Abstract
Repetitive behavior refers to a highly heterogeneous set of responses associated with a wide range of conditions, including normative development. Treatment studies for aberrant repetitive behavior are limited although one promising approach involves conceptualizing such behavior as a generalized inflexibility or lack of variability in responding. Relatively little is known about the neurobiological mechanisms that mediate the development and expression of repetitive behavior, information critical to the design of effective pharmacotherapies, early interventions, and prevention strategies. We will review clinical findings in repetitive behavior as well as findings from animal models highlighting environmental factors and the role of cortical-basal ganglia circuitry in mediating the development and expression of these behaviors. Findings from animal models have included identification of a specific neural pathway important in mediating repetitive behavior. Moreover, pharmacological studies that support the importance of this pathway have led to the identification of novel potential therapeutic targets. Expanding the evidence base for environmental enrichment-derived interventions and focusing on generalized variability in responding will aid in addressing the broader problem of rigidity or inflexibility.
Keywords: Inflexible behavior, Animal models, Cortical-basal ganglia pathway, Stereotypy
Repetitive behaviors (e.g., stereotypies, compulsions, rituals) are diagnostic for autism and common in other neurodevelopmental disorders (e.g., fragile X syndrome, Prader-Willi syndrome, nonsyndromic intellectual disability; Lewis and Bodfish 1998; Moss et al. 2009). Indeed, the first report of autism as a disorder in the medical literature (Kanner 1943) included obsessive desire for sameness, verbal and motor rituals, obsessive questioning, and rigid adherence to routine as key components of the clinical presentation. Although our focus here is neurodevelopmental disorders, it is important to note that repetitive behaviors also manifest in a number of other clinical disorders including Tourette syndrome, Parkinson’s disease, and frontotemporal dementia (Singer 2013). In addition, repetitive behaviors can develop as a consequence of early experiential deprivation including congenital blindness and highly impoverished environments (Fazzi et al. 1999; Rutter et al. 1999). Motor stereotypies persisting beyond what is developmentally normative have also been reported in children that do not meet diagnostic criteria for neurodevelopmental or neurological disorders (Singer 2009). The expression of repetitive behaviors over a number of clinical disorders and conditions suggests that repetitive behavior likely arises from multiple etiologies or sources of central nervous system (CNS) insult. As we will suggest, however, there appears to be a common neural circuitry involved.
Repetitive behavior represents a broad range of responses that include stereotyped motor movements, self-injurious behavior, repetitive manipulation of objects, compulsions, rituals and routines, insistence on sameness, and circumscribed interests (Leekam et al. 2011; Lewis and Bodfish 1998). These forms of repetitive behavior have been shown to cluster as either “lower order” (stereotyped movements, self-injury, repetitive manipulation of objects) or “higher order” behaviors (compulsions, rituals, insistence on sameness), the latter category involving more complex behaviors characterized by rigidity or inflexibility. Other work has provided evidence for a third factor of circumscribed interests (Lam and Aman 2007). One implication of the range or heterogeneity of repetitive behavior is that these behaviors are mediated by a complex circuitry involving multiple brain regions. The more complex the circuitry, the more vulnerable it may be to a wide variety of alterations or perturbations (e.g., early social deprivation, gene by environment interactions) that result in repetitive behavior.
Beyond discrete categories or clusters of repetitive behavior in specific clinical disorders (e.g., hand flapping in autism), research and treatment would benefit from an expanded conceptualization of repetitive behavior. Such expansion would include at least two additional components. The first is the recognition that repetitive behavior is normative in typical development. There is ample evidence that repetitive motor behavior, compulsions, and rituals are commonly observed in typical children’s development (e.g., Evans et al. 1997; Thelen 1979) albeit expressed at lower frequencies or severity levels than those observed in autism spectrum disorders (ASD; Wolff et al. 2014). As repetitive behaviors are already in the repertoire of the individual, greater attention should be paid to the developmental timing of the transition from normative to pathological repetitive behavior. This question has received scant attention (Pietrefesa and Evans 2007), and little is known about the environmental factors or neurobiological mechanisms that mediate this transition (Langen et al. 2014). Understanding the timing and related factors that result in a divergent trajectory of repetitive behavior for an individual with a neurodevelopmental disorder could have important treatment implications.
A second critical expansion of our conceptualization of repetitive behavior comes from the recognition that discrete repetitive behaviors are exemplars of what appears to be a larger, generalized pattern of rigid, inflexible behavior or lack of response variability across multiple behavioral classes (Rodriguez and Thompson 2015). A striking example of this comes from eye-tracking studies that show a restricted and repetitive pattern of visual attention to a pictorial array of nonsocial items in children with ASD compared to typically developing children (Sasson et al. 2008). This pattern appeared to reflect an inflexible, repeated sequence of visual exploration of the stimuli presented. A second example is from our work showing that children with ASD exhibit a more restricted and rigid pattern of engagement with age-appropriate play activities than typically developing matched control children. Other examples, involving children with ASD, include greater sequence regularity in random number generating tasks (Williams et al. 2002; Rinehart et al. 2006) and reduced sequence complexity and use of fewer colors when placing colored stamps on paper (Frith 1972).
Treatment of Repetitive Behavior
There have been few systematic efforts to develop behavioral or pharmacological treatments for repetitive behavior in neurodevelopmental disorders. Boyd et al. (2012) reviewed behavioral interventions for repetitive behavior in individuals with ASD concluding that the field lacks programmatic research designed to address the full range of repetitive behaviors characteristic of ASD and related disorders. The rather small published literature largely reflects behavior analytic approaches, mostly focused on lower order repetitive behaviors (Rapp and Vollmer 2005). As many forms of lower order repetitive behavior may involve automatic reinforcement (self-injurious behavior (SIB) often being an exception), antecedent-based interventions (e.g., modifying the environment, teaching adaptive incompatible behaviors) are frequently employed. There is, however, evidence to support both consequence- and antecedent-based treatment approaches for repetitive behavior (Rapp and Vollmer 2005). Of the smaller number of studies addressing higher order repetitive behaviors, differential reinforcement of variability and both antecedent- and consequent-based use of restricted or circumscribed interests have been used (Boyd et al. 2013). There is a need, however, to establish evidence-based practices to treat higher order repetitive behaviors. One promising approach is a modification of a systematic desensitization treatment used successfully in obsessive-compulsive disorder (OCD) called exposure and response prevention. Boyd et al. (2013) have provided preliminary evidence for its feasibility and efficacy in children with ASD.
