Abstract
Objective
Thrombospondin-4 (TSP-4) is 1 of the 5 members of the thrombospondin protein family. TSP-1 and TSP-2 are potent antiangiogenic proteins. However, angiogenic properties of the 3 other TSPs, which do not contain the domains associated with the antiangiogeneic activity of TSP-1 and TSP-2, have not been explored. In our previous studies, we found that TSP-4 is expressed in the vascular matrix of blood vessels of various sizes and is especially abundant in capillaries. We sought to identify the function of TSP-4 in the regulation of angiogenesis.
Approach and Results
The effect of TSP-4 in in vivo angiogenesis models and its effect on angiogenesis-related properties in cultured cells were assessed using Thbs4−/−; mice, endothelial cells (EC) derived from these mice, and recombinant TSP-4. Angiogenesis was decreased in Thbs4−/−; mice compared with wild-type mice. TSP-4 was detected in the lumen of the growing blood vessels. Mice expressing the P387 TSP-4 variant, which was previously associated with coronary artery disease and found to be more active in its cellular interactions, displayed greater angiogenesis compared with A387 form. Lung EC from Thbs4−/−; mice exhibited decreased adhesion, migration, and proliferation capacities compared with EC from wild-type mice. Recombinant TSP-4 promoted proliferation and the migration of EC. Integrin and gabapentin α2 receptor α2δ-1 were identified as receptors involved in regulation of EC adhesion, migration, and proliferation by TSP-4.
Conclusion
TSP-4, an extracellular matrix protein previously associated with tissue remodeling, is now demonstrated to possess proangiogenic activity.
Keywords: angiogenesis, extracellular matrix, thrombospondin-4
Our progress in understanding the mechanisms underlying vascular diseases has been heavily focused on the cells involved. Yet it is well established that it is not only the individual cell types but also their interactions with other cells and with the extracellular matrix (ECM) that control the initiation and progression of various vascular pathologies ranging from atherogenesis to angiogenesis.1–4 The ECM is clearly an important regulator of vascular pathologies, but it has only recently become appreciated as a target for pharmacotherapy.5
Thrombospondin-4 (TSP-4) belongs to a group of matricellular ECM proteins, which do not provide structural support like collagens or elastins, but instead regulate cell–matrix interactions, and functional responses dependent on these interactions, including adhesion, migration, apoptosis, proliferation, and ECM remodeling/fibrosis.6–13 Remodeling of the ECM in the vascular wall initiates and defines the development of cardiovascular disease, diabetic complications, tumor growth, and many other devastating chronic diseases. Remodeling and growth of the blood vessels is guided and regulated by matricellular ECM proteins, which signal through surface receptors to control numerous vascular cell responses.
The TSP family consists of 5 proteins (TSP-1 through TSP-5).8 Two members, TSP-1 and TSP-2, are potent antiangiogenic proteins.10–17 However, there have been no reports regarding the angiogenesis-related activities of the other 3 members of the family, with the exception of an observation that TSP-3 does not inhibit angiogenesis.18 Of note, TSP-3, TSP-4, and TSP-5 do not harbor the protein domains that mediate the antiangiogenic activities of TSP-1 and TSP-2.8 Despite recent observations of the presence and critical roles of TSP-4 in the heart, blood vessels, and vascularized tissues,9,10,19–24 it has not been reported to have any effect on angiogenesis. Circumstantial evidence suggests that TSP-4 might be involved in the regulation of angiogenesis during tissue remodeling and growth. Existing data document the association between TSP-4 and cancer. Tumor growth and metastasis are responses that depend heavily on angiogenesis. Specifically, increased expression of TSP-4 in cancer tissues is associated with cancer progression, and TSP-4 is in the top 1% of the most upregulated genes in several types of cancer, including gastric cancer,25–27 and especially in breast cancer28–30 (www.oncomine.com).
In view of the intimate association between TSP-4, blood vessels, and angiogenic pathologies, the goal of the present work was to examine the effect of TSP-4 on angiogenesis and the proangiogenic functions of endothelial cells (EC).
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Angiogenesis Is Inhibited in Matrigel Plugs in Thbs4−/−; Mice
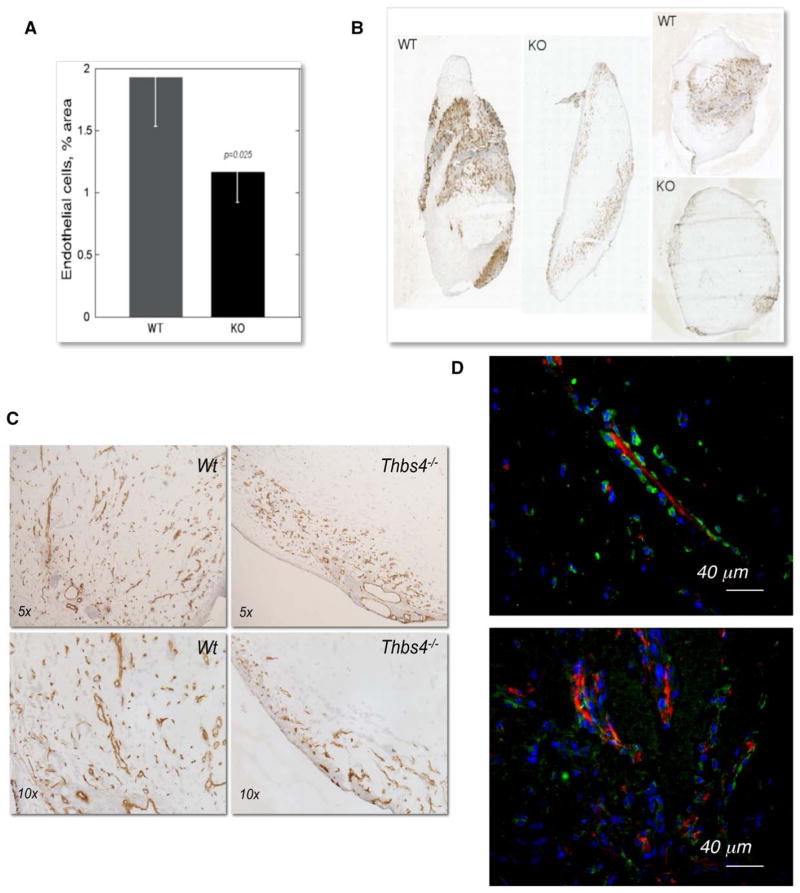
The widely used Matrigel plug model was implemented as an initial approach to assess the effect of TSP-4 deficiency on angiogenesis (Figure 1). Matrigel (750 μl) mixed with 10 ng/mL FGF was injected subcutaneously into wild-type (WT) C57Bl/6 or TSP-4 knockout (Thbs4−/−;) mice (n=10). The plugs were excised 7 days later and processed for immunohistochemistry. Sections were stained with anti-CD31 antibodies, and the stained area was quantified. The level of CD31, a marker of EC, was significantly reduced in the plugs excised from Thbs4−/−; mice (Figure 1A and 1B). Higher power examination of the sections confirmed that the EC staining was associated with the blood vessels (Figure 1C).
Figure 1.
