Synopsis
When approaching the patient with suspected motor neuron disease (MND) the pattern of weakness on exam helps distinguish MND from other diseases of peripheral nerves, the neuromuscular junction, or muscle. MND is a clinical diagnosis supported by findings on electrodiagnostic testing, in the absence of other abnormalities on neuroimaging or serological testing. MNDs exist on a spectrum: from a pure lower motor neuron; to mixed upper and lower motor neuron; to a pure upper motor neuron variant in addition to regional variants restricted to the arms, legs or bulbar region. Amyotrophic lateral sclerosis (ALS) is a progressive mixed upper and lower motor neuron disorder, most commonly sporadic (~85%), which is invariably fatal. The only FDA approved treatments for ALS are riluzole, which prolongs life by about 3 months, and dextromethorphan/quinidine which provides symptomatic relief for pseudobulbar affect (inappropriate bouts of laughter or crying). Here we describe a pattern approach to identifying motor neuron disease, and clinical features of sporadic ALS.
Keywords: Motor neuron disease, Upper motor neuron, Lower motor neuron, Amyotrophic lateral sclerosis, Lou Gehrig's disease
Patterns of Weakness
Neuropathic disorders can be broadly divided into disorders affecting the peripheral nerve processes (neuropathy) or nerve cell body (neuronopathy), can be inherited or acquired, and have different clinical courses.1 Motor neuron diseases are neuronopathies. When approaching the patient with a suspected neuropathies or neuronopathies there are a number of key questions that can help further categorize the disorder:
What parts of the nervous system are involved: motor, sensory, autonomic, or combinations of more than 1 system?
Where is the weakness (proximal, distal, or both) and is it symmetric or asymmetric?
If there is sensory involvement is there pain, or proprioceptive loss?
Over what timeframe did symptoms evolve: acute (<4 weeks), subacute (4-8 weeks), or chronic?
Is there a family history of a similar disorder?
If there is motor involvement, is it upper motor neuron, lower motor neuron, or both?
The neuropathic disorders can be confusing to the clinician first encountering them but a number of key patterns of involvement can help lead to the proper diagnosis (Table 1). For full description of the neuropathic patterns, the reader is referred to “Pattern-recognition approach to neuropathy and neuronopathy.” [Barohn RJ, Amato AA. Pattern-recognition approach to neuropathy and neuronopathy. Neurol Clin. 2013 May;31(2):343-61]. Some of the myopathic patterns, including myopathy pattern 6 (MP6; neck extensor weakness) and MP7 (tongue, pharyngeal or diaphragm), that we previously described also overlap with motor neuron disease [Barohn RJ, Dimachkie MM, Jackson CE. A pattern recognition approach to patients with a suspected myopathy. Neurol Clin. 2014 Aug;32(3):569-93.].
Table 1.
Clinical Patterns of Neuropathic Disorders
| Weakness | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Prox | Dis | Asymm | Symm | Sensory Symptoms |
Severe Proprioceptive Loss |
UMN Signs |
Autonomic Symps/ Signs |
Diagnosis | |
| Pattern 1 - Symmetric prox & Distal weakness w/sensor y loss | + | + | + | + | GBS/CIDP | ||||
| Pattern 2 - Distal sensory loss with/without weakness | + | + | + | CSPN, metabolic, drugs, hereditary | |||||
| Pattern 3 - Distal weakness with sensory loss | + | + | + | Multiple – Vasculitis, HNPP, MADSAM, infection Single – Mononeuropathy, radiculopathy |
|||||
| Pattern 4 - Asymmetric prox & distal weakness w/sensory loss | + | + | + | + | Polyradiculopathy, plexopathy | ||||
| Pattern 5 - Asymmetric distal weakness w/out sensory loss | + | + | +/– | + UMN – ALS Pure UMN - PLS - UMN – MMN, PMA, MAMA, BAD, LAD | |||||
| Pattern 6 - Symmetric sensory loss & upper motor neuron signs | + | + | + | + | + | B12 defic; Copper deficiency, Friedreich's, adrenomyelone uropathy | |||
| Pattern 7* Symmetric weakness without sensory loss | +/– | + | + | Prox & Distal SMA Distal Hereditary motor neuropathy | |||||
| Pattern 8* Focal midline proximal symmetric weakness | + Neck/extensor + Bulbar |
+ + |
+ + |
ALS | |||||
| Pattern 9 - Asymmetric proprioceptive loss w/out weakness | + | + | + | Sensory neuronopathy (ganglionopathy) | |||||
| Pattern 10 - Autonomic dysfunction | + | HSAN, diabetes, GBS, amyloid, porphyria, Fabry's | |||||||
Overlap patterns with myopathy/NMJ disorders
Legend:
ALS amyotrophic lateral sclerosis
BAD brachial amyotrophic diplegia
CIDP chronic inflammatory demyelinating polyneuropathy
CSPN cryptogenic sensory polyneuropathy
GBS Guillain-Barre syndrome
HNPP hereditary neuropathy with liability to pressure palsy
HSAN hereditary sensory and autonomic neuropathy
LAD leg amyotrophic diplegia
LMN lower motor neuron
MADSAM multifocal acquired demyelinating sensory and motor
MAMA multifocal acquired motor axonopathy
MMN multifocal motor neuropathy
PMA progressive muscular atrophy
SMA spinal muscular atrophy
UMN upper motor neuron
MNDs are typically motor syndromes (sensory sparing) which show insidious onset, are chronically progressive, can be distal, proximal, or mixed, and can have different combinations of upper and lower motor neuron findings. They can be inherited or sporadic. There are a number of neuropathic patterns seen in the MNDs.
