Abstract
Background
Vitamin D deficiency is common in cystic fibrosis (CF), but there is no previous data on free 25-hydroxyvitamin D 25(OH)D in CF or in relation to healthy individuals.
Methods
We assessed total serum 25(OH)D concentration by chemiluminescence and serum free 25(OH)D concentration by both direct measurement (ELISA) and calculation, using serum albumin and vitamin D binding protein (VDBP) levels in 80 subjects (28 healthy adults, 25 clinically stable adults and children with CF and 27 adults experiencing a CF exacerbation).
Results
Serum albumin and VDBP concentrations were lower in CF compared to healthy controls. Total serum 25(OH)D concentrations were positively correlated with both calculated and measured free 25(OH)D (p<0.001 for both). Calculated and directly measured serum free 25(OH)D levels were positively correlated (p<0.001)
Conclusions
Serum levels of directly measured free 25(OH)D positively correlated with total 25(OH)D, suggesting that achieving sufficient total serum 25(OH)D may result in adequate free 25(OH)D levels in CF.
Keywords: Free 25-hydroxyvitamin D, vitamin D, vitamin D binding protein, cystic fibrosis
Introduction
The efficacy of vitamin D supplementation is typically determined by measuring total serum 25-hydroxyvitamin D (25(OH)D) concentration, the major circulating form of vitamin D and marker of vitamin D status (1). However, only a small amount of total serum 25(OH)D (10-15% loosely bound to albumin and <1% free in circulation) is considered bioavailable (2). In contrast, 85-90% of circulating 25(OH)D has limited access to target tissues since it is tightly bound by vitamin D binding protein (VDBP) (3), a protein that increases the half-life of 25(OH)D in circulation and aids in the reabsorption of vitamin D filtered by the kidney (4, 5). VDBP binding affinity and concentration are different between races and disease processes (6, 7), thus potentially altering the action of vitamin D at the target cell in certain populations (8, 9). For example, differences in VDBP binding affinity to 25(OH)D are primarily the result of 2 single nucleotide polymorphisms in the globulin-complex (Gc) gene which codes for VDBP (10, 11); certain variants have higher binding affinity to vitamin D than others and may prolong the half-life of vitamin D in circulation (12). In addition, certain protein-catabolic diseases, such as cystic fibrosis (CF) (13) and chronic kidney disease (CKD) (13), and possibly different races (for example, people with African American ancestry (9)), exhibit low VDBP concentrations.
Based on the potential impact of genetic variations in VDBP on total 25(OH)D concentrations, measurements of serum free or bioavailable 25(OH)D may be a better indicator of vitamin D status than total serum 25(OH)D concentration. For example, in VDBP-ablated mice, free 25(OH)D was within normal limits in spite of very low serum concentrations of total 25(OH)D and 1,25(OH)2D (4). In some cross-sectional studies, bioavailable serum 25(OH)D concentrations significantly correlated with bone mineral density (14, 15), parathyroid hormone (PTH) levels (2) and calcium concentration (2) while total 25(OH)D did not (2, 14, 15). Given the high prevalence of vitamin D deficiency in CF (16), an accurate assessment of vitamin D status is crucial in evaluating the need and response to vitamin D supplementation. During periods of pulmonary exacerbation, nutritional challenges are further intensified due to the impairment of protein synthesis and metabolism (17), which may also affect albumin and VDBP levels as well as free vitamin D concentrations. In order to address these nutritional challenges in CF and guide nutritional interventions during both clinically stable periods of CF and pulmonary exacerbations, endogenous vitamin D status must be more accurately assessed.
Past attempts to determine free serum 25(OH)D concentration have relied on equations in which serum concentrations of total 25(OH)D, albumin and VDBP are multiplied by binding affinity constants (18, 19). However, these equations, which were created using healthy adults, do not adjust for potential variations in VDBP affinity and thus may overestimate the directly measured free 25(OH)D concentrations in certain populations (9). Recently, assays have been developed to directly measure free vitamin D. Here, we compared measured and calculated free 25(OH)D levels in healthy adults and in clinically stable adults and children with CF and in a CF cohort studied during pulmonary exacerbation. The primary aims were to assess the relationship between total 25(OH)D and free 25(OH)D concentrations, to determine the relative accuracy of calculated free 25(OH)D compared to directly measured free 25(OH)D, and to determine whether these relationships were altered in CF (clinically stable versus during acute pulmonary exacerbation).
