Abstract
Non-cardiac thoracic manifestations of rheumatoid arthritis (RA) cause significant morbidity and mortality among RA patients. Essentially all anatomic compartments in the chest can be affected including the pleura, pulmonary parenchyma, airway, and vasculature. In addition, treatment-related complications and opportunistic infections are not uncommon. Accurate diagnosis of intra-thoracic disease in an RA patient can be difficult as the radiologic findings may be nonspecific and many of these conditions may coexist. This review article serves to highlight the multitude of RA-related intra-thoracic pathological processes, emphasize differential diagnosis, diagnostic conundrums and discuss how tailoring of CT imaging and image-guided biopsy plays a key role in the management of RA-related pulmonary disease.
Keywords: Rheumatoid arthritis, Interstitial lung disease, Rheumatoid nodule, Pleural disease, Drug toxicity
1. Introduction
Rheumatoid arthritis (RA) is the most common inflammatory arthritis characterized by chronic inflammation, erosions and progressive cartilage damage of multiple joints. In approximately 40% of RA patients, the clinical course can be complicated by a variety of extra-articular manifestations such as rheumatoid nodules, cardiovascular disease, vasculitis, pericarditis, ocular inflammation, and non-cardiac thoracic complications [1]. A high rheumatoid factor (RF) titer and anti-CCP antibodies are predictors of more advanced inflammatory arthritis and extra-articular involvement [1,2]. Non-cardiac thoracic manifestations of RA occur in approximately 5–20% of RA patients and account for approximately 20% of RA-associated mortality [3–5]. All anatomic compartments in the chest can be affected including the pleura, pulmonary parenchyma, airway, and vasculature (Table 1).
Table 1.
Primary thoracic complications of rheumatoid arthritis.
| Anatomic component | Disease |
|---|---|
| Pleura | Pleuritis Pleural effusion Empyema Pleural thickening Pleural fibrosis Bronchopleural fistula Pneumothorax |
| Pulmonary parenchyma and interstitium | Necrobiotic rheumatoid nodule Interstitial lung disease Non-specific interstitial pneumonia Usual interstitial pneumonia Cryptogenic organizing pneumonia Idiopathic lymphocytic interstitial pneumonia Acute interstitial pneumonia Eosinophilic pneumonia Secondary amyloidosis |
| Airway | Cricoarytenoid arthritis Rheumatoid nodule involving the upper airway Bronchiectasis Follicular bronchiolitis Diffuse panbronchiolitis Constrictive or obliterative bronchiolitis |
| Pulmonary vasculature | Pulmonary hypertension Vasculitis Diffuse alveolar hemorrhage (capillaritis) |
Several factors contribute to challenges in the accurate diagnosis of intra-thoracic disease in an RA patient. First, radiologic manifestations may not coincide with the clinical presentation. For instance, extra-articular manifestation such as diffuse lung disease or lung nodules may be the initial presentation on imaging in a patient in whom a diagnosis of RA has not yet been established. Second, these patients are frequently on immunosuppressants, which may predispose them to drug-related toxicity and opportunistic infections (Table 2). Third, many of these conditions and complications may coexist, resulting in a spectrum of radiologic manifestations. Given the degree of complexity, an interdisciplinary approach is necessary in evaluating these patients. This requires understanding of the typical thoracic manifestations of RA as well as possible infectious, drug-related and surgical complications. Histopathologic diagnosis is at times needed to effectively guide management.
Table 2.
Secondary thoracic complications of rheumatoid arthritis.
| Cause | Disease |
|---|---|
| Drug reaction and toxicities | Methotrexate [55,57] Subacute hypersensitivity pneumonitis Non-specific interstitial pneumonia Acute interstitial pneumonitis Pulmonary nodulosis d-penicillamine [58] Obliterative bronchiolitis Obstructive airway dysfunction Leflunomide [18,19] Acute interstitial pneumonitis Progression or exacerbation of preexisting interstitial lung disease Sulphasalazine [58] Eosinophilic pneumonia Rituximab [58] Non-specific interstitial pneumonitis Cryptogenic organizing pneumonia Diffuse alveolar damage Anti-TNF-α agents [56,59,60] Acute interstitial pneumonitis Non-specific interstitial pneumonitis Pulmonary nodulosis Interstitial lung disease with granulomatous component Pleuropericarditis Adenopathy with non-necrotizing granulomas Pulmonary fibrosis |
| Opportunistic infection | Pulmonary tuberculosis Atypical mycobacterial infection Nocardiosis Chronic pulmonary aspergillosis Histoplastomosis Coccidioidomycosis Pneumocystis jiroveci pneumonia Cytomegalovirus pneumonitis |
This article will (1) illustrate typical thoracic manifestations of rheumatoid arthritis and correlate the radiologic findings with clinical and pathological findings when appropriate; (2) emphasize differential diagnosis, diagnostic conundrums and (3) discuss how tailoring of CT imaging and image-guided intervention plays a key role in the management of RA-related pulmonary conditions.
2. Spectrum of pleural disease
Pleural disease is considered the most common thoracic manifestation in RA patients, identified in 38–73% at autopsy [6–9]. Radiographic findings of pleural inflammation, including pleural thickening and pleural effusion, were noted in 20% of RA patients in a study by Jurik et al. [6]. Despite being the most common thoracic manifestation of rheumatoid disease, the majority of patients are asymptomatic and only few present with chest pain or dyspnea.
2.1. Pleural effusion and empyema
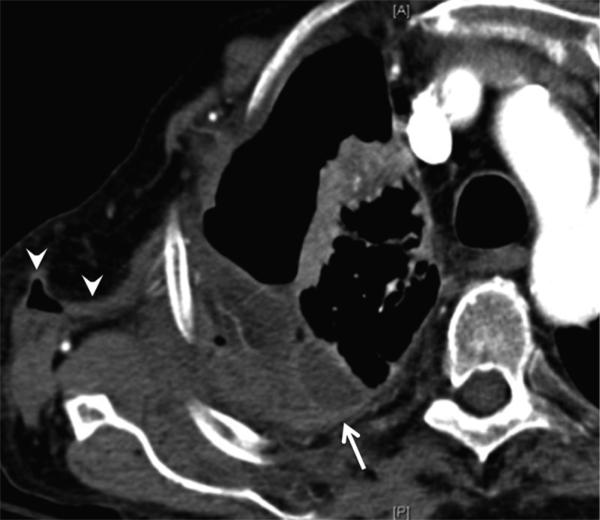
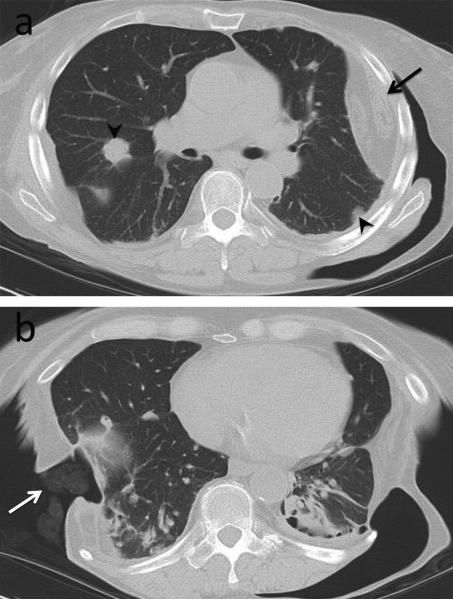
Pleural effusion may result from pleural inflammation, infection or rheumatoid-associated cardiac disease. Effusions secondary to pleural inflammation are typically small and unilateral with associated rim-like pleural enhancement, while those related to cardiac failure are usually bilateral [10,11]. Long-standing sterile pleural effusions with numerous leukocytes and abundant fibrinoid debris may become loculated and surrounded by pleural thickening, simulating the appearance of empyema both radiologically and biochemically, even though it remains sterile. Bacterial staining and culture is needed to differentiate this from true empyema. Chronic pleural effusions may also become milky secondary to cholesterol crystals or lipid-containing inclusions in white blood cells from rupture of necrotic subpleural rheumatoid nodules. This leads to the development of pseudochylous effusions which are distinct from true chylothorax, seen secondary to high triglyceride level [12]. It is also important to realize that pleuropericarditis may represent drug toxicity rather than a primary disease manifestation, as has been described with the use of methotrexate and TNF-α blocking agents [13,14]. Clinical and treatment history should be considered alongside appropriate studies before initiating treatment. In rare cases, chronic empyema becomes recalcitrant to conventional treatment, extending through adjacent chest wall soft tissues to manifest as empyema necessitans (Fig. 1), requiring surgical drainage.
