Background
Disease-specific ankylosing spondylitis (AS) indices, including BASDAI (Bath AS Disease Activity Index), BASFI (Bath AS Functional Index), ASDAS (AS Disease Activity Score), and BASMI (Bath AS Metrology Index), are widely used in clinical trials and in some clinical settings, but not in most routine care. Laboratory tests usually are the only quantitative measures included in routine care of AS patients, but often are poorly informative. Routine Assessment of Patient Index Data 3 (RAPID3) on a Multidimensional Health Assessment Questionnaire (MDHAQ) is feasible and informative in many rheumatic diseases.
Objective
The aim of this study was to compare RAPID3 to BASDAI, BASFI, ASDAS, and BASMI in a cross-sectional analysis of 85 Korean AS patients collected in routine care.
Methods
MDHAQ/RAPID3, BASDAI, and BASFI were completed by patients, and ASDAS and BASMI assessed by health professionals. Indices and individual measures were compared using correlations, cross tabulations, scatter plots, and κ statistics.
Results
RAPID3 scores were correlated significantly with BASDAI (ρ = 0.82) and ASDAS-ESR (erythrocyte sedimentation rate) (ρ = 0.76), at levels similar to the correlation of BASDAI with ASDAS-ESR (ρ = 0.81). All 21 patients with BASDAI scores of 4 or greater, indicating active AS, were among 39 patients who had RAPID3 scores of greater than 12, indicating high severity, whereas 79% of 33 patients with ASDAS of greater than 1.3, indicating high activity, had RAPID3 high severity.
Conclusions
RAPID3 gives similar information to BASDAI and ASDAS in AS patients, in this limited cross-sectional study from 1 setting. Ankylosing spondylitis–specific measures are needed for clinical trials, but poorly feasible in most busy clinical settings. The MDHAQ/RAPID3 offers pragmatic quantitative clinical assessment of AS patients in routine care.
Key Words: ankylosing spondylitis (AS), Ankylosing Spondylitis Disease Activity Score (ASDAS), ASAS core data set, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Multidimensional Health Assessment Questionnaire (MDHAQ), Routine Assessment of Patient Index Data 3 (RAPID3)
Quantitative assessment of patients with rheumatic diseases requires a pooled index of several measures,1 as no single measure can serve as a “gold standard” for all individual patients.2 Over the last 3 decades, many disease-specific self-report questionnaires have been developed to assess different rheumatic diseases.2 In patients with ankylosing spondylitis (AS), disease-specific indices include the Bath AS Disease Activity Index (BASDAI),3 Bath AS Functional Index (BASFI),4 AS Disease Activity Score (ASDAS) of the SpondyloArthritis International Society,5 and Bath AS Metrology Index (BASMI).5,6 These indices are widely used in clinical trials, other clinical research, and a few clinical settings, but not in most routine care in busy clinical settings. Indeed, the only quantitative data found in the medical records of most patients with AS who receive care from most rheumatologists are laboratory tests, which often are not available at the time of the visit and provide limited information in many patients.5
It is relatively simple in busy clinical settings for a receptionist to present a self-report questionnaire to each patient to complete at each visit.7 However, it is not feasible in most such settings to arrange for different patients with different diagnoses to complete different disease-specific questionnaires, such as a BASDAI in AS, Health Assessment Questionnaire in rheumatoid arthritis (RA),8 Western Ontario Questionnaire in osteoarthritis,9 and other indices in other rheumatic diseases.10 Furthermore, new patients without a diagnosis, or patients in whom a definitive diagnosis has not been established, could benefit from availability of a simple questionnaire to help support clinical decisions.11
A Multidimensional Health Assessment Questionnaire/Routine Assessment of Patient Index Data 3 (MDHAQ/RAPID3)10,12,13 was developed from the Health Assessment Questionnaire over many years in a routine clinical setting and administered to all patients with all rheumatic diseases as a continuous quality improvement activity to improve care.12 The MDHAQ/RAPID3 includes scores for physical function, pain, global estimate of status, fatigue, self-report joint count, symptom checklist, and recent medical history. The MDHAQ/RAPID3 has proven useful and informative in regard to patients with many rheumatic diseases, including AS.10,13 However, these reports did not include formal comparisons of RAPID3 scores with scores on AS-specific indices.
