Abstract
Taste avoidance conditioning (TAC) was carried out on the pond snail, Lymnaea stagnalis. The conditional stimulus (CS) was sucrose which elicits feeding behavior; while the unconditional stimulus (US) was a tactile stimulus to the head which causes feeding to be suppressed. The neuronal circuit that drives feeding behavior in Lymnaea is well worked out. We therefore compared the physiological characteristics on 3 classes of neurons involved with feeding behavior especially in response to the CS in conditioned vs. control snails. The cerebral giant cell (CGC) modulates feeding behavior, N1 medial neuron (N1M) is one of the central pattern generator neurons that organizes feeding behavior, while B3 is a motor neuron active during the rasp phase of feeding. We found the resting membrane potential in CGC was hyperpolarized significantly in conditioned snails but impulse activity remained the same between conditioned vs. control snails. There was, however, a significant increase in spontaneous activity and a significant depolarization of N1M’s resting membrane potential in conditioned snails. These changes in N1M activity as a result of training are thought to be due to withdrawal interneuron RPeD11 altering the activity of the CGCs. Finally, in B3 there was: 1) a significant decrease in the amplitude and the frequency of the post-synaptic potentials; 2) a significant hyperpolarization of resting membrane potential in conditioned snails; and 3) a disappearance of bursting activity typically initiated by the CS. These neuronal modifications are consistent with the behavioral phenotype elicited by the CS following conditioning.
Keywords: taste avoidance conditioning, Lymnaea, modulatory neuron, central pattern generator, motor neuron
The pond snail, Lymnaea stagnalis, has been previously used in taste avoidance conditioning (TAC) studies because of a number of attributes that this model system possesses including a very good understanding of the neuronal circuitry that underlies most aspects of feeding behavior. The feeding system of Lymnaea, allows us the opportunity to investigate how electrophysiological changes in identified neurons that play roles in mediating feeding behavior are altered following TAC. Feeding behavior in Lymnaea can undergo either appetitive (appetitive conditioning: APC; 1–5) or avoidance classical conditioning6–14. In APC, sucrose acts as the unconditional stimulus (US); while in TAC, sucrose acts as the conditional stimulus (CS). In the APC procedure, a gentle touch to the lip or application of amyl acetate serves as the CS while sucrose serves as the US. Thus after pairing of the CS with the US, the CS (the gentle touch to the lip or application of amyl acetate) comes to elicit the feeding response, which was initially only elicited by the US (sucrose).
To produce TAC in Lymnaea, a stimulus (CS; e.g. sucrose), which elicits a feeding response, is paired with an aversive stimulus (US; e.g. KCl, quinidine sulfate, electrical shock) that inhibits feeding. After pairing the CS with the US, the CS no longer acts as an appetitive stimulus in that it no longer causes an increase in the feeding response. Typically the US used evokes the whole body withdrawal response, which inhibits feeding. Kojima et al. (1996) found that KCl was the most potent US to use with sucrose as the CS in that there was quicker acquisition and a longer persistence of memory.
Eventually following Lymnaea TAC, training the CS no longer elicits feeding behavior. We can summarized the changes in behavior following TAC training in Lymnaea as follows: 1) Whether the US was a tactile stimulus to the head, KCl application to the lip or electric shocks, after the conditioning the presentation of the CS no longer elicited feeding, 2) Lymnaea TAC was pairing specific. That is, with CS only presentations, US only presentations, US-CS pairing (backward conditioning) the CS continues to elicit feeding, 3) After conditioning, memory retention was accessed 10 min and 24 h later. More than the half of the snails exhibited memory at 10 min later (short-term memory: STM) but not 24 h later (long-term memory: LTM)15. These snails were classified as “poor learners” or “poor performers”, which could acquire STM solely but not develop into LTM, while snails that exhibit memory 24 h later were classified as “good learners” or “good performers”8,16,17, 4) Activation of the α and ε isozymes of protein kinase C (PKC) is crucially involved in the formation of LTM and pre-exposure to the PKC α and ε activator facilitated formation of STM with suboptimal presentation of CS-US pairings14.
As a next step in attempting to elucidate the underlying causal neuronal mechanisms of Lymnaea TAC, we concentrated our effort to characterize the neuronal activities of three classes of neurons involved with feeding.