Fig. 1.
Empyema necessitans (extrapleural transthoracic extension of empyema). 62-year-old female with history of rheumatoid arthritis complicated by empyema, recalcitrant to multiple chest tube drainages. Contrast-enhanced CT shows multi-loculated pleural collection with enhancing pleural abnormality (arrow) extending into the chest wall (arrowheads).
Teaching point: Nombination of pleuritis, underlying parenchymal changes and treatment-related immunosuppression predispose patients to severe empyema.
2.2. Pleural thickening and pleural fibrosis
Rheumatoid pleural involvement may result in thickening of both the parietal and visceral pleurae. Long-standing pleural inflammation can lead to pleural fibrosis, trapped lung and lung restriction [7]. In a few cases, pleural thickening may have a mass-like or nodular appearance, with imaging features indistinguishable from neoplasms such as mesothelioma or metastasis. Core biopsy rather than fine needle aspiration is often required to define a malignant versus benign pathologic diagnosis (Figs. 2 and 3). Pleural biopsy of rheumatoid inflammation typically demonstrates nonspecific inflammatory changes with replacement of normal mesothelial cells by epithelioid cells and multinucleated giant cells [10], rather than specific rheumatoid granulomas.
Fig. 2.
Nodular pleural thickening simulating malignancy. 70-year-old female with history of rheumatoid arthritis (RA) and left breast cancer status post radiation therapy. (a) CT. Nodular left pleural thickening (arrows) in this patient is an indeterminate finding that may represent rheumatoid inflammation, malignancy or infection. (b) Pleural biopsy showed necrotizing granulomatous inflammation without evidence of malignancy. Gram, fungal, and acid-fast stains were negative for organisms.
Teaching point: Nodular pleural thickening in RA patients frequently mimics malignancy such as metastatic disease and mesothelioma.
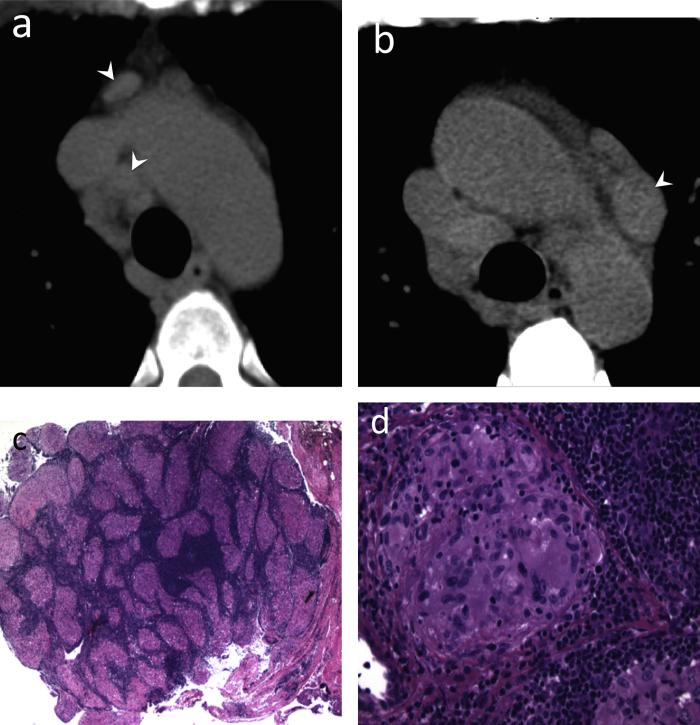
Fig. 3.
Pleural thickening and rheumatoid arthritis associated interstitial lung disease (RA-ILD). 66-year-old female with history of rheumatoid arthritis. (a) CT. Reticular abnormality, traction bronchiectasis and peripheral groundglass opacities in both lower lobes (arrowheads), consistent with RA-ILD. (a and b) CT. A pleural-based ovoid soft tissue mass in the left lower hemithorax (arrows). (c) CT-guided core-needle biopsy was consistent with eosinophillic pleuritis and pleural fibrosis.
Teaching point: Mass-like pleural thickening in rheumatoid arthritis patients may mimic malignancy including primary pleural malignancy and often requires percutaneous core needle biopsy for accurate diagnosis.
2.3. Bronchopleural fistula and pneumothorax
Other less common RA-related pleural diseases are closely related to rheumatoid nodulosis. The rheumatoid nodules, which tend to occur along the subpleural regions and fissures, can cavitate and rupture, essentially providing a portal of entry to the pleural space. The results are complications such as recurrent pneumothorax, bronchopleural fistula (BPF) formation with air leak, abscess and empyema [15] (Fig. 4). The majority of bronchopleural fistulae in pleuroparenchymal disease related to RA are peripheral rather than central due to the subpleural location of necrobiotic pulmonary nodules. Chest CT with thin axial sections has sensitivity of 35–50% for detection of peripheral bronchopleural fistulae [16].
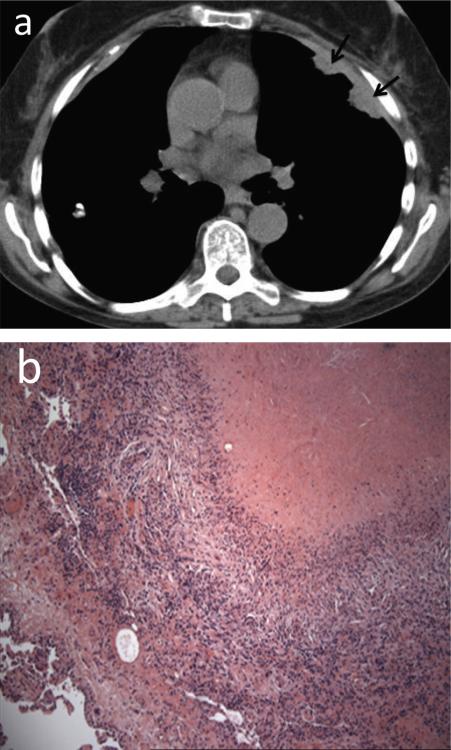
Fig. 4.
Ruptured necrobiotic nodules complicated by hydropneumothorax and empyema. 35-year-old female with rheumatoid arthritis. (a) CT. A ruptured subpleural necrobiotic nodule in the right lower lobe (white arrowhead) with associated small hydropneumothorax (black arrowhead). (b) CT few weeks later. Formation of bronchopleural fistula adjacent to the necrobiotic nodule (black arrow). (c) CT few weeks later following chest tube drainage, open thoracotomy and talc pleurodesis (white arrow). Enlarging hydro-pneumothorax (black arrowheads) secondary to underlying bronchopleural fistula and marked pleural thickening due to empyema resistant to treatments.
Teaching point: Subpleural location of cavitary necrobiotic rheumatoid nodules predisposes the patient to recurrent pneumothoraces and infection within the pleural space.
2.4. Challenges in management of pleural disease
Pleural complications in RA patients can be difficult to treat given the physical characteristics of the underlying pleura and lung parenchyma as well as the patient's immunosuppressed state that predispose to infection and poor healing. Pleural effusions in RA patients should be sampled with caution, as there is a risk of introducing infection within an otherwise sterile pleural space. Furthermore, there is increasing evidence linking specific drugs including methotrexate, etanercept, infliximab and leflunomide with accelerated nodulosis and recurrent pneumothoraces [17–20]. The precise mechanism leading to nodular lung lesions and aseptic non-caseating granulomatous inflammation is unclear and may be variable depending on the drug involved. Histopathological analysis may show rheumatoid like nodules, sarcoid or sarcoid-like reaction or in some patients receiving anti-TNF alpha blockers, a vasculitis like phenomenon can be seen [21]. Knowledge of these potential drug-related complications is important as medications may need to be stopped in such cases.
In a patient with refractory rheumatoid pleural effusions and pneumothoraces, pleurodesis and decortication may be indicated after chest tube drainage fails. However, in cases where an underlying bronchopleural fistula is identified, video-assisted thoracoscopic surgery (VATS) pleurodesis may prove inadequate as the presence of fistula can impede healing and frequently a surgical thoracostomy, thoracomyoplasty or Clagett window creation is needed (Fig. 5). These surgical procedures control both the pleural and parenchymal components of recalcitrant intrathoracic infections, as well as help reduce dead space which does not contribute to gas exchange [22]. Imaging features which indicate successful surgical intervention include resolution of air leak and empyema, gradual obliteration of pleural space and decreasing thickness of pleural rind. On the other hand, persistent on enlarging air fluid levels, visualization of central bronchopleural fistula and thick rind like pleural thickening portend a poor outcome [22].