Three recent reports indicated highly significant correlations of BASDAI with RAPID3,14–16 suggesting that both questionnaires provide similar information. In this report, we present cross-sectional comparisons of RAPID3 with BASDAI, BASFI, ASDAS, and BASMI, collected prospectively in 85 Korean patients with AS in a routine care setting.
PATIENTS AND METHODS
Study Patients
A cross-sectional study was performed in 85 patients who were seen in a usual rheumatology outpatient clinic setting in Daegu, Korea, between May 2012 and July 2012, and who received a diagnosis of AS according to the modified New York criteria.5 The study was reviewed and approved by the ethical review board of the institute prior to its initiation, and written informed consent was received from every patient prior to enrollment. Patients were excluded from the study if they were younger than 18 years and had another rheumatic disease in addition to AS.
Collection of Measures
Each patient seen in the clinic, with any diagnosis, completed a Korean version of MDHAQ, which was translated and validated by Lee et al.17 Each patient with AS also completed a BASDAI and BASFI. An ASDAS and BASMI were recorded by a health professional, either a metrologist or rheumatologist.
RAPID3 is scored on the MDHAQ as the 0- to 30-point sum of three 0- to 10-point patient-reported measures: physical function, pain, and patient global estimate of status.7 Other measures were scored as described in the literature5 (but not included in this report). BASDAI includes 6 measures: fatigue, back pain, pain and swelling of peripheral joints, pain of enthesis, degree of morning stiffness, and duration of morning stiffness.3,5 BASFI includes 10 queries concerning physical function, scored as integers from 1 (“easy”) to 10 (“impossible”) on a Likert scale.4 ASDAS-ESR (erythrocyte sedimentation rate) or ASDAS-CRP (C-reactive protein) are calculated according to a formula that includes patient global status, spinal pain, spinal stiffness, fatigue, physical function, spinal mobility, and acute-phase reactant (ESR or CRP).5 BASMI includes 6 measures: occiput wall distance, modified Schober test, lateral spinal flexion, chest expansion, cervical rotation, and intermalleolar distance.6
Statistical Analysis
Statistical analyses were performed using Stata 12.1 for Windows (College Station, TX). The Shapiro-Wilk W test was used to identify normality of distribution in the study population. Wilcoxon rank-sum test and Spearman correlations were computed for each individual measure and total scores in the composite indices, including deletion of pain scores from each index. Patients were classified for disease activity/severity according to BASDAI (≥4 active vs <4 inactive), ASDAS (≥1.3 active vs <1.3 inactive), and RAPID3 categories that have been developed for RA: more than 12, high; 6.1 to 12, moderate; 3.1 to 6, low severity (the term “severity” is used, as self-report scores do not necessarily distinguish activity from damage); and 0 to 3, remission. It is noted that these categories were developed in comparison to the Disease Activity Score in 28 Joints (DAS28)19 for RA20 and have not been validated in AS, although they may be applicable to other rheumatic diseases.13,21–23 Cross tabulations, κ tests, and scatter plots were used to analyze statistical significance of possible associations between RAPID3, BASDAI, and ASDAS.