Materials and Methods
Study Population
This was a cross-sectional study of 28 healthy controls, 25 clinically stable patients with CF and 27 CF patients during pulmonary exacerbation. Serum samples were obtained from specimens banked during two randomized, double-blind, placebo-controlled trials and one observational study. Samples from the trials (healthy controls and individuals with CF exacerbation) were drawn at baseline, before participants received any intervention, while samples from the observational study were drawn at the patient's annual exam in CF clinic. Study populations were recruited in Atlanta, Georgia and other than CF, study subjects were well-controlled for medications that affect vitamin D concentration or metabolism. Habitual daily vitamin D intake was limited to <1000 IU and <2000 IU per day in the healthy control group and the CF pulmonary exacerbation group, respectively, while the clinically stable CF group had no such limit to daily vitamin D supplementation. Specific details, including the inclusion and exclusion criteria from the study on healthy adults can be found as published in Kearns et al (20). Inclusion criteria for the clinically stable CF group included a clinical diagnosis of CF, primary ciliary dyskinesia, bronchiectasis or CF transmembrane conductance regulator-related metabolic syndrome, and a current patient at any of the adult or pediatric CF clinics at Emory University. Exclusion criteria included history of HIV, and hepatitis B surface antigen or hepatitis C antibody. Inclusion criteria for the CF pulmonary exacerbation group included age ≥ 16 years of age, admission for pulmonary exacerbation within 72 hours and ability to tolerate oral medications. Exclusion criteria were as follows: serum 25(OH)D > 55 ng/mL or <10 ng/mL, pregnancy, parathyroid disease, hypercalcemia, history of nephrolithiasis, chronic kidney disease worse than stage 3, use of oral or intravenous glucocorticoids, lung transplantation, percentage of forced expiratory volume in 1 second (FEV1%) <20%, significant hepatic dysfunction, use of cytotoxic or immunosuppressive drugs, history of AIDS, history of illicit drug abuse, and enrollment in a conflicting study. All three studies were approved by the Emory University Institutional Review Board and both randomized controlled trials were registered at clinicaltrials.gov (NCT01924910 and NCT01426256). All of the included participants gave written consent for additional testing on their banked serum or plasma.
Direct measurement of free vitamin D and other biomarkers
Direct measurement of free 25(OH)D concentrations was performed by immunoassay (Catalog number KARF1991, DiaSource, Louvain-la-Nueve, Belgium), which detects free vitamin D by ELISA on a microtiter plate. The intra assay CV of these measurements were 7.1%, 10.7% for each plate and the inter assay CV was 8.9%, respectively.
Total serum 25(OH)D was measured using a chemiluminescent assay (Catalog Number IS-2700S, IDS-iSYS, Immunodiagnostic Systems, Scottsdale, AZ) on an automated Immunoassay system (IDS-iSYS) in a Vitamin D External Quality Assessment Scheme (DEQAS) certified laboratory. The machine was calibrated using manufacturer controls of known concentration levels; the samples were also run with internal house controls as a quality measure. Total serum 25(OH)D concentrations were used to determine vitamin D status, classified as vitamin D sufficiency (defined as serum 25(OH)D ≥30 ng/mL) and insufficiency (defined as serum 25(OH)D <30 ng/mL) as well as deficiency (serum 25(OH)D <20 ng/mL) and insufficiency/sufficiency (serum 25(OH)D ≥20 ng/mL).
Serum albumin was directly measured in the 28 healthy controls and 25 clinically stable CF samples using an albumin human ELISA kit (Catalog Number Ab108787, Abcam, Cambridge, MA). Serum albumin levels from adults with CF recruited during a pulmonary exacerbation were obtained from patient records at Emory University Hospital with albumin measurements captured ± 3 days from the date of the baseline blood draw. VDBP was measured in all samples using a polyclonal ELISA Kit (Catalog number 30-2314, ALPCO, Salem, NH).