Fig. 5.
Treatment of recurrent hydropneumothorax and pleural effusion with surgical thoracostomy and creation of Clagett window. 57-year-old female with advanced rheumatoid arthritis on methotrexate with recurrent right hydropneumothorax and left rheumatoid pleural effusion, resistant to conventional management. (a) CT. Latissimus dorsi muscle flap in the left chest wall (black arrow). Necrobiotic nodules in both lungs (arrowheads). (b) CT. Packing material in the right thoracic window (white arrow).
Teaching point: Recurrent hydropneumothoraces and empyemas recalcitrant to conservative management may require extensive surgery such as surgical thoracostomy and thoracomyoplasty.
3. Spectrum of parenchymal and interstitial lung disease
3.1. Rheumatoid-associated interstitial lung disease (RA-ILD)
The prevalence of clinically evident RA-ILD is approximately 5% [23], while radiological evidence of ILD is seen in up to 25% of patients [24,25]. The term RA-ILD encompasses a wide spectrum of morphologic changes on HRCT which are associated with different clinical prognosis. In some cases, there maybe co-existence of more than one HRCT pattern. The major CT patterns are usual interstitial pneumonia (UIP) (Fig. 6), non-specific interstitial pneumonia (NSIP) (Fig. 7), and organizing pneumonia (OP) (Fig. 8) [26].
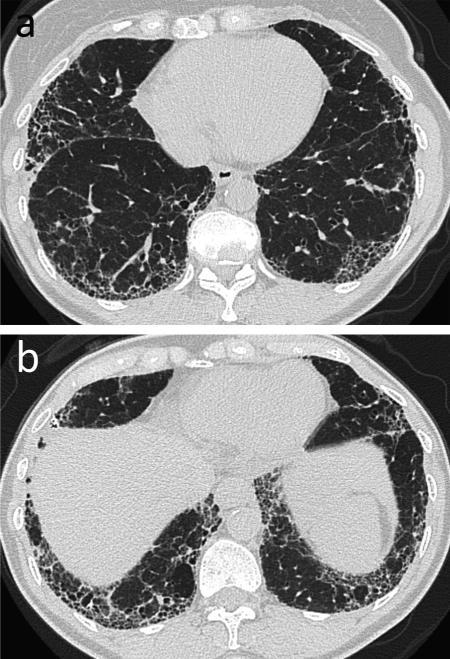
Fig. 6.
Usual interstitial pneumonia (UIP). 63-year-old female with long-standing history of rheumatoid arthritis. (a, b) CT. Bilateral lower lobe predominant subpleural reticular opacity and honeycombing. Biopsy confirmed the diagnosis of UIP. Teaching point: Multilicity of CT patterns may be seen; however, UIP is the most common pattern of interstitial lung disease in rheumatoid arthritis patients.
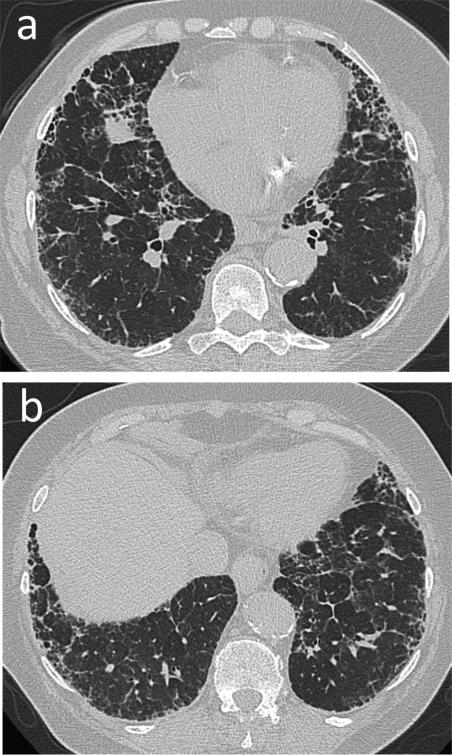
Fig. 7.
Nonspecific interstitial pneumonia (NSIP). 76-year-old male with longstanding history of rheumatoid arthritis with subcutaneous rheumatoid nodules. (a and b) CT. Moderate peripheral reticular opacities and groundglass opacities with minimal honeycombing predominantly in the lower lobes. Biopsy showed changes most consistent with fibrotic NSIP.
Teaching point: NSIP and UIP are common patterns of rheumatoid-associated inter-stitial lung disease. NSIP and UIP can have overlapping imaging features. In such cases, biopsy is required to make the diagnosis.
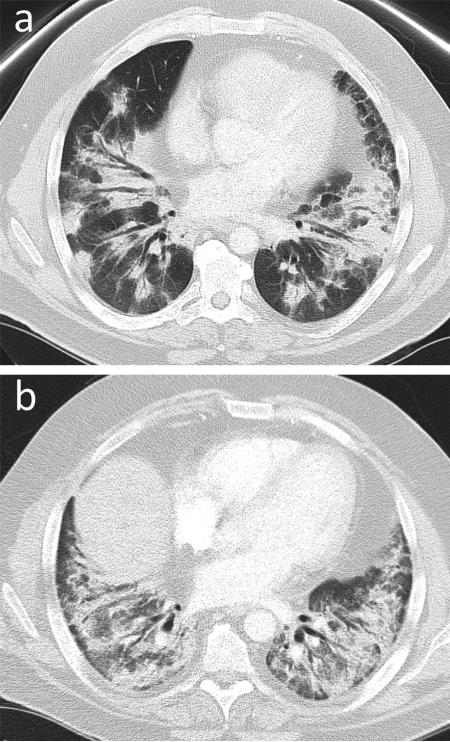
Fig. 8.
Organizing pneumonia. 49-year-old female with RA on etanercept presenting with chest heaviness and nonproductive cough. (a) Initial CT. Bilateral peribronchial and subpleural consolidation with associated architectural distortion and traction bronchiectasis, which did not improve with antibiotics. Bronchoscopy was negative for infection. Wedge biopsy was consistent with organizing pneumonia. (b) CT chest few months later. Significant worsening of disease correlating with patient's declining pulmonary function and respiratory failure.
Teaching point: Organizing pneumonia mimics pneumonia; hence, if the diagnosis is suspected clinically, prompt bronchoscopy should be performed to rule out infection before initiating steroids.
3.2. Correlation between RA-ILD CT pattern and patient prognosis
CT helps in identifying the dominant HRCT morphologic pattern in patients with RA-ILD. Presence of a UIP pattern on HRCT carries a worse prognosis when compared to non-UIP patterns such as NSIP and OP. In a recent study by Walsh et al. both computed tomographic and pulmonary functional indices were assessed as prognostic determinants of connective tissue disease related fibrotic lung disease. The study concluded that traction bronchiectasis, extent of honeycombing and DLco were strongly associated with mortality. The interobserver agreement for traction bronchiectasis was higher than for honeycombing, and was considered to be a more accurate prognostic sign. Additionally the radiological diagnosis had survival implications i.e., patients with discordant UIP had a better prognosis than concordant UIP but worse prognosis than fibrotic NSIP [27].
Overall, RA-ILD carries worse prognosis compared to other collagen vascular disease associated ILD because UIP is more common than NSIP in this subset of patients [23,28,29]. Several patient related and clinical parameters such as male sex, history of smoking, nodular disease, extra-articular disease and disease duration have also been evaluated which may predispose to or be associated with an increased incidence of fibrosing lung disease in patients with RA-ILD. Of these, only smoking has been confirmed as an independent risk factor predicting the development of ILD in RA [30,31].
3.3. Overlap of CT morphologic patterns and multiplicity of HRCT patterns in RA-ILD
There may be frequent and significant overlap between NSIP and UIP patterns. A helpful distinguishing feature is diffuse and extensive nature of groundglass opacity in patients with NSIP and greater basilar predominance of honeycombing and reticulation in patients with definite UIP pattern [32]. Patients with classical UIP rarely undergo biopsy. Given the significant overlap between NSIP and UIP patterns, biopsy may be indicated when a definite UIP pattern is not seen and the patient does not respond to standard treatment regimens.