RESULTS
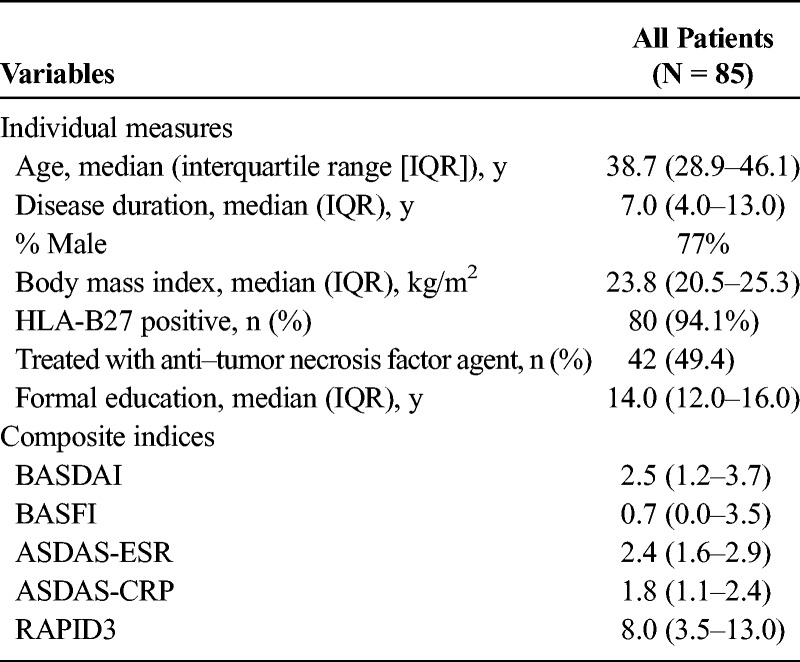
The 85 patients studied included 65 (77%) who were male. Median age was 38.7 years; disease duration, 7.0 years; body mass index, 23.8 kg/m2; level of formal education, 14.0 years; and HLA-B27 positivity, 94.1% (Table 1). These data appear typical for an AS patient cohort.
TABLE 1.
Demographic and Clinical Characteristics of Study Patients
RAPID3 scores were correlated significantly with BASDAI (ρ = 0.82, P < 0.001), BASFI (ρ = 0.66, P < 0.001), and ASDAS (ρ = 0.76, P < 0.001) (Table 2, Fig.). These correlations were in the same range as correlations of BASDAI with ASDAS-ESR (ρ = 0.81) and ASDAS-CRP (ρ = 0.77) (Fig.).
TABLE 2.
Spearman Correlations of RAPID3 With AS Disease-Specific Composite and Individual Measures Including BASDAI, ASDAS, and BASFI
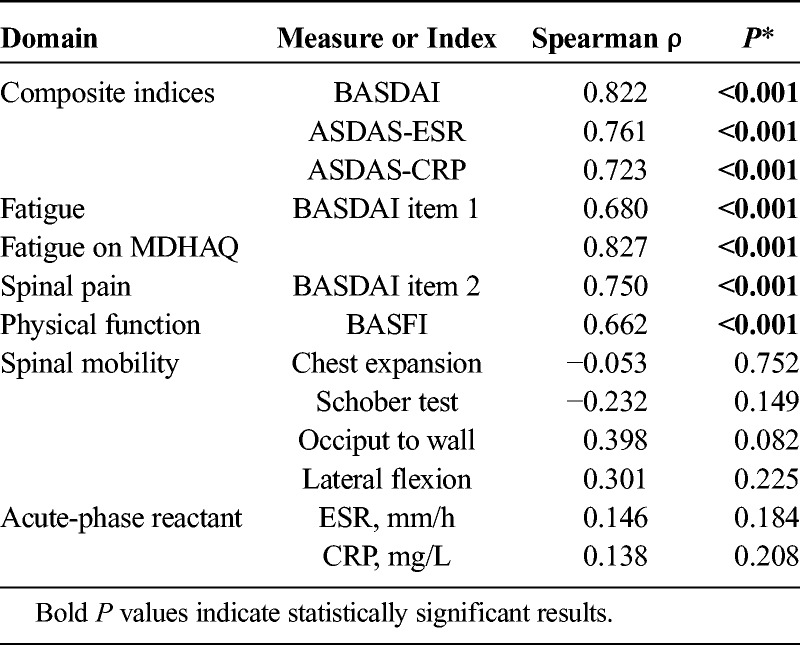
FIGURE.
