Abstract
Background
This study compared safety and efficacy of first- and second-generation DES in an unrestricted, real-life population of diabetic patients undergoing PCI.
Material/Methods
The study was a subanalysis of diabetic patients from the all-comer Katowice-Zabrze Registry of patients undergoing PCI with the implantation of either first- (Paclitaxel-, Sirolimus-eluting stents) or second-generation DES (Zotarolimus-, Everolimus-, Biolimus-eluting stents). Efficacy defined as major adverse cardiac and cerebrovascular events (MACCE: death, myocardial infarction, target vessel revascularization, stroke) and safety defined as stent thrombosis (ST) were evaluated at 1 year.
Results
From the total of 1916 patients, 717 were diabetics. Among them, 257 (36%) were treated with first-generation DES (230 [89%] Paclitaxel-eluting stents, 27 [11%] Sirolimus-eluting stents), 460 with second-generation DES (171 [37%] Zotarolimus-eluting stents, 243 [53%] Everolimus-eluting stents, 46 [10%] Biolimus-eluting stents). Rate of MACCE was equal in both groups (p=0.54). Second-generation DES had a better safety profile than first-generation DES (log-rank for cumulative ST at 1 year p<0.001). First-generation DES was a risk factor for ST (HR 5.75 [1.16–28.47], p=0.03) but not for MACCE (HR 0.89 [0.6–1.32], p=0.57).
Conclusions
In a real-life setting of diabetic patients undergoing PCI, second-generation DES had lower risk of ST and similar MACCE rate compared to first-generation DES.
MeSH Keywords: Coronary Thrombosis, Diabetes Mellitus, Drug-Eluting Stents, Percutaneous Coronary Intervention
Background
Interventional treatment of patients with coronary artery disease and diabetes remains a challenge. This group is known to suffer from greater burden and more rapid progression of coronary atherosclerosis compared to non-diabetic patents. This is the effect of several cardiovascular risk factors associated with diabetes mellitus (DM), which make percutaneous coronary interventions (PCI) more challenging and aggravate the risk for adverse outcome [1–3]. Accordingly, for patients with DM and multivessel and/or complex CAD, coronary artery bypass grafting (CABG) has better performance, and PCI is a valuable alternative in less complex cases [4,5]. The marked improvement in the efficacy and safety of PCI seen in numerous randomized trials was the response to advances in stent technology from bare metal stents (BMS) to early drug-eluting stents (DES) in the general population as well as in diabetics [6–10]. Adverse outcomes after coronary revascularization in patients with DM remain, however, a concern regarding which type of DES to use [11]. This study aimed at comparing long-term safety and efficacy after PCI with first- and second-generation DES in an unrestricted, real-life, 2-center population of diabetic patients.
Material and Methods
Study design
The Katowice-Zabrze Registry is an investigator-initiated all-comer registry of consecutive patients treated with PCI with implantation of DES. The registry was designed to evaluate the differences in outcome between first- and second-generation DES in an unrestricted population, reflecting real clinical conditions. The enrollment was conducted in 2 tertiary high-volume (together 5500 PCI/year) cardiac centers (Upper Silesian Medical Center in Katowice and 2nd Department of Cardiology, Zabrze) from 1 January 2009 to 31 December 2010. The registry retrospectively included all patients in medical records of enrolling centers who had undergone PCI with the implantation of either first- or second-generation DES. The subject for current sub-analysis of the registry was the sub-group of patients with DM, using the same inclusion criteria as for the main registry.