Calculation of Free vitamin D
Previous studies have used the equation as published by Bikle et al (18), which uses serum albumin, VDBP concentrations and total serum 25(OH)D to yield estimates of free 25(OH)D. This equation has been validated in healthy and cirrhotic individuals (18, 21). In this study, we used the formula as shown in Bikle et al (18) based on its simplicity.
Statistics
Descriptive statistics were performed for demographic and clinical characteristics of the study subjects. Continuous variables are presented as mean ± SD for normally distributed variables and median (interquartile range) for non-normally distributed variables. Normally distributed variables were compared across groups using ANOVA while non-normally distributed variables were compared using a Kruskal-Wallis test. Categorical variables are presented as percentages and compared across groups using Fisher's exact test or chi-square test, as appropriate. Multiple comparison testing was done using Tukey's Studentized test on normally distributed continuous variables, Steel-Dwass test for non-normally distributed variables, and a Bonferroni adjustment was made to the pairwise comparisons for categorical variables. Vitamin D measurements within vitamin D status categories were compared with two sample t-tests or a Wilcoxon-Mann-Whitney test (as indicated by normality of the distributions). Multivariable linear regression was used to test the association between measured and calculated free 25(OH)D, measured free and total 25(OH)D, and calculated free and total 25(OH)D while including age, sex, race, BMI, and vitamin D supplementation as a priori covariates. Calculated free 25(OH)D levels and daily amount of vitamin D supplementation were log transformed for regression analyses. All tests were two-sided with a p-value of 0.05 considered statistically significant. Analyses were performed using SAS v. 9.4 (SAS Institute, Cary, NC) and JMP (SAS Institute, Cary, NC).
Results
Demographics
Demographics and clinical characteristics for the three cohorts are shown in Table 1. The three groups were significantly different in all demographic characteristics (e.g. age, % African American, % pancreatic insufficiency, BMI; p<0.05 for all). The daily amount of vitamin D supplementation in subjects habitually taking supplements was similar (Table 1). The majority of participants in all groups were Caucasian. The two CF cohorts had similar proportions of males and females; the healthy control group was predominately female. The healthy control group and the CF exacerbation group were similar in age (mid-twenties to mid-thirties) and percentage of subjects taking daily vitamin D supplementation (18 and 22%, respectively). The clinically stable CF group consisted of younger individuals and had the highest proportion taking vitamin D supplements.
Table 1.
Demographic information and biochemical measures of vitamin D status in subjects with cystic fibrosis and healthy controls
| Healthy Controls | Clinically Stable CF | CF Exacerbation | p-valued | |
|---|---|---|---|---|
| Season of recruitment | Oct-Dec | Mar - Oct | All year | |
| n | 28 | 25 | 27 | |
| Age, y | 29 ± 6.0a | 18.5 ± 14.0b | 27.6 ± 4.9a | <0.001 |
| Male [n(%)] | 6 (21)a | 14 (56)b | 15 (56)b | 0.013 |
| African American [n(%)] | 4 (15)a | 2 (8)a | 2 (8)a | 0.023e |
| BMI (kg/m2) | 22.8 ± 2.6a | 20.1 ± 4.4b | 20.9 ± 3.5b | 0.020 |
| Pancreatic Insufficient [n(%)] | 0 (0)a | 22 (88)b | 23 (85)b | <0.001 |
| Receiving Vitamin D supplements [n(%)] | 5 (18)a | 12 (48)a | 6 (22)a | 0.035e |
| Daily amount of Vitamin D [median (IQR)] | 1000 (500) | 2000 (4272) | 2000 (1000) | 0.073 |
| Albumin (g/dL) | 5.1 ± 0.7a | 4.2 ± 1.7b | 3.4 ± 0.4c | <0.001 |
| Albumin Corrected Calcium (mg/dL) | n/a | n/a | 9.3 ± 0.3 | n/a |
| Vitamin D Binding Protein (mg/dL) | 32.8 ± 10.1a | 19.1 ± 4.3b | 22.1 ± 7.1b | <0.001 |
| Total 25(OH)D (ng/mL) | 17.5 ± 6.2a | 35.5 ± 9.8b | 26 ± 11.0c | <0.001 |
| Total 25(OH)D <30 (ng/mL) n(%) | 26 (93)a | 7 (29)b | 20 (77)a | <0.0001 |
| Total 25(OH)D <20 (ng/mL) n(%) | 21 (75)a | 2 (8)b | 8 (31)b | <0.0001 |
| Measured free 25(OH)D (pg/mL) | 4.7 ± 1.4a,b | 5.9 ± 1.a | 4.6 ± 1.8b | 0.0217 |
| Calculated free 25(OH)D (pg/mL)* | 4.1 (1.9)a | 13.8 (8.4)b | 9.4 (7.6)c | <0.001 |
For each variable, groups not connected by the same letter are significantly different.
