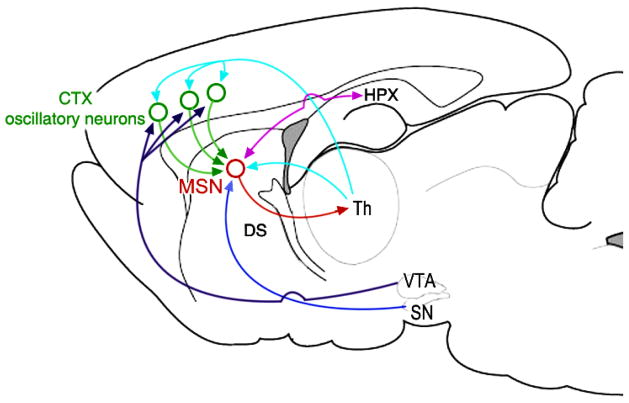
Figure 2.
Diagram of the possible mappings of functional hippocampal connectivity within the cortico-thalamic-basal ganglia circuit of the rat proposed by the striatal beat-frequency (SBF) model of interval timing (Matell & Meck, 2004). In this model, oscillatory neurons in the cortex (CTX) project to medium spiny neurons (MSNs) in the dorsal striatum (DS) which receives dopaminergic input from the substantia nigra pars compacta (SNc). Dopamine projections from the ventral tegmental area (VTA) to the CTX are able to modulate the frequency of CTX oscillations. Bi-directional projections from the hippocampus (HPX) to the DS modulate the firing thresholds of MSNs via either tonic inhibition or phasic excitation. Lesions of the hippocampus would be expected to release the DS from this inhibition, thereby reducing the firing thresholds for MSNs and producing proportional leftward shifts of timing functions (underestimation of duration). The timing circuit is completed by having the CPu project to the thalamus (Th) and back onto the CTX in order to provide feedback control of temporal processing (Lustig & Meck, 2005; Meck, 1988). Adapted from Yin and Troger (2011).
Memory encoding and retrieval processes
While the horizontal leftward shifts observed by Meck, Church, and Olton (1984) were not evaluated for a gradual onset, subsequent work by this group confirmed that lesions of the FFx (Olton et al., 1987), as well as the medial septal area (Meck et al., 1987) produce sustained and gradual effects on temporal control. Such effects are identical in form to those seen following chronic modulation of the cholinergic system, and are consistent with a bias applied during memory encoding (Meck, 1983, 2002a, b; Meck & Church, 1987a, b). Remarkably, there have been no reports of pharmacological or anatomical manipulations that led to immediate and sustained changes in the content of temporal memory, which would be indicative of a bias in memory retrieval. Such a lack of effects is surprising, as it is difficult to imagine that the neural mechanisms underlying memory encoding would evolve in such a way that biases could be induced without a compensatory process to offset them. Indeed, Meck (1983, 1996, 2002a, b) proposed that the memory encoding bias might reflect a storage speed parameter (referred to as K* in scalar timing theory – see Church & Meck, 1988) whereby longer durations, i.e., larger accumulator values, took longer to store in memory due to the need to transfer the pulses in the accumulator into memory at a particular baud rate. However, this notion implies that the stored memory is a proxy for the delay to reinforcement (i.e., the memory is of storage time rather than delay). Because the temporal control of behavior is viewed by scalar timing theory as reflecting a comparison between currently perceived time (i.e., current pulse accumulation) and a sample taken from reference memory (a distribution of previously reinforced times), it is logically necessary to have a process that performs the reverse transformation (from storage time to delay), so that the organism is comparing ‘apples to apples’. However, the neural mechanisms by which such a retrieval process might be implemented largely remain to be determined.
As such, recent work by Matell and colleagues may provide an approach in which such retrieval processes can be specifically addressed. The timing task used by Matell et al. (2011) to examine medial agranular cortical activity produced some novel behavioral effects that suggested that rats engage in ‘retrieval related’ temporal computations. Specifically, they trained the rats that two different modal stimuli (i.e., tone and light) predicted two different times of reinforcement (i.e., 10 s and 20 s, respectively). Presentation of the compound stimulus (tone + light) in extinction led to maximal responding at an intermediate duration (i.e., 16 s). Importantly, this compound peak function was scalar, as was the distribution of responses on individual trials (see also Swanton, Gooch, & Matell, 2009). As such, the authors interpreted this effect as suggesting that the rats generated an average temporal expectation as a result of the simultaneous retrieval of discrepant memories, and then timed this estimate in an otherwise normal manner. Additional research suggested that the form and location of the compound peak function is influenced by the relative probability of reinforcement of the component cues (e.g., Kurti, Swanton, & Matell, 2013; Matell & Henning, 2013; Matell & Kurti, 2013; Swanton & Matell, 2011). Such averaging-like behavior is reminiscent of other effects seen when subjects are required to time multiple durations (e.g., Gu, Jurkowski, Lake, Malapani, & Meck, 2013; Lejeune & Wearden, 2009; Malapani et al., 1998; Meck, Komeily-Zadeh, & Church, 1984), suggesting that the interaction of multiple memories at encoding and retrieval is an important component of normal temporal processing. We anticipate that understanding the form and content of temporal memory and how these temporal memories interact will emerge as a fruitful line of investigation, particularly in the case of developmental changes in the hippocampus and the implantation of false memories (e.g., Buhusi, Lamoureux, & Meck, 2008; Cermak et al., 1999; Jones, Meck, Williams, Wilson, & Swartzwelder, 1999; Meck & Williams, 1997; Meck et al., 2007; Mellott et al., 2004; Ramirez et al., 2013). We hope that future work on these questions will meet the same success as the ‘classic’ team did in terms of generating an appreciation of the importance of ‘internal clocks’ for understanding interval timing and time-based decision making at both short-interval and circadian time scales (e.g., Agostino, Peryer, & Meck, 2008; Agostino, Golombek, & Meck, 2011; Caetano, Guilhardi, & Church, 2011; Cordes & Meck, 2013; Cordes, Williams, & Meck, 2007; Galtress & Kirkpatrick, 2010; Kurti & Matell, 2011; Doyère, & Gruart, 2012).