Organizing pneumonia is the third most common ILD pattern and can be confused with multifocal bacterial pneumonia. Features such as migratory subpleural and peribronchial distribution, a perilobular pattern and architectural distortion in areas of consolidation may help suggest the diagnosis [33].
Bronchoscopy is essential in patients with less characteristic imaging findings to exclude infection, particularly before starting corticosteroids [34,35]. Other less commonly observed CT patterns in RA include diffuse alveolar damage and lymphoid interstitial pneumonia with sparse scattered cysts adjacent to vessels [36].
3.4. Acute airspace disease and acute exacerbation (AE) of ILD in RA
Acute exacerbation occurs not only in patients with IPF but also in patients with connective tissue disease-associated ILDs (CTD-ILDs). It has been reported that RA-associated ILD (RA-ILD) is the most common CTD-ILD associated with AE [37,38]. Patients with acute exacerbations present with subacute respiratory deterioration with worsening hypoxemia in the setting of new radiographic abnormalities. In patients with RA-ILD, acute exacerbations are more common with older age at ILD diagnosis, those who demonstrate a UIP pattern on HRCT and have received methotrexate for RA-ILD [39].
A thorough and comprehensive assessment to exclude respiratory infection, acute interstitial pneumonia, drug toxicity, pulmonary embolism, cardiac dysfunction, and diffuse alveolar hemorrhage is fundamental for the evaluation of such patients. This includes cultures of sputum, blood, urine and lavage fluid, and serological tests for infection as well as echocardiography and pulmonary CT angiogram to rule out left heart failure or pulmonary thromboembolism.
3.5. Rheumatoid nodulosis: diagnostic conundrum
Rheumatoid nodules in the lung are uncommon, usually seen in conjunction with subcutaneous nodules and high rheumatoid factor [40]. Pulmonary rheumatoid nodulosis is characterized by few large discrete subpleural nodules, which may develop cavitation (Figs. 4 and 5). Though usually stable and asymptomatic, cavitation may lead to hemoptysis, spontaneous pneumothoraces or development of bronchopleural fistulae.
The differential diagnosis of rheumatoid nodules includes infection such as septic emboli, mycobacterial and fungal infections as well as malignancy. Rapid evolution and waxing and waning appearance on follow up imaging favors infection or inflammatory etiology. In select cases, PET/CT and or percutaneous biopsy may be needed to rule out malignancy. Disease-modifying antirheumatic drugs (DMARD's) or biologic response modifiers (BRM's) being used for treatment of RA may be the cause of intra-thoracic rheumatoid nodules and may require discontinuation [20,21]. If the nodules are calcified, they often indicate association with pneumoconiosis, a condition known as Caplan's syndrome [41].
4. Spectrum of airway disease
Rheumatoid arthritis can affect the upper larger airways and the smaller distal airways.
4.1. Large airway disease
Cricoarytenoid joint involvement in RA has been shown to occur in 26–86% of RA patients [42]. CT may show cricoarytenoid luxation, erosive changes or abnormal position of the true vocal cords leading to vocal cord paresis, stridor, and dysphagia [43,44]. Aggravation of inflammation leading to airway obstruction has been reported during endotracheal intubation and after extubation [45].
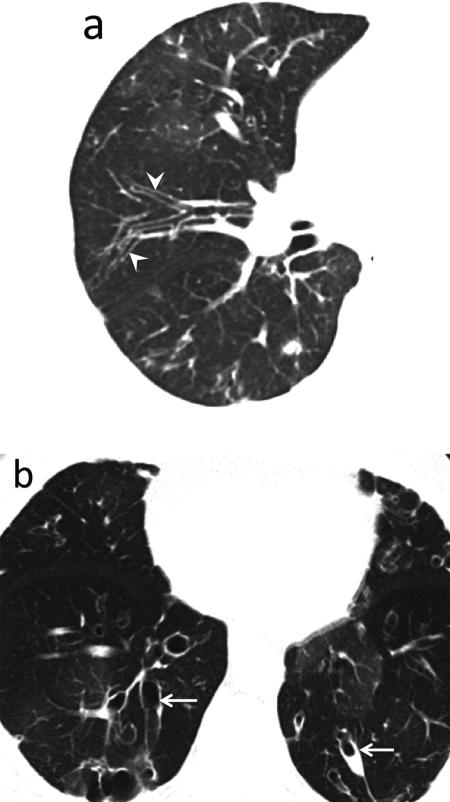
The association between RA and bronchiectasis has long been recognized. On high-resolution CT (HRCT), bronchiectasis has been identified in up to one third of the RA population [24,46–48]. Traction bronchiectasis seen with fibrosing RA-ILD should not be confused with freestanding bronchiectasis seen in many RA patients (Fig. 9). Several hypotheses have been postulated to explain the development of bronchiectasis including autoimmune-related injury, genetic predisposition, DMARD-related and recurrent pulmonary infection [49,50]. Given the increased risk for infections, it is important to recognize the presence of bronchiectasis particularly before initiating treatment with DMARD's or biologic agents.
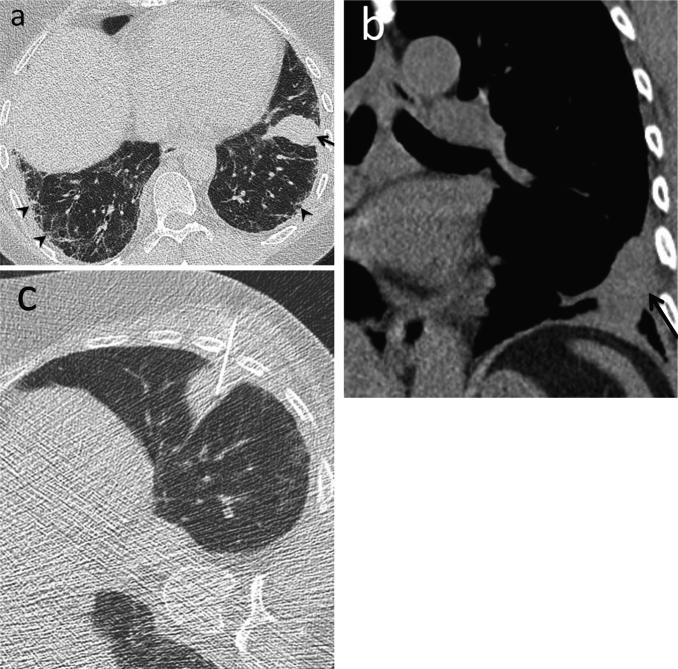
Fig. 9.
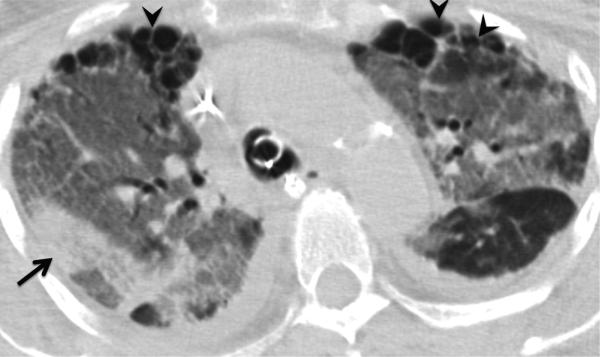
Large airway disease and progressive bronchiectasis. 58-year-old female with RA and severe bronchiectasis with superimposed MAI infection. (a) CT. Cylindrical bronchiectasis (arrowheads) with mosaic attenuation pattern. (b) CT obtained 6 years later. Progression of bronchiectasis, now varicose and cystic (arrows). Persistent areas of mosaic attenuation in both lungs suggest air trapping due to small airway involvement.
Teaching point: Rheumatoid arthritis is an important cause of non-cystic fibrosis bronchiectasis, which can predispose to subsequent infection.
4.2. Small airway disease
The relatively low prevalence of diffuse small airways disease may reflect difficulty in making the diagnosis in patients with mild to moderate disease. Spirometry may show obstruction but standard chest CT may be only suggestive or unrevealing even in symptomatic patients with small airway disease. On HRCT, centrilobular nodular opacities, tree-in-bud opacities, mosaic attenuation and evidence of air trapping are characteristic (Fig. 10). However, evidence of air trapping may only be elucidated on paired inspiratory–expiratory chest CT. If a combination of clinical impression, physiological parameters and advanced tests such as lung clearance index is still not suggestive of a diagnosis, then transbronchial or open lung biopsy may be considered. Histopathologic findings can detect either cellular bronchiolitis(diffuse and follicular subtypes) or fibrosing bronchiolitis (obliterative or constrictive). The histopathologic distinction is crucial as obliterative or constrictive bronchiolitis is a rare irreversible form of small airways disease in RA with associated poor prognosis which may require lung transplantation in severe cases [51].