Basic angiographic characteristics were recorded from the medical records of coronary angiography: location of the lesion, severity of stenosis, the American College of Cardiology/American Heart Association (ACC/AHA) lesion type, thrombus, and calcifications. AHA/ACC classification is a system used for assessment of lesion morphology (length, radial distribution, angulation, accessibility, contour, calcifications, location, branch involvement, and thrombus) and provides information on the probability of procedure success or failure. In every patient, excluding patients after CABG, the severity of coronary artery disease was assessed with the SYNTAX score, a validated tool used for scoring of coronary artery disease complexity. It reflects coronary anatomy, location of the lesion, degree of stenosis, collaterals, length, calcifications, thrombotic component, number of lesions, and number of segments involved. Stents were chosen according to the operator’s decision according to current best practice, the best knowledge, and individual experience and preferences regarding particular stent characteristics suitable to lesion type found on coronary angiogram. Stent types were made of first-generation durable polymer-based DES [Paclitaxel-eluting stents (Taxus, Boston Scientific Corporation, Maple Grove, MN, USA; LucChopin1, LucChopin2, Balton, Poland) or Sirolimus-eluting stents (Cypher, Cordis, USA; Carlo, CarloS, Balton, Poland)] or second-generation DES [Everolimus-eluting stents (Promus, Boston Scientific Corporation; Xience, Xience Prime, Abbott Vascular, Santa Clara, CA, USA), Zotarolimus-eluting stents (Endeavor, Resolute, Medtronic, Minneapolis, MN, USA), and Biolimus-eluting stent (Biolimus A9, Biosensors International, Switzerland)]. In case of implantation of more than 1 stent in 1 patient, the DES implanted to the lesion or to more severe stenosis was considered as the index procedure. When patients received both first- and second-generation stents, they were considered to have received an older-generation DES. Dual antiplatelet therapy (acetylsalicylic acid and P2Y12 subtype of ADP receptor inhibitors) was prescribed for up to 12 months after the procedure in each patient. Baseline clinical, angiographic, and procedure-related data were retrospectively collected from medical records.
Follow-up
Patients were followed-up at 1 year. All information was obtained from medical records of enrolling centers. If no information was available, phone contact was attempted. In case of phone contact failure, information on clinical endpoints was obtained from the National Health Care System. The follow-up was completed in all patients.
The primary efficacy endpoint was a composite of major adverse cardiac and cerebrovascular events (MACCE), including all-cause death, non-fatal myocardial infarction (MI), target vessel revascularization (TVR), and stroke. The secondary endpoints were individual components of the primary endpoint: all-cause death, MI, TVR, stroke, and coronary artery bypass grafting (CABG). The safety of DES was defined as definite ST (acute, subacute, late, and cumulative) and gastrointestinal bleeding rates at 1 year. All endpoints for the sub-analyzed group described above were consistent with endpoints for the main registry. MI was defined according to the universal definition [12]. TVR, definite ST, acute, subacute, and late ST were defined according to the definitions of endpoints for clinical trials [13]. Gastrointestinal bleeding was considered an endpoint if it fulfilled criteria for type 3 or type 5 bleeding according to proposed definitions [14].
The study was approved by the Ethics Committee of Silesian Medical University (No. KNW/0022/KB/59/11).
Statistics
Variables were checked for normality of distribution with Shapiro-Wilks test. Continuous variables are presented as mean ±SD or median (25th; 75th percentile) and were compared with t test or Mann-Whitney test. Categorical variables are presented as percentages and were compared with chi-square test. The Kaplan-Meier method was used to present estimated incidence of endpoints and the long-rank test was used to assess differences between groups. Clinical, hemodynamic, and procedural characteristics that differed significantly between groups were used for univariate Cox regression for assessing the influence on clinical endpoints. Multivariate Cox regression model for primary and secondary endpoints and ST included all variables statistically significant in univariate analysis. All tests were 2-tailed and the value of p<0.05 was considered significant. Statistical analysis was performed with Statistica software, version 10PL (StatSoft Inc., Tulsa, OK, USA) and GraphPad Prism software version 6.00 (GraphPad, La Jolla, California, USA).
Results
A total of 1916 patients were enrolled into the registry. Of them, 717 (37%) patients had diabetes, in which the present analysis was conducted. Within this group, 257 patients (36%) were treated with first-generation DES (of them 230 [89%] Paclitaxel-eluting stents, 27 [11%] Sirolimus-eluting stents) and 460 (64%) with second-generation DES (of them 46 [10%] Biolimus-eluting stents, 243 [53%] Everolimus-eluting stents, 171 [37%] Zotarolimus-eluting stents).
Both groups had comparable baseline demographic profiles, prior revascularization, and cardiovascular risk factors (Table 1). Patients who received second-generation DES had higher EF (54 [45;60] vs. 50 [40;55]%, p=0.004), and more often suffered from renal insufficiency (26% vs. 19%, p=0.03) in comparison to patients with first-generation DES.
Table 1.
