Abstract
Previous work has demonstrated a correlation between serum anti-citrullinated HSP90 antibodies and rheumatoid arthritis-associated interstitial lung disease (RA-ILD). To further investigate this potential pathogenic relationship, we used ELISA-based techniques to assess anti-citrullinated HSP90 antibody profiles in bronchoalveolar lavage fluid (BALF) of patients with different stages of RA-ILD. 9/21 RA-derived BALF specimens demonstrated IgG and/or IgA antibodies targeting citrullinated HSP90 proteins/peptides, highlighting disease specific responses (with a predilection for RA-ILD) that did not occur in IPF patients (0/5) or healthy control subjects (0/5). Comparison of antibody profiles between BALF and matching serum specimens revealed various recognition patterns favoring predominant production of anti-citrullinated HSP90 antibodies within the lung microenvironment—further supporting the connection between this antibody specificity and parenchymal lung disease. Equally important, qualitative as well as quantitative differences in anti-citrullinated HSP90 profiles between BALF and serum indicate that the lung plays a direct role in shaping the immune repertoire of RA/RA-ILD.
Keywords: Rheumatoid arthritis, Interstitial lung disease, Bronchoalveolar lavage fluid, Autoantibodies, HSP90, Citrullination
1. Introduction
Extra-articular disease manifestations represent a significant cause of morbidity and mortality in rheumatoid arthritis (RA) [1–6]. Chief among these complications are infection, cardiovascular disease, and pulmonary abnormalities that include interstitial lung disease (ILD) [6,7]. While the estimated prevalence of RA-ILD varies from 5% to 50% depending on the clinical, functional, and radiographic parameters used to define this entity, the spectrum of pathology includes both non-specific interstitial pneumonia (NSIP) and usual interstitial pneumonia (UIP) [8–20]. Illustrating the significance of these findings, recent epidemiologic data suggest that the clinical course of RA-UIP parallels that of idiopathic pulmonary fibrosis (IPF), with dismal 3-year survival rates of less than 50% [16]. In fact, among all patients with clinically evident RA-ILD (including those with less severe histopathologic variants), the standardized mortality ratio compared to RA alone ranges from 2.5 to 5 [5,6]. Given the negative survival impact of RA-ILD, elucidation of immunopathogenesis and development of more precise/predictive biomarker profiles is of critical importance.
As part of this effort, we recently reported the association between RA-ILD and serum autoantibodies recognizing citrullinated isoforms of heat shock protein 90 (citHSP90α, citHSP90β) [21]. Although the collective sensitivity of anti-citHSP90α and anti-citHSP90β antibodies was relatively modest (~30%), their specificity for RA-ILD exceeded 90%. Of note, this antibody profile not only distinguished RA-ILD from RA alone, but also separated RA-ILD from MCTD (in which antibodies against non-citrullinated HSP90 have been reported) and IPF [21]. The latter finding is of particular interest, as immunohistochemical studies of lung specimens derived from RA-ILD and IPF have revealed evidence of tissue citrullination in both disorders [22]—indicating that citrullination itself (likely induced by tissue damage and/or environmental “danger signals” such as smoking that may concomitantly upregulate HSP90) is not a disease-specific process. Because the immune response to citrullinated proteins such as HSP90α/HSP90β is unique to RA-ILD, however, these observations highlight the distinctive immunogenetic background of RA and its contribution to the formation of anti-citHSP90α and anti-citHSP90β antibodies in the setting of coexisting ILD.
Despite the provocative association between anti-citrullinated HSP90 antibodies and RA-ILD, their contribution to tissue-specific disease manifestations and relationship to overall disease activity remain undefined. Ascertaining the site of initiation for this targeted immune response represents a critical step in addressing these questions, but is limited by the relative lack of lung tissue as well as appropriate reagents for immunohistochemical analysis of in situ HSP90 citrullination. However, as shown by studies in alternative diseases states such as alveolar proteinosis [23,24], bronchoalveolar lavage fluid (BALF) contains cells and humoral factors that reflect immunobiological processes taking place in the lung (e.g., initiation of immune responses versus shaping of extra-pulmonary immune repertoire)—suggesting that BALF can serve as a more specific surrogate of the lung microenvironment than peripheral blood.
Based on this presumption, we examined anti-citrullinated HSP90 antibody profiles in BALF and peripheral blood samples obtained from patients with different stages of RA-ILD. This comparative analysis of both IgG and IgA anti-citrullinated HSP90 antibody specificities yielded a range of epitope recognition patterns, many of which were uniquely associated with BALF. Because the observed differences between BALF and peripheral blood anti-citrullinated HSP90 antibody profiles were often qualitative as well as quantitative, these data provide compelling evidence that the lung is the principal site of initiation and/or repertoire development for citHSP90-targeted immune responses in RA.
2. Methods
2.1. Inclusion criteria and patient samples
In accordance with IRB protocols established through Brigham and Women’s Hospital and the National Human Genome Research Institute (protocols 99-HG-0056 and 04-HG-0211), bronchoalveolar lavage fluid (BALF) and serum samples were obtained from a previously established NIH registry (originally reported as part of the study described in reference [20]) incorporating patients meeting American College of Rheumatology classification criteria for RA [25]. BALF and serum samples for comparator cohorts of healthy controls and idiopathic pulmonary fibrosis (IPF) patients were also obtained through registries at NIH and Brigham and Women’s Hospital (secondary source of samples accrued through the previously referenced NIH protocols).
Additional diagnostic criteria for rheumatoid arthritis-associated interstitial lung disease (RA-ILD) included the presence of pulmonary symptoms (dyspnea, cough), restrictive physiology on pulmonary function testing (Forced Expiratory Volume in 1 s (FEV1), Forced Vital Capacity (FVC), Total Lung Capacity (TLC), and Diffusion Capacity for Carbon Monoxide (DLCO) < 80% predicted; FEV1/FVC > 80% predicted), and radiographic abnormalities on computed tomography scans consisting of reticulation, septal thickening, traction bronchiectasis, honeycombing, and/or ground glass opacification [20,26]. Classification of RA-subclinical ILD (RA-subILD) also required the presence of these radiographic abnormalities (+/− restrictive pulmonary function tests) in the absence of dyspnea, cough, or other clinical features of pulmonary disease.
