Abstract
Patient: Male, 25
Final Diagnosis: Charcot-Marie-Tooth 1
Symptoms: Muscular • spasticity
Medication: Oxandrolone
Clinical Procedure: Neural and muscle biopsies
Specialty: Neurology
Objective:
Unusual setting of medical care
Background:
Anabolic steroids have the clinical effect of increasing protein synthesis in muscle and other tissues. The brain and spinal cord neurons have gonadal steroid receptors and various studies have shown at structural and molecular levels that androgenic steroids have a significant trophic effect on the brain and spinal cord.
Case Report:
We evaluated the effect of Oxandrolone (an FDA-approved anabolic steroid) at the dose of 20 mg/day for 3 months added to concomitant exercise strength training 3 times a week in a patient affected by a demyelinating disease, Charcot-Marie-Toot 1 (CMT1). After the treatment, an increase in muscular strength and walking capacity was observed. Muscle biopsy revealed a significant increase of type grouping of muscle fibers, an expression of regeneration and reinnervation processes.
Conclusions:
Data ensuing from this single case-report suggest that anabolic androgenic steroids have a potential neuroregenerative effect, with an inherent improvement in neuromuscular efficiency through an increased myelin synthesis at peripheral nervous system site.
MeSH Keywords: Charcot-Marie-Tooth Disease, Demyelinating Diseases, Myelin P2 Protein, Oxandrolone
Background
Oxandrolone is a synthetic analog of testosterone, used to preserve or restore muscle mass in different clinical conditions and to promote beneficial clinical outcomes in the treatment of wasting and catabolic disorders [1]. The indications for using oxandrolone, and generally all anabolic drugs, are neuromuscular diseases and catabolic muscular conditions, with the aim of stimulating muscle regeneration, as in the treatment of Duchenne dystrophy [2] and myositis [3], improving muscle mass and strength [4], increasing exercise tolerance [5], and facilitating up-regulation of androgen receptors [6].
Androgens also exert important activities in the central (CNS) and peripheral nervous system, affecting motor function, axon growth and neurite extension in motoneurons [7]. Testosterone and its derivatives play a fundamental role in the regulation of the regenerative process of injured motoneurons by reducing the extent of denervation atrophy and increasing the rate of axonal regeneration [8]. Androgens also exert a stimulatory action on the protein synthesis of peripheral myelin, which might have clinical significance when the rebuilding of myelin is needed, as in peripheral injury and demyelinating diseases [9]. In a patient affected by Charcot-Marie-Toot I (CMT-I) disease, a hereditary motor and sensory neuropathy characterized by a severe demyelinating process [10], we tested the hypothesis that Oxandrolone, added to a physical rehabilitation program, could increase muscle and nerve protein synthesis and improve strength and motor control.
Case Report
A 25-year-old man with a severe demyelinating neuropathy presented with spastic tetraparesis, muscle weakness, spasticity, and instability during deambulation. The subject was 168 cm in height, and 56 kg in weight. A strength test, a walking test, and sensory nerve conduction velocity (SNCV) and compound muscle action potential (CMAP) for the median and sural nerves were assessed. A balanced diet (40 Kcal/kg body weight) and an exercise training program were prescribed throughout the treatment.
Exercise training program
The exercise training program included isotonic exercises with free weights and isotonic machines with a frequency of 3 times a week, training 2 or 3 muscle groups each session under the supervision of a Health and Fitness Instructor. One exercise for each muscular group was programmed, starting with the minimum effort tolerated by the patients. Initially, the patient could exercise each muscle group only with 2 series of 6–8 repetitions due to muscular weakness.
Strength
Maximal voluntary muscle strength was assessed by using the 1-repetition maximum (1-RM) method. In addition, it was administered Oxandrolone 20 mg daily per os for 3 months. Every 15 days, 2000 U β-HCG (human chorionic gonadotropin) was also injected intramuscularly to prevent testicular inhibition.