Lymnaea feeds rhythmically using its radula, with three movements of almost equal duration: protraction of the radula, rasping and swallowing. A rhythmical ‘fictive’ feeding pattern can also be observed in the isolated central nervous system (CNS). Neurons participating in this rhythm generation are located in the buccal and cerebral ganglia. The buccal motor neurons (B1, B2, ..., B10) involved in feeding are located in the buccal ganglia and are driven by a central pattern generator (CPG). The CPG interneurons consist of three types: N1, N2, and N318–21. The N1 type interneurons are activated during protraction phase, the N2 during rasping, and the N3s mainly during swallowing1,22. Although the N1 medial neuron (N1M) was first described by Rose and Benjamin23, the properties of N1M were elucidated by Straub et al. (2002)24. Activity in both the motor neurons and CPG neurons is modulated by identified higher-order interneurons, such as the slow oscillator neuron (SO)19, the cerebral giant cells (CGCs)25, and the cerebral ventral 1 neuron (CV1)26. The cerebral giant cell (CGC) exerts both weak excitatory monosynaptic and strong inhibitory polysynaptic inputs onto N1M. Thus, repetitive firing of CGC results in inhibition of the N1M cells25. Though it is presumed that the inhibitory influence of the CGCs upon the N1M cells might be potentiated in TAC animals to suppress the feeding response, no difference in electrical properties of the cell body of CGC nor the response of the CGC to the chemosensory inputs were observed Kojima et al. (1997)9.
Here we examined the effect that Lymnaea TAC has on the electrophysiological properties of a modulatory neuron (CGC), a CPG neuron (N1M) and a motor neuron (B3) by comparing the properties of each of these cell types between naïve and conditioned snails. In addition, and what is extremely novel, is that we also examined the activity of a RPeD11 neuron that mediates the whole-body withdrawal response as to the possible role played by this neuron in mediating TAC. This neuron has been shown to play a crucial role in raising the ‘alert’ level in the snail to avoid noxious stimuli27–30.
Materials and Methods
Animals
Laboratory-reared fresh water pond snails, Lymnaea stagnalis, (original stocks from Free University of Amsterdam or supplemented with snails from Tokushima Bunri University or the University of Calgary snail rearing facility that were also derived from the same Amsterdam colony) with shell lengths of 20 mm, were maintained at 20°C in well-aerated water, on a 12-h light: 12-h dark cycle (on at 08:00), and fed cabbages and goldfish pellets (Hikari Staple, Kyorin Co. Ltd., Himeji, Hyogo, Japan). Snails larger than 20 mm in shell length are capable of classical conditioning31. The animals were food deprived for 24 h prior to the experiments. This food deprivation is increase the motivation for acquisition of condiioned taste aversion32.
Experimental apparatus for feeding behavior
The Plexiglas container (diameter: 60 mm and height: 20 mm) had a perfusion system with one inlet and one outlet from which the solution inside the container could be entirely replaced within 30 s at a speed of 250 ml/min. The container contained 10 ml fresh aquarium water in which snails were kept. To observe the feeding response, a mirror was placed under the container. The CS (1 ml of 100 mM sucrose) was applied directly to the lip of the animal with a 1-ml syringe. Immediately following the application of sucrose, the number of bites per minute was measured for a minute. This is the ‘feeding response’. A tactile stimulus was applied to the surface of the animal’s head using a hand-held Plexiglas rod. The stimulation was strong enough to always evoke a whole-body withdrawal response, which terminates incompatible behaviors such the ‘feeding response’.