For each variable, groups not connected by the same letter are significantly different.
For each variable, groups not connected by the same letter are significantly different.
ANOVA for normally distributed continuous variables, Kruskal-Wallis test for non-normally distributed continuous variables, and Fisher's exact or Chi-squared test for cateogorial variables, with Tukey's Student's T-test, Steel Dwass test, and Bonferroni adjustment, respectively, for multiple comparisons.
Groups differ significantly overall, but pairwise comparisons were not significant after Bonferroni correction for multiple comparisons.
Vitamin D Binding Protein, Albumin and Total 25(OH)D
Differences in concentrations of serum albumin, VDBP, total, directly measured and calculated 25(OH)D concentrations are shown by group in Table 1. Total 25(OH)D levels differed significantly between all groups (ANOVA p<0.05), with total serum 25(OH)D concentration highest in the clinically stable CF cohort (35.5 ± 9.8 ng/mL, with 71% of participants vitamin D sufficient with 25(OH)D concentration ≥30 ng/mL). In contrast, the healthy control group had the lowest total 25(OH)D concentrations (17.5 ± 6.2 ng/mL). In spite of similar rates of vitamin D supplementation as healthy controls, CF exacerbation patients had a significantly greater mean serum total 25(OH)D concentration (26 ± 11.0 ng/mL, with 23% of participants with 25(OH)D concentrations ≥ 30 ng/mL). Albumin and VDBP concentrations were highest in the healthy control group and lower in both groups with CF (ANOVA p <0.001 for both)
Impact of Vitamin D Sufficiency on Free 25(OH)D
As shown in Table 2, Examining the three groups based on vitamin D status (serum 25(OH)D ≥ 30 ng/ml vs. serum 25(OH)D < 30 ng/ml), the healthy control group showed no difference in calculated or measured free 25(OH)D between the two sub-groups while both CF groups showed a significant difference in measured free 25(OH)D between the vitamin D sufficient and insufficient sub-groups, with sufficient groups having a greater concentration of free serum 25(OH)D. However, when using a serum 25(OH)D cut-off of 20 ng/mL concentration to define vitamin D status, all three groups showed significant differences in measured free 25(OH)D and calculated free 25(OH)D.
Table 2.
Comparison of free vitamin D measures based on vitamin D insufficiency vs. sufficiency
| Healthy Controls | Total 25(OH)D <30 | Total 25(OH)D ≥30 | p-value |
|---|---|---|---|
| n | 26 | 2 | |
| Total 25(OH)D (ng/ml) | 16.5 ± 5.2 | 30.5 ± 0.3 | 0.001 |
| Calculated Free (pg/ml)a | 4.0 (2.0) | 4.9 (0.5) | 0.560b |
| Measured Free (pg/ml) | 4.7 ± 1.5 | 5 ± 1.1 | 0.795 |
| Clinically Stable CF | Total 25(OH)D <30 | Total 25(OH)D >30 | p-value |
|---|---|---|---|
| n | 7 | 17 | |
| Total 25(OH)D (ng/ml) | 24 ± 5.1 | 40.2 ± 6.9 | <0.001 |
| Calculated Free (pg/ml)a | 10.5 (5.1) | 18.2 (7.8) | 0.008b |
| Measured Free (pg/ml) | 4.4 ± 1.2 | 6.6 ± 1.8 | 0.009 |
| CF Exacerbation | Total 25(OH)D <30 | Total 25(OH)D >30 | p-value |
|---|---|---|---|
| n | 20 | 6 | |
| Total 25(OH)D (ng/ml) | 21.3 ± 6.0 | 41.5 ± 9.6 | <0.001 |
| Calculated Free (pg/ml)a | 8.2 (4.6) | 16.5 (4.1) | 0.001b |
| Measured Free (pg/ml) | 3.8 ± 1.1 | 7.4 ± 1.0 | <0.001 |
median(IQR) for non-normally distributed variables
Wilcoxon-Mann-Whitney test
* two sample independent t-test
Calculated and Measured Free Vitamin D
Calculated serum concentrations of free 25(OH)D differed significantly across all groups (Table 1).The calculated free vitamin D measurements overestimated free vitamin D levels in all 3 groups with the largest difference in means in the clinically stable CF group. Both calculated free 25(OH)D and directly measured values were significantly positively associated with total 25(OH)D in both CF populations, after controlling for the following variables; age, sex, race, BMI and vitamin D supplementation. In the healthy control group, calculated free 25(OH)D but not measured free 25(OH)D, was associated with total serum 25(OH)D. In a sensitivity analysis, there were no differences in mean total 25(OH)D and free 25(OH)D concentrations in younger (<16 years) and older (>16 years) subjects with CF.