2.2. Recombinant HSP90 protein and HSP90-derived peptides
Substrate antigens for ELISA (described below) included uncitrullinated and citrullinated HSP90 isoforms as well as peptide pairs consisting of citrullinated versus uncitrullinated sequences derived from HSP90α and HSP90β. Citrullinated forms of recombinant HSP90α (Cell Sciences, Inc., Canton, MA) and HSP90β (Sigma Aldrich, St. Louis, MO) were generated through an in vitro enzymatic reaction involving overnight incubation of protein with rabbit skeletal muscle peptidylarginine deiminase (PAD; Sigma Aldrich, St. Louis, MO) in a buffer containing 20 mM Tris pH 8.8, 0.3 M NaCl, 1 mM EDTA, 10 mM DTT, and 5 mM CaCl2. Corresponding HSP90 peptide sequences were derived from mass spectrometric analysis of citrullinated proteins immunoprecipitated by RA-ILD sera (as previously described in reference [21]) and included the following:
| 1) | lisnasdaldkiRyesltdpsklds | (R55C)1 | 1-C) lisnasdaldkiCyesltdpsklds |
| 2) | lkidiipnpqeRtltlvdtgigmt | (R82C) | 2-C) lkidiipnpqeCtltlvdtgigmt |
| 3) | hlkedqteyleeRrvkevvkkhsqf | (R196C) | 3-C) hlkedqteyleeCrvkevvkkhsqf |
| 4) | qlefrallfiprRapfdlfenkkkk | (R338C) | 4-C) qlefrallfiprCapfdlfenkkkk |
| 5) | vvdsedlplnisRemlqqskilkvi | (R392C) | 5-C) vvdsedlplnisCemlqqskilkvi |
| 6) | gdemtslseyvsRmketqksiyyit | (R475C) | 6-C) demtslseyvsCmketqksiyyit |
| 7) | skeqvansafveRvrkrgfevvymt | (R502C) | 7-C) skeqvansafveCvrkrgfevvymt |
| 8) | anmerimkaqalRdnstmgymmakk | (R612C) | 8-C) anmerimkaqalCdnstmgymmakk |
| 9) | einpdhpivetlRqkaeadkndkav | (R639C) | 9-C) einpdhpivetlCqkaeadkndkav |
| 10) | tkdqvansafveRlrkhgleviy mi | (R510C) | 10-C) tkdqvansafveClrkhgleviy mi |
All sequences were derived from HSP90β, except for 10/10C (HSP90α); position of Arg/Cit residues are indicated in parentheses.
2.3. ELISA
For BALF as well as serum samples, relative levels of IgG versus IgA antibodies targeting peptide/protein derivatives of HSP90α and HSP90β were measured using standard solid phase ELISA according to the following protocols. Ninety six-well microtiter plates (Nunc, Rochester, NY) were coated with citrullinated versus uncitrullinated recombinant human HSP90α (1.0 µg/ml; Cell Sciences, Inc., Canton, MA), citrullinated versus uncitrullinated recombinant human HSP90β (1.0 µg/ml; Sigma, St. Louis, MO), or no antigen (No Ag) in carbonate buffer (100 mM NaHCO3/Na2CO3, pH 9.6) and incubated overnight at 4 °C. After blocking wells with PBS/Tween containing 4% whey protein and 15% goat serum, diluted serum samples (1:500) or BALF (1:4) were added for 2 h. Sequential incubations with horseradish peroxidase-conjugated, anti-human IgG (0.04 mg/ml, Santa Cruz Biotechnology, Santa Cruz, CA) or anti-human IgA (Invitrogen, Camarillo, CA) secondary antibodies (1:10,000 dilution) and 3,3,5,5-tetramethylbenzidine (TMB) (Sigma-Aldrich, St. Louis, MO) substrate then permitted spectrophotometric measurement of OD450 values. Relative titers of anti-citHSP90α and anti-citHSP90β antibodies (minus background reactivity in wells lacking substrate antigen) were scored based on comparison to a reference serum sample obtained from our index case of RA-ILD.
Assessment of IgG and IgA antibodies recognizing HSP90-derived peptides required a modified protocol incorporating biotinylated peptide substrate antigens (CPC Scientific, Inc., Sunnyvale, CA) and streptavidin-coated microplates (R&D Systems, Inc., Minneapolis, MN). ELISA plates were initially coated with peptide pools (consisting of five peptides, each at 0.2 µg/ml) or individual peptides (1.0 µg/ml) for 30 min. Following multiple washes with PBS/0.1% Tween-20, substrate-bound microplates were blocked for 1 h with PBS/Tween containing 4% whey protein and 15% goat serum. Diluted BALF (1:2–1:4) or serum (1:250–1:500) samples were then added for 1 h; subsequent washing steps, application of HRP-conjugated secondary antibodies, addition of TMB substrate, and spectrophotometry were performed as outlined above. While absolute thresholds for positivity in IgG and IgA peptide ELISAs were established based on levels greater than 2 standard deviations above the mean background-adjusted OD450 values for a small population of healthy control subjects (n = 5), relative levels of peptide-specific antibodies (measured in standardized OD450 units) were determined by comparison of mean OD450 readings between BALF/serum samples and various reference sera—with adjusted valuesof ≥1 considered positive.
As a specificity control, sera and BALF were separately assessed with a commercial IgG anti-CCP2 ELISA kit (Axis-Shield Diagnostics, United Kingdom) according to established protocol (for measurement of IgA anti-CCP2 levels, HRP-conjugated anti-human IgA (Invitrogen, Camarillo, CA) secondary antibody (1:10,000 dilution) was substituted for the anti-IgG conjugate). Comparison of serum: BALF anti-tetanus toxoid (TT) antibody levels also required the use of a commercial assay (Genway Biotech, Inc., San Diego, CA) modified for use with appropriately diluted human samples (BALF 1:10, serum 1:1250).
2.4. Statistical analysis
Where applicable, quantitative comparisons involving categorical variables were performed using Fisher’s exact test, with two tailed p-values < 0.05 marking statistical significance. Quantitative assessment of continuous variables (RF, CCP2) relied on non-parametric rank sum analysis (Mann–Whitney U test) and similar thresholds for determining statistical significance (two-tailed p-value < 0.05).
3. Results
3.1. Cohort development
To further investigate the relationship between HSP90-targeted antibody responses and RA-ILD, we obtained BALF and serum specimens from patients comprising a previously described cohort of RA patients with various stages of ILD. As outlined in Section 2, patients were diagnosed with ILD based on well-defined imaging criteria consisting of reticulation, septal thickening, traction bronchiectasis, honeycombing, and/or ground glass opacification detectable by HRCT. Distinction between RA-ILD and RA-subclinical ILD hinged largely on the presence or absence of cough and dyspnea, though comparison of HRCT abnormalities and functional parameters revealed more severe parenchymal involvement and significantly greater restrictive defects in those RA patients with clinically evident ILD [20]. Among the entire cohort of RA patients (n = 21) included in this analysis, no other significant subgroup differences emerged with respect to age, gender, articular disease activity, or smoking (Supplementary Table 1 and data not shown).