The TAC procedure
The conditioning procedure used here was identical to the TAC procedure used by Kawai et al.8. We briefly summarize the main points of the Lymnaea TAC procedure here. Snails were first allowed to acclimatize for 10 min in the training container. Following the acclimatization period the feeding response (i.e. bites/min) to the CS (1 ml of 100 mM sucrose) was recorded. This served as the pre-conditioning test (pre-test). Ten minutes later, the snails received 20 CS-US pairings. Each snail was exposed to the CS followed 5 seconds later by the US. A one minute inter-trial interval was imposed between pairings of the CS-US. It typically took less than 1 minute for the snail to recover from the US. A “ten min post-conditioning test” (10 min post-test) was performed following the 20 paired CS-US presentations. The CS was again applied directly to the lip of the animal with a 1 ml syringe. We also tested the response to the CS 24 h later. This was termed the “24 h post-conditioning test” (24 h post-test). Immediately following the sucrose application, the number of bites per minute (feeding response) was tabulated for 1 min. In order to confirm whether TAC is CS-US temporal specific, snails were received a tactile stimulus (US) first followed by sucrose application (CS) as backward conditioning. The temporal schedule of conditioning is shown in Figure 1. “Good performers” were defined as having the number of bites elicited by CS presented both at 10 min and 24 h post-test. “Poor performers” were defined as snails that acquired STM but did not consolidate it into LTM. Thus, “good performers” were characterized as those that had exhibited memory in the 24 h post-test.
Figure 1.

Time schedule of Taste Aversion Conditioning. Snails were acclimatized for 10 min then the feeding response to CS was recorded as the pre-conditioning test. Ten minutes later, they received 20 CS-US pairings. Each snail was exposed to the CS followed 5 seconds later by the US. A one minute inter-trial interval was imposed between pairings of the CS-US. A “ten min post-conditioning test” was carried out following the 20 paired CS-US presentations. The CS was again presented to the snails. A “24 h post-conditioning test” was again performed at 24 h later. Electro-physiological recordings were made from semi-intact preparations approximately 1h following the memory test.
We only analyzed the electrophysiological properties of neurons in ‘good performers’ and compared their properties to recordings made from naïve snails. We choose this comparison because in the previous study by Kojima et al., they demonstrated that IPSPs recorded in N1M of ‘poor performers’ were not significantly different from those of naive controls16. That is, the recorded activity from naïve snails was expected to be identical to that of the ‘poor performers’16.
Electrophysiology
All experiments were performed at room temperature (20°C) during the daylight portion of the snails’ diurnal cycle. To assess the electrophysiological effects that TAC produces, intracellular recordings were made in semi-intact preparations from three groups of neurons; a modulatory neuron, the cerebral giant cell, CGC; a CPG neuron, N1M; and a motor neuron, B329. In order to examine the hypothesized neurophysiological role of RPeD11 in Lymnaea TAC (i.e. its modulatory influence on the CGCs and B3), we made simultaneous recordings from RPeD11-CGC and/or RPeD11-B329. Semi-intact preparations were prepared within 10 min after the 24 h post-test for memory. It takes approximately 1 h from the time of dissection to the initial recording of electrophysiological activity. Thus, the electrophysiological data presented here were obtained from the animal approximately 1 h after “24 h post-test”.
The resting membrane potential was measured twice: 1) soon after inserting an electrode into the neuron; and 2) after completion of electrophysiological assessment by pulling out the electrode from the neuron. If the membrane potential was differed more than 5 mV, cells were rejected and the data were not used in any analysis. Those who performed the electrophysiological experiments did not know the behavioral state (i.e. good performer or naïve) of each snail beforehand.
The semi-intact preparation consisting of the mouth and the whole central nervous system (CNS), including the cerebral ganglia and buccal ganglia were dissected out from the snail in Lymnaea saline (51.3 mM NaCl; 1.7 mM KCl; 5.0 mM MgCl2; 1.5 mM CaCl2 and 5.0 mM HEPES; pH 7.9–8.l). This preparation was immobilized on a Sylgard-coated culture plate using stainless-steel pins. In order to physically isolate the CNS from the rest of the semi-intact preparation (i.e. mouth and lip area) a Vaseline dam was constructed around the CNS. This enabled us to apply a sucrose solution to the lip/mouth area without the sucrose coming into contact with the saline bathing the CNS. This can be seen in Figure 2A left panel. For the semi-intact preparation, 1 ml of 100 mM sucrose was applied to the mouth/lip area as in the behavioral feeding test.
Figure 2.