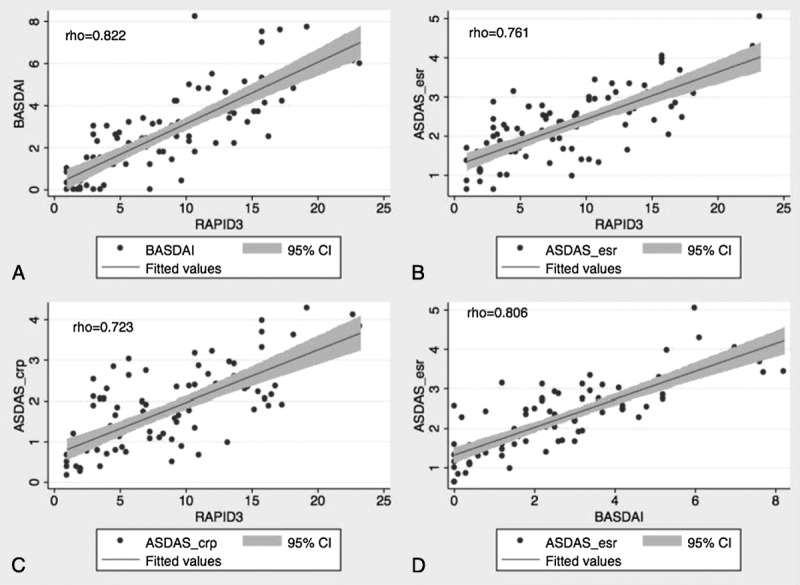
Correlation plots comparing scores for RAPID3 versus (A) BASDAI, (B) ASDAS-ESR, and (C) ASDAS-CRP with RAPID3, and (D) scores for BASDAI versus ASDAS-ESR. Note high correlations of these measures, in the same range for RAPID3 versus AS indices as for BASDAI versus ASDAS.
Correlations of RAPID3 were significant with individual responses on the BASDAI or BASFI for spinal pain (ρ = 0.75, P < 0.001), spinal stiffness (ρ = 0.74, P < 0.001), fatigue (ρ = 0.68, P < 0.001), and physical function (ρ = 0.66, P < 0.001) (Table 1). Correlations of RAPID3 with physical measures of spinal mobility, or with acute-phase reactants ESR and CRP, were not statistically significant (Table 2).
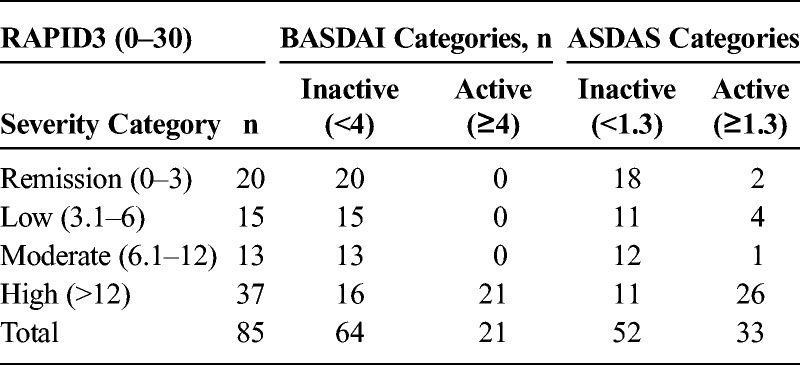
Overall, 20, 25, 23, and 37 patients had RAPID 3 scores indicating high, moderate, low severity, and remission, respectively, according to categories developed initially for RA.18 Interrater agreement of RAPID3 remission or not in remission with ASDAS inactive or active was significant (κ = 0.486, agreement = 77.8%, P < 0.001), but low between RAPID3 remission or not in remission and BASDAI inactive or active (κ = 0.160, agreement = 45.6%, P = 0.002). However, all 20 patients with BASDAI scores of 4 or greater, indicating active AS, had RAPID3 scores of greater than 12, indicating high severity (Table 3). Among 33 patients with ASDAS of greater than 1.3, indicating active disease, 26 (79%) had high severity according to RAPID3 (Table 3).