Angiogenesis is decreased in Thbs4−/−; mouse. A, Quantification of anti-CD31 staining (EC) in Matrigel plugs in WT and Thbs4−/−; mice (n=10). The stained area was quantified using ImagePro6.1. B, Whole plug section image, immunohistochemistry with anti-CD31 antibody. C, CD31 staining in sections of Matrigel plugs harvested from WT and Thbs4−/−; mice. D, TSP-4 expression in the neovasculature in Matrigel plugs. TSP-4 in growing blood vessels in Matrigel plug is localized in the lumen (red, TSP-4 stained with anti–TSP-4, green, EC stained with anti-CD31 antibody, blue, nuclei stained with DAPI). Arrows indicate the blood vessels with TSP-4 in the lumen. EC indicates endothelial cell; KO, knockout; Thbs4−/−;, TSP-4 knockout; and WT, wild-type.
EC were also visualized in Matrigel plug sections using anti–Von Willebrand Factor antibody (Figure 1D, green staining), and TSP-4 was visualized using anti–TSP-4 antibody as described previously9,10,24,31,32 (Figure 1D, red staining). In our previous reports, we described the localization of TSP-4 in blood vessels of different sizes9,13: TSP-4 was found in the tunica adventitia of the larger vessels and on the abluminal side of EC in mature capillaries. In contrast to mature vasculature, TSP-4 was found in the lumen of the neovasculature in the Matrigel plugs (Figure 1D).
Angiogenesis Is Inhibited in the Tumor Model in Thbs4−/−; Mice
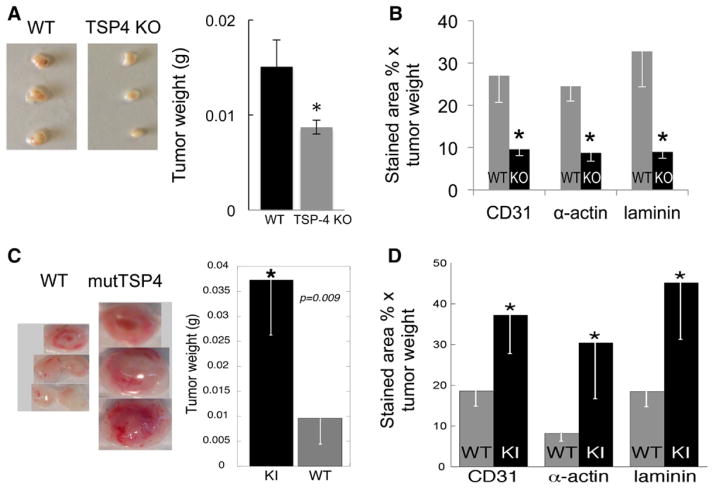
Angiogenesis in WT and Thbs4−/−; mice was examined in a tumor angiogenesis model. EMT6, a mouse breast cancer cell line, was propagated in culture, and 1.5×106 cells were injected into the mammary fat pad of mice. Tumors were harvested on day 14, weighed, and processed for immunohistochemistry.31 Although we did not detect TSP-4 in cultured EMT6 cells by Western blotting of the cell lysates and cell culture supernatants (not shown), it was detected in the EMT6 tumors in vivo. When tumors from Thbs4−/−; mice were stained with anti–TSP-4 antibodies, we have detected TSP-4 staining, suggesting that TSP-4 is produced by EMT6 in in vivo tumors (data not shown). There was no difference in tumor weights between WT and Thbs4−/−; mice (data not shown). To remove the confounding effect of TSP-4 produced by EMT6 cells in vivo from the model system, we transduced EMT6 cells with lentiviral particles containing TSP-4 shRNA. As a control, EMT6 cells transduced with control shRNA were used. EMT6 cells stably expressing either TSP-4 shRNA or the control shRNA were injected into the mammary fat pads of WT and Thbs4−/−; mice, and tumors were collected and processed as described in the Materials and Methods section. In the absence of TSP-4, a significant decrease in tumor weight was detected (Figure 2A). Angiogenesis markers CD31, α-actin, and laminin-1 were visualized by immunostaining with the corresponding antibodies as described in the Materials and Methods section. The levels of all 3 angiogenesis markers were significantly decreased in Thbs4−/−; mice (Figure 2B).
Figure 2.
TSP-4 promotes angiogenesis in a cancer model. A, Mouse breast cancer EMT6 cells with stable TSP-4 knockdown were injected into WT or TSP-4 KO mice. Left, Representative tumors from one of the experiments. Right, Tumor weight, n=10, *P<0.05 compared with WT. B, Angiogenesis markers CD31 (EC), α-actin (SMC), and laminin-1 (basement membrane) were visualized by immunohistochemistry in frozen sections of tumors from WT and Thbs4−/−; mice. Mean stained area, % × mean tumor weight, n=10, *P<0.05 compared with WT. C, Mouse breast cancer EMT6 cells were injected in WT or TSP4 KI mice. Left, Representative tumors from one of the experiments. Right, Tumor weight, n=10, *P<0.05 compared with WT. D, Angiogenesis markers CD31 (EC), α-actin (SMC), and laminin-1 (basement membrane) were visualized by immunohistochemistry in frozen sections of tumors from WT and mutTSP-4 KI mice. Mean stained area, % × mean tumor weight, n=10, *P<0.05 compared with WT. EC indicates endothelial cell; KO, knockout; Thbs4−/−;, TSP-4 knockout; and WT, wild-type.
A387P Mutation in TSP-4 Promotes Angiogenesis In Vivo
Our previous studies have shown that the P387 variant of TSP-4 interacts with cultured cells with high affinity and induces more extensive signaling than A387 WT TSP-4 form.12,33 A387P has been associated with increased susceptibility to early onset myocardial infarction in several studies.34 The growth of EMT6 tumors grown in mutant A387P TSP-4 knock-in (mutTSP-4) KI mice were significantly larger (Figure 2C) than in WT mice that expressed WT A387 TSP-4. Markers of angiogenesis (CD31, α-actin, and laminin-1) were quantified in tumor sections (Figure 2D). In tumors from mutTSP-4 KI mice, all markers of angiogenesis were significantly increased, consistent with the larger tumor mass and higher proliferating and signaling activity of this variant that we reported previously.12,13
Skin Wound Healing Is Delayed in Thbs4−/−; Mice
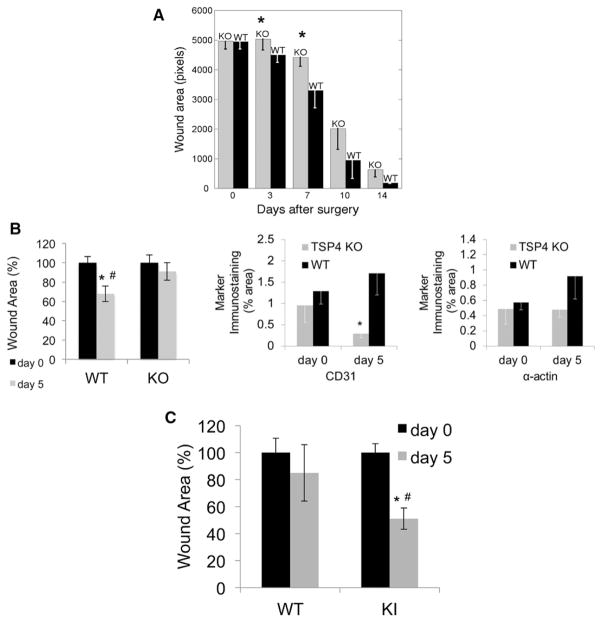
Wound healing critically depends on angiogenesis and vascular remodeling.35 Changes in angiogenic activity influence the rate of wound healing: suppression of angiogenesis delays and accelerated angiogenesis enhances wound healing.36 We performed skin wound healing assays in WT, Thbs4−/−;, and mutTSP-4 KI mice (Figure 3). Full thickness skin wounds (0.7 mm in diameter) were excised on the back of mice and allowed to heal uncovered over time. The sizes of wounds were measured immediately after the surgery and on days 3, 7, 10, and 14 in Thbs4−/−; mice (Figure 3A). Wound healing was delayed starting on day 3 after excision in Thbs4−/−; mice compared with WT mice. The differences between WT and Thbs4−/−; mice were statistically significant on days 3 to 7 when angiogenesis is the most active in the wound.37 At later time points, the wounds still tended to be smaller in the WT mice than in the Thbs4−/−; mice, although with healing the size of the wounds became more variable.