Asymmetric distal weakness without sensory loss (NP5)
If a patient comes in with asymmetric onset of distal weakness or muscle wasting and upper motor neuron findings on examination (brisk reflexes with spread to other regions, Babinski sign, Hoffman reflex, or increased tone) ALS is a main consideration. In particular if the onset was insidious and the weakness is painless. If there are only upper motor neuron findings on examination then Primary Lateral Sclerosis needs to be considered in the differential diagnosis (PLS, see chapter 6). If the patient only has lower motor neuron findings only (muscle wasting or fasciculations) then the differential is broader, and includes a motor neuron disorder like progressive muscular atrophy (PMA, see Chapter 5), acquired diseases of motor neurons, like multifocal motor neuropathy or multifocal acquired motor axonopathy, or para-infectious complications (polio-like illness). As the disease progresses, distal weakness may become symmetric, progress proximally and reflexes may be attenuated as can be seen with MP 2 or NP 7 [Barohn RJ, Dimachkie MM, Jackson CE. A pattern recognition approach to patients with a suspected myopathy. Neurol Clin. 2014 Aug;32(3):569-93.].
Symmetric weakness without sensory loss (NP7)
If weakness is proximal > distal or both then spinal muscular atrophy (SMA)/PMA are considerations. If weakness only includes upper motor neuron signs then PLS. If the weakness is distal only then other heritable conditions like distal SMA, hereditary motor neuropathy, or Charcot Marie Tooth become considerations. However, sensory loss on examination in NP7 is evident for Charcot Marie Tooth disease despite the absence of sensory symptoms but when sensory symptoms are present, it is classified as NP2.
Focal midline proximal symmetric (NP8)
If the neck and trunk are prominently involved ALS is the main consideration also known as MP6. If the onset is mostly bulbar as in MP7 then ALS, and variants that include progressive bulbar palsy, or PLS. ALS can present with isolated respiratory involvement. However care needs to be taken as there is overlap with this pattern of weakness and other MP6 and MP7 disorders of the neuromuscular junction (myasthenia gravis or the Lambert-Eaton myasthenic syndrome) or muscle (occulopharyngeal muscular dystrophy or isolated neck extensor myopathy).
MND have insidious onset, and are chronically progressive
They can be inherited or sporadic
Patterns of weakness include symmetric or asymmetric weakness without sensory loss, or focal midline proximal symmetric
[Tags: Patterns of weakness can help distinguish neuropathic disorders]
Classification of Motor Neuron Diseases
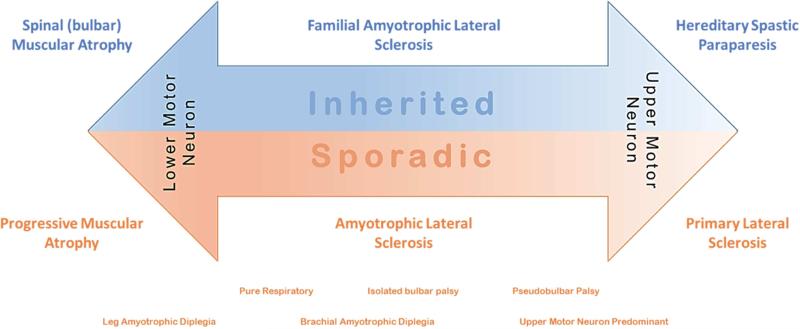
The classification of MNDs is broadly divided into heritable or sporadic, and by the degree of upper or lower motor neuron involvement. MNDs exist on a spectrum from pure lower motor neuron syndromes; to mixed upper and lower motor neuron diseases; to pure upper motor neuron diseases (Figure 1).