TABLE 3.
Cross Tabulation of RAPID3 and BASDAI Severity Groups
DISCUSSION
The BASDAI, ASDAS, BASFI, and BASMI are standard disease-specific indices to assess the status of patients with AS. These indices may include measures from patient self-report, a health professional, and laboratory tests and are collected feasibly in clinical trials and other clinical research, as well as in routine care at a few sites with a special interest in AS. However, most routine clinical rheumatology care settings do not collect an index to assess RA quantitatively24; it appears unlikely that a site would collect a BASDAI in AS patients when RAPID3 is not collected in RA patients.
It is difficult to collect several different patient questionnaires from patients with different diagnoses in busy clinical settings.7 Furthermore, collection of several domains of ASDAS core data set measures and calculation of complex formulas for ASDAS are not feasible in all individual patients seen in routine care, despite an excellent Web site for calculation (http://www.asas-group.org/research/asdas_calculator/asdas.html). By contrast, it is rather simple for the clinic receptionist to distribute the same questionnaire to each patient upon registration at the clinic.7 This practice facilitates completion of the questionnaire by the patient in the waiting area and helps the patient prepare for the visit, so the information is available to both patient and rheumatologist at initiation of the encounter.7 Scoring of RAPID3 on an MDHAQ requires 5 seconds,18 and RAPID3 is useful in all rheumatic diseases in which it has been studied, including RA,11,18,21,25–30 systemic lupus erythematosus,15,22 psoriatic arthritis,13 gout,13 vasculitis,23 osteoarthritis, and AS.14–16 RAPID3 is feasible in assessing disease severity and improvement in routine clinical of RA and is similar to DAS28 and Clinical Disease Activity Index (CDAI) to distinguish active from control treatments in RA trials of adalimumab, abatacept, and certolizumab.31
The limited feasibility of collecting BASDAI and ASDAS as 1 of several questionnaires in routine care usually leaves laboratory tests as the only quantitative data available for clinical decisions in the medical records of most patients with AS. However, laboratory tests often are not available at the time of the encounter and often are normal in patients with active clinical disease.5 Thus, improvement (or worsening) in AS patients is characterized only by narrative descriptions, rather than by quantitative clinical data.32
Several limitations are seen to this study. First, it is cross sectional; longitudinal studies extending a recently published small series including AS over a short period13 would be desirable in clinical trials and usual care. Second, low κ’s between RAPID3 and BASDAI, although statistically significant, were not as strong as might be ideal, explained in part by the presence of joint damage and/or concomitant fibromyalgia in patients who do not have active disease on physical examination. This phenomenon has been documented to be a significant issue with DAS28 and CDAI in RA,33 in which patients with scores indicating moderate or severe activity did not have intensification of therapy, explained in large part by joint damage and fibromyalgia.34 In our study, all 21 patients with BASDAI of 4 or greater, indicating active disease, had RAPID3 of greater than 12, indicating high severity. RAPID3 is thus effective to provide the most important goal of recognizing patients with severe disease activity who may require intensification of treatment, particularly in the era of anti–tumor necrosis factor agents, although some of these patients may not require aggressive anti-inflammatory therapy.
A third possible limitation is that RAPID3 was not correlated significantly with metrology scores or laboratory tests. However, low correlations of patient indices with laboratory tests and imaging data are seen in RA. For example, a CDAI is as effective to document changes in status as a Simplified Disease Activity Index, which includes an ESR or CRP.35 Of course, AS-specific spinal flexibility measures are informative in clinical trials and other research and valuable in development of improved treatments, but may not be required in usual care.