3.2. Detection of IgG and IgA anti-citrullinated HSP90 antibodies in BALF
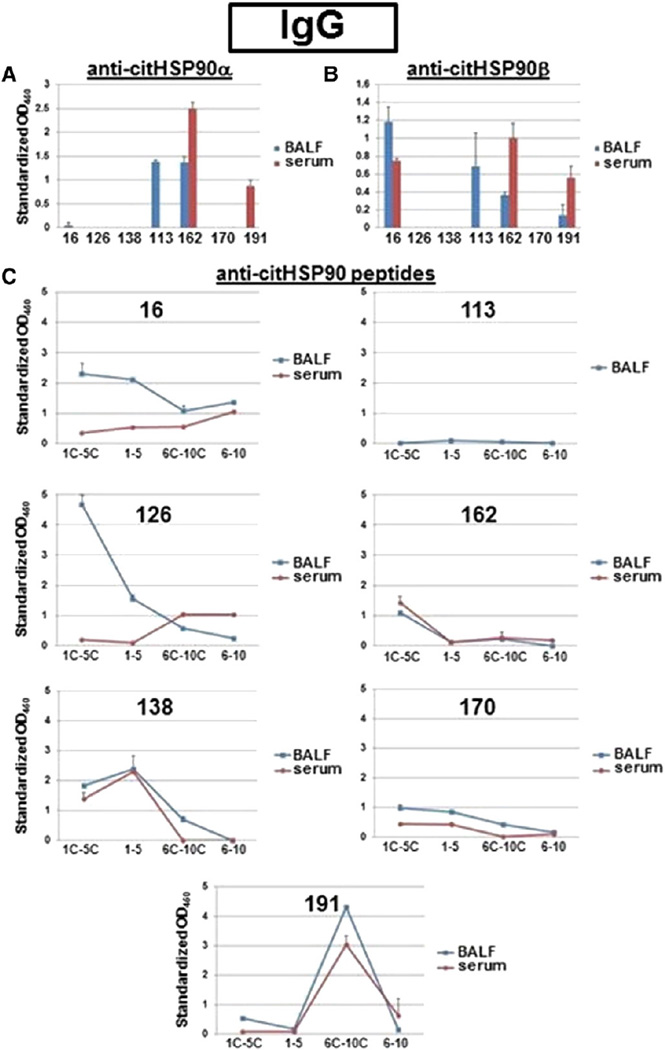
As a first step in this analysis, we examined disease-associated BALF specimens for evidence of IgG antibodies targeting citrullinated versions of recombinant HSP90 or pools of derivative (citrullinated) peptides. Review of Table 1 and Fig. 1 demonstrates that a number of BALF samples obtained from patients with different stages of RA-ILD (n = 7/21) did contain IgG antibodies recognizing citHSP90α, citHSP90β, and/or citrullinated HSP90 peptide pools. Although these data indicated a strong bias toward recognition of citrullinated versions of HSP90-derived sequences (not seen in BALF obtained from IPF patients or healthy control subjects), this specificity was not absolute given the number of RA BALF samples showing at least partial IgG antibody recognition of non-citrullinated HSP90 peptides. Equally intriguing, 5/7 positive BALF specimens yielded discordant IgG antibody responses against full length protein versus peptide (i.e., recognized citHSP90 protein or peptide, but not both; Fig. 1A vs. Fig. 1B), suggesting that some of the relevant epitopes might be discontinuous/ conformational (rather than linear) or masked in the intact protein.
Table 1.
IgG anti-citrullinated HSP90 antibodies in BALF.
| citHSP90αa | citHSP90βa | PP 1C–5Cb | PP 6C–10Cb | |
|---|---|---|---|---|
| RA-ILD (n = 8) | 0 | 1c | 3d | 0 |
| RA-subILD (n = 5) | 2 | 0 | 1e | 0 |
| RA-no ILD (n = 8) | 0 | 0 | 1f | 1 |
| IPF (n = 5) | 0 | 0 | 0 | 0 |
| Healthy control (n = 5) | 0 | 0 | 0 | 0 |
RA-ILD = RA with ILD, RA-subILD = RA with subclinical ILD (absence of cough, dyspnea), RA-no ILD = RA without ILD, IPF = idiopathic pulmonary fibrosis.
Positive responses based on relative ratio of OD450 reading compared to that of standard reference serum, using recombinant citrullinated HSP90α or citrullinated HSP90β as substrate antigen (1 µg/ml).
Positive responses based on relative ratio of OD450 reading compared to that of standard reference serum, using peptide pools (PP) consisting of 1C–5C or 6C–10C (5 peptides/pool, each at 0.2 µg/ml) as substrate antigens.
Sample also positive for uncitrullinated HSP90β and PP 1C–5C.
2/3 samples with similar level of response to uncitrullinated peptide pool 1–5.
Sample also positive for citHSP90α.
Similar level of response to uncitrullinated peptide pool 1–5.
Figure 1.
IgG anti-citrullinated HSP90 antibody levels in BALF versus serum. A) Bar graphs depict relative ELISA-determined IgG anti-citrullinated HSP90α antibody levels (expressed as OD450 units standardized by comparison to serial dilutions of a RA-ILD positive control serum) for BALF (1:4 dilution) and corresponding serum samples (1:500 dilution) derived from patients with RA-ILD (16, 126, 138), RA-subclinical ILD (113 (BALF only), 162), and RA-no ILD (170, 191). B) Bar graphs illustrate relative IgG anti-citrullinated HSP90β antibody levels in BALF (1:4 dilution) and serum samples (1:500 dilution) obtained from the same profile of RA patients, using citHSP90β (1 µg/ml) as substrate antigen for ELISA. Standardized OD450 values were again calculated based on comparison to OD450 readings generated by serial dilutions of a known reference serum. C) In this panel, individual plots demonstrate relative IgG antibody responses against citrullinated (1C–5C, 6C–10C) versus uncitrullinated [1–10] peptide pools derived from HSP90 sequences. While blue curves correspond to BALF samples (1:2 dilution) from designated RA patients with different ILD phenotypes, red curves represent matching serum samples (1:250 dilution) from the same individual. Standardized OD450 units were adjusted based on the ratio of OD450 sample:OD450 reference serum. Error bars represent SEM in all panels.