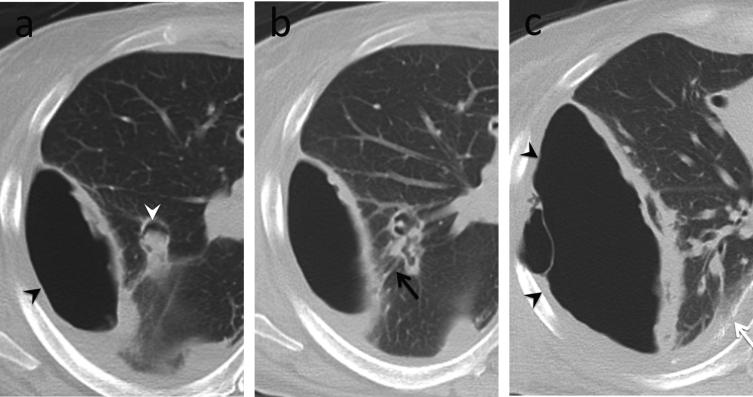
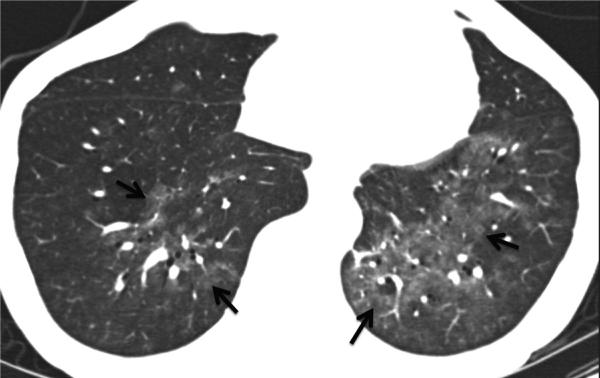
Fig. 10.
Small airway disease, follicular bronchiolitis. 40-year-old female with rheumatoid arthritis whose pulmonary function test showed marked obstructive pattern. (a) CT. Mild bronchial wall thickening and mosaic attenuation in both lungs suggesting air trapping. (b and c), Transbronchial biopsy. Dense peribronchial lymphoid infiltrate with follicular hyperplasia, causing bronchiolar luminal narrowing and obliteration.
Teaching point: Imaging findings in symptomatic patients with small airways disease can be very subtle. Tailoring of CT protocols and use of paired inspiratory and expiratory imaging is helpful to accentuate air trapping.
5. Spectrum of vascular disease
Although historically associated with systemic sclerosis, pulmonary arterial hypertension is being increasingly recognized in other rheumatic diseases, including RA. PAH may be seen in isolation or in association with RA-ILD. Few pathophysiologic mechanisms have been postulated including immune-mediated vasculopathy, hypercoagulability with formation of microthrombi, and intimal proliferation leading to obliterative vasculopathy [52]. On CT, expected findings of pulmonary hypertension include direct signs such as enlarged pulmonary arteries, dilation of right-sided cardiac chambers, engorged inferior vena cava and hepatic veins and indirect signs such as mosaic attenuation.
Small pulmonary vessels can also be affected in RA (Fig. 11). Diffuse alveolar hemorrhage (DAH) is rare and maybe multifactorial. It maybe due to pulmonary capillaritis associated with RA and in some cases drugs such as etanercept have been implicated [53]. Pulmonary capillaritis is a rare small-vessel necrotizing vasculitis recognized by neutrophilic infiltration of the capillary interstitium, leading to destruction of the alveolar capillary network, allowing for red blood cells to enter the alveolar spaces [53]. Therapy typically includes systemic corticosteroid and immune modulators.
Fig. 11.
Mixed small airway and small vessel disease. 44-year-old male with rheumatoid arthritis on prednisone and methotrexate with acute on chronic dyspnea, found to have pulmonary hypertension. (a and b) CT. Predominantly peripheral consolidation and groundglass opacities (arrows). Infection screen was negative. Open lung wedge biopsy demonstrated bronchiolitis with acute and chronic inflammation, significant pulmonary arteriolar disease with medial hypertrophy and intimal proliferation out of proportion to the airway disease. Pulmonary hypertension was thought to be secondary to vascular involvement by RA.
Teaching point: Small vessel disease may manifest as mosaic perfusion and pulmonary arterial hypertension or mixed consolidative and groundglass abnormality due to hemorrhage secondary to capillaritis.
6. Spectrum of drug reaction and toxicity
Multiple drugs are currently used in treating RA. DMARD's are considered the standard of care for most patients [54]. For patients who inadequately respond to or are unable to tolerate adequate trials of conventional DMARD's, biologic agents have provided a major avenue of RA therapy. Common patterns of thoracic drug toxicity are summarized in Table 2. Radiologic manifestations of drug toxicity are often indistinguishable from RA-associated pulmonary disease. Diagnosis is therefore based on combination of clinical, imaging, pathological findings and temporal relationship with initiation and cessation of the drug. There have been case reports of resolution of pulmonary nodules due to withdrawal of MTX [55,56]. However, in RA, nodule formation may fluctuate as part of the course of the disease, making it difficult to ascertain if nodule resolution or attenuation is related to MTX withdrawal or the natural history of RA. Detailed description of all drug-related complications is beyond the scope of this article and only a few examples are discussed. A useful listing of drug-related respiratory complications can be found at www.pneumotox.com
Methotrexate-induced ILD is the archetype of drug-induced pulmonary toxicity in RA patients. A spectrum of pulmonary diseases have been shown to be secondary to methotrexate, including interstitial fibrosis, acute lung injury with non-cardiogenic pulmonary edema, organizing pneumonia, and pleuropericarditis with the most common pattern being acute hypersensitivity pneumonitis as described in rheumatology literature. Radiologically this mimics subacute hypersensitivity pneumonitis and presents as combination of centrilobular groundglass nodules, patchy airspace opacification, irregular linear opacities, and air trapping on expiratory images [57,58] (Fig. 12). As previously mentioned, methotrexate along with other DMARDs and biologic agents have also been associated with accelerated nodulosis and recurrent pneumothoraces [17–20].
Fig. 12.
Methotrexate-induced acute hypersensitivity pneumonitis. 56-year-old woman who was started on methotrexate few weeks prior to presenting with worsening dyspnea. CT. Bilateral poorly defined ground-glass attenuation (arrows). Differential diagnosis includes RA-related interstitial lung disease, opportunistic infection, and adverse drug reaction. Radiologic abnormality and symptoms resolved following withdrawal of methotrexate.
Teaching point: Drug toxicity may mimic infection. Temporal association with treatment history helps in making a diagnosis.
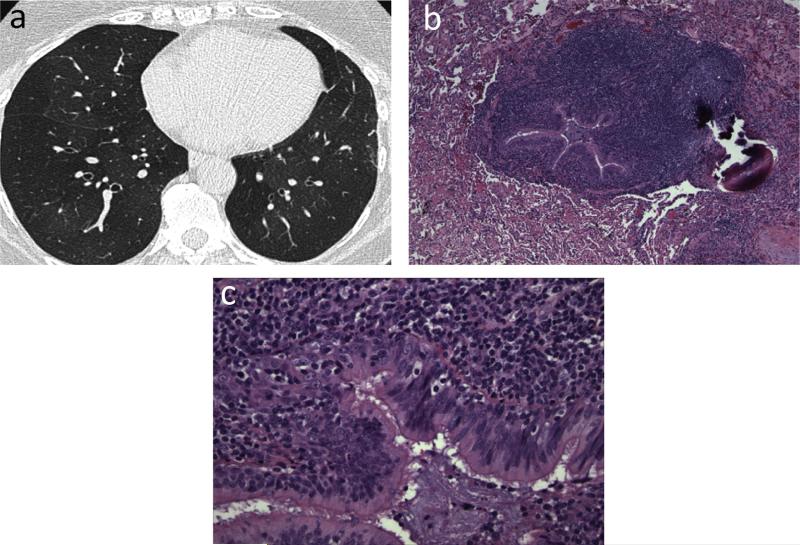
Tumor necrosis factor-α (TNF-α) antagonists, which impair granuloma formation, have been linked with paradoxical development of sarcoidosis-like disease in RA patients, with etanercept being the most commonly implicated agent [59] (Fig. 13). Underlying pathophysiology in such cases is uncertain; however, temporal relationship of granulomatosis with initiation and cessation of TNF-α antagonist suggests that the drug is likely the culprit.