A fourth possible limitation is that highly significant correlations do not necessarily imply identity of information contained in 2 measures, as a line indicating near identity may not go through the origin at zero, but be shifted upward or downward. A shift upward is seen for correlations of RAPID3 (as well as BASDAI) with ASDAS scores. However, correlations of RAPID3 and BASDAI essentially go through the origin at zero. Furthermore, correlations of ρ = 0.8 are at a level seen in test-retest studies of the same measure,36 as high as seen in clinical medicine. Similar high correlations have been observed in 3 other settings in the United States,14 Turkey,15 and Norway,16 indicating great similarity of the information provided by the 2 questionnaires.
The authors do not advocate at all that RAPID3 should replace BASDAI, BASFI, ASDAS, or BASMI. Disease-specific indices are invaluable for research and development of new therapies. Furthermore, RAPID3 (or any index) must be supplemented by a standard medical history, physical examination, laboratory tests, and/or any other clinical information regarded as important by the rheumatologist in formulating clinical decisions. Collection of RAPID3 in no way precludes collection of BASDAI, ASDAS, or any other index. Nonetheless, RAPID3 is feasible in usual care7 and appears preferable to the absence of any quantitative clinical measure at all,32 as is the case for most AS patients seen by rheumatologists at this time. The MDHAQ/RAPID3 might be added to research protocols to provide information to facilitate quantitative clinical assessment in routine care of patients with AS.
Footnotes
S.-H.P., I.C., and T.P. are now with the Division of Rheumatology, Department of Internal Medicine, Rush University Medical Center, Chicago, IL.
This study was supported by the internal institutional funding of the authors.
Dr Pincus is president of Health Report Services, Inc, which owns a copyright for a trademark Multidimensional Health Assessment Questionnaire (MDHAQ)/Routine Assessment of Patient Index Data 3 (RAPID3). No license is needed for clinicians who may freely use MDHAQ/RAPID3 to monitor patient status in usual clinical care. Royalties and license fee are received from for-profit pharmaceutical and electronic medical record companies for the use of MDHAQ/RAPID3, all of which are transferred to medical schools for further development of quantitative measurement in clinical rheumatology care.
REFERENCES
- 1. Goldsmith CH, Smythe HA, Helewa A. Interpretation and power of a pooled index. J Rheumatol. 1993; 20: 575– 578. [PubMed] [Google Scholar]
- 2. Pincus T, Yazici Y, Sokka T. Complexities in assessment of rheumatoid arthritis: absence of a single gold standard measure. Rheum Dis Clin North Am. 2009; 35: 687– 697 v. [DOI] [PubMed] [Google Scholar]
- 3. Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994; 21: 2286– 2291. [PubMed] [Google Scholar]
- 4. Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994; 21: 2281– 2285. [PubMed] [Google Scholar]
- 5. Braun J, Kiltz U, Baraliakos X, et al. Optimisation of rheumatology assessments—the actual situation in axial spondyloarthritis including ankylosing spondylitis. Clin Exp Rheumatol. 2014; 32(5 suppl 85): S-96– S-104. [PubMed] [Google Scholar]
- 6. Jenkinson TR, Mallorie PA, Whitelock HC, et al. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J Rheumatol. 1994; 21: 1694– 1698. [PubMed] [Google Scholar]
- 7. Pincus T, Oliver AM, Bergman MJ. How to collect an MDHAQ to provide rheumatology vital signs (function, pain, global status, and RAPID3 scores) in the infrastructure of rheumatology care, including some misconceptions regarding the MDHAQ. Rheum Dis Clin North Am. 2009; 35: 799– 812, x. [DOI] [PubMed] [Google Scholar]