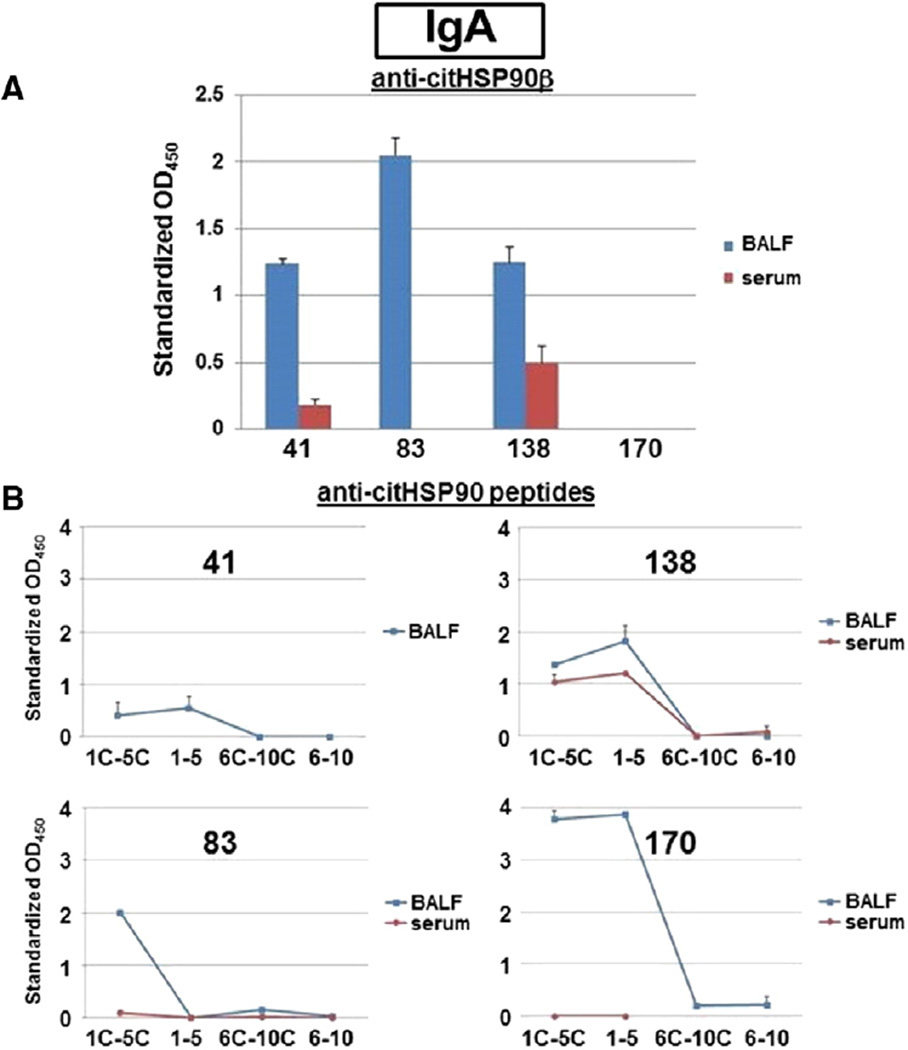
Given the secretory nature of the bronchoalveolar fluid compartment and its potential impact on antibody subtype, we next examined IgA responses in BALF obtained from RA patients and control groups. Again, the presence of IgA anti-citHSP90 protein/peptide antibodies in BALF was fairly specific for RA relative to IPF and healthy controls (occurring in n = 3 RA-ILD, n = 1 RA-no ILD, n = 0 IPF, and n = 0 healthy control subjects; Table 2). Although a precise quantification of sample titer was precluded by the lack of previously defined reference standards, three BALF specimens demonstrated strong recognition of citrullinated HSP90 peptide pools, with background-adjusted OD450 readings > 0.5 and standardized OD450 values well above 1 (Fig. 2B). Paralleling results from analysis of BALF IgG responses against citrullinated versions of HSP90, the overlap between protein and peptide recognition was mixed; while two samples possessed IgA antibodies recognizing both citHSP90β and citrullinated HSP90 peptides, one specimen targeted protein alone and one sample only recognized citrullinated HSP90 peptides (Fig. 2).
Table 2.
IgA anti-citrullinated HSP90 antibodies in BALF.
| citHSP90αa | citHSP90βa | PP 1C–5Cb | PP 6C–10Cb | |
|---|---|---|---|---|
| RA-ILD (n = 8) | 0 | 3c | 2d | 0 |
| RA-subILD (n = 5) | 0 | 0 | 0 | 0 |
| RA-no ILD (n = 8) | 0 | 0 | 1e | 0 |
| IPF (n = 5) | 0 | 0 | 0 | 0 |
| Healthy control (n = 5) | 0 | 0 | 0 | 0 |
RA-ILD = RA with ILD, RA-subILD = RA with subclinical ILD (absence of cough, dyspnea), RA-no ILD = RA without ILD, IPF = idiopathic pulmonary fibrosis.
Positive responses based on OD450 threshold > 2 SD above the mean for healthy control subjects, using recombinant citrullinated HSP90α or citrullinated HSP90β as substrate antigen (1 µg/ml).
Positive responses based on OD450 threshold > 2 SD above the mean for healthy control subjects, using peptide pools (PP) consisting of 1C–5C or 6C–10C (5 peptides/pool, each at 0.2 µg/ml) as substrate antigens.
2/3 specimens also reactive to PP 1C–5C.
1/2 samples also positive for uncitrullinated peptide pool 1–5.
Specimen also positive for uncitrullinated peptide pool 1–5.
Figure 2.
IgA anti-citrullinated HSP90 antibody levels in BALF versus serum. A) Relative titers of IgA anti-citrullinated HSP90β antibodies (determined by ELISA) are shown in this representative graph encompassing patients with RA-ILD (41, 83, 138) as well as RA-no ILD (170); blue and red bars correspond to paired BALF (1:4 dilution) and serum (1:500 dilution) samples, respectively (170 serum not assessed). OD450 units were standardized based on the following ratio: OD450 (sample)/OD450 (BALF 83), with an absolute OD450 threshold for positivity exceeding the mean value for healthy control subjects by more than 2 standard deviations. B) Line plots depict relative levels of IgA antibodies targeting citrullinated (1C–5C, 6C–10C) and uncitrullinated (1–5, 6–10) HSP90 peptides in BALF (blue) and serum (red) for designated patients (170 serum—peptides 6C–10C, 6–10 not assessed); standardization again reflects relative OD450 values compared to those generated by BALF sample 83 (where a threshold value of 1 exceeds the mean adjusted value for healthy control subjects by more than 2 standard deviations). Error bars signify SEM.
3.3. Comparative epitope mapping of BALF- and serum-derived anti-citrullinated HSP90 antibody responses
To better define the role of the lung microenvironment in shaping anti-citrullinated HSP90 antibody repertoire, we evaluated the fine specificity of peptide recognition profiles in BALF and matching serum specimens. In this analysis, relative anti-tetanus toxoid (TT) titers in BALF versus serum served as a control for breach of the blood-alveolar barrier, based on the presumption that plasma cells producing anti-TT antibodies reside outside of the lung parenchyma. As shown in Table 3 detailing IgG antibody responses, BALF derived from RA patients with varying stages of ILD recognized a wide range of peptides, indicating that citHSP90 is not simply an “innocent bystander” target of a cross-reactive immune response directed against an unrelated antigen (a point substantiated by the absence of significant IgG anti-CCP2 antibody titers in 4/7 BALF samples possessing IgG anti-citHSP90 protein/peptide antibodies; Supplementary Table 1). While the epitope specificity was similar between BALF and serum in several cases (138, 162, 191), at least two BALF specimens (16 and 126) yielded qualitatively different IgG antibody profiles relative to their serum counterparts (with preferential recognition of different peptide pools and specific recognition of individual peptides not targeted by corresponding serum samples; Fig. 1B and Table 3, compare peptide recognition profiles as well as relative ratios of reactivity to 1C–5C and 6–10/6C–10C for BALF versus serum in subjects 16, 126). Moreover, in most of the other RA subjects with similar BALF and serum IgG anti-citrullinated HSP90 peptide antibody profiles, significant gradients of serum: BALF anti-TT reactivity (Table 3) supported local production of (anti-citHSP90) antibodies within the lung microenvironment rather than simple breach of the blood-alveolar barrier.