References
- Abela AR, Chudasama Y. Dissociable contributions of the ventral hippocampus and orbitofrontal cortex to decision-making with a delayed or uncertain outcome. European Journal of Neuroscience. 2013;37:640–647. doi: 10.1111/ejn.12071. [DOI] [PubMed] [Google Scholar]
- Abela AR, Dougherty SD, Fagen ED, Hill CJR, Chudasama Y. Inhibitory control deficits in rats with ventral hippocampal lesions. Cerebral Cortex. 2013;23:1396–1409. doi: 10.1093/cercor/bhs121. [DOI] [PubMed] [Google Scholar]
- Agostino PV, Cheng RK, Williams CL, West AE, Meck WH. Acquisition of response thresholds for timed performance is regulated by a calcium-responsive transcription factor, CaRF. Genes, Brain and Behavior. 2013;12:633–644. doi: 10.1111/gbb.12059. [DOI] [PubMed] [Google Scholar]
- Agostino PV, Golombek DA, Meck WH. Unwinding the molecular basis of interval and circadian timing. Frontiers in Integrative Neuroscience. 2011;5:64. doi: 10.3389/fnint.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostino PV, Peryer G, Meck WH. How music fills our emotions and helps us keep time. Behavioral and Brain Sciences. 2008;31:575–576. doi: 10.1017/S0140525X0800530X. [DOI] [Google Scholar]
- Allman MJ, Meck WH. Pathophysiological distortions in time perception and timed performance. Brain. 2012;135:656–677. doi: 10.1093/brain/awr210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman MJ, Pelphrey KA, Meck WH. Developmental neuroscience of time and number: Implications for autism and other neurodevelopmental disabilities. Frontiers in Integrative Neuroscience. 2012;6:7. doi: 10.3389/fnint.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman MJ, Teki S, Griffiths TD, Meck WH. Properties of the internal clock: First- and second-order principles of subjective time. Annual Review of Psychology. 2014;65:xx–yy. doi: 10.1146/annurev-psych-010213-115117. in press. [DOI] [PubMed] [Google Scholar]
- Balci F, Meck WH, Moore H, Brunner D. Timing deficits in aging and neuropathology. In: Bizon JL, Woods A, editors. Animal models of human cognitive aging. Totowa, NJ: Humana Press; 2009. pp. 161–201. [DOI] [Google Scholar]
- Bangasser DA, Waxler DE, Santollo J, Shors TJ. Trace conditioning and the hippocampus: The importance of contiguity. Journal of Neuroscience. 2006;26:8702–8706. doi: 10.1523/JNEUROSCI.1742-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee Y. A timely debate about the brain. Science. 2006;311:596–598. doi: 10.1126/science.311.5761.596. WOS:000235688100024. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, Bussey TJ, Murray EA, Wise SP. Role of the hippocampal system in associtive learning beyond the spatial domain. Brain. 2003;126:1202–1223. doi: 10.1093/brain/awg103. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Aziz D, Winslow D, Carter RE, Swearingten JE, Buhusi MC. Interval timing accuracy and scalar timing in C57BL/6 mice. Behavioral Neuroscience. 2009;123:1102–1113. doi: 10.1037/a0017106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Lamoureux JA, Meck WH. Prenatal choline supplementation increases sensitivity to contextual processing of temporal information. Brain Research. 2008;1237:204–213. doi: 10.1016/j.braines.2008.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Timing for the absence of a stimulus: The gap paradigm reversed. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:305–322. doi: 10.1037//0097-7403.26.3.305. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behavioral Neuroscience. 2002;116:291–297. doi: 10.1037//0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Interval timing with gaps and distracters: Evaluation of the ambiguity, switch, and time-sharing hypotheses. Journal of Experimental Psychology: Animal Behavior Processes. 2006a;32:329–338. doi: 10.1037/0097-7403.32.3.329. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Time sharing in rats: A peak-interval procedure with gaps and distracters. Behavioural Processes. 2006b;71:107–115. doi: 10.1016/j.beproc.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Effect of clozapine on interval timing and working memory for time in the peak-interval procedure with gaps. Behavioural Processes. 2007;74:159–167. doi: 10.1016/j.beproc.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Relative time sharing: New findings and an extension of the resource allocation model of temporal processing. Philosophical Transactions of the Royal Society - London B. 2009a;364:1875–1885. doi: 10.1098/rstb.2009.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Relativity theory and time perception: Single or multiple clocks? PLoS ONE. 2009b;4(7):e6268. doi: 10.1371/journal.pone.0006268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Scalar time contraction following neurotoxic hippocampal lesions is reversed by the D2R antagonist raclopride. 2013 submitted.