Semi-intact preparation used in this study A). The semi-intact preparation consisting of the central ring ganglia along with the buccal ganglia was removed from the snail. In addition the mouth and lips as well as tentacles were left intact. Vaseline dam was constructed around the CNS in order the superfusion of the CS (Sucrose) over the mouth area without coming into contact with neurons in the CNS. Schematic location of N1Ms and B3 motor neurons on the buccal ganglia (right panel). Neuronal circuit involving the feeding behavior, Sensory neurons (SNs), Modulatory neurons, Central Pattern Generator neurons, and Motor neurons B). CGC, N1M and B3 are one of Modulatory neurons, CPG neurons and Motor neurons, respectively. CGC and N1M and B7 are involved in protraction phase (P); SO, N2 and B3 are involved in rasp phase (R); CV1, N3 and B4 are involved in swallow phase (S). Note that in addition to the original scheme by Benjamin et al. RPeD11 has direct inhibitory connection with CGC as shown in Figure 8. Open circles are represented excitatory synapses and closed circles are represented inhibitory synapses. The circuit was modified from Figure 1 of the Benjamin’s review paper (Benjamin et al., 2000).
The CGC, N1M, and B3 were identified according to the location revealed by previous studies as shown in Figure 2A right panel and in some preparations Lucifer Yellow was injected intracellularly for histological identification33. RPeD11 is located in the pedal ganglion lateral to the statocyst as shown in Figure 2 of Sunada et al.30.
The thin connective tissue sheath surrounding the ganglia was partially digested by incubation in protease (type XIV or type VIII, Sigma Chemical, St. Louis, MO, USA) solution (1 mg/ml) for 5 to 6 min at 20°C.
The ‘wiring’ diagram of the feeding circuit is shown in Figure 2B (modified from Benjamin, 2000). As can be seen the circuit is made up of sensory, modulatory, central pattern generator and motor neurons. We recorded from the following neurons: CGC, N1M and B3.
Simultaneous recordings were made from two pairs of neurons from the three neurons (CGC, N1M, and B3) studied here in order to compare ongoing activity and neural connections. Neurons were impaled with a 3 M KCl-filled glass microelectrode with a resistance ranging from 25 to 35 MΩ. The glass microelectrode was fabricated from a borosilicate thick-wall fiber M Systems, Inc., Carlsborg, WA, USA) with a Laser-puller (Model P-2000, Sutter Inst., Co., Novato, CA, USA) connected by a silver chloride wire to a high-input impedance amplifier (Axoclamp 2B, Molecular Devices, Union City, CA, USA). Voltage response were recorded on a storage oscilloscope (5113, Tektronix, Beaverton, OR, USA), and analyzed using a microcomputer by an interface board (Digidata 1322A, Molecular Devices, CA, USA) with analysis software (p-clamp9, Molecular Devices, CA, USA).
Statistics
The multiple comparison of behavioral differences between pre- and post-conditioning (10 min post-test and 24 h post-test) were evaluated with repeated-measures analysis of variance (ANOVA). Scheffe’s F post hoc test was then used to further determine statistical significance. The electrophysiological parameters measured between naïve and conditioned snails in CGC, N1M and B3 were tested with Student t-test. For all analyses, data were considered significant if p<0.05. Data in Results were expressed as mean±standard error (S.E.).
Results
TAC behavior
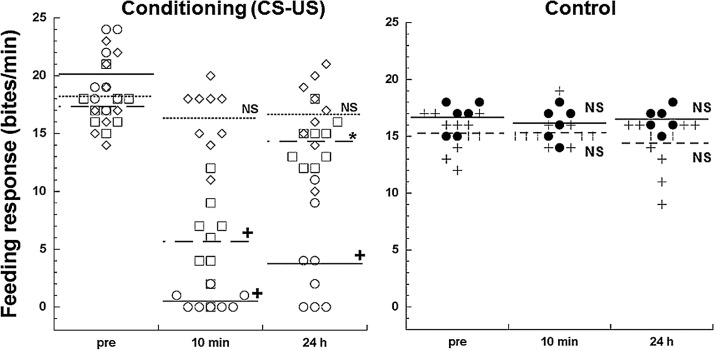
Behavioral experiments (i.e. Lymnaea conditioning) were performed on 44 snails in which 26 forward conditioning, 6 backward -filled conditioning capillary glass (#6020, A- and the rest of 12 naïve snails were included. Figure 3 showed the behavioral feeding scores obtained from TAC conditioned snails (left panel: n=26) and control snails (right panel: 12 naïve snails and 6 backward conditioned snails). There was no significant feeding suppression observed in the backward conditioned group (denoted with filled circles in Fig. 3 right panel: n=6). That is pairing of the US before the CS does not result in suppression of feeding when the CS is presented. In the TAC conditioned snails (left panel) we classifiedthem into three groups (see Methods): 1) good-performers; 2) poor-performers; and 3) no learning. Following the TAC conditioning procedure 17 of the 26 snails exhibited memory for TAC 10 min after the last CS-US pairing, i.e., obtained STM. That is, compared to their pre-test scores the number of bites elicited by the CS decreased significantly. Of these 17 snails when tested for memory 24 h later only 8 snails met our criterion for LTM (i.e. the number of bites was still significantly suppressed). Thus, 8 snails out of 17 (i.e. ∼50%) could be classified as ‘good performers’ while the remaining 9 snails were considered as ‘poor performers’, which could acquire STM solely but not develop into LTM. In the ‘poor performers’ when the scores in 24 h post-test compared to their pre-test scores the number of bites was significantly decreased (*p<0.05 in Fig. 3 left panel).