Clinical characteristics.
| Characteristic | First-generation DES (n=257) | Second-generation DES (n=460) | p value |
|---|---|---|---|
| Male sex | 123 (48) | 253 (55) | 0.07 |
| Age (years) | 66 (60;72) | 67 (60;72) | 0.70 |
| BMI (kg/m2) | 30.9 (27.2;34.5) | 29.8 (27.1;32.9) | 0.15 |
| Obesity | 85 (33) | 142 (31) | 0.54 |
| Renal insufficiency | 48 (19) | 119 (26) | 0.03 |
| Ejection fraction (%) | 50 (40;55) | 54 (45;60) | 0.004 |
| CCS | 3 (2;4) | 3 (2;4) | 0.92 |
| Hypertension | 238 (93) | 434 (94) | 0.36 |
| Dyslipidemia | 175 (68) | 304 (66) | 0.58 |
| Smoker | 40 (16) | 72 (16) | 0.98 |
| Familial history of CAD | 71 (28) | 146 (32) | 0.25 |
| Prior AMI | 118 (46) | 230 (50) | 0.29 |
| Prior PCI | 132 (51) | 265 (58) | 0.11 |
| Prior CABG | 51 (20) | 98 (21) | 0.64 |
| Carotid atherosclerosis | 13 (5) | 36 (8) | 0.16 |
| PAD | 21 (8) | 56 (12) | 0.10 |
| Diagnosis | |||
| ACS | 171 (67) | 331 (72) | 0.13 |
| Unstable Angina | 104 (40) | 244 (53) | 0.001 |
| NSTEMI | 42 (16) | 63 (14) | 0.34 |
| STEMI | 25 (10) | 24 (5) | 0.02 |
Data are presented as n (%) or median (interquartile range). DES – drug-eluting stent; BMI – body mass index; CCS – Canadian Cardiovascular Society; NYHA – New York Heart Association; CAD – coronary artery disease; AMI – acute myocardial infarction; PCI – percutaneous coronary intervention; CABG – coronary artery bypass grafting; PAD – peripheral artery disease; ACS – acute coronary syndrome; NSTEMI – non-ST-elevation myocardial infarction; STEMI - ST-elevation myocardial infarction.
Patients did not differ regarding treated vessel and CAD burden as measured with SYNTAX score (with median score of 15 points in both groups, p=0.4). First-generation DES were implanted to more calcified lesions with lower maximal inflation pressure and were less frequently evaluated with IVUS (Table 2). Procedures did not differ regarding length and diameter of the stent or total number of stents per lesion. Regarding clinical setting, both stent generations were implanted in equal proportions in ACS (67% for first- vs. 72% for second-generation DES, p=0.13), with second-generation predominance in UA (p=0.001) and first-generation in patients with STEMI (p=0.02) (Table 1). Angiographic outcome of the procedure was equal for first- and second-generation DES and final TIMI 3 flow was achieved in 98% and 97% of cases, respectively (p=0.41).
Table 2.
Angiographic and procedural characteristics.
| Characteristic | First-generation DES (n=257) | Second-generation DES (n=460) | p value |
|---|---|---|---|
| Culprit vessel | |||
| LM | 33 (13) | 62 (13) | 0.81 |
| LAD | 215 (84) | 373 (81) | 0.39 |
| Cx | 155 (60) | 270 (59) | 0.67 |
| RCA | 147 (57) | 274 (59) | 0.53 |
| SVG | 31 (12) | 62 (13) | 0.59 |
| AG | 8 (3) | 26 (6) | 0.13 |
| MVD | 83 (32) | 149 (32) | 0.97 |
| AHA/ACC lesion type | |||
| A | 45 (18) | 109 (24) | 0.053 |
| B | 163 (63) | 264 (57) | 0.15 |
| C | 48 (19) | 65 (14) | 0.10 |
| SYNTAX score (n=338) | 15 (8;26) | 15 (7;24) | 0.4 |
| Thrombus | 14 (5) | 17 (4) | 0.34 |
| Ostial lesion | 35 (14) | 64 (14) | 0.72 |
| De novo lesion | 118 (46) | 338 (73) | <0.001 |
| Calcifications | 44 (17) | 29 (6) | <0.001 |
| Stenosis severity (%) | 90 (70;95) | 90 (80;95) | 0.01 |
| No DES per lesion | 1 (1;1) | 1 (1;1) | 0.83 |
| Length DES | |||
| per lesion (mm) | 20 (15;28.5) | 20 (15;28) | 0.98 |
| Stent diameter (mm) | 3.2±0.5 | 3.1±0.5 | 0.22 |
| Predilatation | 132 (51) | 190 (41) | 0.052 |
| Maximal inflation pressure (atm) | 14 (12;18) | 16 (14;18) | <0.001 |
| TIMI 3 flow post-PCI | 252 (98) | 448 (97) | 0.41 |
| GPIIb/IIIa inhibitors | 16 (6) | 20 (4) | 0.27 |
| IVUS | 1 (0.4) | 11 (2.4) | 0.04 |
| Post-procedural dual antiplatelet therapy | 248 (96) | 448 (97) | 0.5 |
Data are presented as n (%), mean±SD or median (25th; 75th percentile). DES – drug-eluting stent; LM – left main; LAD – left anterior descending artery; Cx- circumflex artery; RCA – right coronary artery; SVG – saphenous graft; AG – arterial graft; TIMI – thrombosis in myocardial infarction; PCI – percutaneous coronary intervention; GPIIb/IIIa – glycoprotein IIb/IIIa receptor; IVUS – intravascular ultrasound.