- Buhusi CV, Mocanu M, Meck WH. Abnormal memory consolidation of interval timing in rats with ibotenic lesions of the hippocampus. Abstracts of the Society for Neuroscience. 2004;34:550–18. [Google Scholar]
- Buhusi CV, Oprisan SA. Time-scale invariance as an emergent property in a perceptron with realistic, noisy neurons. Behavioural Processes. 2013;95:60–70. doi: 10.1016/j.beproc.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Perera D, Meck WH. Memory for timing visual and auditory signals in albino and pigmented rats. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:18–30. doi: 10.1037/0097-7403.31.1.18. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Sasaki A, Meck WH. Temporal integration as a function of signal/gap intensity in rats (Rattus norvegicus) and pigeons (Columba livia) Journal of Comparative Psychology. 2002;116:381–390. doi: 10.1037//0735-7036.116.4.381. [DOI] [PubMed] [Google Scholar]
- Buhusi M, Scripa I, Williams CL, Buhusi CV. Impaired interval timing and spatial-temporal integration in mice deficient in CHL1, a gene associated with schizophrenia. Timing & Time Perception. 2013 doi: 10.1163/22134468-00002003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Mauk MD. Neural network model of the cerebellum: Temporal discrimination and timing of motor response. Neural Computation. 1994;6:38–55. doi: 10.1162/neco.1994.6.1.38. [DOI] [Google Scholar]
- Caetano MS, Guilhardi P, Church RM. Stimulus control in multiple temporal discriminations. Learning & Behavior. 2011;40:520–529. doi: 10.3758/s13420-012-0071-9. [DOI] [PubMed] [Google Scholar]
- Cermak JM, Blusztajn JK, Meck WH, Williams CL, Fitzgerald C, Rosene DL, Loy R. Prenatal availability of choline alters the development of acetylcholinesterase in rat hippocampus. Developmental Neuroscience. 1999;21:94–104. doi: 10.1159/000017371. [DOI] [PubMed] [Google Scholar]
- Cheng K, Westwood R. Analysis of single trials in pigeons timing performance. Journal of Experimental Psychology: Animal Behavior Processes. 1993;19:56–67. doi: 10.1037/0097-7403.19.1.56. [DOI] [Google Scholar]
- Cheng RK, Ali YM, Meck WH. Ketamine “unlocks” the reduced clock-speed effect of cocaine following extended training: Evidence for dopamine-glutamate interactions in timing and time perception. Neurobiology of Learning and Memory. 2007;88:149–159. doi: 10.1016/j.nlm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Cheng RK, Etchegaray M, Meck WH. Impairments in timing, temporal memory, and reversal learning linked to neurotoxic regimens of methamphetamine intoxication. Brain Research. 2007;1186:255–266. doi: 10.1016/j.brainres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Cheng RK, Hakak OL, Meck WH. Habit formation and the loss of control of an internal clock: Inverse relationship between the level of baseline training and the clock-speed enhancing effects of methamphetamine. Psychopharmacology. 2007;193:351–362. doi: 10.1007/s00213-007-0783-2. [DOI] [PubMed] [Google Scholar]
- Cheng RK, Jesuthasan S, Penney TB. Time for zebrafish. Frontiers in Integrative Neuroscience. 2011;5:40. doi: 10.3389/fnint.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RK, Jesuthasan S, Penney TB. Zebrafish forebrain and temporal conditioning. Philosophical Transactions of the Royal Society - London B. 2013 doi: 10.1098/rstb.2012.0462. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RK, MacDonald CJ, Meck WH. Differential effects of cocaine and ketamine on time estimation: Implications for neurobiological models of interval timing. Pharmacology Biochemistry and Behavior. 2006;85:114–122. doi: 10.1016/j.pbb.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Cheng RK, Meck WH. Prenatal choline supplementation increases sensitivity to time by reducing non-scalar sources of variance in adult temporal processing. Brain Research. 2007;1186:242–254. doi: 10.1016/j.brainres.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RK, Meck WH, Williams CL. α7 nicotinic acetylcholine receptors and temporal memory: Synergistic effects of combining prenatal choline and nicotine on reinforcement-induced resetting of an interval clock. Learning & Memory. 2006;13:127–134. doi: 10.1101/lm.31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RK, Williams CL, Meck WH. Oscillatory bands, neuronal synchrony and hippocampal function: Implications of the effects of prenatal choline supplementation for sleep-dependent memory consolidation. Brain Research. 2008;1237:176–194. doi: 10.1016/j.brainres.2008.08.077. [DOI] [PubMed] [Google Scholar]
- Church RM. Properties of the internal clock. Annals of The New York Academy of Sciences. 1984;423:566–582. doi: 10.1111/j.1749-6632.1984.tb23459.x. [DOI] [PubMed] [Google Scholar]
- Church RM. A concise introduction to scalar timing theory. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca Raton, FL: CRC Press; 2003. pp. 3–22. [Google Scholar]
- Church RM, Deluty MZ. Bisection of temporal intervals. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:216–228. doi: 10.1037//0097-7403.3.3.216. [DOI] [PubMed] [Google Scholar]
- Church RM, Gibbon J. Temporal generalization. Journal of Experimental Psychology: Animal Behavior Processes. 1982;8:165–186. doi: 10.1037//0097-7403.8.2.165. [DOI] [PubMed] [Google Scholar]
- Church RM, Meck WH. Biological basis of the remembered time of reinforcement. In: Commons ML, Church RM, Stellar JR, Wagner AR, editors. Quantitative analyses of behavior: Biological determinants of reinforcement. Vol. 7. Hillsdale, NJ: Erlbaum; 1988. pp. 103–119. [Google Scholar]
- Church RM, Meck WH, Gibbon J. Application of scalar timing theory to individual trials. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20:135–155. doi: 10.1037//0097-7403.20.2.135. [DOI] [PubMed] [Google Scholar]
- Church RM, Miller KD, Meck WH, Gibbon J. Symmetrical and asymmetrical sources of variance in temporal generalization. Animal Learning & Behavior. 1991;19:207–214. doi: 10.3758/BF03197878. [DOI] [Google Scholar]
- Cordes S, Meck WH. Ordinal judgments in the rat: An understanding of longer and shorter for suprasecond, but not subsecond durations. Journal of Experimental Psychology: General. 2013 doi: 10.1037/a0032439. in press. [DOI] [PubMed] [Google Scholar]