Figure 3.
Skin wound healing is Thbs4−/−; and mutTSP4 KI mice. Skin wounds (0.7×0.7 cm2) were excised in WT, Thbs4−/−; (KO) and mutTSP4 KI (KI) mice. Wound area was measured at the indicated time points. A, Wound healing in Thbs4−/−; mice; n=10. B, Wound area (left panel) and levels of angiogenic markers (CD31, EC; middle panel and α-actin, SMC; right panel) in WT and Thbs4−/−; mice on day 5; n=5. C, Wound area on day 5 in mutTSP4 KI mice; n=5 in WT group, n=10 in mutTSP4 KI group; *P<0.05 in comparison of genotypes, #P<0.05 compared with day 0. EC indicates endothelial cell; KO, knockout; mutTSP4 KI, mutant A387P TSP-4 knock-in; Thbs4−/−;, TSP-4 knockout; and WT, wild-type.
We have examined the levels of angiogenesis markers CD31 and α-actin in skin wound sections of WT and Thbs4−/−; mice on day 5 (Figure 3B). The difference in the wound size was significantly different between WT and Thbs4−/−; mice on day 5 (Figure 3B, left panel). The levels of CD31 and α-actin were both decreased (Figure 3B, middle and right panels), and the difference in the levels of CD31 was statistically significant (Figure 3B, middle panel).
Accelerated Wound Healing in mutTSP-4 KI Mice
The size of wounds was measured on day 5 in WT and mutTSP-4 KI mice (Figure 3C). In mutTSP-4 KI mice, the size of the wounds was significantly different between day 0 and day 5, whereas in WT mice, the difference remained insignificant. The wounds of mutTSP-4 KI mice were significantly smaller than the wounds of WT mice on day 5.
Delayed Postnatal Retinal Vasculature Development in Thbs4−/−; Mice
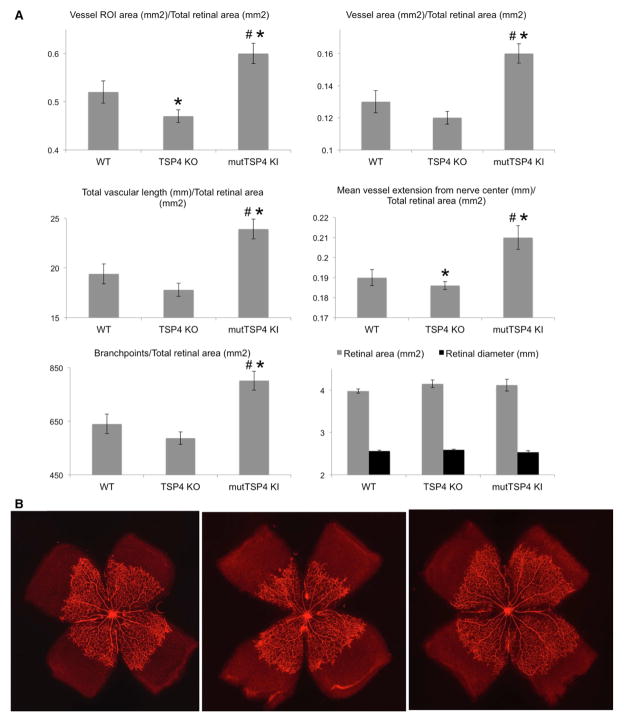
Flat mounts of retinae were prepared and stained with Alexa-568–labeled GS-IB4 as described in the Materials and Methods section to visualize EC. The entire region of vascular outgrowth (region of interest), precise area occupied by vessels, total vascular length, mean radial vessel extension from the optic nerve, the number of branch points, the whole retinal tissue area (vascular and avascular), and diameter were measured as described in Materials and Methods. These indeces characterizing the postnatal development of the retinal vasculature are shown in Figure 4A. The values were normalized to retinal area for each individual flat mount. Retinal area and retinal diameter values were similar between all genotypes. The vascularized area and the mean vessel extension from the center of the optic nerve were significantly smaller in Thbs4−/−; mice, whereas the rest of indeces also tended to be decreased in these mice (Figure 4A), suggesting that the postnatal development of the retinal vasculature is slower in Thbs4−/−; mice.
Figure 4.
TSP-4 regulates the postnatal development of retinal vasculature. The retinae of 5-day-old pups were processed as described in the Materials and Methods section, and the region of vessel area, total vascular length, mean extension of vascularized area form the optic nerve center, and the number of vascular branchpoints were quantified as described in Materials and Methods and normalized to retinal area. A, Analysis of retinal vasculature in flat-mounts stained with Alexa-568 lectin was performed as described in Materials and Methods. Mean±SEM; *Statistically significant difference as compared with WT mice; #Difference significant between TSP4 KO and mutTSP4 KI; n=10 in mutTSP4 KI group; n=16 in WT and TSP4 KO groups. B, Representative images of staining of vessels with Alexa-568 lectin in retinae of 5-day-old mice: Left, WT mice; middle, Thbs4−/−; mice; right, mutTSP-4 KI mice. EC indicates endothelial cell; KO, knockout; mutTSP4 KI, mutant A387P TSP-4 knock-in; ROI, region of interest (region of vessel extension from the optical nerve); Thbs4−/−;, TSP-4 knockout; and WT, wild-type.
Accelerated Postnatal Retinal Vasculature Development in mutTSP-4 KI Mice
All of the measured parameters were significantly increased in mutTSP-4 KI mice as compared with both WT and Thbs4−/−; mice (Figure 4A), consistent with the observations of increased angiogenesis in these mice in 3 other angiogenesis models. Representative images of retinae flat mounts from WT, Thbs4−/−;, and mutTSP-4 KI mice are shown in Figure 4B.
Regulation of Proangiogenic Activities by TSP-4 in Cultured EC
EC were isolated from the lungs of WT, Thbs4−/−;, and mutTSP-4 KI mice,38 and their angiogenic properties (cell adhesion, cell migration, and proliferation) were compared in vitro.