Table 3.
IgG anti-citrullinated HSP90 profiles–BALF versus serum.
| citHSP90αa | citHSP90βa | PP 1C-5Cb | PP 1-5b | PP 6C-10Cb | PP 6-10b | Peptides | Tet Toxoidc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAL | S | BAL | S | BAL | S | BAL | S | BAL | S | BAL | S | BAL | S | BAL | S | |
| RA-ILD | ||||||||||||||||
 |
− | − | 1+ | +/− | 2+ | − | 2+ | − | 1+ | − | 1+ | 1+ | 5,5C 6, 6C 7, 7C 10,10C |
5,5C 6 10Cd |
4+ | 4+ |
 |
− | − | − | − | 3+ | − | 1+ | − | − | 1+ | − | 1+ | 4C | − | +/− | 3+ |
 |
− | − | − | − | 1–2+ | 1 + | 2+ | 2+ | − | − | − | − | 5,5C | 5,5C | 1+ | 3+ |
| RA-subILD | ||||||||||||||||
| 113e | 1+ | NA | − | NA | − | NA | − | NA | − | NA | − | NA | NA | NA | NA | NA |
 |
1+ | 2+ | − | 1+ | 1+ | 1+ | − | − | − | − | − | − | 5C | 5C | − | 3+ |
| RA-no ILD | ||||||||||||||||
 |
− | − | − | − | 1+ | − | +/− | − | − | − | − | − | 5d,5Cd | − | 2+ | 4+ |
 |
− | − | − | − | − | − | − | − | 3+ | 3+ | − | − | 6C,7C | 6C,7C | − | +/− |
Responses scored based on relative ratio of OD450 reading compared to that of standard reference serum (with gradations corresponding to thresholds depicted in Fig. 1A (+/− = relative OD450 0.75–1.0, 1 + =relative OD450 1.0–2.0, 2+ = relative OD450 > 2.0)), using recombinant citrullinated HSP90α or citrullinated HSP90β as substrate antigen (1 µg/ml).
Responses based on relative ratio of OD450 reading compared to that of standard reference serum, using peptide pools (PP) consisting of 1C–5C, 1–5, 6C–10C, or 6–10 (5 peptides/pool, each at 0.2 µg/ml) as substrate antigens; gradations again correspond to thresholds shown in Fig. 1B (+/− = relative OD450 0.75–1.0, 1 + = relative OD450 1.0–2.0, 2+ = relative OD450 2.0–3.0, 3+ = relative OD450 > 3.0).
Relative IgG anti-tetanus toxoid antibody titer (0 = OD450 < 0.25, +/− = OD450 0.25–0.5, 1 + = OD450 0.5–1.0, 2+ = OD450 1.0–1.5, 3+ = OD450 1.5–2.0, 4+ = OD450 > 2.0).
Low positive recognition.
Serum not available; NA = not assessed.
 = +BALF, −serum;
= +BALF, −serum;  = +BALF, inverted serum peptide profile;
= +BALF, inverted serum peptide profile;  = +BALF, +serum, TTS > TTBAL;
= +BALF, +serum, TTS > TTBAL;  = +BALF, +serum, TTS = TTBAL.
= +BALF, +serum, TTS = TTBAL.
In those individuals manifesting IgA anti-citHSP90 protein/peptide antibody responses, the selectivity for BALF was even greater than that seen for IgG antibody profiles (Fig. 2 and Table 4). Specifically, HSP90-targeted antibody responses were unique to BALF for three out of four positive subjects; in the 4th subject (138), the large serum: BALF anti-TT antibody gradient (Table 4) again favored local production of anti-citrullinated HSP90 peptide antibodies within lung-associated lymphoid tissue. More detailed analysis revealed that two of these BALF samples (138, 170) demonstrated equally strong recognition of peptides 5 and 5C, resembling findings in three BALF specimens (two overlapping) manifesting dual IgG recognition of citrullinated and uncitrullinated versions of this HSP90-derived sequence—but differing from other specimens demonstrating more exclusive recognition of alternative citrullinated HSP90 peptides. Of note, although 138 and 170 were the only subjects whose BALF samples contained both IgG and IgA anti-citrullinated HSP90 peptide-specific antibodies (Tables 3–4), they differed with respect to their relative serum IgA responses in which targeting of peptides 5/5C was limited to patient 138. Intriguingly, however, the relative ratio of BALF: serum recognition of this peptide combination was inverted in the IgG and IgA compartments for subject 138 (< 1 for IgG, >1 for IgA), disfavoring breach of the blood alveolar barrier or dilutional error (in which case the ratio of IgA:IgG anti-peptide antibodies in BALF and serum would be parallel rather than discordant) and supporting the possibility of independent repertoire development within the lung microenvironment.
Table 4.
IgA anti-citrullinated HSP90 profiles—BALF versus serum.
| citHSP90αa | citHSP90βa | PP 1C-5Cb | PP 1-5b | PP 6C-10Cb | PP 6–10b | Peptides | Tet Toxoidc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAL | S | BAL | S | BAL | S | BAL | S | BAL | S | BAL | S | BAL | S | BAL | S | |
| RA-ILD | ||||||||||||||||
 |
− | NA | 1+ | − | − | NA | − | NA | − | NA | − | NA | NA | NA | − | +/− |
 |
− | NA | 2+ | − | 2+ | − | − | − | − | − | − | − | 2C,3C | − | +/− | 4+ |
 |
− | NA | 1+ | − | 1+ | 1+ | 1–2+ | 1+ | − | − | − | − | 5,5C | 5,5C | 1+ | 3+ |
| RA-no ILD | ||||||||||||||||
 |
− | NA | NA | 3+ | 3+ | NA | NA | 5,5C | 2+ | 4+ | ||||||
Positive responses based on OD450 threshold > 2 SD above the mean for healthy control subjects, using recombinant citrullinated HSP90α or citrullinated HSP90β as substrate antigen (1 µg/ml); numerical grading of responses determined by relative ratios of OD450 readings to those of BALF sample 138 (1 + = relative OD450 1.0–2.0, 2+ = relative OD450 > 2.0), as shown in Fig. 2A.
Positive responses based on OD450 threshold > 2 SD above the mean for healthy control subjects, using peptide pools (PP) consisting of 1C-5C or 6C-10C (5 peptides/pool, each at 0.2 µg/ml) as substrate antigens; numerical grading of responses again determined by relative ratios of OD450 readings to those of serum sample 138 (1 + = relative OD450 1.0–2.0, 2+ = relative OD450 2.0–3.0, 3+ = relative OD450 > 3.0 (Fig. 2B)).