- Cordes S, Williams CL, Meck WH. Common representations of abstract quantities. Current Directions in Psychological Science. 2007;16:156–161. doi: 10.1111/j.1467-8721.2007.00495.x. [DOI] [Google Scholar]
- Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Barba G, La Corte V. The hippocampus, a time machine that makes errors. Trends in Cognitive Sciences. 2013;17:102–104. doi: 10.1016/j.tics.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Allen JD. Functional dissociation of the prefrontal cortex and the hippocampus in timing behavior. Behavioral Neuroscience. 1998;112:1043–1047. doi: 10.1037/0735-7044.112.5.1043. [DOI] [PubMed] [Google Scholar]
- Drane DL, Lee GP, Loring DW, Meador KJ. Time perception following unilateral amobarbital injection in patients with temporal lobe epilepsy. Journal of Clinical and Experimental Neuropsychology. 1999;21:385–396. doi: 10.1076/jcen.21.3.385.922. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S, Meck WH. How emotions colour our perception of time. Trends in Cognitive Sciences. 2007;11:504–513. doi: 10.1016/j.tics.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Ellen P, Powell EW. Timing behavior after lesions of zona incerta and mammillary body. Science. 1963;141:828–830. doi: 10.1126/science.141.3583.828. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Memory on time. Trends in Cognitive Sciences. 2013;17:81–88. doi: 10.1016/j.tics.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidalgo C, Conejo NM, González-Pardo H, Arias JL. Functional interaction between the dorsal hippocampus and the striatum in visual discrimination learning. Journal of Neuroscience Research. 2012;90:715–720. doi: 10.1002/jnr.22774. [DOI] [PubMed] [Google Scholar]
- Fouquet C, Babayan BM, Watilliaux A, Bontempi B, Tobin C, Rondi-Reig L. Complementary roles of the hippocampus and the dorsomedial striatum during spatial and sequence-based navigation behavior. PLoS ONE. 2013 doi: 10.1371/journal.pone.0067232. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtress T, Kirkpatrick K. The role of the nucleus assumbens core in impulsive choice, timing, and reward processing. Behavioral Neuroscience. 2010;124:26–43. doi: 10.1037/a0018464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber’s law in animal timing. Psychological Review. 1977;84:279–325. doi: 10.1037//0033-295X.84.3.279. [DOI] [Google Scholar]
- Gibbon J. Origins of scalar timing. Learning and Motivation. 1991;22:3–38. doi: 10.1016/0023-9690(91)90015-Z. [DOI] [Google Scholar]
- Gibbon J, Church RM. Sources of variance in an information processing theory of timing. In: Roitblat HL, Bever TG, Terrace HS, editors. Animal Cognition. Hillsdale, NJ: Erlbaum; 1984. [Google Scholar]
- Gibbon J, Church RM. Representation of time. Cognition. 1990;37(1–2):23–54. doi: 10.1016/0010-0277(90)90017-E. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. Annals of the New York Academy of Sciences. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Malapani C, Dale CL, Gallistel C. Toward a neurobiology of temporal cognition: Advances and challenges. Current Opinion in Neurobiology. 1997;7:170–184. doi: 10.1016/S0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Quigley WA, Stebbins W. Effect of frontal and parietal lesions on timing behavior in monkeys. Psychonomic Science. 1964;1:265–266. [Google Scholar]
- Gu BM, Cheng RK, Yin B, Meck WH. Quinpirole-induced sensitization to noisy/sparse periodic input: Temporal synchronization as a component of obsessive-compulsive disorder. Neuroscience. 2011;179:143–150. doi: 10.1016/j.neuroscience.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Gu B-M, Jurkowski AJ, Lake JI, Malapani C, Meck WH. Bayesian models of interval timing and distortions in temporal memory as a function of Parkinson’s disease and dopamine-related error processing. In: Vatakis A, Allman MJ, editors. Time distortions in mind: Temporal processing in clinical populations. Boston, MA: Brill Academic Publishers; 2013. in press. [Google Scholar]
- Gu B-M, Laubach M, Meck WH. Oscillatory mechanisms supporting interval timing and working memory in prefrontal-striatal-hippocampal circuits. 2013 submitted. [Google Scholar]
- Gu BM, Meck WH. Neural oscillations and spiking activity in prefrontal-striatal-hippocampal circuits during temporal reproduction. Society for Neuroscience Abstracts. 2012;42:808–09. [Google Scholar]
- Haritha AT, Wood KH, Ver Hoef LW, Knight DC. Human trace fear conditioning: Right-lateralized cortical activity supports trace-interval processes. Cognitive, Affective, & Behavioral Neuroscience. 2013;13:225–237. doi: 10.3758/s13415-012-0142-6. [DOI] [PubMed] [Google Scholar]
- Hata T. Glutamate – a forgotten target for interval timing. Frontiers in Integrative Neuroscience. 2011;5:27. doi: 10.3389/fnint.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton SC, Meck WH. How time flies: Functional and neural mechanisms of interval timing. Advances in Psychology. 1997a;120:409–457. doi: 10.1016/S0166-4115(97)80062-3. [DOI] [Google Scholar]
- Hinton SC, Meck WH. The “internal clocks” of circadian and interval timing. Endeavour. 1997b;21:82–87. doi: 10.1016/S0160-9327(97)01043-0. [DOI] [PubMed] [Google Scholar]
- Jaldow EJ, Oakley DA. Performance on a differential reinforcement of low-rate schedule in neodecorticated rats and rats with hippocampal-lesions. Psychobiology. 1990;18:394–403. [Google Scholar]
- Jaldow EJ, Oakley DA, Davey GC. Performance on two fixed-interval schedules in the absence of neocortex in rats. Behavioral Neuroscience. 1990;104:763–777. doi: 10.1037//0735-7044.104.5.763. [DOI] [PubMed] [Google Scholar]
- Jones CR, Jahanshahi M. Dopamine modulates striato-frontal functioning during temporal processing. Frontiers in Integrative Neuroscience. 2011;5:70. doi: 10.3389/fnint.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JP, Meck WH, Williams CL, Wilson WA, Swartzwelder SH. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Developmental Brain Research. 1999;118:159–167. doi: 10.1016/S0165-3806(99)00103-0. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Ludvig EA, Sutton RS. Magnitude and timing of conditioned responses in delay and trace classical conditioning of the nictitating membrane response of the rabbit (Oryctolagus cuniculus) Behavioral Neuroscience. 2009;123:1095–1101. doi: 10.1037/a0017112. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Neural mediation of memory for time: Role of the hippocampus and medial prefrontal cortex. In: Fountain SB, Bunsey MD, Danks JH, McBeath MK, editors. Animal cognition and sequential behavior: Behavioral, biological, and computational perspectives. Boston, MA: Kluwer Academic Press; 2002. pp. 213–237. [DOI] [Google Scholar]