A small but compelling literature has demonstrated empirically that it is possible to shape variable, flexible behavior using reinforcement procedures (e.g., Neuringer 2004). Promoting the development of flexibility and variability in behavior may be a more effective and generalizable treatment approach than targeting a particular repetitive behavior for modification. Moreover, this approach may have an important impact on brain and behavior development when conducted with very young children with neurodevelopmental disorders.
There are few pharmacological interventions with established efficacy for the treatment of repetitive behavior in neurodevelopmental disorders (Tanimura et al. 2011). Commonly prescribed medications such as selective serotonin reuptake inhibitors or SSRIs (e.g., Prozac) have been shown to lack efficacy for repetitive behavior in individuals with ASD as well as exhibit significant adverse effects (Carrasco et al. 2012; King et al. 2009). Similarly, although atypical antipsychotics (e.g., Risperdal) have been reported to have some efficacy, FDA approval for the use of two of these drugs in autism is to treat irritability not repetitive behavior. In addition, atypical antipsychotics are associated with significant weight gain and, potentially, metabolic syndrome with little evidence of efficacy for repetitive behavior.
Clinical Neuroscience of Repetitive Behavior
The lack of effective pharmacological treatments for repetitive behavior is due, at least in part, to the lack of knowledge of the underlying neurobiology. Genetic studies have provided some, albeit very limited, information. For example, in autism, there is evidence for the heritability of repetitive behavior based on monozygotic twin data (e.g., Bailey et al. 1995). In addition, specific genetic syndromes such Prader-Willi (e.g., skin picking, compulsive eating) and Rett (e.g., stereotypic midline hand clasping) include specific repetitive behaviors that are a readily identifiable feature of their clinical presentation. As the genetic loci (e.g., 15q11-13 for Prader-Willi) or gene (e.g., MECP2 for Rett) for these genetic syndromes is known, this provides important information about what candidate gene or genes may be involved in repetitive behavior. Despite this, there has been very limited progress in identifying alterations in specific genes associated with repetitive behavior. Some exceptions include a neurotransmitter receptor subtype located on the same region of chromosome 15 that is mutated in Prader-Willi and Angelman syndromes. This receptor subtype binds the neurotransmitter GABA and was shown to be altered in families sharing high insistence on sameness factor score (Shao et al. 2003). An association between a subtype of the serotonin transporter gene (SLC6A4) and repetitive sensory motor behaviors has also been shown (Brune et al. 2006).
Few studies have attempted to identify neuropathological changes associated with repetitive behavior using postmortem tissue from individuals with neurodevelopmental disorders. A notable exception is the postmortem examination of brains from individuals with Lesch-Nyhan disease, all of whom exhibit SIB (Saito and Takashima 2000). A small number of neuroimaging studies have addressed the association between regional brain volumetric changes and repetitive behavior. These studies have implicated changes in the basal ganglia (see Fig. 2), particularly the caudate-putamen, brain regions important in the mediating movement and movement disorders such as Parkinson’s disease and Tourette syndrome. For example, studies with medication-naive Tourette syndrome patients have indicated reductions in caudate-putamen volume (Bloch et al. 2005) although increased (Fredericksen et al. 2002) and similar volumes (Zimmerman et al. 2000) have also been reported. In addition, smaller caudate volumes in children predicted increased severity of Tourette symptoms in adulthood (Bloch et al. 2005; Hyde et al. 1995). For OCD, Radua and Mataix-Cols (2009) reviewed 12 MRI studies which showed increased gray matter volume in the basal ganglia, including the putamen and caudate. This analysis also indicated a correlation between OCD severity and the increased magnitude of basal ganglia volume. Volumetric reductions in the left and right caudate nuclei as well as in frontal white matter have been reported in boys with complex stereotypies but no identifiable neurological or psychiatric disorder (Kates et al. 2005).
Fig. 2.
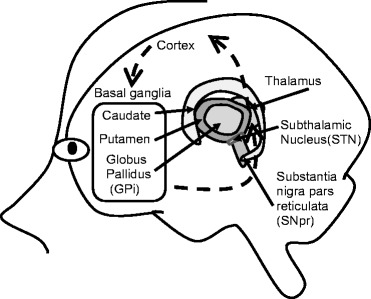
Schematic diagram of cortical-basal ganglia circuitry
In ASD, caudate/putamen volume has been both negatively and positively correlated with routines, compulsions, and rituals (Sears et al. 1999; Hollander et al. 2005; Rojas et al. 2006). In two studies using a wider age range (7–25 years) and high-functioning ASD individuals, Langen et al. (2007, 2009) reported either no significant correlations with Autism Diagnostic Interview (ADI) repetitive behavior scores or a negative correlation with the insistence on sameness cluster of ADI scores. In a more recent work, Langen et al. (2012) found greater growth in the caudate nucleus over a 2.4-year period in ASD children compared to control participants with caudate growth per year exhibiting a significant positive correlation with insistence on sameness behavior.
In very young (3–4 years old) children with ASD, Estes et al. (2011) observed no systematic relationship between caudate volumes and restricted, repetitive behavior. This is at odds with the results of Wolff et al. (2013) who reported that the compulsive and ritual subscales of the Repetitive Behavior Scale-Revised were significantly positively associated with caudate volumes in 3–6-year-old ASD children.
There has been little use of functional neuroimaging (fMRI) or diffusion tensor imaging (DTI) to determine the neurocircuitry of repetitive behavior in neurodevelopmental disorders. Only one study (Langen et al. 2012) has related white matter organization of frontostriatal tracts using DTI measures and found no significant association with repetitive behavior. The relative paucity of clinical neuroimaging studies combined with inconsistent findings results in very limited knowledge about the neurobiology of repetitive behavior.