Endpoints
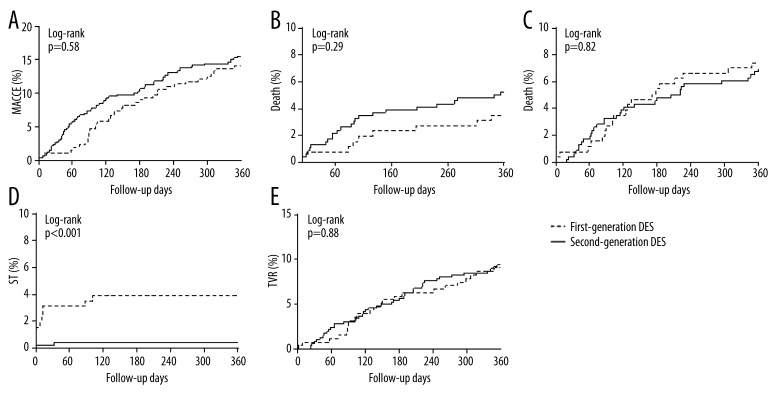
Procedures with first- and second-generation DES were equally efficient, with no significant difference in the incidence of the primary and secondary endpoint at 1 year (Table 3). The Kaplan-Meier curves, presented in Figure 1, show the incidence of MACCE. In univariate Cox regression model, significant factors for prediction of MACCE were renal insufficiency (HR 1.82 [1.23–2.7], p=0.003), ejection fraction (HR 0.97 [0.96–0.98], p<0.001), maximal concentration of troponin (1.1 [1.04–1.18], p=0.001) and CK-MB (HR 1.003 [1.001–1.01], p=0.002), and the diagnosis of STEMI (HR 2.0 [1.12–3.56], p=0.02). After adjustment, only renal insufficiency (HR 1.69 [1.13–2.52], p=0.01) and ejection fraction (HR 0.98 [0.96–0.99] p=0.003) remained statistically significant predictors of MACCE (Table 4). Regarding the incidence of death, significant predictors in univariate analysis were renal insufficiency (HR 4.07 [2.09–7.91], p<0.001), ejection fraction (HR 0.92 [0.9–0.95], p<0.001), NYHA (HR 1.89 [1.28–2.8], p=0.001), maximal concentration of troponin (HR 1.16 [1.09–1.24], p<0.001) and CK-MB (HR 1.005 [1.003–1.008], p<0.001), and the diagnosis of STEMI (HR 3.66 [1.6–8.39], p=0.002). After adjustment, in the multivariate model, factors statistically significant for the prediction of death were renal insufficiency (HR 3.32 [1.65–6.68], p<0.001) and ejection fraction (HR 0.93 [0.91–0.96], p<0.001) (Table 4). The safety profile in acute and subacute setting was better after implantation of second-generation DES when compared to first-generation DES (0.2% vs. 1.9%, p=0.02 for acute and 0% vs. 1.2%, p=0.02 for subacute ST). This advantage was not further observed in 1-year follow-up, with no statistically significant difference in late ST (0.2% vs. 0.8%, p=0.27) (Figure 2). The incidence of ST over time is presented with Kaplan-Meier curves (Figure 1D). There was an early and continuous separation of curves in favor of second-generation DES. The generation of DES was an independent risk factor in Cox regression model for cumulative ST at 1 year (HR 9.07 [1.99–41.39], p=0.004). Other factors predictive for cumulative ST were the diagnosis of STEMI (HR 7.04 [2.12–23.39], p=0.001), ejection fraction (HR 0.95 [0.91–0.99], p=0.03), de novo lesion (HR 0.99 [0.97–0.998], p=0.02), and maximal inflation pressure (HR 0.79 [0.65–0.95], p=0.01). In multivariate Cox analysis, the generation of DES remained a predictive factor for cumulative ST (HR 5.75 [1.16–28.47], p=0.03) together with the diagnosis of STEMI (HR 4.38 [1.21–15.9], p=0.02) (Table 4). The rates of gastrointestinal bleeding were low and did not differ between groups (p=0.5) (Table 3).