Cell Migration
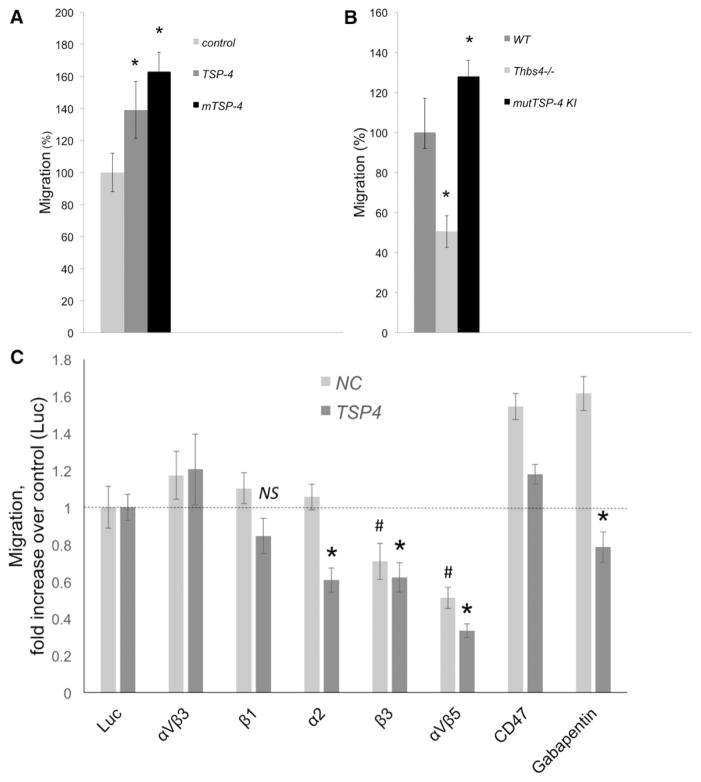
The effect of r-TSP-4 and mTSP-4 on EC migration was measured in Boyden chambers.12,39 The bottom of the upper chamber was coated with either recombinant TSP-4 (r-TSP-4) or r-mTSP-4 or left uncoated as a control. Both r-TSP-4 and r-mTSP-4 increased the migration of WT EC (Figure 5A). Consistent with the observed effects of r-TSP-4 and recombinant mut-TSP-4 (r-mut-TSP-4), Thbs4−/−; EC exhibited decreased migration, and mutTSP-4 KI EC had higher migratory activity compared with WT EC (Figure 5B).
Figure 5.
TSP-4 increases migration of EC. A, Migration of EC in noncoated Boyden chambers with 8 μm pores was compared with that in chambers coated with r-TSP-4 or mTSP-4. DNA in the lower chamber was measured after 4 h at 37°C. The values are expressed as percent of average migration in control chambers. *P<0.05 compared with control (uncoated wells), n=3. B, EC isolated from WT, Thbs4−/−;, and mutTSP-4 KI mice were plated into Boyden chambers with 8 μm pores. DNA amount in the lower chamber was measured after 4 h incubation at 37°C. Migration of TSP-4 KO and TSP-4 KI cells were compared with WT EC (100%). *P<0.05 compared with WT cell migration, n=3. C, EC from WT mice were seeded into noncoated chambers or chambers coated with r-TSP-4. The blocking antibodies against the potential receptors for TSP-4 were added to the chambers, and the cells were incubated at 37°C for 4 h. Then, the DNA in lower chambers was quantified using the CyQUANT reagent. *P<0.05 compared with the effect of a control Ab (anti-luciferase) in r-TSP-4–coated wells; #P≤0.05 compared with the effect of a control Ab in uncoated wells; n=3. EC indicates endothelial cell; KO, knockout; mutTSP4 KI, mutant A387P TSP-4 knock-in; r-TSP-4, recombinant TSP-4; Thbs4−/−;, TSP-4 knockout; and WT, wild-type.
We performed EC migration assays with the function blocking antibodies against known receptors of TSPs as described in Materials and Methods (Figure 5C). All antibodies were well tested blocking antibodies reacting with mouse proteins. As a control, an unrelated anti-luciferase antibody, which did not have any effect on EC migration (not shown), was used (Figure 6C, Luc). As was observed in previous experiments (Figure 5A), coating with r-TSP-4 increased migration of EC by 39%±9% (P=0.0076). We searched for an antibody that produces the specific inhibitory effect of inhibiting the migration on r-TSP-4 without inhibiting the migration on the uncoated surface. Two reagents produced the specific inhibition of TSP-4–induced migration: anti-integrin α2 and gabapentin, a ligand of α2δ-1 receptor that prevents TSP-4 binding to this receptor.23 Both antbodies decreased migration on TSP-4 (P<0.05 as compared with the effect of a control antibody on migration in the presence of TSP-4), but did not affect the migration in control uncoated wells (no decrease as compared with the effect of a control antibody on migration of EC in control uncoated wells). The difference between the values not normalized to the corresponding controls was also statistically significant for the effects of these 2 antibodies in the presence of TSP-4 and in uncoated wells (P=0.039 for α2 integrin antibody; P=0.0009 for α2δ-1 receptor antibody).
Figure 6.
TSP-4 promotes proliferation of EC. A, 12-well plates were coated with fibronectin or fibronectin mixed with r-TSP4 or mTSP4 overnight at 4°C. EC from WT, Thbs4−/−;, and mutTSP4 KI mice were added and allowed to proliferate at 37°C for 24, 48, and 72 h. *P<0.05 compared with fibronectin-only wells, n=3. B, EC isolated from WT, Thbs4−/−;, and mutTSP4 KI mice were plated onto fibronectin-coated plates. The amount of DNA in the wells was measured at 24, 48, and 72 hours after seeding the cells. *P<0.05 compared with WT cells, n=3. The average values of fluorescence are presented as % of average values of fluorescence in control wells coated with fibronectin alone. C, EC from WT mice were seeded into noncoated chambers or chambers coated with r-TSP-4. The blocking antibodies against the potential receptors for TSP-4 were added to the chambers, and the cells were incubated at 37°C for 48 h. Then, the DNA in lower chambers was quantified using the CyQUANT reagent. *P<0.05 compared with the effect of a control Ab (anti-luciferase) in r-TSP-4–coated wells. EC indicates endothelial cell; KO, knockout; mutTSP4 KI, mutant A387P TSP-4 knock-in; r-TSP-4, recombinant TSP-4; Thbs4−/−;, TSP-4 knockout; and WT, wild-type.
Antibodies against integrin αVβ3, β1 integrin subunit, and CD47 did not have significant effects on EC migration (Figure 5C). Antibodies against β3 subunit and αVβ5 inhibited both the TSP-4–dependent and TSP-4–independent migration (P<0.05 when the effect of each of 2 antibodies in the wells coated with TSP-4 was compared with the effect of a control antibody in the wells coated with TSP-4 and when the effect of each of the 2 antibodies on migration in uncoated wells was compared with the effect of a control antibody in the uncoated wells). There was no difference in values not normalized to the corresponding controls: both antibodies reduced migration to the same absolute value both in presence of TSP-4 and in uncoated wells, indicating that the inhibition of migration is not matrix-dependent.
Cell Proliferation
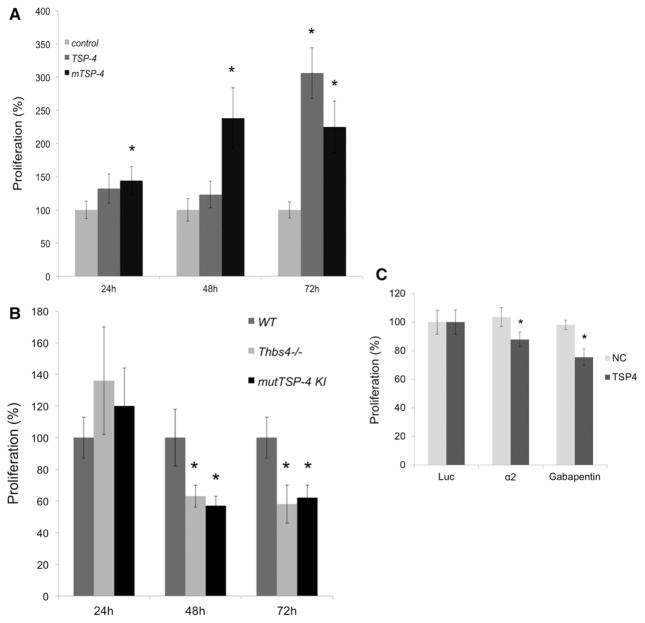
The proliferation of EC from WT mice was measured using the CyQUANT reagent cell proliferation kit in EC growth medium supplemented with 5% FCS with cells plated onto a fibronectin substrate alone or supplemented with either r-TSP-4 or r-mutTSP-4 (Figure 6A). Both r-TSP-4 and r-mutTSP-4 stimulated EC proliferation. The effect of r-mutTSP-4 was statistically significant as early as 24 hours after the cells were plated (Figure 6A). The effect of r-TSP-4 became significant by 72 hours after EC were seeded.