Figure 1.
The spectrum of motor neuron diseases
Pure lower motor neuron disease
Heritable diseases include SMA (NP7) and spinobulbar muscular atrophy (SBMA; NP8/MP7, or MP1 [proximal symmetric weakness without sensory loss or upper motor neuron signs]). SMA is characterized by progressive initially proximal weakness that can present at birth, in early childhood, or as an adult.2 SMA is loosely divided based on whether they develop the ability to walk or sit, and the vast majority are autosomal recessive due to deletion of the survival motor neuron gene. SBMA on the other hand typically presents in adulthood (3-5th decade), is characterized by prominent bulbar involvement, but can have sensory loss with decreased reflexes, and is X-linked due to CAG repeat expansion in the androgen receptor gene.3 Sporadic progressive weakness confined to lower motor neurons is termed PMA, and is more common in men, with slower disease progression than ALS (Chapter 5) and presents initially at NP5 without upper motor neuron signs followed in 40% by bulbar symptoms, but occasionally starts as MP1.4 In addition certain ALS regional variants present initially with isolated lower motor neuron involvement, including both leg (NP5 without upper motor neuron signs) and brachial (NP5 without upper motor neuron signs) amyotrophic diplegia (ALS Regional Variants, Chapter 7).
Mixed upper and lower motor neuron diseases
When the combination of both upper and lower motor neuron disease is present, and ancillary work up fails to provide an alternative explanation, this almost invariably turns out to be ALS (NP5). Both familial and sporadic ALS present in a clinically similar fashion, and cannot be distinguished by clinical history or signs alone with the exception of family history. In addition isolated respiratory involvement (NP8/MP7) can have both upper and lower motor neuron components, and isolated bulbar palsy (NP8/MP7), also presents with mixed upper and lower motor neuron findings.5
Pure upper motor neuron diseases
Both hereditary spastic paraplegias and PLS fall under NP5 with some distinctive clinical features. Patients who present with a pure upper motor neuron syndrome, with symmetric symptoms or signs largely confined to the legs, usually at an early age, with foot deformities (pes cavus or hammertoes), with or without a known family history, likely fall into the category of hereditary spastic paraplegias – these disorders can be dominant or recessive and over 60 genes have been described. Mutations in spastin are the most common dominant disorder, accounting for 30-50% families; and mutations in spatacsin are the most common recessive disorder with thinning of the corpus callosum, accounting for ~50% of cases.6, 7 PLS, on the other hand is progressive, occurs at a later age, typically in the 5-6th decade, and often involves the arms or bulbar region, in addition to the legs asymmetrically.8 In addition an upper motor neuron predominant presentation of ALS has been described.
[Tags: Motor neuron disease occurs of spectrum on involvement of upper and lower motor nerves, and can be inherited or sporadic]
Amyotrophic Lateral Sclerosis (ALS)
ALS is a progressive disorder of motor neurons in the brain and spinal cord that is invariably fatal. It can present with equal frequency as NP 5 in the arms, NP of the legs or bulbar NP8/MP7 onset. Clinically patients have mixed upper and lower motor neuron findings on exam. The majority of patients have sporadic disease (~85%) – the heritable causes of ALS will be described elsewhere (Familial ALS Chapter 11). ALS is fundamentally a clinical diagnosis, supported by neuro-physiological testing. There are no cures for ALS – but there are evidence-based guidelines for standards of care (Symptom Management Chapter 9). Here we describe the epidemiology, clinical presentation, diagnosis, and progression of ALS.