- Knudsen EB, Flint RD, Moxon KA. Encoding of temporal intervals in the rat hindlimb sensorimotor cortex. Frontiers in Systems Neuroscience. 2012;6:67. doi: 10.3389/fnsys.2012.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurti AN, Matell MS. Nucleus accumbens dopamine modulates response rate but not response timing in an interval timing task. Behavioral Neuroscience. 2011;125:215–225. doi: 10.1037/a0022892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurti A, Swanton DN, Matell MS. The potential link between temporal averaging and drug-taking behavior. In: Lloyd D, Arstila V, editors. Subjective time: The philosophy, psychology, and neuroscience of temporality. Cambridge, MA: MIT Press; 2013. pp. xx–yy. in press. [Google Scholar]
- Lake JI, Meck WH. Differential effects of amphetamine and haloperidol on temporal reproduction: Dopaminergic regulation of attention and clock speed. Neuropsychologia. 2013;51:284–292. doi: 10.1016/j.neuropsychologia.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Lee AS, Duman RS, Pittenger C. A double dissociation revealing bidirectional competition between striatum and hippocampus during learning. Proceedings of the National Academy of Sciences, USA. 2008;105:17163–17168. doi: 10.1073/pnas.0807749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune H, Wearden JH. Vierordt’s The Experimental Study of the Time Sense (1868) and its legacy. European Journal of Cognitive Psychology. 2009;21(6):941–960. doi: 10.1080/09541440802453006. [DOI] [Google Scholar]
- Lewis PA, Meck WH. Time and the sleeping brain. The Psychologist. 2012;25:594–597. [Google Scholar]
- Lustig C, Matell MS, Meck WH. Not “just” a coincidence: Frontal-striatal synchronization in working memory and interval timing. Memory. 2005;13:441–448. doi: 10.1080/09658210344000404. [DOI] [PubMed] [Google Scholar]
- Lustig C, Meck WH. Chronic treatment with haloperidol induces working memory deficits in feedback effects of interval timing. Brain and Cognition. 2005;58:9–16. doi: 10.1016/j.bandc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Lustig C, Meck WH. Book review of The Overflowing Brain: Information Overload and the Limits of Working Memory by Torkel Klingberg. The New England Journal of Medicine. 2009;360:1469. doi: 10.1056/NEJMbkrev0809181. [DOI] [Google Scholar]
- MacDonald CJ, Cheng RK, Meck WH. Acquisition of “Start” and “Stop” response thresholds in peak-interval timing is differentially sensitive to protein synthesis inhibition in the dorsal and ventral striatum. Frontiers in Integrative Neuroscience. 2012;6:10. doi: 10.3389/fnint.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Cheng RK, Williams CL, Meck WH. Combined organizational and activational effects of short and long photoperiods on spatial and temporal memory in rats. Behavioural Processes. 2007;74:226–233. doi: 10.1016/j.beproc.2006.08.001. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Meck WH. Systems-level integration of interval timing and reaction time. Neuroscience and Biobehavioral Reviews. 2004;28:747–769. doi: 10.1016/j.neubiorev.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Malapani C, Deweer B, Gibbon J. Separating storage from retrieval dysfunction of temporal memory in Parkinson’s disease. Journal of Cognitive Neuroscience. 2002;14:311–322. doi: 10.1162/089892902317236920. [DOI] [PubMed] [Google Scholar]
- Malapani C, Rakitin B, Levy R, Meck WH, Deweer B, Dubois B, Gibbon J. Coupled temporal memories in Parkinson’s disease: A dopamine-related dysfunction. Journal of Cognitive Neuroscience. 1998;10:316–331. doi: 10.1162/089892998562762. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Church RM. The differential effects of haloperidol and methamphetamine on time estimation in the rat. Psychopharmacology. 1983;79:10–15. doi: 10.1007/BF00433008. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Roberts S, Church RM. Methamphetamine and time estimation. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:18–30. doi: 10.1037//0097-7403.7.1.18. [DOI] [PubMed] [Google Scholar]
- Matell MS. Searching for the Holy Grail: Temporally informative firing patterns in the rat. In: Merchant H, de Lafuente V, editors. Neurobiology of interval timing. New York NY: Springer-Verlag; 2013. pp. xx–yy. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matell MS, Bateson M, Meck WH. Single-trials analyses demonstrate that increases in clock speed contribute to the methamphetamine-induced horizontal shifts in peak-interval timing functions. Psychopharmacology. 2006;188:201–212. doi: 10.1007/s00213-006-0489-x. [DOI] [PubMed] [Google Scholar]
- Matell MS, Henning AM. Temporal memory averaging and post-encoding alterations in temporal expectation. Behavioural Processes. 2013 doi: 10.1016/j.beproc.2013.02.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matell MS, King GR, Meck WH. Differential modulation of clock speed by the administration of intermittent versus continuous cocaine. Behavioral Neuroscience. 2004;118:150–156. doi: 10.1037/0735-7044.118.1.150. [DOI] [PubMed] [Google Scholar]
- Matell MS, Kurti AN. Reinforcement probability modulates temporal memory selection and integration processes. Acta Psychologica. 2013 doi: 10.1016/j.actpsy.2013.06.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Reinforcement-induced within-trial resetting of an internal clock. Behavioural Processes. 1999;45:159–171. doi: 10.1016/S0376-6357(99)00016-9. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Neuropsychological mechanisms of interval timing behavior. Bioessays. 2000;22:94–103. doi: 10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: Coincidence detection of oscillatory processes. Cognitive Brain Research. 2004;21:139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH, Nicolelis MAL. Integration of behavior and timing: Anatomically separate systems or distributed processing? In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca Raton, FL: CRC Press; 2003a. pp. 371–391. [Google Scholar]