The proliferation of EC derived from 3 strains of mice (WT, Thbs4−/−;, and mutTSP-4 KI) was measured at 24, 48, and 72 hours. Proliferation of EC derived from either Thbs4−/−; or mutTSP-4 KI mice was slower than those obtained from WT mice (Figure 6B). This pattern was observed with 3 separate isolates of EC from each mouse strain.
To ensure that proliferation rather than DNA content is changing with genotype, we have counted cells 72 hours after plating the cells in the conditions identical to the conditions described earlier. Equal numbers of cells of all 3 genotypes were plated per culture dish area in the beginning of the experiment. Cells were washed with phosphate buffered saline, fixed with 4% paraformaldehyde, stained with Trypan Blue, and photographed. Stained cell areas were quantified using Adobe Photoshop CS3. The number of pixels of cell staining, which reflects the actual number of cells in the cell culture dish at 72 hours, is presented in Figure I in the online-only Data Supplement. Similar to our data obtained using the CyQUANT reagent cell proliferation kit, both WT r-TSP-4 and r-mutTSP-4 increased proliferation (Figure IA in the online-only Data Supplement), and EC from either Thbs4−/−; or mutTSP-4 KI mice proliferated more slowly than WT EC (Figure IB in the online-only Data Supplement).
To test whether α2 integrin and α2δ-1 contribute to EC proliferation in response to TSP-4, we cultured EC for 72 hours in the presence of either 10 μg/mL neutralizing anti-α2 integrin antibody or 10 μg/mL gabapentin, a ligand of α2δ-1 added to the media 6 hours after plating the cells. Antiluciferase antibody was used as a control. Both the anti-α2 integrin antibody and gabapentin, a ligand of α2δ-1, significantly decreased proliferation of EC in cell culture plates coated with r-TSP-4 but not in control cell culture plates coated with bovine serum albumin (Figure 6C).
Cell Adhesion
Cell adhesion was measured using the CyQUANT reagent cell proliferation kit to quantify the adherent at 1 hour after plating EC in cell culture plates coated with fibronectin mixed with Bovine serum albumin, r-TSP-4, or r-mutTSP-4. r-TSP-4 did not have any effect on EC adhesion (Figure 7A). r-mutTSP-4 was less adhesive for the mouse EC, similar to its effect on human EC as we previously reported.13 When EC from the 3 mouse strains were assessed for their adhesive activity, both Thbs4−/−; and mutTSP-4 KI EC adhered poorly compared with WT EC (Figure 7B).
Figure 7.
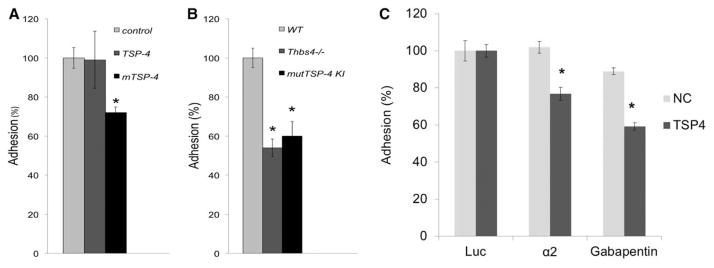
TSP-4 increases EC adhesion. A, MLEC from WT mice were added 24-well plates coated with fibronectin (control) or fibronec-tin mixed with r-TSP4 or mTSP4 and incubated at 37°C for 1 h. Unattached cells were removed by washing, and the remaining DNA in the wells was measured. Adhesion of MLEC on each substrate was compared with the adhesion in control wells coated with fibronectin only (100%).*P<0.05 compared with control wells, n=3. B, EC from WT, Thbs4−/−;, and mutTSP4 KI mice were plated onto fibronectin-coated plates. Plates were incubated at 37°C for 1 h. Unattached cells were washed away, and the DNA in the wells was measured using the CyQUANT reagent. Adhesion capacity of TSP-4 KO and TSP-4 KI EC was compared with WT EC (100%). *P<0.05 compared with WT cells, n=3. C, EC from WT mice were seeded into noncoated chambers or chambers coated with r-TSP-4. The blocking antibodies against the potential receptors for TSP-4 were added to the chambers, and the cells were incubated at 37°C for 1 h. Then, the DNA in lower chambers was quantified using the CyQUANT reagent. *P<0.05 compared with the effect of a control Ab (anti-luciferase) in r-TSP-4–coated wells. *P<0.05 compared with WT cells, n=3. EC indicates endothelial cell; KO, knockout; MLEC, mouse lung endothelial cells; mutTSP4 KI, mutant A387P TSP-4 knock-in; r-TSP-4, recombinant TSP-4; Thbs4−/−;, TSP-4 knockout; and WT, wild-type.
Quantification of the Trypan Blue staining of cells attached to the cell culture plates coated with fibronectin or fibronectin mixed with r-TSP-4 or r-mutTSP-4 resulted in similar differences between experimental conditions (Figure IIA and IIB in the online-only Data Supplement), although quantification of DNA appeared to be more sensitive in detecting the differences between cells in different conditions.
Discussion
Our results identify TSP-4 as a novel regulator of angiogenesis. TSP-4 is a member of the thrombospondin family that includes 4 other proteins: TSP-1, TSP-2, TSP-3, and TSP-5 (COMP).6,8 TSP-1 and TSP-2 have evolved more recently and are potent antiangiogenic proteins.16,40–43 Their antiangiogenic properties have been documented in vitro and in vivo and are attributed to the TSP repeats where the binding site for the cell receptor CD36 and the sequence involved in regulation of matrix metalloproteinase activity reside.44,45 TSP-3, TSP-4, and TSP-5 belong to the more ancient TSP subgroup,8 and they have not been reported to be regulators of angiogenesis. None of the latter 3 TSPs has the TSP repeats domain associated with the antiangiogeneic activity of TSP-1 and TSP-2.8,17,46
TSP-4 has attracted significant interest recently and has been associated with the remodeling of vasculature and myocardium,9,12,21,22,47 control of the organization and function of tendon and skeletal muscle,24 risk for the cardiovascular disease,10,13,21,22,24,47,48 inflammation,10 and synaptogenesis in the central nervous system.23 The existing data document the association between TSP-4 and cancer,25–27 especially with the breast cancer.28–30 However, the mechanisms of these associations remain unknown.
In this report, we describe a novel and unexpected function of TSP-4: stimulation of angiogenesis. We documented the proangiogenic activity of TSP-4 in several complementary models: in vivo Matrigel angiogenesis, in vivo tumor angiogenesis, postnatal retinal vasculature development model, skin wound healing, and in several in vitro assays, including cultured EC adhesion, migration, and proliferation assays. The deficiency in TSP-4 resulted in decreased angiogenesis or reduced proangiogenic functions. Both r-TSP-4 and A387P r-mutTSP-4, which is more active in its cellular interactions, increased proangiogenic properties in vitro and both stimulated angiogenesis. Throughout the present study, we did not observe any sex-specific effects of either TSP-4 or mutTSP-4 P387 on angiogenesis. Although we did not systematically examine the age-dependence of the effects, our results indicate that the effect of TSP-4 on angiogenesis can be detected at different ages, from 5-day-old pups (postnatal retinal vasculature development) to 27-week-old mice (Matrigel plug assay).