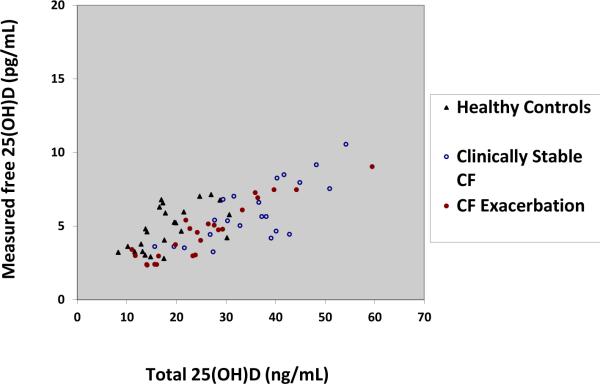
Overall, total serum 25(OH)D was significantly and positively associated with both calculated free 25(OH)D and measured free 25(OH)D, independent of study group. The only other variable to significantly associate with measures of free 25(OH)D was group assignment. Figure 1 is a graphical representation of the raw values of total serum 25(OH)D and measured free 25(OH)D in non African Americans(beta=0.14, p<0.001).There were only 8 subjects who were African-American and thus were excluded from the figure.
Figure 1. Total 25(OH)D vs. Measured free 25(OH)D in non African Americans with and without cystic fibrosis.
Total 25(OH)D concentrations are plotted on the x-axis and directly measured free 25(OH)D concentrations are plotted on the y-axis. Both variables were normally distributed. The closed triangles represent non-African healthy control subjects, the open circles represent non-African clinically stable CF subjects and the closed circles represent non-African CF exacerbation subjects. Total 25(OH)D concentrations were significantly associated with direct measures of free 25(OH)D with an adjusted β-coefficient of 0.14 (p<0.001).
Discussion
In this study, we compared different measures of serum 25(OH)D concentrations (total 25(OH)D, calculated free 25(OH)D and directly measured free 25(OH)D) in addition to concentrations of proteins that bind vitamin D in circulation (albumin and VDBP) between individuals from three populations: healthy adults, adults and children with clinically stable CF and adults with CF admitted to the hospital with a pulmonary exacerbation. All measures of 25(OH)D (total, free and calculated) were significantly associated with each other while controlling for group assignment, age, sex, BMI and vitamin D supplementation. Both CF groups exhibited lower levels of albumin and VDBP than healthy controls, likely due to decreased synthesis of these proteins associated with increased chronic inflammation and intermittent pulmonary infection, coupled with increased protein catabolism in the individuals with CF.
In the individual groups, total 25(OH)D was positively and significantly associated with directly measured free 25(OH)D before and after controlling for age, race, sex and BMI with the exception of the healthy control group. In this group, total 25(OH)D was not significantly associated with measured free after controlling for the aforementioned variables. In our healthy control group, the average total 25(OH)D concentration was <30 ng/mL with only 2 subjects >30 ng/mL. Limited studies have measured these vitamin D metabolites together in healthy populations and most healthy groups studied have a total 25(OH)D level <30 ng/mL similar to our group. One study showed a significant, direct positive relationship between total 25(OH)D and measured free 25(OH)D in the healthy comparator group (9). The healthy controls in this group, however also had a sub-optimal total 25(OH)D concentration (26.2 ± 11.4 ng/mL) (9). Another study also showed significant positive relationships between total 25(OH)D and free 25(OH)D in otherwise healthy postmenopausal black and white women (22). However, the mean total overall 25(OH)D in this group was also below 30 ng/mL (23.26 ± 6.71 ng/mL) (22).