- 8. Fries JF, Spitz P, Kraines RG, et al. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980; 23: 137– 145. [DOI] [PubMed] [Google Scholar]
- 9. Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988; 15: 1833– 1840. [PubMed] [Google Scholar]
- 10. Pincus T, Askanase AD, Swearingen CJ. A Multi-dimensional Health Assessment Questionnaire (MDHAQ) and routine assessment of patient index data (RAPID3) scores are informative in patients with all rheumatic diseases. Rheum Dis Clin North Am. 2009; 35: 819– 827, x. [DOI] [PubMed] [Google Scholar]
- 11. Pincus T, Castrejón I. MDHAQ/RAPID3 scores: quantitative patient history data in a standardized “scientific” format for optimal assessment of patient status and quality of care in rheumatic diseases. Bull NYU Hosp Jt Dis. 2011; 69: 201– 214. [PubMed] [Google Scholar]
- 12. Pincus T, Maclean R, Yazici Y, et al. Quantitative measurement of patient status in the regular care of patients with rheumatic diseases over 25 years as a continuous quality improvement activity, rather than traditional research. Clin Exp Rheumatol. 2007; 25(6 suppl 47): 69– 81. [PubMed] [Google Scholar]
- 13. Castrejón I, Bergman MJ, Pincus T. MDHAQ/RAPID3 to recognize improvement over 2 months in usual care of patients with osteoarthritis, systemic lupus erythematosus, spondyloarthropathy, and gout, as well as rheumatoid arthritis. J Clin Rheumatol. 2013; 19: 169– 174. [DOI] [PubMed] [Google Scholar]
- 14. Danve A, Reddy A, Vakil-Gilani K, et al. Routine Assessment of Patient Index Data 3 score (RAPID3) correlates well with Bath Ankylosing Spondylitis Disease Activity index (BASDAI) in the assessment of disease activity and monitoring progression of axial spondyloarthritis. Clin Rheumatol. 2015; 34: 117– 124. [DOI] [PubMed] [Google Scholar]
- 15. Cinar M, Yilmaz S, Cinar FI, et al. A patient-reported outcome measures-based composite index (RAPID3) for the assessment of disease activity in ankylosing spondylitis. Rheumatol Int. 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16. Michelsen B, Fiane R, Diamantopoulos AP, et al. A comparison of disease burden in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis. PLoS One. 2015; 10:e0123582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee SS, Park MJ, Yoon HJ, et al. Evaluating the Korean version of the Multidimensional Health Assessment Questionnaire in patients with rheumatoid arthritis. Clin Rheumatol. 2006; 25: 353– 357. [DOI] [PubMed] [Google Scholar]
- 18. Pincus T, Swearingen CJ, Bergman MJ, et al. RAPID3 (Routine Assessment of Patient Index Data) on an MDHAQ (Multidimensional Health Assessment Questionnaire): agreement with DAS28 (Disease Activity Score) and CDAI (Clinical Disease Activity Index) activity categories, scored in five versus more than ninety seconds. Arthritis Care Res (Hoboken). 2010; 62: 181– 189. [DOI] [PubMed] [Google Scholar]
- 19. Prevoo ML, van’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995; 38: 44– 48. [DOI] [PubMed] [Google Scholar]
- 20. Pincus T, Chung C, Segurado OG, et al. An index of patient reported outcomes (PRO-Index) discriminates effectively between active and control treatment in 4 clinical trials of adalimumab in rheumatoid arthritis. J Rheumatol. 2006; 33: 2146– 2152. [PubMed] [Google Scholar]
- 21. Pincus T, Bergman MJ, Yazici Y. RAPID3-an index of physical function, pain, and global status as “vital signs” to improve care for people with chronic rheumatic diseases. Bull NYU Hosp Jt Dis. 2009; 67: 211– 225. [PubMed] [Google Scholar]
- 22. Askanase AD, Castrejón I, Pincus T. Quantitative data for care of patients with systemic lupus erythematosus in usual clinical settings: a patient Multidimensional Health Assessment Questionnaire and physician estimate of noninflammatory symptoms. J Rheumatol. 2011; 38: 1309– 1316. [DOI] [PubMed] [Google Scholar]