Table 3.
Clinical outcomes at 1 year.
| Characteristic | First-generation DES (n=257) | Second-generation DES (n=460) | p value |
|---|---|---|---|
| Stent thrombosis (ST) | |||
| Acute ST | 5 (1.9) | 1 (0.2) | 0.02 |
| Subacute ST | 3 (1.2) | 0 (0) | 0.046 |
| Late ST | 2 (0.8) | 1 (0.2) | 0.29 |
| Cumulative ST | 10 (3.9) | 2 (0.4) | 0.001 |
| Primary end point | |||
| MACCE | 37 (14.4) | 74 (16.1) | 0.54 |
| Secondary end point | |||
| Death | 10 (3.9) | 25 (5.4) | 0.36 |
| AMI | 16 (6.2) | 27 (5.9) | 0.84 |
| TVR | 23 (8.9) | 44 (9.6) | 0.79 |
| Stroke | 2 (0.8) | 4 (0.9) | 0.90 |
| CABG | 5 (1.9) | 8 (1.7) | 0.84 |
| Gastrointestinal bleeding | 5 (1.9) | 6 (1.3) | 0.50 |
Data are presented as n (%).DES – drug-eluting stent; ST – stent thrombosis; MACCE – major adverse cardiovascular events; AMI – acute myocardial infarction; TVR – target vessel revascularization; CABG – coronary artery bypass grafting.
Figure 1.
Kaplan-Meier curves for the incidence of major adverse cardiac events (MACE) (A), death (B), myocardial infarction (MI) (C), stent thrombosis (ST), (D) target vessel revascularization (TVR), (E) DES (drug-eluting stent).
Table 4.
Outcomes of Cox regression for the prediction of MACCE, death, and cumulative ST. Data are presented as HR (95% CI).
| Univariate | Multivariate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MACCE | death | Cumulative ST | MACCE | death | Cumulative ST | |||||||
| HR | p | HR | p | HR | p | HR | p | HR | p | HR | p | |
| First-generation DES | 0.89 (0.6–1.32) | 0.57 | 0.71 (0.34–1.48) | 0.36 | 9.07 (1.99–41.39) | 0.004 | – | – | – | – | 5.75 (1.16–28.47) | 0.03 |
| Renal insufficiency | 1.82 (1.23–2.7) | 0.003 | 4.07 (2.09–7.91) | <0.001 | 1.09 (0.3–4.04) | 0.9 | 1.69 (1.13–2.52) | 0.01 | 3.32 (1.65–6.68) | <0.001 | – | – |
| Ejection fraction | 0.97 (0.96–0.98) | <0.001 | 0.92 (0.9–0.95) | <0.001 | 0.95 (0.91–0.99) | 0.03 | 0.98 (0.96–0.99) | 0.003 | 0.93 (0.91–0.96) | <0.001 | 0.97 (0.92–1.02) | 0.22 |
| NYHA | – | – | 0.89 (1.28–2.8) | 0.001 | – | – | – | – | 0.94 (0.6–1.48) | 0.79 | – | – |
| Troponin mx | 1.1 (1.04–.18) | 0.001 | 1.16 (1.09–1.24) | <0.001 | 1.09 (0.97–1.24) | 0.15 | 1.07 (0.97–1.17) | 0.16 | 1.08 (0.97–1.19) | 0.15 | – | – |
| CK-MB mx | 1.003 (1.001–1.01) | 0.002 | 1.005 (1.003–1.008) | <0.001 | 1.003 (0.998–1.009) | 0.22 | 1.001 (0.998–1.004) | 0.43 | 1.0 (0.999–1.007) | 0.14 | – | – |
| UA | 1.21 (0.83–.76) | 0.31 | 0.98 (0.51–1.94) | 0.99 | 0.76 (0.24–2.38) | 0.63 | – | – | – | – | – | – |
| STEMI | 2.0 (1.12–.56) | 0.02 | 3.66 (1.6–8.39) | 0.001 | 7.04 (2.12–23.39) | 0.001 | 1.17 (0.58–2.35) | 0.66 | 1.15 (0.4–3.29) | 0.8 | 4.38 (1.21–15.9) | 0.02 |
| De novo lesion | 1.0 (0.99–1.004) | 0.99 | 1.0 (0.996–1.02) | 0.46 | 0.99 (0.97–0.998) | 0.02 | – | – | – | – | 0.97 (0.98–1.01) | 0.59 |
| Calcifications | 0.8 (0.44–.56) | 0.52 | 0.95 (0.47–1.95) | 0.9 | 0.99 (0.81–1.22) | 0.95 | – | – | – | – | – | – |
| Severity of stenosis | 1.006 (0.99–.02) | 0.44 | 1.0 (0.97–1.03) | 0.9 | 1.0 (0.95–1.05) | 0.9 | – | – | – | – | – | – |
| Maximal inflation pressure | 1.0 (0.93–.07) | 0.92 | 0.99 (0.88–1.11) | 0.82 | 0.79 (0.65–0.95) | 0.01 | – | – | – | – | 0.84 (0.68–1.04) | 0.12 |
HR – hazard ratio; CI – confidence interval; MACCE - major adverse cardiovascular events; ST – stent thrombosis; DES – drug-eluting stents; NYHA – New York Heart Association; CK-MB – creatine kinase myocardial bound; UA – unstable angina; STEMI – ST-segment elevation myocardial infarction.