Muscle and nerve biopsy
We obtained signed informed consent from the patient for nerve and muscle biopsies. Before treatment, the sural nerve biopsy showed peripheral neuropathy involving the myelinic axons (Figure 1). Onion bulbs in the nerves of patients with CMT1 are characteristic of the disease and are present in several presentations of CMT1 [11]. The name is due to their resemblance to the layers of an onion sliced in half. After the program, a muscle biopsy was obtained from the quadriceps femoris. The specimen was frozen in isopentane (BDH) and cooled to −160°C in liquid nitrogen. Serial 10-μm-thick frozen sections were prepared for histological and histochemical examination, as previously reported [12]. Stains used included hematoxylin-eosin (H&E), modified Gomori trichrome, myofibrillar adenosine triphosphatase (ATPase) at pH of 9.4, 4.6, and 4.35, NADH-tetrazolium reductase (NADH-Tr), succinic dehydrogenase (SDH), lactic dehydrogenase (LDH), cytochrome c oxidase (COX), Oil red O, myoadenylate deaminase (MAD), and periodic acid Schiff with and without diastase. NADH (in black) show aspects of type grouping: enlargement ×40 and ×100, reaction ATPase at pH 9.4×100 e ×40. It is evidence of an extensive type grouping. The fibres are hypotrophic and others are atrophic and angulate (Figures 2, 3). An extensive type-grouping consisting of cluster of type II fibres is suggestive of a reinnervation process. In histochemical stains, such motor units appear as groups of myofibers of the same histochemical type (fiber type grouping).
Figure 1.
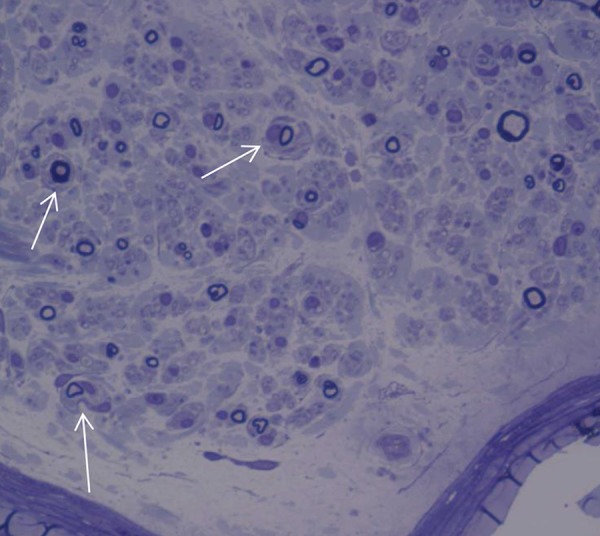
Sural nerve biopsy. Semi-thin section. Note rarefaction of myelinated fibers, foldings of myelin, and onion bulb proliferations of Schwann cells indicated by the arrows (enlargement ×40).
Figure 2.
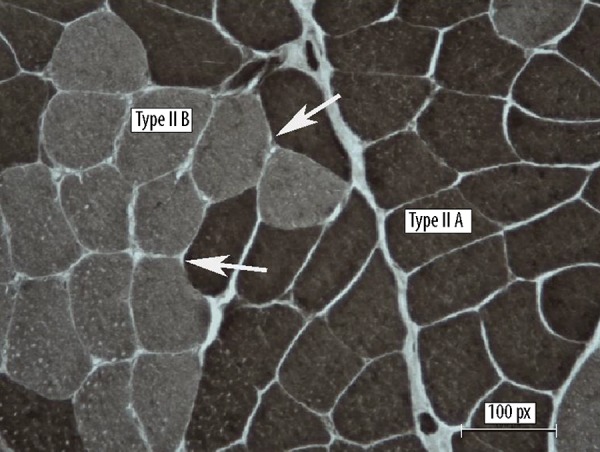
Muscle biopsy. Pattern of type grouping, reaction ATPasi pH 9,4 NADH-Tr (Hematoxylin-eosin (H&E), NADH (in black) show aspects of type grouping (enlargement ×100, Barr 1 μ). The arrows indicate clusters of new fibres. Fibres II A (black), Fibres II B (green).