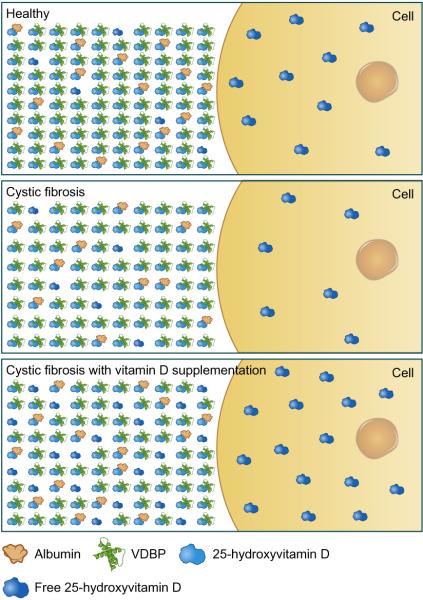
However, the two groups with CF had the highest total serum 25(OH)D concentration, likely due to the emphasis on vitamin D supplementation as part of medical management of CF, as both CF groups had twice the median daily intake of vitamin D than the healthy controls. The total serum 25(OH)D concentration strongly positively correlated with free serum 25(OH)D concentrations in CF populations suggesting that supplementing patients with CF to achieve serum 25(OH)D concentrations ≥ 30 ng/mL) may result in a parallel increase in free 25(OH)D, though the exact concentration of free vitamin D necessary to confer optimal health outcomes in CF is unknown. This suggests that a sufficient total 25(OH)D level may correlate well with free vitamin D levels thereby decreasing the potential need to directly measure free 25(OH)D. Our data suggest that total serum 25(OH)D concentrations in CF may be an adequate indicator of vitamin D status and bioavailability and that despite lower levels of VDBP in CF, vitamin D supplementation may appropriately increase the fraction of free vitamin D in many individuals (Figure 2)
Figure 2. Total and Free 25-hydroxyvitamin D in circulation.
The majority of circulating 25-hydroxyvitamin D is bound to vitamin D binding protein (VDBP) and albumin with <1% in a free or unbound form (figure not drawn to scale). The free 25(OH)D can enter cells by diffusion or facilitated transport in healthy individuals (top panel). In CF, there are lower levels of both albumin and VDBP and thus lower concentrations of total serum 25(OH)D. Lower total 25(OH)D concentrations results in less free 25(OH)D transport into the cell (middle panel). However, vitamin D supplementation in CF may adequately increase the amount of circulating free 25(OH)D and thus increase the amount of free 25(OH)D entering the cell (bottom panel).
According to the free hormone hypothesis, the biological activity of a hormone is dependent on the unbound, bioavailable form of hormone rather than the protein-bound concentration (3). This hypothesis has been proposed for steroid hormones such as vitamin D, which is lipophilic and largely protein-bound. These properties require 4 different mechanisms to transport 25(OH)D into the cell where it is converted into its biologically active form (1,25-dihydroxyvitamin D) by the enzyme CYP27B1. Two mechanisms transport the VDBP-bound 25(OH)D (megalin mediated endocytotic uptake of VDBP and receptor-facilitated diffusion) while 2 mechanisms transport free, unbound vitamin D (passive diffusion and megalin-facilitated diffusion) (3). In a majority of extra-renal cells, which do not express megalin or similar VDBP-25(OH)D complex transporters, free 25(OH)D may be the major method of transport into the cell (3). Thus measures of free 25(OH)D may be more reflective of the bioavailable vitamin D involved in extra-renal synthesis.