Animal Models of Repetitive Behavior
Because information about neurobiological mechanisms that mediate repetitive behavior from clinical studies is limited, animal models with the requisite validity provide an important opportunity to identify the relevant brain circuitry. Bechard and Lewis (2012) organized animal model studies into four categories. The first includes studies of repetitive behavior induced by specific brain insults, most of which involved genetic manipulations such as knocking out a gene. One striking example involved deleting a gene in mice that codes for a synaptic associated protein (SAP-AP3) important in stabilizing synaptic connections between the cortex and striatum (caudate-putamen) (Welch et al. 2007). This gene deletion resulted in mice that groomed excessively, to the point of removing fur and causing skin lesions (i.e., SIB). A second category of animal model studies includes repetitive behavior induced by administration of a specific pharmacological agent. It is well known that drugs like amphetamine and cocaine, in sufficient doses, will induce repetitive behavior in animals and humans (Cooper and Dourish 1990). More recently, studies employing administration of selective pharmacological agents into specific brain regions to induce or block repetitive behavior have been useful in identifying key brain regions and neurotransmitters involved in repetitive behavior (Bechard and Lewis 2012). The third category involves repetitive behavior observed in specific inbred mouse strains such as the BTBR and C58 strains (Amodeo et al. 2012; Muehlmann et al. 2012; McFarlane et al. 2008; Ryan et al. 2010). Mice of these strains exhibit excessive grooming (BTBR) or vertical jumping/backward somersaulting (C58) without any perturbation such as a genetic manipulation, drug, CNS lesion, or other intervention needed. The environment (standard laboratory caging) in which these behaviors develop and are expressed, however, is likely a key factor in addition to genetic background. This leads to the fourth category, which involves repetitive behavior induced by environmental restriction or confinement. As but one of many examples, deer mice (Peromyscus) exhibit high rates of stereotyped motor behavior as a consequence of being reared in standard laboratory cages (Powell et al. 2000). Our work uses both the C58 and deer mouse models so environmental factors play a particularly important role in the development and expression of repetitive behavior as well as correlated brain changes in these animals.
Role of the Environment in Repetitive Behavior
Early experiential deprivation has been strongly associated with the induction of repetitive behavior in both humans and animals (Lutz et al. 2007; Bechard and Lewis 2012; Devine 2014). For example, almost half of adoptees from Romanian orphanages exhibited stereotyped body rocking (Beckett et al. 2002). High levels of repetitive behavior in nonhuman primates were shown to be an invariant consequence of early social deprivation (Harlow and Harlow 1962). Rearing animals in confined or restricted environments predictably induces stereotypic behavior across a variety of species maintained in zoos, farms, and laboratories (Mason and Rushen 2006). We have shown that deer mice (Peromyscus) as well as C58 mice (Mus) develop high levels of stereotyped motor behaviors over development as a consequence of being reared in standard laboratory cages (Muehlmann et al. 2012; Powell et al. 2000; Tanimura et al. 2010a, b).
Compelling evidence for the causative role of environmental restriction in the induction of repetitive behavior comes from studies of environmental enrichment (EE). These studies using multiple species have consistently shown that animals reared in larger, more complex environments show less repetitive behavior than their environmentally restricted counterparts (Mason and Rushen 2008). Our work has shown that EE in both deer mice and C58 inbred mice markedly attenuates repetitive behavior (Powell et al. 2000; Turner et al. 2002, 2003; Muehlmann et al. 2012). Figure 1 depicts the enriched environment currently used in our lab.
Fig. 1.

Picture of enriched environment kennels used in Lewis lab mouse studies
Findings from Two Mouse Models of Repetitive Behavior
C58 mice exhibit high levels of spontaneous stereotyped behavior in the form of hindlimb jumping and backward somersaulting (Ryan et al. 2010; Muehlmann et al. 2012). We have found that these behaviors reach frequencies exceeding 10,000 individual stereotypic responses during a 12-h dark cycle (active period for nocturnal animals) by the mouse equivalent of young adulthood (Muehlmann et al. 2012). Housing C58 mice in an enriched environment from weaning to young adulthood largely eliminated their repetitive behaviors (Muehlmann et al. 2012).
As discussed earlier, repetitive behaviors in individuals with ASD have been shown to aggregate into two clusters: repetitive sensory motor behaviors (lower order) and behaviors that reflect resistance to change or insistence on sameness (higher order; see Bishop et al. 2013; Mooney et al. 2009). We have assessed whether C58 mice demonstrate greater resistance to change relative to C57BL/6 control mice by using reversal learning and extinction of an appetitive operant nose-poke right-left positional discrimination task. Moreover, we assessed whether performance of C58 mice during reversal learning and extinction was related to their rates of repetitive motor behavior. C58 mice made more perseverative errors during the first session of reversal learning than did C57BL/6 mice and fewer regressive errors. C58 mice also exhibited a greater increase in responding during the first session of extinction compared to C57BL/6 mice. We found a positive correlation between stereotyped motor behaviors and the number of responses required to reach criterion for reversal learning. We also found positive correlations between repetitive motor behaviors and total errors and perseverative errors during the first session of reversal learning. These findings suggest that C58 mice exhibit both higher order as well as lower order repetitive behaviors, which increases the translational value of this model.
Our findings with C58 mice have largely recapitulated our earlier results with deer mice. Deer mice develop the same two forms of repetitive behavior (vertical jumping and backward somersaulting) with the same general developmental trajectory. As deer mice are outbred and exhibit greater individual variability, we were able to identify three discrete developmental trajectory groups (Tanimura et al. 2010b ). Deer mice also exhibit higher order repetitive behaviors as well. We established this by demonstrating that high repetitive motor behavior mice showed greater deficits in reversal learning using a water T-maze task with one arm of the T having a platform which allowed an escape response. Errors in reversal learning were significantly correlated with frequency of stereotyped motor behaviors (Tanimura et al. 2008). In addition, EE for a period of 6 weeks after weaning markedly reduced repetitive behavior in deer mice as it did in C58 mice (Hadley et al. 2006).