Figure 3.
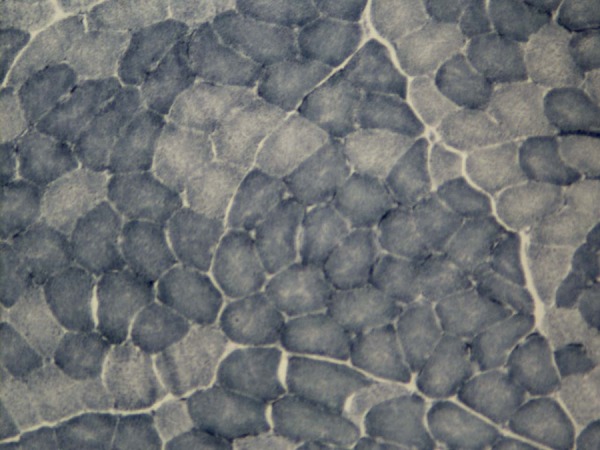
Same muscle biopsy. The type grouping is more evident, showing an increased clustering of muscle fibers of the same metabolic type (enlargement ×40).
Results
After the 3-month therapy, the patient gained 5.0 kg of lean body mass. Muscular strength increased significantly: maximum voluntary contraction at bench press increased from 10 to 25 kg, dumbbell fly capacity increased from 2 to 8 kg, and leg press capacity increased from 15 to 40 kg. The Walking Index (WISCI) [13] improved from level 6 to 14. The SNCV increased from 20.3 to 22 m/s in the medial nerve and 19.6 to 21.5 m/s in the tibial nerve, respectively. Similarly, the CMAP increased from 3.1 to 3.4mV and from 1.0 mV to 1.3 mV in medial and tibial nerves, respectively. The muscle biopsy showed an extensive type-grouping, consisting of cluster of types, suggesting a reinnervation process.
Discussion
Androgens exert trophic effects on the spinal cord and motoneuron, at both structural and molecular levels, and stimulate nerve regeneration. The population of neurons within the brain and spinal cord, including spinal motor neurons, contain gonadal steroid receptors [14]. Androgens increase protein synthesis in CNS and peripheral nervous system, as well, and stimulate the mRNA, either in vitro and in vivo Schwann cells [7–9]. Jones et al. [15] explored the trophic capabilities of steroids as therapeutic agents in neuronal injury and repair and demonstrated that androgens act either directly or indirectly on the injured motoneurons, by enhancing neuronal reparative response and successful functional recovery. Androgens may also modulate the central glia response to nerve damage. This stimulatory action on the proteins of peripheral myelin affects many aspects of neuronal functioning, including nerve-cell survival, growth and metabolism, elaboration of processes, synaptogenesis and neurotransmission [15]. In our patient affected by CMT1, sensory loss, muscle weakness and atrophy were due to axonal loss [16]. The genes causing the CMT1 disease encode myelin-related proteins: peripheral myelin protein 22 (PMP22), myelin protein zero (P0) and connexin 32 (Cx32) [10] and the PMP22 gene is found in most patients with demyelinating CMT1A [17]. Magnaghi et al. [18] demonstrated that Po and PMP22 proteins play a crucial physiological role in the maintenance of the multilamellar structure of peripheral myelin and are androgen dependent. They demonstrated the positive effects of sex steroid hormones on the gene expressions of Po and PMP22, suggest that a treatment with these molecules or their synthetic agonists may be useful in cases in which the rebuilding of myelin is necessary [19].