The in vivo Matrigel plug assay is a simple and convenient model to study the growth of new blood vessels in the absence of influences from surrounding tissue. Angiogenesis was evaluated by the levels of 3 angiogenesis-related markers (CD31, a marker of EC; α-actin, a marker of smooth muscle cells/pericytes and of maturation of the growing capillaries; and laminin-1, a marker of basement membrane). Angiogenesis was reduced in Thbs4−/−; mice, indicating that TSP-4 stimulates angiogenesis. TSP-4 was detected in growing blood vessels, but its localization was different compared with its localization in mature blood vessels where TSP-4 was present on the abluminal side of the EC monolayer in the vascular wall.10 In new vessels growing in the Matrigel plug, TSP-4 was detected in the lumen. This localization suggests that TSP-4 may be secreted by EC or pave a path for EC to form the new vessel. Although TSP-4 was found in a proximity to EC in growing vessels, we did not address its source systematically. Smooth muscle cells can be producing TSP-4 as we reported in the past,13 and the blood cells as a source of TSP-4 cannot be excluded: although we did not detect any production of TSP-4 protein by the blood cells (data not shown), and others never reported its production by the blood cells, we did detect TSP-4 mRNA in the cellular fraction of blood (data not shown).
In the mouse breast cancer angiogenesis model, host TSP-4 deficiency alone did not reduce tumor mass or the levels of the angiogenesis markers. We found that the cancer cells produced TSP-4 in vivo. TSP-4 is a secreted protein that becomes available to all cell types after it is released. Therefore, we considered whether TSP-4 produced by the cancer cells could have provided sufficient signals to EC and other vascular cells to maintain angiogenesis within the tumors. When we used EMT6 stably transduced with lentiviral particles expressing TSP-4 shRNA that did not produce TSP-4 in the in vivo cancer angiogenesis model, the weights of tumors were significantly decreased in the absence of TSP-4 as compared with the weights of tumors in WT mice. The levels of angiogenesis markers were also significantly decreased, indicating that TSP-4 facilitates tumor angiogenesis and growth. The source of TSP-4 (vascular- or cancer-cell–produced) does not seem to be important, rather the level of TSP-4 in tumors seems to be sufficient to support tumor angiogenesis and growth based on the lack of the effect in our experiments that used WT EMT6 in Thbs4−/−; mice.
A387P TSP-4 has been associated with cardiovascular disease in several patient cohorts.13,34,49–53 We have reported that A387P TSP-4 is more active in interactions with cells and produces more pronounced effects in cell culture.12,13,33 Thus, the enhanced angiogenesis and cancer growth observed in the KI mice expressing A387P TSP-4 compared with WT TSP-4 is consistent with its increased bioactivity.
Wound healing is a process of tissue remodeling and is dependent on angiogenesis.35,37 In skin wounds, angiogenesis is clearly detected by day 3 and is active until at least day 7.37 The healing at this stage is accelerated if angiogenesis is increased and delayed if angiogenesis is inhibited.35,37 We have tested Thbs4−/−; mice in an excisional skin wound healing assay. Healing was significantly delayed in Thbs4−/−; mice, consistent with the proangiogenic effects of TSP-4 in other models. The delay was detectable on the day 3 and remained statistically significant until day 7. Wound healing was accelerated in mutTSP-4, once again consistent with the greater activity of P387 TSP-4 in cellular and physiological processes.
Each angiogenesis model examined has advantages and limitations. The Matrigel model allows analyses of the formation of new vessels in the absence of surrounding tissue, but the plug induces an inflammatory response in the host. The cancer angiogenesis models involve complex interactions of the vascular cells with the surrounding tissue, but the vessels formed in a tumor are different from the vessels developing during the normal physiological processes of remodeling. The outcomes in the skin wound healing model depend not only on angiogenesis, but also on an inflammatory response and the function of fibroblasts and keratinocytes.
The postnatal retinal development model is another way to address the effects of protein knockout or overexpression on angiogenesis. In this model, the angiogenesis can be observed in the absence of proinflammatory signals and external interventions. In this model, TSP-4 knockout resulted in delayed development of retinal vasculature, whereas mutTSP-4 accelerated the process.
In the in vivo models that we used to study the role of TSP-4 in angiogenesis, TSP-4 knockout and mutTSP-4 expression in KI mice produced opposite effects that we could expect based on our previous studies of the effects of TSP-4 and mutTSP-4.12,13 Although the effects of compensation can never be completely excluded when working with transgenic mice, the effects of compensation seem to be less likely when the knockout of a protein and an overexpression of a mutant protein with a known increased activity result in opposite and predicted effects. We previously examined the expression and localization of TSP-3 and TSP-5, 2 proteins highly homologous to TSP-4, in tissues and blood vessels of Thbs4−/−; mice, and we found that their expression was not changed and that they displayed a distinct nonoverlapping localization in tissues.9,10,24
Our in vitro experiments performed using EC from Thbs4−/−; and mutTSP-4 KI mice and recombinant TSP-4 and mutTSP-4 complement and support the conclusions obtained in the in vivo models. Although the in vitro approach had its limitations (eg, the source of TSP-4 may affect its function, EC are studied in isolation from other cell types, etc), it complemented the in vivo approach, lead to similar conclusions, and allowed to begin to investigate the cellular mechanisms of the observed effects.
Invasion of EC into tissue and formation of a vessel lumen is a multistep process. A variety of activities of EC and extracellular matrix proteins are involved in this complex response. We have examined the effect of TSP-4 and A387P TSP-4 on EC adhesion to the matrix, EC proliferation, and EC migration. In all 3 in vitro models, we observed an effect of TSP-4 consistent with its proangiogenic activity. r-TSP-4 increased EC proliferation, whereas the proliferation was inhibited in Thbs4−/−; EC. Thbs4−/−; EC demonstrated decreased adhesion, although r-TSP-4 did not have any effect on WT EC adhesion. Differences in the effects of endogenous and exogenously introduced TSP is a known phenomenon: the effects of a TSP on cells greatly depends on its origin (endogenous versus exogenous).54 TSPs have multiple protein ligands that modify their effects, and some of the binding events occur during the production and secretion of TSPs.55 r-TSP-4 increased the migration of EC, and the migratory capacity was reduced in EC from Thbs4−/−; mice.
The effects of r-mutTSP-4 were more complex in the in vitro assays. r-mutTSP-4 increased EC migration, and EC from mice expressing mutTSP-4 demonstrated increased migration as well. Adhesion was inhibited by r-mutTSP-4, both in the in vitro experiments with the recombinant mutTSP-4 and with EC from mutTSP-4 KI mice. This observation is consistent with our previous report on the effect of r-mTSP-4 on human EC.13 Although r-mutTSP-4 increased EC proliferation, there was a significant decrease in proliferation of mutTSP-4 KI EC. This discrepancy may be because of a complexity of the mutTSP-4 effects on EC that are not fully reproduced in an in vitro proliferation assay or to a compensation of function(s) in the transgenic mouse. In either case, we conclude that mutTSP-4 may acquire additional properties compared with WT TSP-4 rather than just being more potent in cellular interactions.
In sum, we have identified a novel function for TSP-4: its capacity to regulate angiogenesis. TSP-4 is a new proangiogenic ECM protein. It influences multiple EC responses in vitro that translate into enhanced blood vessel formation in vivo.