Prevalence and epidemiology
The first major attempt to identify overall national US prevalence data for ALS utilized major national administrative databases (Medicare, Medicaid, Veterans Health Administration, and Veterans Benefits Administration), including over 12 thousand ALS cases identified by a standard algorithm (ICD9 codes, VHB codes, neurologist visits, and prescriptions for riluzole) between October 19, 2010, and December 31, 2011.9 Using 2011 Census data they estimated a US population prevalence for ALS of 3.9/100,000. The prevalence differed by age with highest prevalence between 70-79 years (17/100,000) and lowest between 18-39 years (0.5/100,000). There was a higher prevalence of ALS in men compared to women with a male to female ratio of 1.56. Overall ALS was twice as common in whites as blacks. Worldwide prevalence rates for ALS have varied – a meta-analysis of studies published since 1991 found 26 studies quoting prevalence rates between 1.07-11.31 per 100,000.10 Most studies report a male predominance with male to female ratios between 1.2-1.5.11, 12 There is less incidence data on ALS rates in the US. But one large study in 3 states and 8 large metropolitan areas, identified 5883 unique ALS cases (74.8% white, 9.3% African American, and 3.6% Asian). Most (77.5%) were non-hispanic.13 They estimated an average annual incidence rate of 1.52 per 100,000 person-years (an age adjusted rate of 1.44 per 100,000 person-years). Worldwide incidence rates have varied between 0.42-5.3 per 100,000 person years.10 The average age of onset is later in life, with means from 60.7-64.3 years, and an average time from symptom onset to diagnosis between 10.8-16.9 months.9, 11, 14-16 In the US African Americans may be affected slightly younger, with median age of onset of 58 years, and over half of African Americans diagnosed before 60 years.13
Clinical Findings
Although there is considerable clinical variability in the presentation of ALS. A number of key patterns and clinical features are highly suggestive.
Insidious onset of painless weakness and muscle wasting, which starts in one limb then spreads typically to the contralateral limb
Insidious onset of problems with speech or swallowing, followed by weakness or muscle wasting in the limbs
Progressive muscle stiffness and spasticity with muscle cramps and fasciculations
Unexplained restrictive respiratory disease, with a pattern suggestive of diaphragmatic weakness
Head drop with weakness of trunk or paraspinal musculature with upper motor neuron signs on exam.
The classic ALS presentation is insidious onset of symptoms which are progressive, and have a combination of both upper and lower motor neuron findings on clinical examination. The most common presentation is limb-onset; bulbar onset accounting for between 30-32.5% of cases. Lower motor neuron findings include muscle wasting, weakness, and fasciculations. Upper motor neuron findings include spasticity, pathologically brisk reflexes, and weakness in anti-gravity muscles. Bulbar symptoms can include difficulty speaking (dysarthria), difficulty swallowing (dysphagia), and subsequent excess saliva (sialorrhea). Patients typically have a diaphragmatic pattern of respiratory weakness, and may complain of feeling short of breath with activities, or when lying on their back. In addition to the muscular complications of ALS, patients can also experience pseudobulbar affect, which is characterized by excessive laughter or crying with minimal stimulation. Cognitive and behavioral changes have been described in up to 50% of patients with ALS, typically in the spectrum of changes seen with frontotemporal degeneration, with a smaller percentage meeting the frank diagnostic criteria for frontotemporal dementia.17
[Tags: ALS presents with insidious onset of painless muscle wasting which progresses to include other regions]
Diagnosis
According the revised El Escorial Criteria, the diagnosis of ALS18 is suggested by the presence of:
Lower motor neuron dysfunction by physical, electrophysiological, or neuropathological examination
Upper motor neuron dysfunction by clinical examination
Progression of symptoms or signs over 6 months, as demonstrated by spread within a region or to other spinal regions
In the absence of:
Electrophysiological or pathological evidence of other disease processes
Neuroimaging evidence of other disease processes
The diagnosis is largely based on clinical history and physical examination findings, and supported by electrodiagnostic testing. Evidence for widespread active denervation on electromyography includes fibrillations and positive sharp waves. Evidence for re-innervation on electromyography include large motor units, polyphasic motor unit morphology, reduced interference pattern with increased firing rates, or unstable motor unit potentials. The clinical diagnosis of ALS is often broken down by probabilities and the revised El Escorial criteria, which were developed for clinical trials, are widely used in clinic.18
Patients are considered clinically definite by the presence of upper and lower motor neuron signs in the bulbar region, and two spinal cord levels, or in 3 spinal cord levels
Clinically probable if they have upper and lower motor neuron signs in two spinal cord levels, with some upper motor neuron signs rostral to the lower motor neuron signs
Clinically probable-laboratory supported if they have upper and lower motor neuron signs in only one spinal cord level, or when upper motor neuron signs are present alone in one region, and lower motor neuron signs are present on electromyography in two regions.
Clinically possible patients have upper and lower motor neuron findings in only one region, or upper motor neuron sings are found alone in two regions, or lower motor neuron sings are found rostral to upper motor neuron signs.