The increased lean body mass, muscular strength, after Oxandrolone therapy suggests a recovery of motor neuronal function. The motor control and function were increased and thus a remyelination process could be inferred. The muscle biopsy showed an extensive type-grouping and angular atrophic fibers that were scattered or in small groups, findings commonly described as neuropathic [20]. Type-grouping typically occurs after crush injury and after autograft or single-lumen nerve graft repair [21], whereas the number of nerve fibers and the number of alpha-nerve fibers increased in this group and can be explained by increased regenerating axons branches traveling along the periphery. Rhrich-Haddout et al. [22] demonstrated that, after a local spinal injury, typical alpha-motoneurons can reinnervate a skeletal muscle by regenerating axons after peripheral nerve graft. The emerging branches are kept together by Schwann cell basal lamina scaffolds, suggesting an increase in the synthesis of myelin proteins [23]. Neurophysiological studies showed an active denervation-reinnervation process in CMT 1 [24] that changes considerably during lifetime. To distinguish between a diseased and a normal muscle, quantitative data on the fibers-type arrangement is required. In our patient, the extensive type-grouping showed a prevalence of reinnervation. The neural amyelinic degeneration state in this patient is a condition, that exercise training alone could not be responsible for the observed recovery. In fact, exercise improve modestly the muscular strength in multiple sclerosis (4.5–36%) [25] and in patients with mild to moderate disability [26]; but in CMT1 patients small trials of exercise have shown no significant benefits [27] to lower myelination. The loss of myelin of the axons, evidenced by neural biopsy, explains the severity of clinical disease and the axonal dysfunction related to the myelination status. Motor axonal dysfunction is the major cause of muscle dysfunction, impaired motor control, muscle weakness, atrophy, sensory loss, and reduced strength [28]. The increase in muscle mass, strength, and motor control induced by Oxandrolone may be due to the increased protein synthesis in the muscles and in myelin of the motoneurons. Neuroactive steroids, like progesterone, dihydroprogesterone, tetrahydroprogesterone testosterone, and dihydrotestosterone are involved in the control of gene expression of myelin proteins in the peripheral nervous system, and are also capable to influence peripheral glial elements and their specific products like myelin membranes [29]. In the adult injured spinal cord and motoneurons, either by disease or by experimentally-induced trauma, an axonal repair (due to myelin associated inhibitors) is observed [30] and the axons may extend over very long distances; these capabilities persist even in neurons reprogrammed from very aged human cells [31]. This process appears to be responsive to exogenous and/or endogenous androgen. This finding might be of relevance for new therapeutic approaches for recovery of motoneuron function and muscle reinnervation, rebuilding the peripheral myelin in demyelinating diseases.
Conclusions
In conclusion, in the patient of this case-report, treatment with Oxandrolone added to exercise improves neuromuscular function, likely as a consequence of reinnervation of the muscle, as evidenced by type-grouping and recovery of motoneuron function. This observation suggests that treatment with Oxandrolone may be useful to restore functional axon and motoneuron efficiency and could be considered within rehabilitation programs in patients with neuromuscular damages, such as demyelinating diseases, hereditary neuropathy, aging, and after peripheral injury. Furthermore, type grouping can also be applied to investigate the accuracy of reinnervation.
Limitations of the study
This is a standalone case report and a case series of a treatment would have obviously strengthened our findings. However, we have treated other patients with similar diseases and paraplegic subjects after spinal cord injury and in our experience, all patients benefit from androgen therapy. In addition, muscle biopsy immunohistochemistry could be more appropriate to evaluate the various types of protein in the muscle. The aim of this study is to increase the interest to other authors in further research because studies in this field are clearly warranted and useful for clinical practice.
Footnotes
Conflict of interest
There is no conflict of interest to declare.