Relative IgG anti-tetanus toxoid antibody titer (0 = OD450 < 0.25, +/− = OD450 0.25–0.5, 1 + = OD450 0.5–1.0, 2+ = OD450 1.0–1.5, 3+ = OD450 1.5–2.0, 4+ = OD450 > 2.0).
NA = not assessed.
 = + BALF, −serum;
= + BALF, −serum;  = +BALF, inverted serum peptide profile;
= +BALF, inverted serum peptide profile;  = +BALF, +serum, TTS > TTBAL;
= +BALF, +serum, TTS > TTBAL;  = +BALF, +serum, TTS = TTBAL.
= +BALF, +serum, TTS = TTBAL.
3.4. Classification of anti-citrullinated HSP90 protein/peptide antibody recognition patterns
Coupled with data obtained from assessment of BALF and serum IgG antibody recognition profiles, these results pertaining to IgA antibody targeting of citHSP90β and/or derivative peptides strongly favored the occurrence of antigen-specific immune responses within the lung microenvironment. In fact, based on different patterns of reactivity outlined in Tables 3–4, citHSP90-driven antibody responses could be loosely segregated into different categories highlighting their tissue specificity. For example, of the nine BALF specimens with identifiable IgG or IgA anti-citHSP90 protein/peptide antibodies, three had corresponding serum samples that were completely devoid of HSP90-specific antibodies (category A). Extending this theme, sample 126 (category B) demonstrated a somewhat unique pattern of BALF antibody recognition in which the relative ratio of reactivity to HSP90 peptide pools 1C–5C and 6C–10C was inverted with respect to serum (strongly favoring 1C–5C in BALF, with weaker selectivity for 6C–10C in serum; Fig. 1B)— effectively highlighting BALF-specific antibodies targeting peptide 4C. In those cases where both BALF- and serum-derived antibodies recognized specific citrullinated HSP90 peptides, most were associated with anti-TT antibody gradients that disfavored breach of the blood–alveolar barrier—strongly suggesting independent antibody production/secretion in BALF (Category C). Only one subject (16a, Category D) displayed partially overlapping BALF and serum IgG anti-citHSP90 peptide responses in the setting of significant blood-alveolar exchange (marked by equivalent anti-TT antibody titers in serum and BALF); even in this instance, however, the discrepancy in individual as well as pooled peptide reactivity between BALF and serum indicated at least a component of lung-specific antibody production marked by increased relative affinity for selected peptide combinations (Table 3).
3.5. Clinical and demographic characteristics of patients distinguished by the presence of anti-citrullinated HSP90 antibodies within BALF
To determine the phenotypic significance of these collective findings, we compared the clinical, demographic, and serologic features of patients with and without citHSP90-targeted antibody responses in BALF (Table 5). With the caveat that limited patient numbers restricted the power of this analysis, no significant differences emerged between BALF antibody-positive and BALF antibody-negative individuals for disease subset, gender, race, smoking, medication usage, level of RF, or mean serum/BALF CCP2 titers. Strikingly, however, 6/9 subjects with evidence of BALF anti-citHSP90 antibodies lacked significant levels of BALF IgG or IgA anti-CCP2 antibodies (Supplementary Table 1), including each of the four RA patients with BALF-exclusive citHSP90 antibody responses (Categories A and B). Further supporting the specific connection between lung-initiated immune responses and the development of anti-citHSP90 antibodies, 7/9 BALF specimens with substantial levels of IgG or IgA anti-citHSP90 antibodies were derived from individuals with clinical and/or radiographic evidence of RA-ILD.
Table 5.
Clinical and demographic characteristics of RA patients with BALF anti-citHSP90 antibodies.
| BALF+ (n = 9) | BALF− (n = 12) | p-value | |
|---|---|---|---|
| Disease subgroup | |||
| RA-ILD | 5 | 3 | NS |
| RA-subILD | 2 | 3 | NS |
| RA-no ILD | 2 | 6 | NS |
| Gender (M:F) | 3:6 | 4:8 | NS |
| Race | |||
| Caucasian | 7 | 11 | NS |
| African American | 1 | 0 | NS |
| Other | 1 | 1 | NS |
| Smoking (ever %) | 6 (67) | 8 (67) | NS |
| Medication | |||
| Prednisone | 6 | 8 | NS |
| Methotrexate | 0 | 4 | NS |
| TNF-inhibitor | 2 | 4 | NS |
| RF—Serum | |||
| Mean ± SD | 70.2 ± 60.2 | 105.5 ± 146.2 | NS |
| (Range) | (10–183) | (10–522) | |
| CCP2 (IgG)—Seruma | |||
| Mean ± SD | 58.1 ± 108.2 | 84.4 ± 90.2 | NS |
| (Range) | (0–254) | (0–248) | |
| CCP2(IgG)—BALF | |||
| Mean ± SD | 61.8 ± 99.4 | 41.3 ± 51.2 | NS |
| (Range) | (0–256) | (0–125) | |
| CCP2 (IgA)—BALF | |||
| Mean ± SD | 20.9 ± 28.8 | 29.2 ± 57.2 | NS |
| (Range) | (1–70) | (1–191) |
One sample missing from both BALF+ (n = 8) and BALF− (n = 11) groups.
4. Discussion
Overall, these results clearly demonstrate BALF-associated antibody responses against citHSP90 and/or derivative peptides in patients with various stages of RA-ILD. Comparison of antibody profiles between BALF and corresponding serum samples reveals several examples of “lung-specific” IgG and IgA antibody responses targeting these proteins. As detailed above, more precise peptide epitope mapping highlights a variety of recognition patterns, some of which are qualitatively as well as quantitatively distinct from serum antibody profiles. These data therefore support a paradigm in which the lung plays an active role in shaping the immune repertoire against resident citrullinated autoantigens contributing to the pathogenesis of RA-ILD.
From a diagnostic perspective, the composite sensitivity and specificity of BALF screening for RA-ILD were relatively high, with 5/8 RA-ILD, 2/5 RA-subclinical ILD, and 2/8 RA-no ILD BALF specimens showing reactivity against citHSP90α, citHSP90β, or citrullinated HSP90 peptides (yielding a combined sensitivity for ILD of 7/13 = 0.54 and a specificity of 6/8 = 0.75). These performance characteristics compare favorably to those of serum, where 4/8 RA-ILD and 5/10 RA-subclinical ILD samples from the same cohort recognize citHSP90 protein and/or peptide ([21] and data not shown). Interestingly, 2/3 RA-ILD subjects lacking anti-citHSP90 protein/peptide antibodies in BALF demonstrate such antibodies in serum. While these examples of preferential serum responses appear to challenge the notion that anti-citHSP90 antibodies are formed in the lung, alternative explanations include sampling bias of bronchoalveolar lavage, the existence of alternative, untested epitope specificities in BALF, or transient/self-limited lung-derived immune responses that are perpetuated in extra-pulmonary tissues.