For the patient meeting clinically definite criteria who also has bulbar involvement neuroimaging is not essential for the diagnosis. For other patients additional evaluations which may be important include:
MRI of the brain and/or spine
Complete metabolic profile, blood count and differential, and Thyroid studies
Autoimmune testing (e.g. GM1 antibodies, serum protein electrophoresis)
Screening for malignancy (e.g. lymphoma, lung cancer)
Cerebrospinal fluid testing
Infectious work up (e.g. HIV, HTLV1, lyme)
Toxins (e.g. heavy metals)
The mimics of ALS include multifocal motor neuropathy, endocrinopathies, post-poliomyelitis syndromes, heavy metal toxicity, and paraneoplastic syndromes. Not all patients will meet formal ALS criteria, and patients with motor neuron involvement and failure to find an alternative explanation should be followed in ALS multidisciplinary clinics.
[Tags: The Revised El Escorial criteria were developed for clinical trials, but are largely used diagnostically in clinic. For clinically definite ALS and clinically probable ALS, no additional testing is required besides expert clinical evaluation. Otherwise, some testing may be helpful and is guided by the clinical phenotype and medical history as discussed in other chapters of this issue.]
History of ALS Diagnostic Criteria
Dr. Edward Lambert published in 1957 the first attempt to help physicians diagnose ALS with electromyographic guidelines. 19 These criteria included 1) normal sensory nerve conduction studies, 2) motor nerve conduction velocities that are normal when recording from relatively unaffected muscles and not less than 70% of the age-based average normal value when recording from severely affected muscles, 3) fibrillation and fasciculation potentials in muscles of the upper and lower extremities or in the muscles of the extremities and the head, and 4) motor unit potentials which are reduced in number and increased in duration and amplitude. A three day workshop on “The Clinical Limits of ALS” was convened in El Escorial, Spain in 1990 by the World Federation of Neurology Subcommittee on Motor Neuron Disease and developed the El Escorial Criteria (EEC). 20 In the original EEC criteria, when there are clear upper and lower motor neuron findings in 2-3 regions, EMG is not required for a patient to enter a study (Table 2). If EMG is required, fibrillation potentials must be present. For the EEC diagnostic categories, the reader is referred to Table 3.
Table 2.
The EEC diagnosis of Amyotrophic Lateral Sclerosis (ALS) requires
| presence of: (A:1) signs of lower motor neuron degeneration by clinical, electrophysiological or neuropathology examination, (A:2) signs of upper motor neuron degeneration by clinical examination, and (A:3) progressive spread of signs within a region or to other regions |
| together with the absence of: (B:1) electrophysiological evidence of other disease processes that might explain the signs of lower motor neuron and/or upper motor neuron degeneration, and (B:2) neuroimaging evidence of other disease processes that might explain the observed clinical and electrophysiological signs. |
Table 3.
EEC Diagnostic Categories
| Definite ALS: is defined on clinical grounds alone by the presence of UMN as well as LMN signs in the bulbar region and at least two of the other spinal regions or the presence of UMN and LMN signs in three spinal regions. |
| Probable ALS: is defined on clinical grounds alone by UMN and LMN signs in at least two regions. While the regions may be different, some UMN signs must be rostral (above) the LMN signs. Multiple different combinations of UMN and LMN signs may be present in patients with probable ALS. |
| Possible ALS: is defined on clinical grounds alone when the UMN and LMN signs are in only one region or UMN signs alone are present in 2 or more regions or LMN signs are rostral to UMN signs. |
| Suspected ALS: will manifest only LMN signs in 2 or more regions, although UMN pathology might be demonstrated at autopsy. However, only clinical signs are considered pertinent to the classification the time of diagnostic evaluation. |
In 1998, the Western ALS (WALS) Study Group modified World Federation of Neurology diagnostic criteria for ALS to facilitate early diagnosis and used these criteria for enrollment of ALS patients in the rhCNTF study trial. 21 The WALS criteria developed required lower motor neuron involvement in at least two limbs and upper motor neuron involvement in at least one region (bulbar, cervical, or lumbosacral). The EMG finding of fibrillation potentials was required for evidence of LMN involvement. Electrodiagnostic studies, neuroimaging, and laboratory studies were also used to exclude disorders that might mimic ALS. If no bulbar signs were present, a cervical MRI was needed for the patients to meet study criteria.
The revised El Escorial criteria which are described above under “Diagnosis”were very specific although insensitive for early diagnosis. In 2006, a group of ALS researchers met in Awaji-shima, Japan to revise ALS diagnostic criteria.22 They made two significant changes to the El Escorial system (Table 4). First, signs of denervation on EMG were regarded as equivalent to clinical lower motor neuron signs and it was suggested to delete “laboratory supported probable ALS” and use only “probable ALS.” Second, fasciculation potentials in muscles with chronic neurogenic EMG changes, in a clinical context fitting ALS, counted as a sign of active denervation, even in the absence of fibrillation potentials and positive sharp waves (Table 4). The Awaji Criteria (AC) improved the sensitivity of EMG studies considerably without increasing the rate of false positive diagnoses, especially in bulbar onset patients. One weakness of (AC) was the requirement for 2 upper motor neuron signs for the clinically probable category. Though patients with upper motor neuron signs in one segment would be allowed into research studies by the revised El Escorial Clinically Probable-Laboratory Supported category, they would be downgraded to clinically possible by the AC requirement for 2 regions with UMN involvement.