Figure 3.
A scatter plot of feeding scores of TAC conditioned (n=26) and control snails (n=18) are shown. Scores were obtained from pre-test and post-test at 10 min or 24 h after conditioning procedure. Snails were conditioned with 20 paired presentations of sucrose (CS) first then a tactile stimulus to the head (US) or vice versa as forward or backward conditioning, respectively. Naïve animals (n=12) were placed in the experimental chamber for the same period of conditioning. In the CS-US conditioning group (left panel) open circles represented scores obtained from good-performers (n=8), while open squares, open diamond represented poor-performer (n=9) and animals without acquisition of learning (no memory) (n=9), respectively. Control (right panel) was included snails of 12 naïve (+) and 6 backward conditioning (•) with US-CS pairs. Horizontal lines denoted each average scores (Conditioned group: line- good performer; dash- poor performer; dash- no memory| Control group: line-backward; dot-naïve). Note that the behavioral scores of animal without memory, backward conditioner and naïve were statistically identical. Statistical test was performed between the score of pre-test and that of post-test (10 min and 24 h). Even poor-performers showed significant *p<0.05, +p<0.0001, NS: not significant
Nine out of 26 snails did not show any conditioning effect, that is, their feeding behavior elicited by the CS was not modified after repeated pairings of CS-US. The behavioral scores of animals (denoted with diamonds in the left panel of Fig. 3) without memory were not statistically different as those of the backward conditioned group and naïve animals as shown in the right panel of Figure 3. There was no statistical difference in the pre- and post-test feeding scores in these snails (snails without memory, backward conditioned and naïve) in the post-10 min/24 h test.
Resting membrane potentials and spontaneous activities of CGC, N1M, and B3
Semi-intact preparations were made from snails and we recorded intracellularly from pairs of neurons in each preparation. We recorded from CGC, N1M, and B3 in both good performers and naïve preparations. Thus we could compare and contrast the properties of the three types of neurons in snails possessing LTM and naïve snails.