Fig. 13.
Etanercept causing mediastinal adenopathy. 56-year-old male with severe rheumatoid arthritis (RA) on etarnercept. (a and b) CT. Multiple moderately enlarged mediastinal lymph nodes (arrowheads). Lymph node biopsy was performed to exclude lymphoproliferative disease. (c and d) Low power (20×) & high power (200×) images of mediastinal nodes showed non-caseating granulomatous inflammation.
Teaching point: Mediastinal nodal enlargement may be seen in RA patients due to underlying connective tissue disorder and RA-related interstitial lung disease; however, progressive nodal enlargement raises suspicion for malignancy or sarcoidosis. Knowledge of treatment history with drugs such as TNF-α antagonists may avoid invasive procedures in select patients.
7. Spectrum of opportunistic infections
In addition to underlying immunosuppressed state of RA patients, the immunomodulatory properties of agents used to treat RA render them more susceptible to activation of latent infections and opportunistic infections (Table 2). Diagnosis of infection may be delayed by nonspecific or atypical clinical presentation. CT is often required to detect superimposed infection in the setting of chronic RA-associated pulmonary disease (Fig. 14) and provides an accurate roadmap for the pulmonologist to obtain tissue samples. Even then, imaging features are often nonspecific, and bronchoalveolar lavage or percutaneous biopsy combined with serologic testing is usually required to make accurate diagnosis (Figs. 14 and 15).
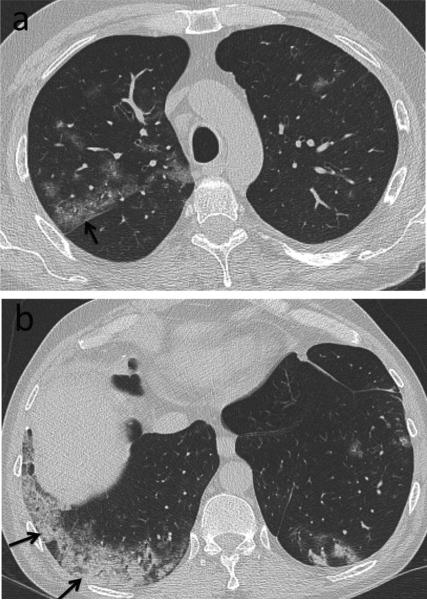
Fig. 14.
Aspergillosis. 57-year-old female with history of rheumatoid arthritis (RA). CT. Focal right upper lobe consolidation (arrow) represents invasive aspergillosis. Aspergillus was isolated from bronchoalveolar lavage. Peripheral subpleural reticulation and honeycombing (arrowheads) is consistent with RA-related interstitial lung disease (RA-ILD). Diffuse groundglass opacity in both lungs was thought to be due to pulmonary edema.
Teaching point: A variety of processes can be seen in RA patients, including severe RA-ILD, superimposed infection related to immunosuppression, all contributing to respiratory failure.
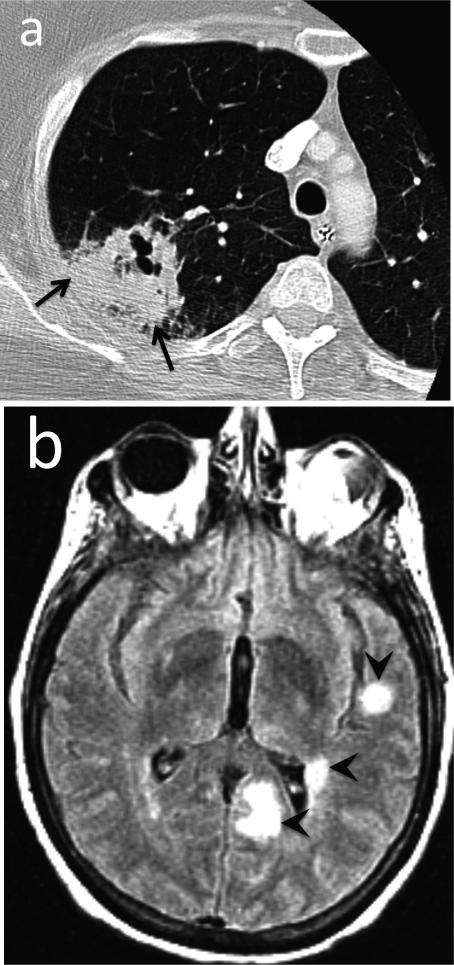
Fig. 15.
Angioinvasive aspergillosis. 52-year-old female with history of rheumatoid arthritis (RA) with fever, shortness of breath and seizure. (a) CT. Consolidation with cavitation in the right upper lobe represents invasive aspergillosis (arrows). Aspergillus was isolated from bronchoalveolar lavage. (b) Contrast-enhanced T1-weighted MR image of the brain. Multiple enhancing cerebral foci (arrowheads) were presumed to represent angioinvasive aspergillosis.
Teaching point: Opportunistic infection secondary to immunosuppression is not uncommon among RA patients.
Biologic agents, particularly TNF-α antagonists have been shown to increase the risk of infections including reactivation of latent tuberculosis infection and new-onset tuberculosis, as well as increased incidence of non-tuberculous mycobacterial infections [60,61]. Prior to the initiation of biologic agents, patients should be screened for latent tuberculosis, and anti-tuberculosis prophylaxis should be considered for patients at risk [61] (Fig. 16).
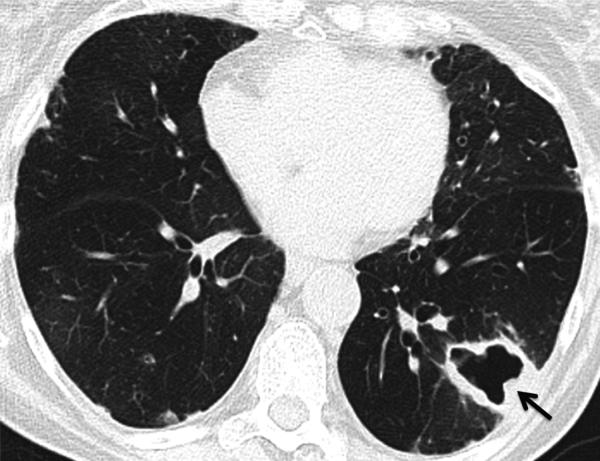
Fig. 16.
Mycobacterium avium-intracellulare (MAI) infection. CT. Thick-walled cavitary lesion in the left lower lobe (arrow) in an asymptomatic patient. Biopsy showed abscess with necrotizing granulomata and associated acid-fast bacilli. Note also mosaic attenuation suggesting air trapping, consistent with the patient's severe obstructive ventilatory deficit (FEV1 <35% predicted).
Teaching point: New large cavitary lesion in a rheumatoid arthritis patient is suspicious for infection such as tuberculosis and atypical mycobacterial infection. Multiple small cavitary nodules may be due to rheumatoid nodules, drug-related nodulosis or septic emboli.
8. Tailoring of CT protocols to clinical scenario
Problem-specific protocols are useful problem solving tools. In patients with severe dyspnea and abnormal pulmonary function tests due to suspected small airways disease and bronchiolitis, a dual phase static airway protocol maybe used. This includes acquisition of paired end-inspiratory and end-expiratory images to assess for air trapping, an imaging marker for small airway disease such as constrictive bronchiolitis. 3D reformations including virtual bronchoscopy, minimum intensity projection (minIP) images and curved planar reformats of the central airways are a useful adjunct to multiplanar reformations (MPR's) to delineate focal airway narrowing, irregularity or abnormal collapsibility. In addition, virtual bronchoscopic images and minIP images create a roadmap for the pulmonologist and surgeon for sampling of focal or multifocal pulmonary abnormalities in patients with RA, and assist the surgeon in planning surgery or stent placement for treatment of complications such as bronchopleural fistulae. The widespread use of HRCT has increased the detection of subtle interstitial lung abnormalities. Prone HRCT images can be obtained to eliminate changes secondary to dependent atelectasis and demonstrate subtle or early interstitial lung disease. This may be an area of interest for future studies focusing on novel therapies to target early stages of disease [62].