Table 4.
Awaji Criteria
| Clinically definite | UMN + LMN signs in bulbar region + > or = 2 spinal regions; or UMN + LMN signs in 3 spinal regions |
| Clinically probable | UMN + LMN signs in > or = 2 spinal regions and “with some UMN signs necessarily rostral to the LMN signs” |
| Clinically possible | UMN + LMN signs in 1 spinal region; or UMN signs in > or = 2 spinal regions; or LMN signs are found rostral to UMN signs, Only after the appropriate neuroimaging and laboratory tests are performed to exclude other possible differential diagnosis that may mimic ALS |
Clinical Course
ALS is a progressive disease which is invariably fatal. The median time from diagnosis to death ranges depending on the study, but is generally reported to be approximately 2.5-3 years.15,23,24 Due to delays in diagnosis most patients will show some respiratory involvement at the time of diagnosis, with over half having a forced vital capacity less than 75% of predicted for age and gender at the time of first clinic visit.15 A number of factors have been associated with faster progression in ALS,15,23-25 and these include:
Bulbar onset of symptoms
Older age at symptom onset
Shorter duration of symptoms
Reduced forced vital capacity
Patients who present with symptoms isolated to either end of motor neuron disease spectrum and regional ALS variants can have slower progression. Patients with PMA, or lower motor neuron variants of ALS (brachial and leg amyotrophic diplegia) are more frequently male, and have slower disease progression. On the other end of the spectrum patients with upper motor neuron dominant ALS also have slower progression than classic ALS.26
[Tags: ALS is progressive and invariably fatal, but regional variants can demonstrate slower progression]
Therapeutic Strategies
The only FDA approved therapy for ALS is riluzole, which is believed to act by decreasing glutamate toxicity on at risk motor neurons, and prolongs survival by approximately 3 months.27 The main side effects of riluzole therapy are gastrointestinal upset, but it can cause elevation in liver transaminases. When starting riluzole therapy it is recommended to check liver profile at baseline, then 1 month after starting therapy, then every 3 months for the first year. The only other FDA approved therapy for ALS is the use of neudexta for pseudobulbar affect (bouts of sustained laughter or crying). Neudexta is a combination medication containing dextromethorphan and quinidine. It is generally well tolerated but is contra-indicated for use in patients taking monamine oxidase inhibitors, and caution should be used in patients on serotonin specific reuptake inhibitors due to a slight increase in risk of serotonin syndrome with the dextromethorphan. For a detailed description of other ALS symptom management options with a variety of medications, the reader is referred to the chapter in this issue titled “Symptoms Management and End of Life Care”. The bottom line is that other than riluzole, there has not been a drug that has been shown to slow ALS disease progression and that has led to FDA approval. Symptomatic management for ALS will be discussed elsewhere (Chapter 9), but we recommend all patients with ALS be followed in a multidisciplinary clinic.28,29 Regular surveillance and areas of symptom management include:
Respiratory therapy to monitor respiratory function – possible interventions include non-invasive positive pressure ventilation, cough assist, and oral suctioning
Speech therapy to monitor swallow and speech – possible interventions include percutaneous gastrostomy tube for nutrition, and assistive communication devices
Assessment for excess salivation – possible interventions include anticholinergic medications, botulism toxin and irradiation.
Physical and occupational therapy to asses for the need for bracing, assistive devices or power wheelchair
Psychosocial counseling for patients and family members to deal with the emotional impact of ALS.
Case manager to review health benefits available to Veterans and others
[Tags: The mainstays of therapy for ALS are riluzole, neudexta, and a multidisciplinary approach to symptom management]
Summary and Future Directions
Motor neuron disease exist on a spectrum of involvement with varying degrees of upper and lower motor neuron involvement. MNDs can be distinguished from other neuropathies or neuronopathies by the pattern of motor and/or sensory involvement. ALS is the most prevalent MND and can be inherited or sporadic. ALS is characterized by mixed upper and lower motor neuron disease, with insidious onset of painless muscle wasting which spreads from one region of the body to another. While variability exists based on the site of symptom onset, the regions of the body involved and rate of progression, ALS is an invariably fatal disease. The only FDA approved therapy prolongs survival by a few months. All patients with ALS should be followed in a multidisciplinary care clinic which improve the quality of life in people with ALS. A better understanding of the genetics and pathophysiology in familial ALS, along with a better understanding of regional variants with faster or slower progression, may help develop more effective future therapies.