In several studies conducted in our lab, we attempted to determine how EE was changing the brain to result in attenuation of repetitive behavior in deer mice (Turner et al. 2002, 2003; Turner and Lewis 2003). These studies, which used a general marker of activation of neurons as well as neuroanatomical and neurochemical measures of neuroplasticity, pointed to changes in areas associated with cortical-basal ganglia circuitry but not in other brain regions. In addition to evidence for the importance of cortical-basal ganglia circuitry, these brain changes were evident only in those animals that “benefited” from enrichment as defined by a significant reduction in repetitive behavior.
Cortical-Basal Ganglia Circuitry
Cortical-basal ganglia circuitry (depicted in Fig. 2) refers to a set of connections among brain regions that originates with neurons in specific areas of the cortex sending projections to the striatum (caudate/putamen). Projections from striatal neurons, in turn, converge on the basal ganglia nuclei globus pallidus (internal aspect)/substantia nigra (pars reticulata) otherwise referred to as GPi/SNpr. GPi/SNpr neurons, in turn, project to the thalamus, and the circuit is then completed by thalamic projections returning to the cortex (Lewis and Kim 2009). This cortical-basal ganglia circuitry is thought to be comprised of multiple, parallel loops that although interacting are anatomically distinct and serve different functions (Alexander et al. 1986; Langen et al. 2011). Although five loops have been described, three loops are generally considered to have clear and discrete functional roles: the sensorimotor (motor and oculomotor cortex), associative (dorsolateral prefrontal cortex), and limbic (lateral orbitofrontal and anterior cingulate cortex) loops. These loops mediate motor, cognitive, and affective functions, respectively. Of these, the motor circuit has been the most studied and emerges as the best candidate for mediation of repetitive motor movements. The limbic loop may be the best candidate for mediation of some higher order repetitive behaviors, particularly compulsions, and the associative loop may be important for rigidity.
The motor loop makes use of two distinct basal ganglia pathways in transmitting information from the striatum to the GPi/SNpr (see Fig. 3). Approximately half of striatal neurons express the neuropeptide dynorphin as well as D1 dopamine receptors and A1 adenosine receptors and constitute direct pathway neurons. These neurons send projections from the striatum directly to the GPi/SNpr. Striatal neurons that express the neuropeptide enkephalin, as well as D2 dopamine receptors and A2A adenosine receptors, constitute indirect pathway neurons. Indirect pathway neurons project to the external segment of the globus pallidus (GPe) and then to the subthalamic nucleus (STN) before converging on GPi/SNpr. The classic view has been that the direct pathway facilitates movement by disinhibition of thalamocortical neurons and thus increasing excitation of the cortex, whereas the indirect pathway inhibits ongoing movement via inhibition of thalamocortical neurons and thus decreased excitation of the cortex (Gerfen et al. 1990). Thus, basal ganglia-mediated behaviors depend on the balance of activity from these two antagonistic yet dynamically interacting pathways to regulate initiation and suppression of various motor programs that allow for smooth selection and execution of planned movements.
Fig. 3.
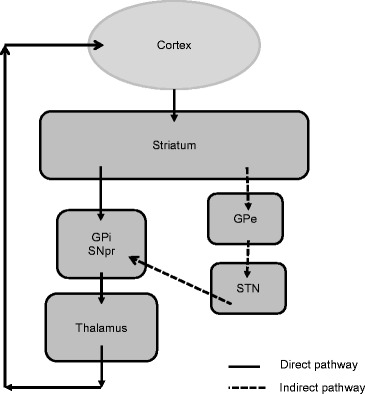
Schematic diagram of direct (solid arrows) and indirect (dotted arrows) basal ganglia pathways
Within an operant conditioning framework, automatic reinforcement has been identified via functional analyses as a maintaining variable for repetitive behavior. Interestingly, recent work has suggested that the direct and indirect basal ganglia pathways may mediate reward and punishment, respectively (Kravitz and Kreitzer 2012). For example, it has been shown that mice quickly learned to contact a touch sensor that resulted in striatal direct pathway neurons being stimulated, whereas they avoided contact with a touch sensor that resulted in striatal indirect pathway neurons being stimulated (Kravitz et al. 2012). As the role of striatal dopamine in reward is well established, it may be that dopamine release can mediate reinforcement through two independent basal ganglia pathways: D1 receptor-mediated activation of direct pathway neurons and D2 receptor-mediated inhibition of indirect pathway neurons (Kravitz and Kreitzer 2012).
Indirect Basal Ganglia Pathway and Repetitive Behavior
Traditionally, movement disorders (hypokinetic such as Parkinson’s disease and hyperkinetic such as Huntington’s disease) reflected an imbalance in the activity of the direct and indirect basal ganglia pathways. In an early study from our lab using deer mice, we tested the hypothesis that high levels of repetitive behavior were associated with such an imbalance (Presti and Lewis 2005). Our findings confirmed this imbalance, but surprisingly, it was reduced indirect basal ganglia pathway activation that was associated with the expression of high levels of repetitive behavior. We have extended these findings using different neurochemical methods by demonstrating that neuronal activation is reduced in key brain regions of the indirect pathway (e.g., STN) in mice exhibiting high versus low levels of repetitive behavior. Moreover, our measure of neuronal activation was significantly negatively correlated with the frequency of repetitive motor behavior (Tanimura et al. 2010a; Tanimura et al. 2011). We have also shown decreased functioning of the indirect basal ganglia pathway in C58 compared to C57BL/6 control mice (Muehlmann et al. 2013). C58 mice had significantly less functional activation of the STN with no significant differences from C57BL/6 mice in neuronal activation in control brain regions (e.g., hippocampus).