Figure 2.
The incidence of stent thrombosis (ST) by type of drug-eluting stent (DES).
Discussion
Based on the subanalysis of diabetic patients from the Katowice-Zabrze registry in a real-life setting, the implantation of second-generation DES proved to be equally efficient and to have better safety profile when compared to first-generation DES. The trend for lower rates of ST in second-generation DES was most pronounced early after stent placement and was sustained for up to 1 year. Diabetes mellitus is known to enhance the risk of ST and restenosis after PCI, already elevated by eosinophilia [15], by promoting neointimal hyperplasia, smooth muscle cell proliferation, increased platelet reactivity, local inflammatory process, and plaque growth [16]. Despite better in-stent performance of second- (Everolimus-eluting) than first-generation (Sirolimus-, Paclitaxel-eluting) DES with lower in-stent late lumen loss for Everolimus-eluting stents described in the literature [17–20], we observed equal combined event rates regardless of the type of eluting drug. Indeed, second-generation DES were safer than first-generation DES and significantly reduced the rate of ST. Thus, our results confirm superiority of second- vs. first-generation DES in patients with DM in terms of ST, first reported by Simsek et al. [21] It is also known that the presence of DM worsens the prognosis [22,23], aggravating the 10-year risk of adverse events in patients with CAD to 75% [24]. We report a relatively high rate of MACCE when compared with previous reports from RCTs on patients with DM [25,26]. First, it could reflect the high percentage of acute coronary syndrome in the population. Second, it occurred despite low median risk according to the SYNTAX score. This fact could be regarded as confirmation of the thesis that DM is a strong risk factor for adverse events, regardless of lesion complexity [25]. However, the performance of different types of DES in this setting is unknown [27]. Regarding this, the fact of equal incidence of overall MACCE in diabetic patients, regardless of the type of stent, seems interesting. A previous report suggested that the role of secondary prevention is more important than the choice of a particular DES [28]. We observed no differences in rates of individual components of MACCE (including AMI and TVR), contrary to what was reported previously in large RCTs [9,29–31], but confirmed, on the other hand, in a pooled analysis of SPIRIT II, SPIRIT III, SPIRIT IV, and COMPARE studies [22]. This observation is of great value, as most of the available data came from RCTs conducted in well-developed, Western-European countries and our registry is the first analysis in this field from central/eastern Europe on such a large population. It shows comparable outcomes, thus strengthening the recommendation for the use of second-generation DES. Moreover, to date, there have been few studies evaluating the use of first- vs. second-generation DES in diabetic patients in real-life, all-comer settings. Our study is comparable to only 1 registry [28], which concluded there was no difference between first- and second-generation DES in diabetics. Our study adds information showing the better safety of PCI with second-generation DES in an unrestricted population of diabetics, reflecting circumstances met in everyday clinical practice, with the potential for direct implementation of the outcomes in real life.
In studies (mostly RCTs) on diabetic patients that account for baseline characteristics to improve the precision of risk estimates, the reduction in ST rate with similar MACE rate shows the advantage of use of second-generation DES [29]. In light of this fact, the advantage of this real-life registry is the similarity in baseline profile between both groups, although not matched in-pair. This enables relatively thorough comparison of outcomes from previous studies, which are in line with those presented in our study.