- Matell MS, Meck WH, Nicolelis MAL. Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behavioral Neuroscience. 2003b;117:760–773. doi: 10.1037/0735-7044.117.4.760. [DOI] [PubMed] [Google Scholar]
- Matell MS, Shea-Brown E, Gooch C, Wilson AG, Rinzel J. A heterogeneous population code for elapsed time in rat medial agranular cortex. Behavioral Neuroscience. 2011;125:54–73. doi: 10.1037/a0021954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Tseng W, Disterhoft JF. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. Journal of Neuroscience. 2003;23:1535–1547. doi: 10.1523/JNEUROSCI.23-04-01535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH. Selective adjustment of the speed of internal clock and memory processes. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:171–201. doi: 10.1037/0097-7403.9.2.171. [DOI] [PubMed] [Google Scholar]
- Meck WH. Hippocampus and “general” mnemonic function: Only time will tell. Behavioral and Brain Sciences. 1985;8:509–510. [Google Scholar]
- Meck WH. Hippocampal function is required for feedback control of an internal clock’s criterion. Behavioral Neuroscience. 1988;102:54–60. doi: 10.1037//0735-7044.102.1.54. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Cognitive Brain Research. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH. Interval timing and genomics: What makes mutant mice tick? International Journal of Comparative Psychology. 2001;14:211–231. [Google Scholar]
- Meck WH. Choline uptake in the frontal cortex is proportional to the absolute error of a temporal memory translation constant in mature and aged rats. Learning and Motivation. 2002a;33:88–104. doi: 10.1006/lmot.2001.1101. [DOI] [Google Scholar]
- Meck WH. Distortions in the content of temporal memory: Neurobiological correlates. In: Fountain SB, Bunsey MD, Danks JH, McBeath MK, editors. Animal cognition and sequential behavior: Behavioral, biological, and computational perspectives. Boston, MA: Kluwer Academic Press; 2002b. pp. 175–200. [DOI] [Google Scholar]
- Meck WH. Functional and neural mechanisms of interval timing. Boca Raton, FL: CRC Press LLC; 2003. [Google Scholar]
- Meck WH. Neuropsychology of timing and time perception. Brain and Cognition. 2005;58:1–8. doi: 10.1016/j.bandc.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Meck WH. Frontal cortex lesions eliminate the clock speed effect of dopaminergic drugs on interval timing. Brain Research. 2006a;1108:157–167. doi: 10.1016/j.brainres.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuroanatomical localization of an internal clock: A functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Research. 2006b;1109:93–107. doi: 10.1016/j.brainres.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Meck WH. Temporal memory in mature and aged rats is sensitive to choline acetyltransferase inhibition. Brain Research. 2006c;1108:168–175. doi: 10.1016/j.brainres.2006.06.047. [DOI] [PubMed] [Google Scholar]
- Meck WH, Cheng RK, MacDonald CJ, Gainetdinov RR, Caron MG, Çevik MÖ. Gene-dose dependent effects of methamphetamine on interval timing in dopamine-transporter knockout mice. Neuropharmacology. 2012;62:1221–1229. doi: 10.1016/j.neuropharm.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM. Abstraction of temporal attributes. Journal of Experimental Psychology: Animal Behavior Processes. 1982a;8:226–243. doi: 10.1037/0097-7403.8.3.226. [DOI] [Google Scholar]
- Meck WH, Church RM. Discrimination of fixed intertrial intervals in cross-modal transfer of duration. Bulletin of the Psychonomic Society. 1982b;19:234–236. [Google Scholar]
- Meck WH, Church RM. A mode control model of counting and timing processes. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:320–334. doi: 10.1037/0097-7403.9.3.320. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM. Simultaneous temporal processing. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:1–29. doi: 10.1037/0097-7403.10.1.1. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM. Cholinergic modulation of the content of temporal memory. Behavioral Neuroscience. 1987a;101:457–464. doi: 10.1037//0735-7044.101.4.457. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM. Nutrients that modify the speed of internal clock and memory storage processes. Behavioral Neuroscience. 1987b;101:465–475. doi: 10.1037/0735-7044.101.4.465. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM, Olton DS. Hippocampus, time, and memory. Behavioral Neuroscience. 1984;98:3–22. doi: 10.1037/0735-7044.98.1.3. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM, Wenk GL. Arginine vasopressin inoculates against age-related increases in sodium-dependent high affinity choline uptake and discrepancies in the content of temporal memory. European Journal of Pharmacology. 1986;130:327–331. doi: 10.1016/0014-2999(86)90287-6. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM, Wenk GL, Olton DS. Nucleus basalis magnocellularis and medial septal area lesions differentially impair temporal memory. Journal of Neuroscience. 1987;7:3505–3511. doi: 10.1523/JNEUROSCI.07-11-03505.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Doyère V, Gruart A. Interval timing and time-based decision making. Frontiers in Integrative Neuroscience. 2012;6:13. doi: 10.3389/fnint.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Komeily-Zadeh FN, Church RM. Two-step acquisition: Modification of an internal clock’s criterion. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:297–306. doi: 10.1037//0097-7403.10.3.297. [DOI] [PubMed] [Google Scholar]
- Meck WH, MacDonald CJ. Amygdala inactivation reverses fear’s ability to impair divided attention and make time stand still. Behavioral Neuroscience. 2007;121:707–720. doi: 10.1037/0735-7044.121.4.707. [DOI] [PubMed] [Google Scholar]
- Meck WH, N’Diaye K. Un modèle neurobiologique de la perception et de l’estimation du temps. Psychologie Francaise. 2005;50:47–63. doi: 10.1016/j.psfr.2004.10.009. [DOI] [Google Scholar]