Supplementary Material
Significance.
Thrombospondin-4 (TSP-4) was found to be abundantly expressed in vasculature and in several cancers and to play an important role in tissue remodeling. However, the significance of its expression in blood vessels and its effect on angiogenesis remained unknown. The TSP protein family has been associated with regulation of angiogenesis, but the effects of TSP-1 and TSP-2, 2 known angiogenesis regulators, are antiangiogenic. The differences in the structure of TSP-1/TSP-2 and TSP-4 suggest that they may have distinct or even opposite functions in vasculature. The results of our work described in this report revealed that TSP-4 is a novel proangiogenic ECM protein that promotes EC adhesion, migration, and proliferation and increases angiogenesis in in vivo Matrigel and cancer models. Thus, we have identified a novel protein that promotes angiogenesis.
Acknowledgments
Sources of Funding
This work was supported by National Institute of Health (NIH) R01HL117216 (to O. Stenina-Adognravi and E. Plow); NIH R01CA177771 (to O. Stenina-Adognravi); and NIH DK067532 (to O. Stenina-Adognravi).
Nonstandard Abbreviations and Acronyms
- EC
endothelial cells
- ECM
extracellular matrix
- mutTSP4 KI
mutant A387P TSP-4 knock-in
- PBS
phosphate buffered saline
- r-TSP-4
recombinant TSP-4
- r-mut-TSP-4
recombinant mut-TSP-4
- TSP
thrombospondin
- TSP-4
thrombospondin-4
- Thbs4−/−
TSP-4 knockout
- WT
wild-type
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.115.305912/-/DC1.
Disclosures
None.
References
- 1.Eble JA, Niland S. The extracellular matrix of blood vessels. Curr Pharm Des. 2009;15:1385–1400. doi: 10.2174/138161209787846757. [DOI] [PubMed] [Google Scholar]
- 2.Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 2010;86:226–235. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Germain S, Monnot C, Muller L, Eichmann A. Hypoxia-driven angiogenesis: role of tip cells and extracellular matrix scaffolding. Curr Opin Hematol. 2010;17:245–251. doi: 10.1097/MOH.0b013e32833865b9. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol Biol Cell. 2010;21:687–690. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Järveläinen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev. 2009;61:198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest. 2001;107:929–934. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiPietro LA, Nebgen DR, Polverini PJ. Downregulation of endothelial cell thrombospondin 1 enhances in vitro angiogenesis. J Vasc Res. 1994;31:178–185. doi: 10.1159/000319585. [DOI] [PubMed] [Google Scholar]
- 8.Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frolova EG, Sopko N, Blech L, Popovic ZB, Li J, Vasanji A, Drumm C, Krukovets I, Jain MK, Penn MS, Plow EF, Stenina OI. Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload. FASEB J. 2012;26:2363–2373. doi: 10.1096/fj.11-190728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frolova EG, Pluskota E, Krukovets I, Burke T, Drumm C, Smith JD, Blech L, Febbraio M, Bornstein P, Plow EF, Stenina OI. Thrombospondin-4 regulates vascular inflammation and atherogenesis. Circ Res. 2010;107:1313–1325. doi: 10.1161/CIRCRESAHA.110.232371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raman P, Krukovets I, Marinic TE, Bornstein P, Stenina OI. Glycosylation mediates up-regulation of a potent antiangiogenic and proatherogenic protein, thrombospondin-1, by glucose in vascular smooth muscle cells. J Biol Chem. 2007;282:5704–5714. doi: 10.1074/jbc.M610965200. [DOI] [PubMed] [Google Scholar]
- 12.Pluskota E, Stenina OI, Krukovets I, Szpak D, Topol EJ, Plow EF. Mechanism and effect of thrombospondin-4 polymorphisms on neutrophil function. Blood. 2005;106:3970–3978. doi: 10.1182/blood-2005-03-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenina OI, Desai SY, Krukovets I, Kight K, Janigro D, Topol EJ, Plow EF. Thrombospondin-4 and its variants: expression and differential effects on endothelial cells. Circulation. 2003;108:1514–1519. doi: 10.1161/01.CIR.0000089085.76320.4E. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol. 2000;19:597–614. doi: 10.1016/s0945-053x(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 15.Carpizo D, Iruela-Arispe ML. Endogenous regulators of angiogenesis–emphasis on proteins with thrombospondin–type I motifs. Cancer Metastasis Rev. 2000;19:159–165. doi: 10.1023/a:1026570331022. [DOI] [PubMed] [Google Scholar]
- 16.Bonnefoy A, Moura R, Hoylaerts MF. The evolving role of thrombospondin-1 in hemostasis and vascular biology. Cell Mol Life Sci. 2008;65:713–727. doi: 10.1007/s00018-007-7487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol. 2000;12:634–640. doi: 10.1016/s0955-0674(00)00143-5. [DOI] [PubMed] [Google Scholar]
- 18.Volpert OV, Tolsma SS, Pellerin S, Feige JJ, Chen H, Mosher DF, Bouck N. Inhibition of angiogenesis by thrombospondin-2. Biochem Biophys Res Commun. 1995;217:326–332. doi: 10.1006/bbrc.1995.2780. [DOI] [PubMed] [Google Scholar]
- 19.Congote LF, Difalco MR, Gibbs BF. The C-terminal peptide of thrombospondin-4 stimulates erythroid cell proliferation. Biochem Biophys Res Commun. 2004;324:673–678. doi: 10.1016/j.bbrc.2004.09.107. [DOI] [PubMed] [Google Scholar]
- 20.Mustonen E, Ruskoaho H, Rysä J. Thrombospondin-4, tumour necrosis factor-like weak inducer of apoptosis (TWEAK) and its receptor Fn14: novel extracellular matrix modulating factors in cardiac remodelling. Ann Med. 2012;44:793–804. doi: 10.3109/07853890.2011.614635. [DOI] [PubMed] [Google Scholar]
- 21.Lynch JM, Maillet M, Vanhoutte D, et al. A thrombospondin-dependent pathway for a protective ER stress response. Cell. 2012;149:1257–1268. doi: 10.1016/j.cell.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cingolani OH, Kirk JA, Seo K, Koitabashi N, Lee DI, Ramirez-Correa G, Bedja D, Barth AS, Moens AL, Kass DA. Thrombospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circ Res. 2011;109:1410–1414. doi: 10.1161/CIRCRESAHA.111.256743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eroglu C, Allen NJ, Susman MW, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frolova EG, Drazba J, Krukovets I, Kostenko V, Blech L, Harry C, Vasanji A, Drumm C, Sul P, Jenniskens GJ, Plow EF, Stenina-Adognravi O. Control of organization and function of muscle and tendon by thrombospondin-4. Matrix Biol. 2014;37:35–48. doi: 10.1016/j.matbio.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho JY, Lim JY, Cheong JH, et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011;17:1850–1857. doi: 10.1158/1078-0432.CCR-10-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Errico M, de Rinaldis E, Blasi MF, Viti V, Falchetti M, Calcagnile A, Sera F, Saieva C, Ottini L, Palli D, Palombo F, Giuliani A, Dogliotti E. Genome-wide expression profile of sporadic gastric cancers with microsatellite instability. Eur J Cancer. 2009;45:461–469. doi: 10.1016/j.ejca.