These findings led to the idea that if we could increase indirect basal ganglia pathway activation, we should be able to reduce repetitive behavior. We decided to achieve such activation by selecting a drug targeted to a neurotransmitter receptor that was expressed selectively on striatal indirect pathway neurons. We targeted A2A adenosine receptors as these are highly enriched in the striatum and expressed on indirect, but not direct, pathway striatal neurons (DeMet and Chicz-DeMet 2002). Our efforts to reduce repetitive behavior by administering an A2A agonist were unsuccessful, however. This failure was explained by the work of Karcz-Kubicha et al. (2006), who showed that an A2A agonist alone did not generally induce activation in striatal neurons. When they added an A1 agonist to the A2A agonist, they now observed striatal neuronal activation, and importantly, this activation was seen in indirect but not direct pathway neurons. This led us to test whether combining a selective A2A receptor agonist with a selective A1 agonist would attenuate repetitive behavior in deer mice. As described in Tanimura et al. (2010a), this drug combination selectively and substantially reduced repetitive behavior in a dose-dependent manner. We have since extended these findings to the C58 mouse model. By using oil as a vehicle, we have been able to employ a slow release drug preparation that suppresses repetitive behavior for up to 6 h.
Striatal neurons of both direct and indirect pathways express complexes of neurotransmitter receptors that are structurally and functionally linked (Fuxe et al. 2010). Indirect pathway neurons express a receptor complex that includes D2, A2A, and mGluR5 receptors. We evaluated the effects of drugs that target this receptor complex to elucidate their individual and combined roles in modulating repetitive behavior. As stated previously, we knew that stimulating the A2A receptor alone would be insufficient to reduce repetitive behavior. We also knew from other work that the A2A and D2 receptors are functionally antagonistic or negatively coupled, whereas the A2A and mGluR5 receptor are positively coupled. We, therefore, tested whether an A2A agonist, a D2 antagonist, and an mGluR5 agonist, in combination, would reduce repetitive behavior by presumably maximizing stimulation of the indirect pathway. Consistent with our hypothesis, we found that this three drug combination or cocktail substantially and selectively reduced repetitive behavior. Administration of any of these drugs alone or any combination of two drugs had no significant effects on repetitive behavior. These findings were clearly demonstrated in both the deer mouse and C58 mouse models of repetitive behavior.
One important question posed by our biochemical and pharmacological findings is whether decreased indirect basal ganglia pathway plays an important role in repetitive behavior beyond our two mouse models. Happily for us and for the translational value of our work, the short answer is yes. For example, the enhanced motor movements characteristic of Huntington’s disease have been attributed to the differential degeneration of indirect pathway striatal neurons (Deng et al. 2004; Starr et al. 2008). Deep brain stimulation (DBS) applied to the STN, a key relay station in the indirect pathway, reduced symptom severity in previously treatment refractory OCD patients (Burdick et al. 2009). Grabli et al. (2004) have reported that stereotyped and self-injurious behavior (e.g., licking and biting of fingers) was induced in monkeys when a GABA receptor antagonist was injected into the external aspect of the GPe, also a brain region that is part of the indirect pathway. This repetitive behavior was reduced by DBS applied to STN (Baup et al. 2008), further supporting a role for the indirect pathway. It should be stressed, however, that there is no evidence yet for mediation of repetitive behavior in neurodevelopmental disorders by the indirect basal ganglia pathway.
Implications
We believe there are important implications of our work with animal models for persons with neurodevelopmental disorders exhibiting repetitive behaviors. First, we can develop animal models that exhibit robust lower order and higher order repetitive behaviors. This is critical if we are to try to understand the neurobiology of the broad range of repetitive behavior. Second, we have shown in two different models that environmental factors or early experience plays a critical role in the development and attenuation of repetitive behavior. Understanding how such experience affects brain structure and function will inform development of more effective or targeted early interventions. A very promising example of this, although not directed specifically at repetitive behavior, comes from the work of Woo and Leon (2013). In this study, sensorimotor enrichment, analogous to our use of EE in mice, was shown to be effective in ameliorating some of the symptoms of autism in children.
Our work identifying the role of the indirect pathway is hopefully of translational value in several ways. It provides evidence of a delineated brain pathway that mediates repetitive behavior in an animal model relevant to autism and related disorders. Based on this and other work, there is reason to think that alterations in this pathway may be clinically important. This generalization could be explored using sophisticated neuroimaging techniques such as DTI which allows visualization and quantification of brain pathways.
Our pharmacological studies here are important in at least two ways. First, the results of these experiments provide strong evidence to support the hypothesis that repetitive behavior is mediated by reduced indirect pathway activation. Second, these studies provide novel potential therapeutic targets that can be utilized in drug development. This is particularly important as there are currently no clinically available medications that effectively treat repetitive behavior in neurodevelopmental disorders. Successful development of medications of proven efficacy will more likely come from “bottom-up” efforts that delineate the underlying neurobiology. This work will identify novel potential therapeutic targets, which will then provide the basis for drug development efforts that will hopefully culminate in safe and effective medications to treat repetitive behaviors.
We have shown in two different models that environmental factors or early experience plays a critical role in the development and attenuation of repetitive behavior. Understanding how such experience affects brain structure and function will inform development of more effective or targeted early interventions. Thus, expanding the evidence base for the effectiveness of EE-derived interventions for both lower order and higher order repetitive behaviors in children with neurodevelopmental disorders will be critical. In addition, continued translation into clinical practice of studies that conceptualize variability as an operant and differentially reinforce response variance (e.g., Miller and Neuringer 2000) will provide a behavioral technology to address effectively the broader problem of generalized rigidity or inflexibility.