9. Conclusion
Myriad pulmonary manifestations of RA are well documented. However, the diagnosis of the pulmonary manifestations and treatment-associated complications remains challenging due to a combination of factors. Treatment-related immunosuppression and complications may further cause imaging abnormalities, which confound diagnosis. Usually, a combination of follow-up imaging, biopsy in select cases, temporal association of findings with respect to treatment regimens and multidisciplinary clinical input can point to the correct diagnosis.
Footnotes
Conflict of interest
Neither the authors nor authors’ institutions have conflicts of interest. This includes financial or personal relationships that inappropriately influence (bias) their actions within 3 years of the work beginning submitted. All authors have participated sufficiently, approved the manuscript and this submission.
References
- 1.Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann. Rheum. Dis. 2003;62:722–727. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris ED., Jr. Rheumatoid arthritis. Pathophysiology and implications for therapy. New Engl. J. Med. 1990;322:1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 3.Lynch DA. Lung disease related to collagen vascular disease. J. Thorac. Imaging. 2009;24:299–309. doi: 10.1097/RTI.0b013e3181c1acec. [DOI] [PubMed] [Google Scholar]
- 4.Minaur NJ, Jacoby RK, Cosh JA, Taylor G, Rasker JJ. Outcome after 40 years with rheumatoid arthritis: a prospective study of function, disease activity, and mortality. J. Rheumatol. Suppl. 2004;69:3–8. [PubMed] [Google Scholar]
- 5.Tansey D, Wells AU, Colby TV, Ip S, Nikolakoupolou A, du Bois RM, et al. Variations in histological patterns of interstitial pneumonia between connective tissue disorders and their relationship to prognosis. Histopathology. 2004;44:585–596. doi: 10.1111/j.1365-2559.2004.01896.x. [DOI] [PubMed] [Google Scholar]
- 6.Jurik AG, Davidsen D, Graudal H. Prevalence of pulmonary involvement in rheumatoid arthritis and its relationship to some characteristics of the patients. A radiological and clinical study. Scand. J. Rheumatol. 1982;11:217–224. doi: 10.3109/03009748209098194. [DOI] [PubMed] [Google Scholar]
- 7.Sahn SA. State of the art. The pleura. Am. Rev. Respir. Dis. 1988;138:184–234. doi: 10.1164/ajrccm/138.1.184. [DOI] [PubMed] [Google Scholar]
- 8.Talbott JA, Calkins E. Pulmonary involvement in rheumatoid arthritis. JAMA: J. Am. Med. Assoc. 1964;189:911–913. doi: 10.1001/jama.1964.03070120033008. [DOI] [PubMed] [Google Scholar]
- 9.Toyoshima H, Kusaba T, Yamaguchi M. [Cause of death in autopsied RA patients] Ryumachi [Rheumatism] 1993;33:209–214. [PubMed] [Google Scholar]
- 10.Balbir-Gurman A, Yigla M, Nahir AM, Braun-Moscovici Y. Rheumatoid pleural effusion. Semin. Arthritis Rheum. 2006;35:368–378. doi: 10.1016/j.semarthrit.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Walker WC, Wright V. Rheumatoid pleuritis. Ann. Rheum. Dis. 1967;26:467–474. doi: 10.1136/ard.26.6.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen PU, Blair JL. Cholesterol crystals causing falsely elevated automated cell count. Am. J. Clin. Pathol. 2006;125:358–363. [PubMed] [Google Scholar]
- 13.Abu-Shakra M, Nicol P, Urowitz MB. Accelerated nodulosis, pleural effusion, and pericardial tamponade during methotrexate therapy. J. Rheumatol. 1994;21:934–937. [PubMed] [Google Scholar]
- 14.Ozkan H, Cetinkaya AS, Yildiz T, Bozat T. A rare side effect due to TNF-alpha blocking agent: acute pleuropericarditis with adalimumab. Case Rep. Rheumatol. 2013;2013:985914. doi: 10.1155/2013/985914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hindle W, Yates DA. Pyopneumothorax complicating rheumatoid lung disease. Ann. Rheum. Dis. 1965;24:57–60. doi: 10.1136/ard.24.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricci ZJ, Haramati LB, Rosenbaum AT, Liebling MS. Role of computed tomography in guiding the management of peripheral bronchopleural fistula. J. Thorac. Imaging. 2002;17:214–218. doi: 10.1097/00005382-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Cunnane G, Warnock M, Fye KH, Daikh DI. Accelerated nodulosis and vasculitis following etanercept therapy for rheumatoid arthritis. Arthritis Rheum. 2002;47:445–449. doi: 10.1002/art.10535. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Yoo WH. Recurrent pneumothorax associated with pulmonary nodules after leflunomide therapy in rheumatoid arthritis: a case report and review of the literature. Rheumatol. Int. 2011;31:919–922. doi: 10.1007/s00296-009-1240-9. [DOI] [PubMed] [Google Scholar]
- 19.Rozin A, Yigla M, Guralnik L, Keidar Z, Vlodavsky E, Rozenbaum M, et al. Rheumatoid lung nodulosis and osteopathy associated with leflunomide therapy. Clin. Rheumatol. 2006;25:384–388. doi: 10.1007/s10067-005-0024-1. [DOI] [PubMed] [Google Scholar]
- 20.van Ede A, den Broeder A, Wagenaar M, van Riel P, Creemers MC. Etanercept-related extensive pulmonary nodulosis in a patient with rheumatoid arthritis. J. Rheumatol. 2007;34:1590–1592. [PubMed] [Google Scholar]
- 21.Toussirot E, Berthelot JM, Pertuiset E, Bouvard B, Gaudin P, Wendling D, et al. Pulmonary nodulosis and aseptic granulomatous lung disease occurring in patients with rheumatoid arthritis receiving tumor necrosis factor-alpha-blocking agent: a case series. J. Rheumatol. 2009;36:2421–2427. doi: 10.3899/jrheum.090030. [DOI] [PubMed] [Google Scholar]
- 22.Madan R, Chick JF. Spectrum of radiologic appearances of surgical thoracostomy and thoracoplasty in the treatment of pleuroparenchymal infections. AJR Am. J. Roentgenol. 2014;202:W123–W132. doi: 10.2214/AJR.13.10879. [DOI] [PubMed] [Google Scholar]
- 23.Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, Dawson J, Sathi N, Ahmad Y, Koduri G, Young A, British Rheumatoid Interstitial Lung (BRILL) Network Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics — a large multicentre UK study. Rheumatology (Oxford) 2014;53(9):1676–1682. doi: 10.1093/rheumatology/keu165. [DOI] [PubMed] [Google Scholar]
- 24.McDonagh J, Greaves M, Wright AR, Heycock C, Owen JP, Kelly C. High resolution computed tomography of the lungs in patients with rheumatoid arthritis and interstitial lung disease. Br. J. Rheumatol. 1994;33:118–122. doi: 10.1093/rheumatology/33.2.118. [DOI] [PubMed] [Google Scholar]
- 25.Sokka T, Kautiainen H, Toloza S, Makinen H, Verstappen SM, Lund Hetland M, et al. QUEST-RA: quantitative clinical assessment of patients with rheumatoid arthritis seen in standard rheumatology care in 15 countries. Ann. Rheum. Dis. 2007:1491–1496. doi: 10.1136/ard.2006.069252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka N, Kim JS, Newell JD, Brown KK, Cool CD, Meehan R, et al. Rheumatoid arthritis-related lung diseases: CT findings. Radiology. 2004;232:81–91. doi: 10.1148/radiol.2321030174. [DOI] [PubMed] [Google Scholar]
- 27.Walsh SL, Sverzellati N, Devaraj A, Keir GJ, Wells AU, Hansell DM. Connective tissue disease related fibrotic lung disease: high resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax. 2014;69:216–222. doi: 10.1136/thoraxjnl-2013-203843. [DOI] [PubMed] [Google Scholar]
- 28.Kim EJ, Collard HR, King TE., Jr. Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest. 2009;136:1397–1405. doi: 10.1378/chest.09-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim EJ, Elicker BM, Maldonado F, Webb WR, Ryu JH, Van Uden JH, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur. Respir. J. 2010;35:1322–1328. doi: 10.1183/09031936.00092309. [DOI] [PubMed] [Google Scholar]