References:
- 1.Orr R, Fiatarone Singh M. The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: review of efficacy and safety. Drugs. 2004;64(7):725–50. doi: 10.2165/00003495-200464070-00004. [DOI] [PubMed] [Google Scholar]
- 2.Fenichel GM, Griggs RC, Kissel J, et al. A randomized efficacy and safety trial of oxandrolone in the treatment of Duchenne dystrophy. Neurology. 2001;56(8):1075–79. doi: 10.1212/wnl.56.8.1075. [DOI] [PubMed] [Google Scholar]
- 3.Rutkove SB, Parker RA, Nardin RA, et al. A pilot randomized trial of oxandrolone in inclusion body myositis. Neurology. 2002;58(7):1081–87. doi: 10.1212/wnl.58.7.1081. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder ET, Terk M, Sattler FR. Androgen therapy improves muscle mass and strength but not muscle quality: results from two studies. Am J Physiol Endocrinol Metab. 2003;285(1):E16–24. doi: 10.1152/ajpendo.00032.2003. [DOI] [PubMed] [Google Scholar]
- 5.Tamaki T, Uchiyama S, Uchiyama Y, et al. Anabolic steroids increase exercise tolerance. Am J Physiol Endocrinol Metab. 2001;280(6):E973–81. doi: 10.1152/ajpendo.2001.280.6.E973. [DOI] [PubMed] [Google Scholar]
- 6.Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, et al. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab. 2004;89(10):5245–55. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- 7.Fargo KN, Galbiati M, Foecking EM, et al. Androgen regulation of axon growth and neurite extension in motoneurons. Horm Behav. 2008;53(5):716–28. doi: 10.1016/j.yhbeh.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kujawa KA, Jacob JM, Jones KJ. Testosterone regulation of the regenerative properties of injured rat sciatic motor neurons. J Neurosci Res. 1993;35(3):268–73. doi: 10.1002/jnr.490350306. [DOI] [PubMed] [Google Scholar]
- 9.Melcangi RC, Mensah-Nyagan AG. Neuroprotective effects of neuroactive steroids in the spinal cord and peripheral nerves. J Mol Neurosci. 2006;28(1):1–2. doi: 10.1385/jmn:28:1:1. [DOI] [PubMed] [Google Scholar]
- 10.Hattori N, Yamamoto M, Yoshihara T, et al. Demyelinating and axonal features of Charcot-Marie-Tooth disease with mutations of myelin-related proteins (PMP22, MPZ and Cx32): a clinicopathological study of 205 Japanese patients. Brain. 2003;126(Pt 1):134–51. doi: 10.1093/brain/awg012. [DOI] [PubMed] [Google Scholar]
- 11.Gabreels-Festen A, Wetering RV. Human nerve pathology caused by different mutational mechanisms of the PMP22 gene. Ann NY Acad Sci. 1999;883:336–43. [PubMed] [Google Scholar]
- 12.Padykula HA, Herman E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955;3(3):170–95. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- 13.Morganti B, Scivoletto G, Ditunno P, et al. Walking index for spinal cord injury (WISCI): criterion validation. Spinal Cord. 2005;43(1):27–33. doi: 10.1038/sj.sc.3101658. [DOI] [PubMed] [Google Scholar]
- 14.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 15.Jones KJ, Coers S, Storer PD, et al. Androgenic regulation of the central glia response following nerve damage. J Neurobiol. 1999;40(4):560–73. doi: 10.1002/(sici)1097-4695(19990915)40:4<560::aid-neu11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Lawson VH, Gordon Smith A, Bromberg MB. Assessment of axonal loss in Charcot-Marie-Tooth neuropathies. Exp Neurol. 2003;184(2):753–57. doi: 10.1016/S0014-4886(03)00293-0. [DOI] [PubMed] [Google Scholar]
- 17.Timmerman V, Nelis E, Van Hul W, et al. The peripheral myelin protein gene PMP-22 is contained within the Charcot-Marie-Tooth disease type 1A duplication. Nat Genet. 1992;1(3):171–75. doi: 10.1038/ng0692-171. [DOI] [PubMed] [Google Scholar]