Key Points.
Patterns of weakness can be useful when approaching the patient with suspected motor neuron disease
Motor neuron diseases exist on a spectrum: pure lower motor neuron ⇔ mixed upper and lower motor neuron ⇔ pure upper motor neuron
Amyotrophic lateral sclerosis (ALS) is a progressive mixed upper and lower motor neuron disease that is most commonly sporadic and invariably fatal
A better understanding of the genetics and pathophysiology of heritable ALS may yield insights into possible therapies for sporadic ALS
Acknowledgments
This publication was also supported by an Institutional Clinical and Translational Science Award, NIH/NCATS Grant Numbers UL1TR000001. J.S. work on this project supported by a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # KL2TR000119. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement:
Dr. Barohn has served as a consultant and received consulting fees from Baxter, CSL Behring, Genzyme, Grifols, Novartis and NuFactor. He has received research grants from Biomarin, Cytokinetics, Eli Lilly, FDA/OPD, GSK, MDA, MGFA, Neals, NIH, NINDS, Novartis, PTC, Sanofi/Genzyme, and Teva.
Dr. McVey has received research grants from Cytokinetics.
J.K. is a consultant and has received consulting fees from NuFactor. He has received research grants from ALSA, FDA, MDA and Synapse
Dr. Statland has no disclosures.
Dr. Dimachkie is on the speaker's bureau or is a consultant for Baxter, Biomarin, Catalyst, CSLBehring, Depomed, Genzyme, Merck, NuFactor and Pfizer. He has also received grants from Catalyst, CSL-Behring, FDA/OPD, GSK, MDA, NIH and TMA.
References
- 1.Barohn RJ, Amato AA. Pattern-recognition approach to neuropathy and neuronopathy. Neurologic clinics. 2013;31(2):343–361. doi: 10.1016/j.ncl.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. Journal of child neurology. 2007;22(8):1027–1049. doi: 10.1177/0883073807305788. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes LE, Freeman BK, Auh S, et al. Clinical features of spinal and bulbar muscular atrophy. Brain : a journal of neurology. 2009;132(Pt 12):3242–3251. doi: 10.1093/brain/awp258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norris F, Shepherd R, Denys E, et al. Onset, natural history and outcome in idiopathic adult motor neuron disease. Journal of the neurological sciences. 1993;118(1):48–55. doi: 10.1016/0022-510x(93)90245-t. [DOI] [PubMed] [Google Scholar]
- 5.Burrell JR, Vucic S, Kiernan MC. Isolated bulbar phenotype of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2011;12(4):283–289. doi: 10.3109/17482968.2011.551940. [DOI] [PubMed] [Google Scholar]
- 6.Hazan J, Fontaine B, Bruyn RP, et al. Linkage of a new locus for autosomal dominant familial spastic paraplegia to chromosome 2p. Human molecular genetics. 1994;3(9):1569–1573. doi: 10.1093/hmg/3.9.1569. [DOI] [PubMed] [Google Scholar]
- 7.Martinez Murillo F, Kobayashi H, Pegoraro E, et al. Genetic localization of a new locus for recessive familial spastic paraparesis to 15q13-15. Neurology. 1999;53(1):50–56. doi: 10.1212/wnl.53.1.50. [DOI] [PubMed] [Google Scholar]
- 8.Zhai P, Pagan F, Statland J, et al. Primary lateral sclerosis: A heterogeneous disorder composed of different subtypes? Neurology. 2003;60(8):1258–1265. doi: 10.1212/01.wnl.0000058900.02672.d2. [DOI] [PubMed] [Google Scholar]
- 9.Mehta P, Antao V, Kaye W, et al. Prevalence of amyotrophic lateral sclerosis - United States, 2010-2011. Morbidity and mortality weekly report Surveillance summaries. 2014;63(Suppl 7):1–14. doi: 10.15585/mmwr.ss6508a1. [DOI] [PubMed] [Google Scholar]
- 10.Deenen J, Horlings C, Verschuuren J, et al. The Epidemiology of Neuromuscular Disorders: A Comprehensive Overview of the Literature. Journal of Neuromuscular Diseases. 2015;2:73–85. [PubMed] [Google Scholar]
- 11.Abhinav K, Stanton B, Johnston C, et al. Amyotrophic lateral sclerosis in South-East England: a population-based study. The South-East England register for amyotrophic lateral sclerosis (SEALS Registry). Neuroepidemiology. 2007;29(1-2):44–48. doi: 10.1159/000108917. [DOI] [PubMed] [Google Scholar]