- 23. Annapureddy N, Elsallabi O, Baker J, et al. Patient-reported outcomes in ANCA-associated vasculitis. A comparison between Birmingham Vasculitis Activity Score and routine assessment of patient index data 3. Clin Rheumatol. 2015. [DOI] [PubMed] [Google Scholar]
- 24. Anderson J, Caplan L, Yazdany J, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken). 2012; 64: 640– 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pincus T, Swearingen CJ, Bergman M, et al. RAPID3 (Routine Assessment of Patient Index Data 3), a rheumatoid arthritis index without formal joint counts for routine care: proposed severity categories compared to disease activity score and Clinical Disease Activity Index categories. J Rheumatol. 2008; 35: 2136– 2147. [DOI] [PubMed] [Google Scholar]
- 26. Pincus T, Yazici Y, Bergman MJ. RAPID3, an index to assess and monitor patients with rheumatoid arthritis, without formal joint counts: similar results to DAS28 and CDAI in clinical trials and clinical care. Rheum Dis Clin North Am. 2009; 35: 773– 778 viii. [DOI] [PubMed] [Google Scholar]
- 27. Pincus T. Can RAPID3, an index without formal joint counts or laboratory tests, serve to guide rheumatologists in tight control of rheumatoid arthritis in usual clinical care? Bull NYU Hosp Jt Dis. 2009; 67: 254– 266. [PubMed] [Google Scholar]
- 28. Pincus T, Furer V, Keystone E, et al. RAPID3 (Routine Assessment of Patient Index Data 3) severity categories and response criteria: similar results to DAS28 (Disease Activity Score) and CDAI (Clinical Disease Activity Index) in the RAPID 1 (Rheumatoid Arthritis Prevention of Structural Damage) clinical trial of certolizumab pegol. Arthritis Care Res (Hoboken). 2011; 63: 1142– 1149. [DOI] [PubMed] [Google Scholar]
- 29. Pincus T. RAPID3, an index of only 3 patient self-report core data set measures, but not ESR, recognizes incomplete responses to methotrexate in usual care of patients with rheumatoid arthritis. Bull Hosp Jt Dis (2013). 2013; 71: 117– 120. [PubMed] [Google Scholar]
- 30. Castrejón I, Pincus T. Assessing remission in rheumatoid arthritis on the basis of patient reported outcomes—advantages of using RAPID3/MDHAQ in routine care. Bull Hosp Jt Dis (2013). 2014; 72: 136– 141. [PubMed] [Google Scholar]
- 31. Pincus T, Richardson B, Strand V, et al. Relative efficiencies of the 7 rheumatoid arthritis Core Data Set measures to distinguish active from control treatments in 9 comparisons from clinical trials of 5 agents. Clin Exp Rheumatol. 2014;( 32 suppl 85): 47– 54. [PubMed] [Google Scholar]
- 32. Pincus T, Wolfe F. Patient questionnaires for clinical research and improved standard patient care: is it better to have 80% of the information in 100% of patients or 100% of the information in 5% of patients? J Rheumatol. 2005; 32: 575– 577. [PubMed] [Google Scholar]
- 33. Bergman MJ, Castrejón I, Pincus T. RHEUMDOC: a one-page RHEUMatology DOCtor form with four physician global estimates for overall status, inflammation, damage, and symptoms based on neither inflammation nor damage. Bull Hosp Jt Dis (2013). 2014; 72: 142– 147. [PubMed] [Google Scholar]
- 34. Tymms K, Zochling J, Scott J, et al. Barriers to optimal disease control for rheumatoid arthritis patients with moderate and high disease activity. Arthritis Care Res (Hoboken). 2014; 66: 190– 196. [DOI] [PubMed] [Google Scholar]
- 35. Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005; 23(5 suppl 39): S100– S108. [PubMed] [Google Scholar]
- 36. Uhlig T, Kvien TK, Pincus T. Test-retest reliability of disease activity core set measures and indices in rheumatoid arthritis. Ann Rheum Dis. 2009; 68: 972– 975. [DOI] [PubMed] [Google Scholar]