- 30.Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax. 2001;56:622–627. doi: 10.1136/thorax.56.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saag KG, Kolluri S, Koehnke RK, Georgou TA, Rachow JW, Hunninghake GW, et al. Rheumatoid arthritis lung disease. Determinants of radiographic and physiologic abnormalities. Arthritis Rheum. 1996;39:1711–1719. doi: 10.1002/art.1780391014. [DOI] [PubMed] [Google Scholar]
- 32.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JS, Lynch DA, Sharma S, Brown KK, Muller NL. Organizing pneumonia: prognostic implication of high-resolution computed tomography features. J. Comput. Assisted Tomogr. 2003;27:260–265. doi: 10.1097/00004728-200303000-00027. [DOI] [PubMed] [Google Scholar]
- 34.Lee HK, Kim DS, Yoo B, Seo JB, Rho JY, Colby TV, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest. 2005;127:2019–2027. doi: 10.1378/chest.127.6.2019. [DOI] [PubMed] [Google Scholar]
- 35.Webb WR, Müller NL, Naidich DP. High-resolution CT of the Lung. 4th ed. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia: 2009. [Google Scholar]
- 36.Gutsche M, Rosen GD, Swigris JJ. Connective tissue disease-associated interstitial lung disease: a review. Curr. Respir. Care Rep. 2012;1:224–232. doi: 10.1007/s13665-012-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park IN, Kim DS, Shim TS, Lim CM, Lee SD, Koh Y, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132:214–220. doi: 10.1378/chest.07-0323. [DOI] [PubMed] [Google Scholar]
- 38.Suda T, Kaida Y, Nakamura Y, Enomoto N, Fujisawa T, Imokawa S, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir. Med. 2009;103:846–853. doi: 10.1016/j.rmed.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hozumi H, Nakamura Y, Johkoh T, Sumikawa H, Colby TV, Kono M, et al. Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: a retrospective case control study. BMJ Open. 2013;3:e003132. doi: 10.1136/bmjopen-2013-003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capobianco J, Grimberg A, Thompson BM, Antunes VB, Jasinowodolinski D, Meirelles GS. Thoracic manifestations of collagen vascular diseases. Radiogr.: Rev. Publ. Radiol. Soc. North Am. Inc. 2012;32:33–50. doi: 10.1148/rg.321105058. [DOI] [PubMed] [Google Scholar]
- 41.Schreiber J, Koschel D, Kekow J, Waldburg N, Goette A, Merget R. Rheumatoid pneumoconiosis (Caplan's syndrome) Eur. J. Intern. Med. 2010;21:168–172. doi: 10.1016/j.ejim.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Hakala P, Randell T. Intubation difficulties in patients with rheumatoid arthritis. A retrospective analysis. Acta Anaesthesiol. Scand. 1998;42:195–198. doi: 10.1111/j.1399-6576.1998.tb05108.x. [DOI] [PubMed] [Google Scholar]
- 43.Chen JJ, Branstetter BF, Myers EN. Cricoarytenoid rheumatoid arthritis: an important consideration in aggressive lesions of the larynx. AJNR Am. J. Neuroradiol. 2005;26:970–972. [PMC free article] [PubMed] [Google Scholar]
- 44.Montgomery WW. Cricoarytenoid arthritis. Laryngoscope. 1963;73:801–836. doi: 10.1288/00005537-196307000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Kolman J, Morris I. Cricoarytenoid arthritis: a cause of acute upper airway obstruction in rheumatoid arthritis. Can. J. Anaesth. = Journal Canadien d'anesthesie. 2002;49:729–732. doi: 10.1007/BF03017454. [DOI] [PubMed] [Google Scholar]
- 46.Cortet B, Perez T, Roux N, Flipo RM, Duquesnoy B, Delcambre B, et al. Pulmonary function tests and high resolution computed tomography of the lungs in patients with rheumatoid arthritis. Ann. Rheum. Dis. 1997;56:596–600. doi: 10.1136/ard.56.10.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bilgici A, Ulusoy H, Kuru O, Celenk C, Unsal M, Danaci M. Pulmonary involvement in rheumatoid arthritis. Rheumatol. Int. 2005;25:429–435. doi: 10.1007/s00296-004-0472-y. [DOI] [PubMed] [Google Scholar]
- 48.Mohd Noor N, Mohd Shahrir MS, Shahid MS, Abdul Manap R, Shahizon Azura AM, Azhar Shah S. Clinical and high resolution computed tomography characteristics of patients with rheumatoid arthritis lung disease. Int. J. Rheum. Dis. 2009;12:136–144. doi: 10.1111/j.1756-185X.2009.01376.x. [DOI] [PubMed] [Google Scholar]
- 49.Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA, et al. An investigation into causative factors in patients with bronchiectasis. Am. J. Respir. Crit. Care Med. 2000;162:1277–1284. doi: 10.1164/ajrccm.162.4.9906120. [DOI] [PubMed] [Google Scholar]
- 50.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity. Arthritis Rheum. 2012;64:1756–1761. doi: 10.1002/art.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlesinger C, Meyer CA, Veeraraghavan S, Koss MN. Constrictive (obliterative) bronchiolitis: diagnosis, etiology, and a critical review of the literature. Ann. Diagn. Pathol. 1998;2:321–334. doi: 10.1016/s1092-9134(98)80026-9. [DOI] [PubMed] [Google Scholar]
- 52.Shahane A. Pulmonary hypertension in rheumatic diseases: epidemiology and pathogenesis. Rheumatol. Int. 2013;33:1655–1667. doi: 10.1007/s00296-012-2659-y. [DOI] [PubMed] [Google Scholar]
- 53.Schwarz MI, Zamora MR, Hodges TN, Chan ED, Bowler RP, Tuder RM. Isolated pulmonary capillaritis and diffuse alveolar hemorrhage in rheumatoid arthritis and mixed connective tissue disease. Chest. 1998;113:1609–1615. doi: 10.1378/chest.113.6.1609. [DOI] [PubMed] [Google Scholar]
- 54.Block SR. Hidden hazards and practical problems: comment on the 2002 update of the American College of Rheumatology Guidelines for the Management of Rheumatoid Arthritis. Arthritis Rheum. 2002;46:3102–3103. doi: 10.1002/art.10707. author reply 3–4. [DOI] [PubMed] [Google Scholar]
- 55.Balbir-Gurman A, Guralnik L, Best LA, Vlodavsky E, Yigla M, Menahem Nahir A, et al. Accelerated pulmonary nodulosis and sterile pleural effusion in a patient with psoriatic arthropathy during methotrexate therapy: a case report. J. Clin. Rheumatol.: Pract. Rep. Rheum. Musculoskeletal Dis. 2009;15:29–30. doi: 10.1097/RHU.0b013e31817de10b. [DOI] [PubMed] [Google Scholar]
- 56.Watson P, Simler N, Screaton N, Lillicrap M. Management of accelerated pulmonary nodulosis following etanercept therapy in a patient with rheumatoid arthritis. Rheumatology. 2008;47:928–929. doi: 10.1093/rheumatology/ken102. [DOI] [PubMed] [Google Scholar]
- 57.Cannon GW. Methotrexate pulmonary toxicity. Rheum. Dis. Clin. North Am. 1997;23:917–937. doi: 10.1016/s0889-857x(05)70366-5. [DOI] [PubMed] [Google Scholar]
- 58.Roubille C, Haraoui B. Interstitial lung diseases induced or exacerbated by DMARDS and biologic agents in rheumatoid arthritis: a systematic literature review. Semin. Arthritis Rheum. 2014;43(5):613–626. doi: 10.1016/j.semarthrit.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Tong D, Manolios N, Howe G, Spencer D. New onset sarcoid-like granulomatosis developing during anti-TNF therapy: an under-recognised complication. Intern. Med. J. 2012;42:89–94. doi: 10.1111/j.1445-5994.2011.02612.x. [DOI] [PubMed] [Google Scholar]
- 60.Hochberg MC, Lebwohl MG, Plevy SE, Hobbs KF, Yocum DE. The benefit/risk profile of TNF-blocking agents: findings of a consensus panel. Semin. Arthritis Rheum. 2005;34:819–836. doi: 10.1016/j.semarthrit.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 61.Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. New Engl. J. Med. 2006;355:704–712. doi: 10.1056/NEJMct055183. [DOI] [PubMed] [Google Scholar]
- 62.Doyle TJ, Dellaripa PF, Batra K, Frits ML, Iannaccone CK, Hatabu H, et al. Functional impact of a spectrum of interstitial lung abnormalities in rheumatoid arthritis. Chest. 2014;146:41–50. doi: 10.1378/chest.13-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]