Although our data suggest that calculated estimates of free serum 25(OH)D are representative of directly measured free 25(OH)D in our healthy control cohort, a group in which these equations were validated, these calculated estimates appear to vary considerably from directly measured free 25(OH)D levels in individuals with CF. Calculated estimates may be an alternative to measuring total 25(OH)D during acute illness as total 25(OH)D has been shown to vary considerably hour by hour in acute illness (23). In addition, it has also been shown that critically ill patients have a large reduction in VDBP and albumin which may make calculated free 25(OH)D a more accurate biomarker of vitamin D status than total 25(OH)D (24). Similarly, in chronic illness, poor protein nutritional status may result in lower levels of VDBP and albumin. However, in both acute and chronic illness, calculated estimates may differ considerably from measured free 25(OH)D levels in part due to the variability of VDBP binding affinity for 25(OH)D, which is not taken into account in current equations which assume a single VDBP affinity constant (7 × 108 M−1) (11,12). In addition, various genetic polymorphisms may also contribute to varying levels of VDBP, which, in one study, appeared to independently explain 79.4% and 9.9% of the variation in levels of VDBP and total 25(OH)D (25). This same study also demonstrated that black Americans had lower levels of VDBP compared to white Americans suggesting that VDBP concentrations may vary based on ethnic differences (25). In another study comparing measured and calculated free 25(OH)D in different clinical populations, a post-hoc analysis was used to adjust the binding coefficient based on Gc frequencies in race which improved, but did not entirely rectify previous estimates (9). It is important to note, however, that the initial paper investigating VDBP in black and white Americans used a monoclonal VDBP assay (rather than a polyclonal VDBP assay), which only recognizes one epitope on an antigen. This monoclonal antibody primarily binds to the Gc1S variant of VDBP, and not the Gc1F variant primarily found in black people, possibly contributing to their results (26). A recent study showed that VDBP levels were much higher using a polyclonal antibody compared to a monoclonal antibody as the monoclonal ELISA is very specific and less able to recognize the various protein isoforms of VDBP (22).
This is the first study, to our knowledge, to explore bioavailable 25(OH)D in CF. Strengths of this study include having multiple populations, including healthy adults with few confounding variables and the two CF cohorts (clinically stable versus those with concurrent pulmonary exacerbation). The study was limited by: small sample sizes, having populations with significantly different characteristics (e.g., age, sex, race, BMI, pancreatic insufficiency) and variations in vitamin D supplementation in each population. Another limitation included estimates of free 25(OH)D based solely on the Bilke et al (18) method and not the modification of the Vermuelen method (17) (an equation originally intended to estimate free testosterone) although both methods use identical binding affinity constants to adjust serum albumin and VDBP concentrations and yield nearly identical results (2).
In conclusion, we found a strong correlation between total 25(OH)D and free 25(OH)D in CF, suggesting that vitamin D supplementation that increases total 25(OH)D levels into a range considered sufficient will also significantly increase free 25(OH)D. As of yet, the exact utility of determining free serum 25(OH)D is not entirely clear. While using free 25(OH)D may be a fairly good indicator of vitamin D status, particularly in individuals with CF as shown in our study, it may be unnecessary to employ this new and expensive test in order to most accurately assess vitamin D status. Further investigation is necessary to assess whether measuring free serum 25(OH)D, which is best correlated with several markers of vitamin D sufficiency and bone health, is useful in monitoring the vitamin D status of individuals with CF.
Aknowledgements
This work was supported in part by National Institutes of Health grants T32 DK007734 (ES), K24 DK096574 (TRZ), T32 DK007298-32S1 and K01 DK102851 (JAA), UL1 TR000454 (Atlanta Clinical and Translational Science Institute), and McCart145R0 (Biobank of CF specimens)
Footnotes
Conflict of Interest: The authors have no conflicts of interest to report.
Author Contributions:
ML, MDK, TRZ, JAA and VT were responsible for the design of the study; ML, MDK, and JAA recruited subjects and coordinated the study; ML, MDK, LH were responsible for data collection; ML, EMS, VT were responsible for data analysis; ML, MDK wrote the draft of the manuscript; and all authors contributed to revisions of the manuscript.