- Meck WH, Penney TB, Pouthas V. Cortico-striatal representation of time in animals and humans. Current Opinion in Neurobiology. 2008;18:145–152. doi: 10.1016/j.conb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuro Report. 1997;8:2831–2835. doi: 10.1097/00001756-199709080-00005. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Frontiers in Integrative Neuroscience. 2008;1:7. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melgire M, Ragot R, Samson S, Penney TB, Meck WH, Pouthas V. Auditory/visual duration bisection in patients with left or right medial-temporal lobe resection. Brain and Cognition. 2005;58:119–124. doi: 10.1016/j.bandc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB Journal. 2004;18:545–547. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- Merchant H, Harrington DL, Meck WH. Neural basis of the perception and estimation of time. Annual Review of Neuroscience. 2013;36:313–336. doi: 10.1146/annurev-neuro-062012-170349. [DOI] [PubMed] [Google Scholar]
- Miller JP, McAuley JD, Pang KCH. Effects of the NMDA receptor antagonist MK-801 on short-interval timing in rats. Behavioral Neuroscience. 2006;120:162–172. doi: 10.1037/0735-7044.120.1.162. [DOI] [PubMed] [Google Scholar]
- Morell V. Setting a biological stopwatch. Science. 1996;271:905–906. doi: 10.1126/science.271.5251.905. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behavioral Neuroscience. 1990;104:243–252. doi: 10.1037/0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Noulhiane M, Pouthas V, Hasboun D, Baulac M, Samson S. Role of the medial temporal lobe in time estimation in the range of minutes. Neuro Report. 2007;18:1035–1038. doi: 10.1097/WNR.0b013e3281668be1. [DOI] [PubMed] [Google Scholar]
- Ogden RS, Wearden JH, Gallagher DT, Montgomery C. Acta Psychologica. 2011;138:254–262. doi: 10.1016/j.actpsy.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Olton DS. Frontal cortex, timing and memory. Neuropsychologia. 1989;27:121–130. doi: 10.1016/0028-3932(89)90094-8. [DOI] [PubMed] [Google Scholar]
- Olton DS, Becker JT, Handelman GE. Hippocampus, space, and memory. Behavoural and Brain Sciences. 1979;2:313–365. doi:10/1017/S0140525X00062713. [Google Scholar]
- Olton DS, Meck WH, Church RM. Separation of hippocampal and amygdaloid involvement in temporal memory dysfunctions. Brain Research. 1987;404:180–188. doi: 10.1016/0006-8993(87)91369-2. [DOI] [PubMed] [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Olton DS, Samuelson RJ. Remembrance of places passed: Spatial memory in rats. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2:97–116. doi: 10.1037/0097-7403.2.2.97. [DOI] [PubMed] [Google Scholar]
- Olton DS, Wenk GL, Church RM, Meck WH. Attention and the frontal cortex as examined by simultaneous temporal processing. Neuropsychologia. 1988;26:307–318. doi: 10.1016/0028-3932(88)90083-8. [DOI] [PubMed] [Google Scholar]
- Onoda K, Sakata S. An ERP study of temporal discrimination in rats. Behavioural Processes. 2006;71:235–240. doi: 10.1016/j.beproc.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Onoda K, Takahashi E, Sakata S. Event-related potentials in the frontal cortex, hippocampus, and cerebellum during a temporal discrimination task in rats. Cognitive Brain Research. 2003;17:380–387. doi: 10.1016/S0926-6410(03)00139-3. [DOI] [PubMed] [Google Scholar]
- Oprisan SA, Buhusi CV. Modeling pharmacological clock and memory patterns of interval timing in a striatal beat-frequency model with realistic, noisy neurons. Frontiers in Integrative Neuroscience. 2011;5:52. doi: 10.3389/fnint.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprisan SA, Buhusi CV. How noise contributes to time-scale invariance of interval timing. Physical Review E. 2013;87:052717. doi: 10.1103/PhysRevE.87.052717. http://link.aps.org/doi/10.1103/PhysRevE.87.052717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, White NM. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behavioral Neuroscience. 1991;105:295–306. doi: 10.1037/0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule MG, Meck WH, McMillan DE, McClure GYH, Bateson M, Popke EJ, Chelonis JJ, Hinton SC. The use of timing behaviors in animals and humans to detect drug and/or toxicant effects. Neurotoxicology and Teratology. 1999;21:491–502. doi: 10.1016/S0892-0362(99)00015-X. [DOI] [PubMed] [Google Scholar]
- Penney TB, Gibbon J, Meck WH. Categorical scaling of duration bisection in pigeons (Columba livia), mice (Mus musculus), and humans (Homo sapiens) Psychological Science. 2008;19:1103–1109. doi: 10.1111/j.1467-9280.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- Perbal S, Ehrle N, Samson S, Baulac M, Pouthas V. Time estimation in patients with right or left medial-temporal lobe resection. Neuro Report. 2001;12:939–942. doi: 10.1097/00001756-200104170-00015. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/S0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Wilson AG, Matell MS. Behavioral sensitivity of temporally modulated striatal neurons. Frontiers in Integrative Neuroscience. 2011;5:30. doi: 10.3389/fnint.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakitin BC, Gibbon J, Penney TB, Malapani C, Hinton SC, Meck WH. Scalar expectancy theory and peak-interval timing in humans. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:15–33. doi: 10.1037//0097-7403.24.1.15. [DOI] [PubMed] [Google Scholar]
- Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- Rawlins JN, Winocur G, Gray JA. The hippocampus, collateral behavior, and timing. Behavioral Neuroscience. 1983;97:857–872. doi: 10.1037//0735-7044.97.6.857. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV. Topographic organization of the striatal and thalamic connections of rat medial agranular cortex. Brain Research. 1999;841:43–52. doi: 10.1016/S0006-8993(99)01779-5. [DOI] [PubMed] [Google Scholar]
- Roberts S, Church RM. Control of an internal clock. Journal of Experimental Psychology: Animal Behavior Processes. 1978;4:318–337. doi: 10.1037//0097-7403.4.4.318. [DOI] [Google Scholar]
- Sakata S, Onoda K. Electrophysiological correlates of interval timing. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca Raton, FL: CRC Press; 2003. pp. 339–349. [Google Scholar]