Acknowledgments
This manuscript was based on a B.F. Skinner science lecture delivered at the Association for Behavior Analysis International annual meeting, Chicago, May 2014. We gratefully acknowledge the support provided by NIH grants MH080055 and MH091554 and the Gatorade Trust through funds distributed by the University of Florida, Department of Medicine.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:9357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6 J mice on probabilistic reversal learning and stereotyped behaviors. Behavioural Brain Research. 2012;227(1):64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P. Autism as a strongly genetic disorder: evidence from a British twin study. Psychological Medicine. 1995;25(1):63–77. doi: 10.1017/S0033291700028099. [DOI] [PubMed] [Google Scholar]
- Baup N, Grabli D, Karachi C, Mounayar S, François C, Yelnik J, Tremblay L. High-frequency stimulation of the anterior subthalamic nucleus reduces stereotyped behaviors in primates. The Journal of Neuroscience. 2008;28(35):8785–8788. doi: 10.1523/JNEUROSCI.2384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechard A, Lewis MH. Modeling restricted repetitive behavior in animals. Autism. 2012;S1:006. [Google Scholar]
- Beckett C, Bredenkamp D, Castle J, Groothues C, O’Connor TG, Rutter M. Behavior patterns associated with institutional deprivation: a study of children adopted from Romania. Journal of Developmental and Behavioral Pediatrics. 2002;23(5):297–303. doi: 10.1097/00004703-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Hus V, Duncan A, Huerta M, Gotham K, Pickles A, Lord C. Subcategories of restricted and repetitive behaviors in children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013;43(6):1287–1297. doi: 10.1007/s10803-012-1671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65(8):1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd BA, McDonough SG, Bodfish JW. Evidence-based behavioral interventions for repetitive behaviors in autism. Journal of Autism and Developmental Disorders. 2012;42(6):1236–1248. doi: 10.1007/s10803-011-1284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, B., Woodard, C., & Bodfish, J. M. (2013). Feasibility of exposure response prevention to treat repetitive behaviors of children with autism and an intellectual disability: A brief report. Autism, 17(2), 196--204. doi:10.1177/1362361311414066. [DOI] [PMC free article] [PubMed]
- Brune CW, Kim S, Salt J, Leventhal BL, Lord C, Cook EJ. 5-HTTLPR genotype-specific phenotype in children and adolescents with autism. The American Journal of Psychiatry. 2006;163(12):2148–2156. doi: 10.1176/appi.ajp.163.12.2148. [DOI] [PubMed] [Google Scholar]
- Burdick A, Goodman WK, Foote KD. Deep brain stimulation for refractory obsessive-compulsive disorder. Frontiers in Bioscience. 2009;14:1880–1890. doi: 10.2741/3348. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Volkmar FR, Bloch MH. Pharmacologic treatment of repetitive behaviors in autism spectrum disorders: evidence of publication bias. Pediatrics. 2012;129(5):e1301–e1310. doi: 10.1542/peds.2011-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SJ, Dourish CT. Neurobiology of stereotyped behaviour. New York: Clarendon/Oxford University Press; 1990. [Google Scholar]
- DeMet EM, Chicz-DeMet A. Localization of adenosine A2A-receptors in rat brain with [3H] ZM-241385. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2002;366(5):478–481. doi: 10.1007/s00210-002-0613-3. [DOI] [PubMed] [Google Scholar]
- Deng YP, Albin RL, Penney JB, Young AB, Anderson KD, Reiner A. Differential loss of striatal projection systems in Huntington’s disease: a quantitative immunohistochemical study. Journal of Chemical Neuroanatomy. 2004;27:143–164. doi: 10.1016/j.jchemneu.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Devine DP. Self-injurious behaviour in autistic children: a neuro-developmental theory of social and environmental isolation. Psychopharmacology. 2014;231(6):979–997. doi: 10.1007/s00213-013-3279-2. [DOI] [PubMed] [Google Scholar]
- Estes A, Shaw DW, Sparks BF, Friedman S, Giedd JN, Dawson G, Dager SR. Basal ganglia morphometry and repetitive behavior in young children with autism spectrum disorder. Autism Research. 2011;4(3):212–220. doi: 10.1002/aur.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DW, Leckman JF, Carter A, Reznick JS, Henshaw D, King RA, Pauls D. Ritual, habit, and perfectionism: the prevalence and development of compulsive-like behavior in normal young children. Child Development. 1997;68(1):58–68. doi: 10.2307/1131925. [DOI] [PubMed] [Google Scholar]
- Fazzi E, Lanners J, Danova S, Ferrarri-Ginevra O, Gheza C, Luparia A, Lanzi G. Stereotyped behaviours in blind children. Brain & Development. 1999;21(8):522–528. doi: 10.1016/S0387-7604(99)00059-5. [DOI] [PubMed] [Google Scholar]
- Fredericksen KA, Cutting LE, Kates WR, Mostofsky SH, Singer HS, Cooper KL, Lanham DC, Kaufmann WE. Disproportionate increases of white matter in right frontal lobe in Tourette syndrome. Neurology. 2002;58(1):85–89. doi: 10.1212/WNL.58.1.85. [DOI] [PubMed] [Google Scholar]
- Frith U. Cognitive mechanisms in autism: experiments with color and tone sequence production. Journal of Autism & Childhood Schizophrenia. 1972;2(2):160–173. doi: 10.1007/BF01537569. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Borroto-Escuela DO, Guescini M, Fernández-Dueñas V, Tanganelli S, Agnati LF. Adenosine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neuroscience & Therapeutics. 2010;16(3):e18–e42. doi: 10.1111/j.1755-5949.2009.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Grabli D, McCairn K, Hirsch EC, Agid Y, Féger J, François C, Tremblay L. Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain: A Journal of Neurology. 2004;127(9):2039–2054. doi: 10.1093/brain/awh220. [DOI] [PubMed] [Google Scholar]