Beyond this composite analysis, more focused examination of anti-citHSP90 protein versus anti-citHSP90 peptide responses in BALF reveals a level of discordance that potentially impacts the sensitivity of these individual serological parameters for RA-ILD. In fact, the incongruity between anti-citHSP90 protein and peptide reactivity in BALF mirrors that found in serum where only one subclinical RA-ILD specimen (162) recognizes both citHSP90 protein and peptide (Table 3). From a structural point of view, the lack of precise correlation between anti-citHSP90 protein and peptide responses in BALF as well as serum suggests that some peptide epitopes are masked in whole protein, while other protein-specific epitopes are either discontinuous/conformational or underrepresented in relatively limited peptide pools that do not fully encompass the range of potential citrullinated HSP90 epitopes.
Within this cohort, the overall specificity of BALF-associated anti-citHSP90 antibody responses for RA/RA-ILD was excellent, as 0/5 IPF and 0/5 healthy control subjects manifested such reactivity. On the other hand, these serological responses were not exclusive to RA-ILD, occurring in two samples obtained from patients with RA alone (170, 191; Tables 3–4). Of interest, the pattern of epitope recognition in one of these BALF samples (191) was not found in any of the positive RA-ILD samples; coupled with the parallel observation that several alternative peptides were uniquely recognized by RA-ILD-derived BALF (Tables 3–4), this result suggested that fine epitope specificity in BALF might correlate with disease status. Consistent with this hypothesis, the repertoire of serum anti-citHSP90 peptide antibodies detected in a limited number of positive RA-no ILD samples is far more restricted relative to profiles defined for RA-subclinical ILD and RA-ILD (data not shown).
Notwithstanding these questions regarding specificity for ILD, the presence of anti-citHSP90 antibodies in BALF derived from RA patients 170 and 191 remains compatible with an underlying paradigm in which the lung is an important site of initiation for antigen-specific immune responses in RA. In the case of 170, for example, both IgA and IgG anti-citHSP90 peptide responses are highly selective for BALF (and therefore lung), effectively illustrating the capacity of the lung to shape immune repertoire. With 191, on the other hand, the presence of high level anti-citHSP90 peptide reactivity in both serum and BALF clouds this interpretation, though the anti-TT antibody gradient (negligible titers in BALF relative to serum) suggests concomitant production of anti-citHSP90 antibodies in the lung and extra-pulmonary sites rather than breach of the blood–alveolar barrier (which should not occur in the absence of active lung disease).
Apart from these global issues of disease specificity, more detailed molecular characterization of BALF-associated antibody responses reveals that several BALF specimens (16, 138, 170) recognize uncitrullinated as well as citrullinated versions of HSP90-derived peptides. In each case, this dual recognition involves peptides 5 and 5C (and for RA-ILD subject 16, this paired specificity extends to 6/6C, 7/7C, and 10/10C). Intriguingly, structural modeling facilitated by Molecular Dynamics simulation indicates that citrullination does not change the overall conformation of these peptide combinations—providing a rational explanation for equivalent antibody responses against modified and unmodified versions of these sequences. Although such observations suggest primary recognition of non-citrullinated epitopes shared by individual peptide combinations (particularly when compared to cases where serum/BALF more selectively target citrullinated versions of these peptides), it is conceivable that citrullination breaks T cell tolerance with subsequent expansion of polyclonal B cell responses against non-citrullinated (as well as citrullinated) structures.
Viewed more broadly, this work provides direct support for the hypothesis that the lung may serve as an initiating site for antigen-specific immune responses directed against citrullinated protein targets in RA—or, at the very least, may play a significant role in shaping anti-citrullinated protein antibody repertoire. Perhaps this framework of lung-directed immune responses is best illustrated by alveolar proteinosis, a disease in which autoantibodies against a component of GM-CSF alter surfactant metabolism to the extent that alveoli become congested with lipoproteinaceous material [23,24]. While examples of similar lung-oriented humoral immune responses occurring in the context of underlying systemic autoimmune disease have been far more limited [27], investigators have recently demonstrated enhanced titers of IgG as well as IgA anti-CCP antibodies in RA-associated saliva and BALF [28,29]. However, none of these previous studies identified specific protein targets or focused on fine epitope specificity; consequently, these analyses could not demonstrate qualitative as well as quantitative differences between BALF and serum antibody profiles. The latter point is critical, as shifts in relative affinity and/or epitope specificity between BALF and serum—even in a relatively limited number of specimens—provide the most compelling evidence supporting non-stochastic, lung-derived immune responses (i.e., these variables are less biased by the imprecise normalization of BALF and serum dilution factors used for quantitative comparisons). At the same time, the emergence of several BALF specimens with IgA anti-citHSP90 protein/peptide responses is equally consistent with this lung-centric view of autoantibody derivation given the predilection of this antibody subclass for secretory compartments such as the bronchoalveolar tree.
5. Conclusions
Although this study reinforces the concept that anti-citHSP90 antibody responses originate and/or mature in the lung, systematic analysis of BALF from larger cohorts of RA patients with different stages of ILD will be required to fully substantiate these findings. Additional autoantibody/ autoantigen markers will also be needed to generate composite profiles that may capture a greater number of RA patients, particularly those with subclinical forms of RA-ILD. Ultimately, however, direct proof of this mechanistic paradigm will hinge on immunohistochemical demonstration of citrullinated HSP90 isoforms within lung tissue as well as isolation and comparative analysis of antigen-specific lymphocytes/plasma cells residing within the lung parenchyma and synovium.
Supplementary Material
Acknowledgments
The authors take full responsibility for the contents of this paper, which do not represent the views of the Department of Veterans Affairs or the United States Government. The work encompassed by this manuscript was supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health as well as VA Merit Review 1I01BX000788 (DPA) and ACR/REF Within Our Reach Foundation (CVO, DPA) grants.
Abbreviations
- RA
rheumatoid arthritis
- ILD
interstitial lung disease
- BALF
bronchoalveolar lavage fluid
- IPF
idiopathic pulmonary fibrosis
- HSP90
heat shock protein 90 kDa
Footnotes
Funding: Dana P. Ascherman: VA Merit Review 1I01BX000788, ACR/REF Within Our Reach Foundation.
Conflict of interest
The authors declare that there are no conflicts of interest.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.clim.2014.08.004.