- 12.Chio A, Calvo A, Moglia C, et al. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. Journal of neurology, neurosurgery, and psychiatry. 2011;82(7):740–746. doi: 10.1136/jnnp.2010.235952. [DOI] [PubMed] [Google Scholar]
- 13.Rechtman L, Jordan H, Wagner L, et al. Racial and ethnic differences among amyotrophic lateral sclerosis cases in the United States. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(1-2):65–71. doi: 10.3109/21678421.2014.971813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chio A, Mora G, Calvo A, et al. Epidemiology of ALS in Italy: a 10-year prospective population-based study. Neurology. 2009;72(8):725–731. doi: 10.1212/01.wnl.0000343008.26874.d1. [DOI] [PubMed] [Google Scholar]
- 15.Traxinger K, Kelly C, Johnson BA, et al. Prognosis and epidemiology of amyotrophic lateral sclerosis: Analysis of a clinic population, 1997-2011. Neurology Clinical practice. 2013;3(4):313–320. doi: 10.1212/CPJ.0b013e3182a1b8ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Traynor BJ, Codd MB, Corr B, et al. Incidence and prevalence of ALS in Ireland, 1995-1997: a population-based study. Neurology. 1999;52(3):504–509. doi: 10.1212/wnl.52.3.504. [DOI] [PubMed] [Google Scholar]
- 17.Giordana MT, Ferrero P, Grifoni S, et al. Dementia and cognitive impairment in amyotrophic lateral sclerosis: a review. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2011;32(1):9–16. doi: 10.1007/s10072-010-0439-6. [DOI] [PubMed] [Google Scholar]
- 18.Brooks BR, Miller RG, Swash M, et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 19.Lambert EH, Mulder DW. Electromyographic studies in amyotrophic lateral sclerosis. Mayo Clinic. 1957;32:441–446. [PubMed] [Google Scholar]
- 20.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases. J Neurol Sci. 1994 Jul;124(Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 21.Ross MA, Miller RG, Berchert L, Parry G, Barohn RJ, et al. Toward earlier diagnosis of amyotrophic lateral sclerosis: revised criteria. rhCNTF ALS Study Group. Neurology. 1998 Mar;50(3):768–772. doi: 10.1212/wnl.50.3.768. [DOI] [PubMed] [Google Scholar]
- 22.de Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, Mills K, Mitsumoto H, Nodera H, Shefner J, Swash M. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008 Mar;119(3):497–503. doi: 10.1016/j.clinph.2007.09.143. [DOI] [PubMed] [Google Scholar]
- 23.Chancellor AM, Slattery JM, Fraser H, et al. The prognosis of adult-onset motor neuron disease: a prospective study based on the Scottish Motor Neuron Disease Register. Journal of neurology. 1993;240(6):339–346. doi: 10.1007/BF00839964. [DOI] [PubMed] [Google Scholar]
- 24.del Aguila MA, Longstreth WT, Jr., McGuire V, et al. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003;60(5):813–819. doi: 10.1212/01.wnl.0000049472.47709.3b. [DOI] [PubMed] [Google Scholar]
- 25.Kollewe K, Mauss U, Krampfl K, et al. ALSFRS-R score and its ratio: a useful predictor for ALS-progression. Journal of the neurological sciences. 2008;275(1-2):69–73. doi: 10.1016/j.jns.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Gordon PH, Cheng B, Katz IB, et al. Clinical features that distinguish PLS, upper motor neuron-dominant ALS, and typical ALS. Neurology. 2009;72(22):1948–1952. doi: 10.1212/WNL.0b013e3181a8269b. [DOI] [PubMed] [Google Scholar]
- 27.Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). The Cochrane database of systematic reviews. 2012;3:CD001447. doi: 10.1002/14651858.CD001447.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley WG, Anderson F, Bromberg M, et al. Current management of ALS: comparison of the ALS CARE Database and the AAN Practice Parameter. The American Academy of Neurology. Neurology. 2001;57(3):500–504. doi: 10.1212/wnl.57.3.500. [DOI] [PubMed] [Google Scholar]
- 29.Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73(15):1227–1233. doi: 10.1212/WNL.0b013e3181bc01a4. [DOI] [PMC free article] [PubMed] [Google Scholar]