- Hadley C, Hadley B, Ephraim S, Yang M, Lewis MH. Spontaneous stereotypy and environmental enrichment in deer mice (Peromyscus maniculatus): reversibility of experience. Applied Animal Behaviour Science. 2006;97(2–4):312–322. doi: 10.1016/j.applanim.2005.08.006. [DOI] [Google Scholar]
- Harlow HF, Harlow MK. Social deprivation in monkeys. Scientific American. 1962;207:136–146. doi: 10.1038/scientificamerican1162-136. [DOI] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, Buchsbaum M. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biological Psychiatry. 2005;58(3):226–232. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Stacey ME, Coppola R, Handel SF, Rickler KC, Weinberger DR. Cerebral morphometric abnormalities in Tourette’s syndrome: a quantitative MRI study of monozygotic twins. Neurology. 1995;45(6):1176–1182. doi: 10.1212/WNL.45.6.1176. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:2217–250. [PubMed] [Google Scholar]
- Karcz-Kubicha M, Ferré S, Díaz-Ruiz O, Quiroz-Molina C, Goldberg SR, Hope BT, Morales M. Stimulation of adenosine receptors selectively activates gene expression in striatal enkephalinergic neurons. Neuropsychopharmacology. 2006;31(10):2173–2179. doi: 10.1038/sj.npp.1301035. [DOI] [PubMed] [Google Scholar]
- Kates WR, Lanham DC, Singer HS. Frontal white matter reductions in healthy males with complex stereotypies. Pediatric Neurology. 2005;32(2):109–112. doi: 10.1016/j.pediatrneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- King BH, Hollander E, Sikich L, McCracken JT, Scahill L, Bregman JD, Ritz L. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Archives of General Psychiatry. 2009;66(6):583–590. doi: 10.1001/archgenpsychiatry.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Kreitzer AC. Striatal mechanisms underlying movement, reinforcement, and punishment. Physiology. 2012;27(3):167–177. doi: 10.1152/physiol.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature Neuroscience. 2012;15(6):816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KS, Aman MG. The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2007;37:855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Langen M, Durston S, Staal WG, Palmen SC, van Engeland H. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biological Psychiatry. 2007;62(3):262–266. doi: 10.1016/j.biopsych.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Langen M, Schnack HG, Nederveen H, Bos D, Lahuis BE, de Jonge MV, Durston S. Changes in the developmental trajectories of striatum in autism. Biological Psychiatry. 2009;66(4):327–333. doi: 10.1016/j.biopsych.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Langen M, Kas MH, Staal WG, van Engeland H, Durston S. The neurobiology of repetitive behavior: of mice…. Neuroscience and Biobehavioral Reviews. 2011;35(3):345–355. doi: 10.1016/j.neubiorev.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Langen M, Leemans A, Johnston P, Ecker C, Daly E, Murphy CM, Murphy DM. Fronto-striatal circuitry and inhibitory control in autism: findings from diffusion tensor imaging tractography. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. 2012;48(2):183–193. doi: 10.1016/j.cortex.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Langen M, Bos D, Noordermeer SS, Nederveen H, van Engeland H, Durston S. Changes in the development of striatum are involved in repetitive behavior in autism. Biological Psychiatry. 2014;76(5):405–411. doi: 10.1016/j.biopsych.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Leekam SR, Prior MR, Uljarevic M. Restricted and repetitive behaviors in autism spectrum disorders: a review of research in the last decade. Psychological Bulletin. 2011;137(4):562–593. doi: 10.1037/a0023341. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Bodfish JW. Repetitive behavior disorders in autism. Mental Retardation and Developmental Disabilities Research Reviews. 1998;4:80–89. doi: 10.1002/(SICI)1098-2779(1998)4:2<80::AID-MRDD4>3.0.CO;2-0. [DOI] [Google Scholar]
- Lewis MH, Kim SJ. The pathophysiology of restricted repetitive behavior. Journal of Neurodevelopmental Disorders. 2009;1:114–132. doi: 10.1007/s11689-009-9019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz CK, Davis EB, Ruggiero AM, Suomi SJ. Early predictors of self-biting in socially-housed rhesus macaques (Macaca mulatta) American Journal of Primatology. 2007;69(5):584–590. doi: 10.1002/ajp.20370. [DOI] [PubMed] [Google Scholar]
- Mason G, Rushen J, editors. Stereotypic animal behaviour: fundamentals and applications to welfare. Wallingford: CABI; 2006. [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism‐like behavioral phenotypes in BTBR T+ tf/J mice. Genes, Brain, and Behavior. 2008;7(2):152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Miller N, Neuringer A. Reinforcing variability in adolescents with autism. Journal of Applied Behavior Analysis. 2000;33(2):151–165. doi: 10.1901/jaba.2000.33-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney EL, Gray KM, Tonge BJ, Sweeney DJ, Taffe JR. Factor analytic study of repetitive behaviours in young children with pervasive developmental disorders. Journal of Autism and Developmental Disorders. 2009;39(5):765–774. doi: 10.1007/s10803-008-0680-5. [DOI] [PubMed] [Google Scholar]
- Moss J, Oliver C, Arron K, Burbidge C, Berg K. The prevalence and phenomenology of repetitive behavior in genetic syndromes. Journal of Autism and Developmental Disorders. 2009;39:572–88. doi: 10.1007/s10803-008-0655-6. [DOI] [PubMed] [Google Scholar]
- Muehlmann AM, Edington G, Mihalik AC, Buchwald Z, Koppuzha D, Korah M, Lewis MH. Further characterization of repetitive behavior in C58 mice: developmental trajectory and effects of environmental enrichment. Behavioral Brain Research. 2012;235(2):143–149. doi: 10.1016/j.bbr.2012.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlmann, A.M., Buchwald, Z., Edington, G., Lewis, M.H. (2013). Neuronal hypoactivation of the subthalamic nucleus in an inbred model of restricted, repetitive behavior. Society for Neuroscience Abstracts, 43.