References
- 1.Korkmaz C, Us T, Kasifoglu T, Akgun Y. Anti-cyclic citrullinated peptide (CCP) antibodies in patients with long-standing rheumatoid arthritis and their relationship with extra-articular manifestations. Clin. Biochem. 2006;39(10):961–965. doi: 10.1016/j.clinbiochem.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Turesson C, Jacobsson LT. Epidemiology of extra-articular manifestations in rheumatoid arthritis. Scand. J. Rheumatol. 2004;33(2):65–72. doi: 10.1080/03009740310004621. [DOI] [PubMed] [Google Scholar]
- 3.Young A, Koduri G. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2007;21(5):907–927. doi: 10.1016/j.berh.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Young A, Koduri G, Batley M, Kulinskaya E, Gough A, Norton S, et al. Mortality in rheumatoid Increased in the early course of disease arthritis in ischaemic heart disease and in pulmonary fibrosis. Rheumatology (Oxford) 2007;46(2):350–357. doi: 10.1093/rheumatology/kel253. [DOI] [PubMed] [Google Scholar]
- 5.Brown KK. Rheumatoid lung disease. Proc. Am. Thorac. Soc. 2007;4(5):443–448. doi: 10.1513/pats.200703-045MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population based study. Arthritis Rheum. 2010;62:1583–1591. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naz SM, Symmons DP. Mortality in established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2007;21(5):871–883. doi: 10.1016/j.berh.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Afeltra A, Zennaro D, Garzia P, Gigante A, Vadacca M, Ruggiero A, et al. Prevalence of interstitial lung involvement in patients with connective tissue diseases assessed with high-resolution computed tomography. Scand. J. Rheumatol. 2006;35(5):388–394. doi: 10.1080/03009740600844381. [DOI] [PubMed] [Google Scholar]
- 9.Ayhan-Ardic FF, Oken O, Yorgancioglu ZR, Ustun N, Gokharman FD. Pulmonary involvement in lifelong non-smoking patients with rheumatoid arthritis and ankylosing spondylitis without respiratory symptoms. Clin. Rheumatol. 2006;25(2):213–218. doi: 10.1007/s10067-005-1158-x. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Shi Y, Wang X, Huang H, Ascherman D. Asymptomatic preclinical rheumatoid arthritis-associated interstitial lung disease. Clin. Dev. Immunol. 2013;2013:406927. doi: 10.1155/2013/406927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortet B, Flipo RM, Remy-Jardin M, Coquerelle P, Duquesnoy B, Remy J, et al. Use of high resolution computed tomography of the lungs in patients with rheumatoid arthritis. Ann. Rheum. Dis. 1995;54(10):815–819. doi: 10.1136/ard.54.10.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demir R, Bodur H, Tokoglu F, Olcay I, Ucan H, Borman P. High resolution computed tomography of the lungs in patients with rheumatoid arthritis. Rheumatol. Int. 1999;19(1–2):19–22. doi: 10.1007/s002960050093. [DOI] [PubMed] [Google Scholar]
- 13.Gabbay E, Tarala R, Will R, Carroll G, Adler B, Cameron D, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am. J. Respir. Crit. Care Med. 1997;156(2 Pt 1):528–535. doi: 10.1164/ajrccm.156.2.9609016. [DOI] [PubMed] [Google Scholar]
- 14.Georgiadis AN, Metafratzi ZM, Drosos AA. Pulmonary abnormalities in patients with early and longstanding rheumatoid arthritis. J. Rheumatol. 2009;36(2):444–445. doi: 10.3899/jrheum.080882. (author reply 5–6). [DOI] [PubMed] [Google Scholar]
- 15.Kelly C, Saravanan V. Treatment strategies for a rheumatoid arthritis patient with interstitial lung disease. Expert. Opin. Pharmacother. 2008;9(18):3221–3230. doi: 10.1517/14656560802591430. [DOI] [PubMed] [Google Scholar]
- 16.Kim EJ, Elicker BM, Maldonado F, Webb WR, Ryu JH, Van Uden JH, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur. Respir. J. 2009;35:1322–1328. doi: 10.1183/09031936.00092309. [DOI] [PubMed] [Google Scholar]
- 17.Mori S, Cho I, Koga Y, Sugimoto M. Comparison of pulmonary abnormalities on high-resolution computed tomography in patients with early versus longstanding rheumatoid arthritis. J. Rheumatol. 2008;35(8):1513–1521. [PubMed] [Google Scholar]
- 18.Metafratzi ZM, Georgiadis AN, Ioannidou CV, Alamanos Y, Vassiliou MP, Zikou AK, et al. Pulmonary involvement in patients with early rheumatoid arthritis. Scand. J. Rheumatol. 2007;36(5):338–344. doi: 10.1080/03009740701393957. [DOI] [PubMed] [Google Scholar]
- 19.Nannini C, Ryu JH, Matteson EL. Lung disease in rheumatoid arthritis. Curr. Opin. Rheumatol. 2008;20(3):340–346. doi: 10.1097/BOR.0b013e3282f798ed. [DOI] [PubMed] [Google Scholar]
- 20.Gochuico BR, Avila NA, Chow CK, Novero LJ, Wu HP, Ren P, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch. Intern. Med. 2008;168(2):159–166. doi: 10.1001/archinternmed.2007.59. [DOI] [PubMed] [Google Scholar]
- 21.Harlow L, Rosas IO, Gochuico BR, Mikuls TR, Dellaripa PF, Oddis CV, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. 2013;65(4):869–879. doi: 10.1002/art.37881. [DOI] [PubMed] [Google Scholar]
- 22.Bongartz T, Cantaert T, Atkins SR, Harle P, Myers JL, Turesson C, et al. Citrullination in extra-articular manifestations of rheumatoid arthritis. Rheumatology (Oxford) 2007;46(1):70–75. doi: 10.1093/rheumatology/kel202. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura T, Tanaka N, Watanabe J, Kanegasaki Uchida S, Yamada Y, et al. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1999;190(6):875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nei T, Urano S, Motoi N, Takizawa J, Kaneko C, Kanazawa H, et al. IgM-type GM-CSF autoantibody is etiologically a bystander but associated with IgG-type autoantibody production in autoimmune pulmonary alveolar proteinosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302(9):L959–L964. doi: 10.1152/ajplung.00378.2011. [DOI] [PubMed] [Google Scholar]
- 25.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 26.Richards TJ, Eggebeen A, Gibson K, Yousem S, Fuhrman C, Gochuico BR, et al. Characterization and peripheral blood biomarker assessment of anti-Jo-1 antibody-positive interstitial lung disease. Arthritis Rheum. 2009;60(7):2183–2192. doi: 10.1002/art.24631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inui N, Matsui T, Suda T, Chida K. Anti-endothelial cell antibodies in patients with sarcoidosis. Chest. 2008;133(4):955–960. doi: 10.1378/chest.07-0850. [DOI] [PubMed] [Google Scholar]
- 28.Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol. 2014;66(1):31–39. doi: 10.1002/art.38201. [DOI] [PubMed] [Google Scholar]
- 29.Willis VC, Demoruelle MK, Derber LA, Chartier-Logan CJ, Parish MC, Pedraza IF, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum. 2013;65(10):2545–2554. doi: 10.1002/art.38066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.