Conclusions
The present study adds to the available data on safety and efficacy of different types of DES for PCI in diabetic patients, leading to the conclusion that the performance of second-generation DES in real-life setting of DM is advantageous in terms of safety of the procedure, especially early after stent placement. Based on this, patients with DM after implantation of first-generation of DES should undergo restrictive follow-up, especially early after the procedure, focused on signs and symptoms suggestive of ST. The implantation of second-generation of DES should be considered in every case of PCI in diabetic patients in order to reduce the rate of ST.
Limitations
Assuming that insulin-dependent DM provokes more attenuated general and in situ negative effects and plaque burden in vessels by the mechanism evolved by insulin resistance, the division in insulin-dependent and non-insulin-dependent DM patients could enrich the study and provide additional guidance on optimal choice between first- and second-generation DES for PCI in each group; however, this was not done because we did not want to lower the size of compared groups with low number of end-points (ST) in the population.
Footnotes
Conflict of interest
The authors have no financial conflicts of interest.
Source of support: Departmental sources
References
- 1.Mathew V, Holmes DR. Outcomes in diabetics undergoing revascularization: the long and the short of it. J Am Coll Cardiol. 2002;40:424–27. doi: 10.1016/s0735-1097(02)02025-9. [DOI] [PubMed] [Google Scholar]
- 2.Van Belle E, Perie M, Braune D, et al. Effects of coronary stenting on vessel patency and long-term clinical outcome after percutaneous coronary revascularization in diabetic patients. J Am Coll Cardiol. 2002;40:410–17. doi: 10.1016/s0735-1097(02)01971-x. [DOI] [PubMed] [Google Scholar]
- 3.Smith SC, Jr, Faxon D, Cascio W, et al. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group VI: revascularization in diabetic patients. Circulation. 2002;105(18):e165–69. doi: 10.1161/01.cir.0000013957.30622.05. [DOI] [PubMed] [Google Scholar]
- 4.Mohr FW, Morice MC, Kappetein A, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–38. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 5.Farkouh ME, Domanski M, Sleeper LA, et al. FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–84. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 6.Di Lorenzo E, Sauro R, Varricchio A, et al. Benefits of drug-eluting stents as compared to bare metal stent in ST-segment elevation myocardial infarction: four year results of the PaclitAxel or Sirolimus-Eluting stent vs. bare metal stent in primary angiOplasty (PASEO) randomized trial. Am Heart J. 2009;158(4):e43–50. doi: 10.1016/j.ahj.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Kirtane AJ, Ellis SG, Dawkins KD, et al. Paclitaxel-eluting coronary stents in patients with diabetes mellitus: pooled analysis from 5 randomized trials. J Am Coll Cardiol. 2008;51:708–15. doi: 10.1016/j.jacc.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 8.Sinning JM, Baumgart D, Werner N, et al. SCORPIUS Study. Five-year results of the multicenter randomized controlled open-label study of the CYPHER sirolimus-eluting stent in the treatment of diabetic patients with de novo native coronary artery lesions (SCORPIUS) study: a German multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patients. Am Heart J. 2012;163:446–453. 453.e1. doi: 10.1016/j.ahj.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Briguori C, Airoldi F, Visconti G, et al. Novel approaches for preventing or limiting events in diabetic patients (NAPLES-DIABETES) trial: a randomized comparison of 3 drug-eluting stents in diabetic patients. Circ Cardiovasc Interv. 2011;4:121–29. doi: 10.1161/CIRCINTERVENTIONS.110.959924. [DOI] [PubMed] [Google Scholar]
- 10.Cassese S, Byrne RA, Tada T, et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2014;100(2):153–59. doi: 10.1136/heartjnl-2013-304933. [DOI] [PubMed] [Google Scholar]
- 11.Bangalore S, Toklu B, Feit F. Outcomes with coronary artery bypass graft surgery versus percutaneous coronary intervention for patients with diabetes mellitus can newer generation drug-eluting stents bridge the gap? Circ Cardiovasc Interv. 2014;7:518–25. doi: 10.1161/CIRCINTERVENTIONS.114.001346. [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K, Alpert JS, Jaffe AS, et al. Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–67. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 13.Cutlip DE, Windecker S, Mehran R, et al. Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 14.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 15.Yagi H, Amiya E, AndoIn J, et al. Stent restenosis exacerbated by drug-induced severe eosinophilia after second-generation drug-eluting stent implantation. Am J Case Rep. 2014;15:397–400. doi: 10.12659/AJCR.891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstron EJ, Rutledge JC, Rogers JH. Coronary artery revascularization in patients with diabetes mellitus. Circulation. 2013;128:1675–85. doi: 10.1161/CIRCULATIONAHA.113.002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone GW, Midei M, Newman M, et al. SPIRIT III Investigators. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299(16):1903–13. doi: 10.1001/jama.299.16.1903. [DOI] [PubMed] [Google Scholar]