- 18.Magnaghi V, Ballabio M, Gonzalez LC, et al. The synthesis of glycoprotein Po and peripheral myelin protein 22 in sciatic nerve of male rats is modulated by testosterone metabolites. Brain Res Mol Brain Res. 2004;126(1):67–73. doi: 10.1016/j.molbrainres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Melcangi RC, Magnaghi V, Galbiati M, et al. The action of steroid hormones on peripheral myelin proteins: a possible new tool for the rebuilding of myelin? J Neurocytol. 2000;29(5–6):327–39. doi: 10.1023/a:1007105121765. [DOI] [PubMed] [Google Scholar]
- 20.Ericson U, Ansved T, Borg K. Charcot-Marie-Tooth disease – muscle biopsy findings in relation to neurophysiology. Neuromuscul Disord. 1998;8(3–4):175–81. doi: 10.1016/s0960-8966(98)00018-2. [DOI] [PubMed] [Google Scholar]
- 21.Bovenberg MS, Degeling MH, de Ruiter GC, et al. Type grouping in rat skeletal muscle after crush injury. J Neurosurg. 2011;114(5):1449–56. doi: 10.3171/2010.9.JNS091656. [DOI] [PubMed] [Google Scholar]
- 22.Rhrich-Haddout F, Kassar-Duchossoy L, Bauchet L, et al. Alpha-motoneurons of the injured cervical spinal cord of the adult rat can reinnervate the biceps brachii muscle by regenerating axons through peripheral nerve bridges: combined ultrastructural and retrograde axonal tracing study. J Neurosci Res. 2001;64(5):476–86. doi: 10.1002/jnr.1099. [DOI] [PubMed] [Google Scholar]
- 23.Vleggeert-Lankamp CL, de Ruiter GC, Wolfs JF, et al. Type grouping in skeletal muscles after experimental reinnervation: another explanation. Eur J Neurosci. 2005;21(5):1249–56. doi: 10.1111/j.1460-9568.2005.03954.x. [DOI] [PubMed] [Google Scholar]
- 24.Borg K, Ericson-Gripenstedt U. Muscle biopsy abnormalities differ between Charcot-Marie-Tooth type 1 and 2: reflect different pathophysiology? Exerc Sport Sci Rev. 2002;30(1):4–7. doi: 10.1097/00003677-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Cruickshank TM, Reyes AR, Ziman MR. A systematic review and meta-analysis of strength training in individuals with multiple sclerosis or Parkinson disease. Medicine (Baltimore) 2015;94(4):e411. doi: 10.1097/MD.0000000000000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latimer-Cheung AE, Pilutti LA, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil. 2013;94(9):1800–28e3. doi: 10.1016/j.apmr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Young P, De Jonghe P, Stogbauer F, Butterfass-Bahloul T. Treatment for Charcot-Marie-Tooth disease. Cochrane Database Syst Rev. 2008;(1):CD006052. doi: 10.1002/14651858.CD006052.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhamme C, van Schaik IN, Koelman JH, et al. Clinical disease severity and axonal dysfunction in hereditary motor and sensory neuropathy Ia. J Neurol. 2004;251(12):1491–97. doi: 10.1007/s00415-004-0578-x. [DOI] [PubMed] [Google Scholar]
- 29.Magnaghi V, Cavarretta I, Galbiati M, et al. Neuroactive steroids and peripheral myelin proteins. Brain Res Brain Res Rev. 2001;37(1–3):360–71. doi: 10.1016/s0165-0173(01)00140-0. [DOI] [PubMed] [Google Scholar]
- 30.Lee JK, Zheng B. Role of myelin-associated inhibitors in axonal repair after spinal cord injury. Exp Neurol. 2012;235(1):33–42. doi: 10.1016/j.expneurol.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu P, Woodruff G, Wang Y, et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83(4):789–96. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