References
- 1.Tangpricha V, Kelly A, Stephenson A, Maguiness K, Enders J, Robinson KA, Marshall BC, Borowitz D. Cystic Fibrosis Foundation Vitamin D Evidence-Based Review Committee. An update on the screening, diagnosis, management, and treatment of vitamin D deficiency in individuals with cystic fibrosis: evidence-based recommendations from the Cystic Fibrosis Foundation. J Clin Endocrinol Metab. 2012 Apr;97(4):1082–93. doi: 10.1210/jc.2011-3050. [DOI] [PubMed] [Google Scholar]
- 2.Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney international. 2012;82(1):84–9. doi: 10.1038/ki.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: The free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2013 doi: 10.1016/j.jsbmb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103(2):239–51. doi: 10.1172/JCI5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96(4):507–15. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 6.Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93(9):3381–8. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Constans J, Hazout S, Garruto RM, Gajdusek DC, Spees EK. Population distribution of the human vitamin D binding protein: anthropological considerations. Am J Phys Anthropol. 1985;68(1):107–22. doi: 10.1002/ajpa.1330680110. [DOI] [PubMed] [Google Scholar]
- 8.Berg JP. Vitamin D-binding protein prevents vitamin D deficiency and presents vitamin D for its renal activation. European journal of endocrinology / European Federation of Endocrine Societies. 1999;141(4):321–2. doi: 10.1530/eje.0.1410321. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz JB, Lai J, Lizaola B, Kane L, Markova S, Weyland P, et al. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99(5):1631–7. doi: 10.1210/jc.2013-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li F, Jiang L, Willis-Owen SA, Zhang Y, Gao J. Vitamin D binding protein variants associate with asthma susceptibility in the Chinese Han population. BMC medical genetics. 2011;12:103. doi: 10.1186/1471-2350-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Human genetics. 1993;92(2):183–8. doi: 10.1007/BF00219689. [DOI] [PubMed] [Google Scholar]
- 12.Lauridsen AL, Vestergaard P, Hermann AP, Brot C, Heickendorff L, Mosekilde L, et al. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcified tissue international. 2005;77(1):15–22. doi: 10.1007/s00223-004-0227-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26(7):1609–16. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnsen MS, Grimnes G, Figenschau Y, Torjesen PA, Almas B, Jorde R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scandinavian journal of clinical and laboratory investigation. 2014;74(3):177–83. doi: 10.3109/00365513.2013.869701. [DOI] [PubMed] [Google Scholar]
- 16.Wolfenden LL, Judd SE, Shah R, Sanyal R, Ziegler TR, Tangpricha V. Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol (Oxf) 2008 Sep;69(3):374–81. doi: 10.1111/j.1365-2265.2008.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shepherd RW, Holt TL, Cleghom G, Ward LC, Isles A, Francis P. Short-term nutritional supplementation during management of pulmonary exacerbations in cystic fibrosis: a controlled study, including effects of protein turnover. Am J Clin Nutr. 1988;48(2):235–9. doi: 10.1093/ajcn/48.2.235. [DOI] [PubMed] [Google Scholar]
- 18.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63(4):954–9. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 19.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 20.Kearns MD, Binongo JN, Watson D, Alvarez JA, Lodin D, Ziegler TR, et al. The effect of a single, large bolus of vitamin D in healthy adults over the winter and following year: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2015;69(2):193–7. doi: 10.1038/ejcn.2014.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985;61(5):969–75. doi: 10.1210/jcem-61-5-969. [DOI] [PubMed] [Google Scholar]
- 22.Aloia J, Mikhail M, Dhaliwal R, Shieh A, Usera G, Stolberg A, et al. Free 25(OH)D and the vitamin D paradox in african americans. J Clin Endocrinol Metab. 2015:JC20152066. doi: 10.1210/JC.2015-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatesh B, Davidson B, Robinson K, Pascoe R, Appleton C, Jones M. Do random estimations of vitamin D3 and parathyroid hormone reflect the 24-h profile in the critically ill? Intensive Care Med. 2012;38:177–179. doi: 10.1007/s00134-011-2415-x. [DOI] [PubMed] [Google Scholar]
- 24.Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, et al. Alterations in vtiamin D status and antimicrobial peptide levels in patients in the intesive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D-binding protein and vitamin D status of black americans and white americans. N Engl J Med. 2013;369.21:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollis BW, Bikle DB. [letter to the editor] Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370.9.:879–880. doi: 10.1056/NEJMc1315850#SA4. [DOI] [PMC free article] [PubMed] [Google Scholar]