- Neuringer A. Reinforced variability in animals and people: implications for adaptive action. American Psychologist. 2004;59(9):891–906. doi: 10.1037/0003-066X.59.9.891. [DOI] [PubMed] [Google Scholar]
- Pietrefesa AS, Evans DW. Affective and neuropsychological correlates of children’s rituals and compulsive-like behaviors: continuities and discontinuities with obsessive-compulsive disorder. Brain and Cognition. 2007;65(1):36–46. doi: 10.1016/j.bandc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Powell SB, Newman HA, McDonald TA, Bugenhagen P, Lewis MH. Development of spontaneous stereotyped behavior in deer mice: effects of early and late exposure to a more complex environment. Developmental Psychobiology. 2000;37(2):101–108. doi: 10.1002/1098-2302(200009)37:2<100::AID-DEV5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Presti MF, Lewis MH. Striatal opioid peptide content in an animal model of spontaneous stereotypic behavior. Behavioral Brain Research. 2005;157:363–368. doi: 10.1016/j.bbr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. The British Journal of Psychiatry. 2009;195(5):393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Rapp JT, Vollmer TR. Stereotypy I: a review of behavioral assessment and treatment. Research in Developmental Disabilities. 2005;26(6):527–547. doi: 10.1016/j.ridd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Bradshaw JL, Moss SA, Brereton AV, Tonge BJ. Pseudo-random number generation in children with high-functioning autism and Asperger’s disorder: further evidence for a dissociation in executive functioning? Autism. 2006;10(1):70–85. doi: 10.1177/1362361306062011. [DOI] [PubMed] [Google Scholar]
- Rodriguez NM, Thompson RH. Behavioral variability and autism spectrum disorders. Journal of Applied Behavior Analysis. 2015;48:1–21. doi: 10.1002/jaba.164. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, Tregellas JR. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry. 2006;6:56. doi: 10.1186/1471-244X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Andersen‐Wood L, Beckett C, Bredenkamp D, Castle J, Groothues C, O’Connor TG. Quasi‐autistic patterns following severe early global privation. Journal of Child Psychology and Psychiatry. 1999;40(4):537–549. doi: 10.1111/1469-7610.00472. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS. Social deficits, stereotypy, and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behavioral Brain Research. 2010;208(1):178. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Takashima S. Neurotransmitter changes in the pathophysiology of Lesch-Nyhan syndrome. Brain & Development. 2000;22(Suppl1):S122–S131. doi: 10.1016/S0387-7604(00)00143-1. [DOI] [PubMed] [Google Scholar]
- Sasson NJ, Turner‐Brown LM, Holtzclaw TN, Lam KL, Bodfish JW. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research. 2008;1(1):31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1999;23(4):613–624. doi: 10.1016/S0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Shao Y, Cuccaro M, Hauser E, Raiford K, Menold M, Wolpert CM, Pericak-Vance M. Fine mapping of autistic disorder to chromosome 15q11-q13 by use of phenotypic subtypes. American Journal of Human Genetics. 2003;72(3):539. doi: 10.1086/367846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer HS. Motor stereotypies. Seminars in Pediatric Neurology. 2009;16(2):77–81. doi: 10.1016/j.spen.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Singer HS. Motor control, habits, complex motor stereotypies, and Tourette syndrome. Annals of the New York Academy of Sciences. 2013;1304(1):22–31. doi: 10.1111/nyas.12281. [DOI] [PubMed] [Google Scholar]
- Starr PA, Kang GA, Heath S, Shimamoto S, Turner RS. Pallidal neuronal discharge in Huntington’s disease: support for selective loss of striatal cells originating the indirect pathway. Experimental Neurology. 2008;211(1):227–233. doi: 10.1016/j.expneurol.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura Y, Yang MC, Lewis MH. Procedural learning and cognitive flexibility in a mouse model of restricted, repetitive behaviour. Behavioural Brain Research. 2008;189(2):250–256. doi: 10.1016/j.bbr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Tanimura Y, Vaziri S, Lewis MH. Indirect basal ganglia pathway mediation of repetitive behavior: attenuation by adenosine receptor agonists. Behavioural Brain Research. 2010;210(1):116–122. doi: 10.1016/j.bbr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura Y, Yang MK, Ottens AK, Lewis MH. Development and temporal organization of repetitive behavior in an animal model. Developmental Psychobiology. 2010;52(8):813–824. doi: 10.1002/dev.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura Y, King MA, Williams DK, Lewis MH. Development of repetitive behavior in a mouse model: roles of indirect and striosomal basal ganglia pathways. International Journal of Developmental Neuroscience. 2011;29(4):461–467. doi: 10.1016/j.ijdevneu.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E. Rhythmical stereotypies in normal human infants. Animal Behaviour. 1979;27(3):699–715. doi: 10.1016/0003-3472(79)90006-X. [DOI] [PubMed] [Google Scholar]
- Turner CA, Lewis MH. Environmental enrichment: effects on stereotyped behavior and neurotrophin levels. Physiology & Behavior. 2003;80(2–3):259–266. doi: 10.1016/j.physbeh.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Turner CA, Yang MC, Lewis MH. Environmental enrichment: effects on stereotyped behavior and regional neuronal metabolic activity. Brain Research. 2002;938(1–2):15–21. doi: 10.1016/S0006-8993(02)02472-1. [DOI] [PubMed] [Google Scholar]
- Turner CA, Lewis MH, King MA. Environmental enrichment: effects on stereotyped behavior and dendritic morphology. Developmental Psychobiology. 2003;43(1):20–27. doi: 10.1002/dev.10116. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding J, Feng G. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448(7156):894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Moss SA, Bradshaw JL, Rinehart NJ. Random number generation in autism. Journal of Autism and Developmental Disorders. 2002;32(1):43–47. doi: 10.1023/A:1017904207328. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Hazlett HC, Lightbody AA, Reiss AL, Piven J. Repetitive and self-injurious behaviors: associations with caudate volume in autism and fragile X syndrome. Journal of Neurodevelopmental Disorders. 2013;5(1):12. doi: 10.1186/1866-1955-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Botteron KN, Dager SR, Elison JT, Estes AM, Gu H, Piven J. Longitudinal patterns of repetitive behavior in toddlers with autism. Journal of Child Psychology And Psychiatry. 2014;55(8):945–953. doi: 10.1111/jcpp.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CC, Leon M. Environmental enrichment as an effective treatment for autism: a randomized controlled trial. Behavioral Neuroscience. 2013;127(4):487–497. doi: 10.1037/a0033010. [DOI] [PubMed] [Google Scholar]
- Zimmerman AM, Abrams MT, Giuliano JD, Denckla MB, Singer HS. Subcorticol volumes in girls with Tourette syndrome: support for a gender effect. Neurology. 2000;54(12):2224–2229. doi: 10.1212/WNL.54.12.2224. [DOI] [PubMed] [Google Scholar]