- 18.Grube E, Chevalier B, Guagliumi G, et al. The SPIRIT V diabetic study: a randomized clinical evaluation of the XIENCE V everolimus-eluting stent vs. the TAXUS Liberté paclitaxel-eluting stent in diabetic patients with de novo coronary artery lesions. Am Heart J. 2012;163:867–875.e1. doi: 10.1016/j.ahj.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Otake H, Ako J, Yamasaki M, et al. Comparison of everolimus- versus paclitaxel-eluting stents implanted in patients with diabetes mellitus as evaluated by three-dimensional intravascular ultrasound analysis. Am J Cardiol. 2010;106:492–97. doi: 10.1016/j.amjcard.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 20.Kim WJ, Lee SW, Park SW, et al. ESSENCE-DIABETES Study Investigators. Randomized comparison of everolimus-eluting stent versus sirolimus-eluting stent implantation for de novo coronary artery disease in patients with diabetes mellitus (ESSENCE-DIABETES): results from the ESSENCE-DIABETES trial. Circulation. 2011;124:886–92. doi: 10.1161/CIRCULATIONAHA.110.015453. [DOI] [PubMed] [Google Scholar]
- 21.Simsek C, Räber L, Magro M, et al. Long-term outcome of the unrestricted use of everolimus-eluting stents compared to sirolimus-eluting stents and paclitaxel-eluting stents in diabetic patients: The Bern-Rotterdam diabetes cohort study. Int J Cardiol. 2013;170:36–42. doi: 10.1016/j.ijcard.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Stone GW, Kedhi E, Kereiakes DJ, et al. Differential clinical responses to everolimus-eluting and paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124:893–900. doi: 10.1161/CIRCULATIONAHA.111.031070. [DOI] [PubMed] [Google Scholar]
- 23.Kereiakes DJ, Cutlip DE, Applegate RJ, et al. Outcomes in diabetic and nondiabetic patients treated with everolimus- or paclitaxel-eluting stents results from the SPIRIT IV clinical trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system) J Am Coll Cardiol. 2010;56:2084–89. doi: 10.1016/j.jacc.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 25.Kedhi E, Généreux P, Palmerini T, et al. Impact of coronary lesion complexity on drug-eluting stent outcomes in patients with and without diabetes mellitus analysis from 18 pooled randomized trials. J Am Coll Cardiol. 2014;63:2111–18. doi: 10.1016/j.jacc.2014.01.064. [DOI] [PubMed] [Google Scholar]
- 26.Jensen LO, Thayssen P, Junker A, et al. Comparison of outcomes in patients with versus without diabetes mellitus after revascularization with everolimus- and sirolimus-eluting stents (from the SORT OUT IV trial) Am J Cardiol. 2012;110(11):1585–91. doi: 10.1016/j.amjcard.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Bangalore S, Kumar S, Fusaro M, et al. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: mixed treatment comparison analysis of 22,844 patient years of follow-up from randomized trials. BMJ. 2012;345:e5170. doi: 10.1136/bmj.e5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Amico G, Fabris T, Mojoli M, et al. Impact of drug-eluting stent generation on patient- and stent-related adverse events of diabetic patients treated by percutaneous coronary intervention. Minerva Cardioangiol. 2014;62(1):9–18. [PubMed] [Google Scholar]
- 29.Jensen LO, Thayssen P, Junker A, et al. Comparison of outcomes in patients with versus without diabetes mellitus after revascularization with everolimus- and sirolimus-eluting stents (from the SORT OUT IV Trial) Am J Cardiol. 2012;110:1585–91. doi: 10.1016/j.amjcard.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Maeng M, Jensen LO, Tilsted HH, et al. Outcomes of sirolimus-eluting versus zotarolimus-eluting coronary stent implantation in patients with and without diabetes mellitus (a SORT OUT III substudy) Am J Cardiol. 2011;108:1232–37. doi: 10.1016/j.amjcard.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 31.Yan P, Dong P, Li Z. Second- versus first-generation drug-eluting stents for diabetic patients: a meta-analysis. Arch Med Sci. 2014;10(2):213–21. doi: 10.5114/aoms.2014.42571. [DOI] [PMC free article] [PubMed] [Google Scholar]