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 27.Singh D, Febbo PG, Ross K, Jackson DG, Manola J, Ladd C, Tamayo P, Renshaw AA, D’Amico AV, Richie JP, Lander ES, Loda M, Kantoff PW, Golub TR, Sellers WR. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell. 2002;1:203–209. doi: 10.1016/s1535-6108(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 28.Ma XJ, Wang Z, Ryan PD, et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5:607–616. doi: 10.1016/j.ccr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Curtis C, Shah SP, Chin SF, et al. METABRIC Group. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu X, Lu X, Wang ZC, Iglehart JD, Zhang X, Richardson AL. Predicting features of breast cancer with gene expression patterns. Breast Cancer Res Treat. 2008;108:191–201. doi: 10.1007/s10549-007-9596-6. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharyya S, Sul K, Krukovets I, Nestor C, Li J, Adognravi OS. Novel tissue-specific mechanism of regulation of angiogenesis and cancer growth in response to hyperglycemia. J Am Heart Assoc. 2012;1:e005967. doi: 10.1161/JAHA.112.005967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharyya S, Marinic TE, Krukovets I, Hoppe G, Stenina OI. Cell type-specific post-transcriptional regulation of production of the potent antiangiogenic and proatherogenic protein thrombospondin-1 by high glucose. J Biol Chem. 2008;283:5699–5707. doi: 10.1074/jbc.M706435200. [DOI] [PubMed] [Google Scholar]
- 33.Stenina OI, Ustinov V, Krukovets I, Marinic T, Topol EJ, Plow EF. Polymorphisms A387P in thrombospondin-4 and N700S in thrombospondin-1 perturb calcium binding sites. FASEB J. 2005;19:1893–1895. doi: 10.1096/fj.05-3712fje. [DOI] [PubMed] [Google Scholar]
- 34.Topol EJ, McCarthy J, Gabriel S, Moliterno DJ, Rogers WJ, Newby LK, Freedman M, Metivier J, Cannata R, O’Donnell CJ, Kottke-Marchant K, Murugesan G, Plow EF, Stenina O, Daley GQ. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation. 2001;104:2641–2644. doi: 10.1161/hc4701.100910. [DOI] [PubMed] [Google Scholar]
- 35.Thompson WD, Harvey JA, Kazmi MA, Stout AJ. Fibrinolysis and angiogenesis in wound healing. J Pathol. 1991;165:311–318. doi: 10.1002/path.1711650406. [DOI] [PubMed] [Google Scholar]
- 36.Matias MA, Saunus JM, Ivanovski S, Walsh LJ, Farah CS. Accelerated wound healing phenotype in Interleukin 12/23 deficient mice. J Inflamm (Lond) 2011;8:39. doi: 10.1186/1476-9255-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107–114. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- 38.Mahabeleshwar GH, Somanath PR, Byzova TV. Methods for isolation of endothelial and smooth muscle cells and in vitro proliferation assays. Methods Mol Med. 2006;129:197–208. doi: 10.1385/1-59745-213-0:197. [DOI] [PubMed] [Google Scholar]
- 39.Soloviev DA, Pluskota E, Plow EF. Cell adhesion and migration assays. Methods Mol Med. 2006;129:267–278. doi: 10.1385/1-59745-213-0:267. [DOI] [PubMed] [Google Scholar]
- 40.Chatila K, Ren G, Xia Y, Huebener P, Bujak M, Frangogiannis NG. The role of the thrombospondins in healing myocardial infarcts. Cardiovasc Hematol Agents Med Chem. 2007;5:21–27. doi: 10.2174/187152507779315813. [DOI] [PubMed] [Google Scholar]
- 41.Sezaki S, Hirohata S, Iwabu A, Nakamura K, Toeda K, Miyoshi T, Yamawaki H, Demircan K, Kusachi S, Shiratori Y, Ninomiya Y. Thrombospondin-1 is induced in rat myocardial infarction and its induction is accelerated by ischemia/reperfusion. Exp Biol Med (Maywood) 2005;230:621–630. doi: 10.1177/153537020523000904. [DOI] [PubMed] [Google Scholar]
- 42.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 43.Schroen B, Heymans S, Sharma U, et al. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res. 2004;95:515–522. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- 44.Qian X, Wang TN, Rothman VL, Nicosia RF, Tuszynski GP. Thrombospondin-1 modulates angiogenesis in vitro by up-regulation of matrix metalloproteinase-9 in endothelial cells. Exp Cell Res. 1997;235:403–412. doi: 10.1006/excr.1997.3681. [DOI] [PubMed] [Google Scholar]
- 45.Jiménez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong LC, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 2003;22:63–71. doi: 10.1016/s0945-053x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 47.Mustonen E, Aro J, Puhakka J, Ilves M, Soini Y, Leskinen H, Ruskoaho H, Rysä J. Thrombospondin-4 expression is rapidly upregulated by cardiac overload. Biochem Biophys Res Commun. 2008;373:186–191. doi: 10.1016/j.bbrc.2008.05.164. [DOI] [PubMed] [Google Scholar]
- 48.Mustonen E, Ruskoaho H, Rysä J. Thrombospondins, potential drug targets for cardiovascular diseases. Basic Clin Pharmacol Toxicol. 2013;112:4–12. doi: 10.1111/bcpt.12026. [DOI] [PubMed] [Google Scholar]
- 49.Yamada Y, Izawa H, Ichihara S, Takatsu F, Ishihara H, Hirayama H, Sone T, Tanaka M, Yokota M. Prediction of the risk of myocardial infarction from polymorphisms in candidate genes. N Engl J Med. 2002;347:1916–1923. doi: 10.1056/NEJMoa021445. [DOI] [PubMed] [Google Scholar]
- 50.Wessel J, Topol EJ, Ji M, Meyer J, McCarthy JJ. Replication of the association between the thrombospondin-4 A387P polymorphism and myocardial infarction. Am Heart J. 2004;147:905–909. doi: 10.1016/j.ahj.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 51.Cui J, Randell E, Renouf J, Sun G, Han FY, Younghusband B, Xie YG. Gender dependent association of thrombospondin-4 A387P polymorphism with myocardial infarction. Arterioscler Thromb Vasc Biol. 2004;24:e183–e184. doi: 10.1161/01.ATV.0000147304.67100.ee. [DOI] [PubMed] [Google Scholar]
- 52.Cui J, Randell E, Renouf J, Sun G, Green R, Han FY, Xie YG. Thrombospondin-4 1186G>C (A387P) is a sex-dependent risk factor for myocardial infarction: a large replication study with increased sample size from the same population. Am Heart J. 2006;152:543 e1–543.e5. doi: 10.1016/j.ahj.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 53.McCarthy JJ, Parker A, Salem R, Moliterno DJ, Wang Q, Plow EF, Rao S, Shen G, Rogers WJ, Newby LK, Cannata R, Glatt K, Topol EJ GeneQuest Investigators. Large scale association analysis for identification of genes underlying premature coronary heart disease: cumulative perspective from analysis of 111 candidate genes. J Med Genet. 2004;41:334–341. doi: 10.1136/jmg.2003.016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Subramanian A, Wayburn B, Bunch T, Volk T. Thrombospondin-mediated adhesion is essential for the formation of the myotendinous junction in Drosophila. Development. 2007;134:1269–1278. doi: 10.1242/dev.000406. [DOI] [PubMed] [Google Scholar]
- 55.Hecht JT, Hayes E, Snuggs M, Decker G, Montufar-Solis D, Doege K, Mwalle F, Poole R, Stevens J, Duke PJ. Calreticulin, PDI, Grp94 and BiP chaperone proteins are associated with retained COMP in pseudoachon-droplasia chondrocytes. Matrix Biol. 2001;20:251–262. doi: 10.1016/s0945-053x(01)00136-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.