- Sakata S. Timing and hippocampal theta in animals. Reviews in Neurosciences. 2006;17:157–162. doi: 10.1515/REVNEURO.2006.17.1-2.157. [DOI] [PubMed] [Google Scholar]
- Schmaltz LW, Isaacson RL. Effects of caudate and frontal lesions on retention and relearning of a DRL schedule. Journal of Comparative and Physiological Psychology. 1968;65:343–348. doi: 10.1037/h0025534. [DOI] [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, O’Dowd BF, George SR, Perreault ML, Männistö PT, Robinson S, Palmiter RD, Tallerico T. Dopamine supersensitivity correlates with D2 High states, implying many paths to psychosis. Proceedings of the National Academy of Sciences, USA. 2005;102:3513–3518. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML. Memory time. Neuron. 2011;71:571–573. doi: 10.1016/j.neuron.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Ferbinteanu J. Relative spike timing in pairs of hippocampal neurons distinguishes the beginning and end of journeys. Proceedings of the National Acedemy of Sciences, USA. 2006;103:4287–4292. doi: 10.1073/pnas.0508688103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Church RM, Meck WH. Bayesian optimization of time perception. Trends in Cognitive Sciences. 2013 doi: 10.1016/j.tics.2013.09.009. submitted. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100:729–744. doi: 10.1037/0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Sørensen K, Witter M. Entorhinal efferents reach the caudato-putamen. Neuroscience Letters. 1983;35:259–264. doi: 10.1016/0304-3940(83)90327-0. [DOI] [PubMed] [Google Scholar]
- Spiegel EA, Wycis HT, Orchinik CW, Freed H. The thalamus and temporal orientation. Science. 1955;121(3152):771–772. doi: 10.1126/science.121.3152.771. [DOI] [PubMed] [Google Scholar]
- Squire LR. Declarative and nondeclarative memory: Multiple brain systems supporting learning and memory. Journal of Cognitive Neuroscience. 1992;4:232–243. doi: 10.1162/jocn.1992.4.3.232. [DOI] [PubMed] [Google Scholar]
- Swanton DN, Gooch CM, Matell MS. Averaging of temporal memories by rats. Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:434–439. doi: 10.1037/a0014021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanton DN, Matell MS. Stimulus compounding in interval timing: the modality-duration relationship of the anchor durations results in qualitatively different response patterns to the compound cue. Journal of Experimental Psychology: Animal Behavior Processes. 2011;37:94–107. doi: 10.1037/a0020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swearingen JE, Buhusi CV. The pattern of responding in the peak-interval procedure with gaps: An individual-trials analysis. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:443–455. doi: 10.1037/a0019485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SK, Bonardi C. Dorsal hippocampal involvement in appetitive trace conditioning and interval timing. Behavioral Neuroscience. 2012a;126:258–269. doi: 10.1037/a0027164. [DOI] [PubMed] [Google Scholar]
- Tam SKE, Bonardi C. Dorsal hippocampal lesions disrupt Pavlovian delay conditioning and conditioned-response timing. Behavioural Brain Research. 2012b;230:259–267. doi: 10.1016/j.bbr.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Tam SK, Jennings DJ, Bonardi C. Dorsal hippocampal involvement in conditioned-response timing and maintenance of temporal information in the absence of the CS. Experimental Brain Research. 2013;227:547–559. doi: 10.1007/s00221-013-3530-4. [DOI] [PubMed] [Google Scholar]
- Treisman M. The information-processing model of timing (Treisman, 1963): Its sources and further history. Timing & Time Perception. 2013 in press. [Google Scholar]
- van Rijn H, Gu B-M, Meck WH. Dedicated clock/timing-circuit theories of interval timing. In: Merchant H, de Lafuente V, editors. Neurobiology of interval timing. New York NY: Springer-Verlag; 2013. pp. xx–yy. in press. [Google Scholar]
- van Rijn H, Kononowicz TW, Meck WH, Ng KK, Penney TB. Contingent negative variation and its relation to time estimation: A theoretical evaluation. Frontiers in Integrative Neuroscience. 2011;5:91. doi: 10.3389/fnint.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidalaki VN, Ho MY, Bradshaw CM, Szabadi E. Interval timing performance in temporal lobe epilepsy: Differences between patients with left and right hemisphere foci. Neuropsychologia. 1999;37:1061–1070. doi: 10.1016/S0028-3932(98)00155-9. [DOI] [PubMed] [Google Scholar]
- Wallenstein GV, Hasselmo ME, Eichenbaum H. The hippocampus as an associator of discontigous events. Trends in Neuroscience. 1998;21:317–323. doi: 10.1016/S0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- Wiener M, Turkeltaub P, Coslett HB. The image of time: A voxel-wise meta-analysis. Neuro Image. 2010;49:1728–1740. doi: 10.1016/j.neuroimage.2009.09.064. [DOI] [PubMed] [Google Scholar]
- Williamson LL, Cheng RK, Etchegaray M, Meck WH. “Speed” warps time: Methamphetamine’s interactive roles in drug abuse, habit formation, and the biological clocks of circadian and interval timing. Current Drug Abuse Reviews. 2008;1:203–212. doi: 10.2174/1874473710801020203. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Disterhoft JF. Where is the trace in trace conditioning? Trends in Neuroscience. 2008;31:105–112. doi: 10.1016/j.tins.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Yin B, Meck WH. Behavioral phenotyping of dorsal hippocampal lesioned mice: Potential links to striatal dopamine release and D2 receptor sensitivity. Society for Neuroscience Abstracts. 2012;42:808–04. [Google Scholar]
- Yin B, Meck WH. Comparison of interval timing behaviour in mice following dorsal or ventral hippocampal lesions with mice having δ opioid receptor gene deletion. Philosophical Transactions of the Royal Society - London B. 2013 doi: 10.1098/rstb.2012.0466. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin B, Troger AB. Exploring the 4th dimension: Hippocampus, time, and memory revisited. Frontiers in Integrative Neuroscience. 2011;5:36. doi: 10.3389/fnint.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakay D, Block RA. Prospective and retrospective duration judgments: An executive-control perspective. Acta Neurobiologiae Experimentalis. 2004;64:319–328. doi: 10.55782/ane-2004-1516. [DOI] [PubMed] [Google Scholar]