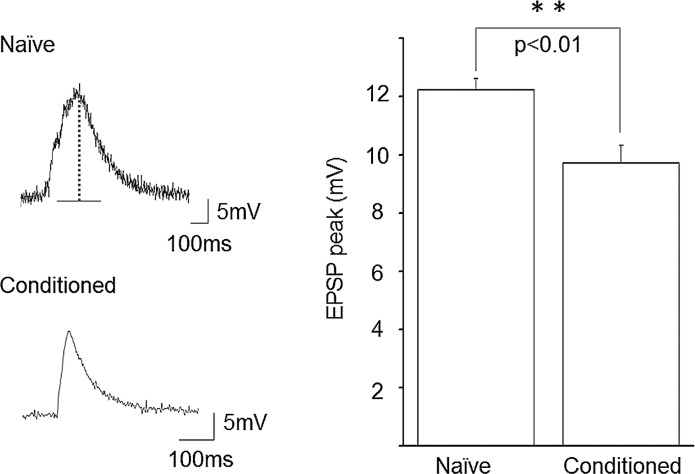
Representative data are presented in Figure 4 and 5 showing on-going spontaneous activities in CGC, N1M, and B3 neurons in both naïve snails (Figs. 4 and 5-A) and good performers (i.e. snails exhibiting LTM: Figs. 4 and 5-B). These data and the combined data presented in Table 1 illustrate that not all types of neurons were changed in the same way (or in any way) following training and LTM formation. For example, a significant difference (p<0.05) was observed between the naïve and good performers in the resting membrane potential (RMP) of the CGCs; −50.0±2.96 mV (n=10) vs. −58.6±2.55 mV (n=11), respectively. In other words the RMP in this cell type was significantly hyperpolarized by TAC and the subsequent LTM formation. However, (see Table 1) in the N1M cell type the RMP from snails exhibiting LTM was statistically more depolarized than in the naïve controls (naïve vs. good performers; −46.3±3.7 mV vs. −38.2±0.9 mV; p<0.05). On the other hand in the B3 motor neuron, the RMP in the snails showing memory was statistically more hyperpolarized than in the naïve preparations (naïve vs. good performers; −63.9±1.9 mV vs. −71.3±1.2 mV; p<0.01). Thus, in preparations exhibiting LTM the RMP in the CGC modulatory neuron was significantly hyperpolarized (p<0.05), while in the CPG neuron N1M exhibiting LTM it was significantly depolarized, and finally in the B3 motor neuron of LTM was correlated with a significant hyperpolarization. In a similar manner the rate of spontaneous activity was also differentially affected in the 3 neuronal types. There was no statistical change in the level of spontaneous activity in CGC between good performers and naive preparations even though the RMP was significantly hyperpolarized after acquisition of learning. In the preparations exhibiting LTM the level of spontaneous activity in N1M was significantly increased compared to naive preparations (naïve vs. good performers; 3.3±1.1 Hz vs. 16.3±3.5 Hz; p<0.01). Finally, in the B3 motor neurons (Fig. 6) the amplitude of the spontaneously occurring excitatory post-synaptic potentials (EPSPs) was significantly reduced in the naïve vs. good performer preparations, (naïve vs. good performers; 12.2±0.3 mV vs. 9.8±0.5 mV; p<0.01). This significant reduction in the amplitude of the EPSPs occurred in spite of the fact that in the good performer preparations the RMP of these motor neurons was significantly hyperpolarized compared to the naïve preparations. A more hyperpolarized RMP should result in larger amplitude EPSPs everything else being equal. In addition, the frequency of the spontaneous EPSPs to the B3 motor neuron was also significantly reduced in the good performers compared to the naïve preparations (naïve vs. good performers; 69.1±3.1 Hz vs. 7.3±0.4 Hz; p<0.01).
Figure 4.
Representative spontaneous activities of CGC, and B3 were displayed in naïve A) and good performer preparations B). CGC and B3 were recorded simultaneously. Notice that B3 had spontaneous EPSP activities in naïve preparations while they were not relatively less active in the good performer preparation. The CGC activity was not different in good performer vs. naïve preparations though after-hyperpolarization in naïve became smaller. Calibration bars represented 20 mV and 20 second.
Figure 5.
Representative spontaneous activities of N1M, and B3 were displayed in naïve A) and good performer preparations B). Each response was recorded simultaneously. Note that spontaneous impulse generation in N1M after conditioning was obviously more frequent (naïve vs. good performers; 3.3±1.1 Hz vs. 16.3±3.5 Hz) while spontaneous EPSPs in the good performer B3 became less frequent and the amplitude became significantly smaller. Calibration bars represented 20 mV and 20 s.
Table 1.
Statistical measures obtained from 3 neurons of CGC, N1M and B3 in naïve and conditioned (good performer) preparations
| CGC | N1M | B3 | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| N (n=10) | C (n=11) | N (n=8) | C (n=7) | N (n=9) | C (n=7) | |
| RMP (mean±SE; mV) | −50.0±3.0 | −58.6±2.6* | −46.3±3.7 | −38.2±0.9* | −63.9±1.9 | −71.3±1.2** |
| SIF (mean±SE; Hz) | 0.57±0.06 | 0.52±0.06 | 3.3±1.1 | 16.3±3.5** | 0 | 0 |
| S-EPSP (mean±SE; mV) | – | – | – | – | 12.2±0.3 | 9.8±0.5** |
| (mean±SE; Hz) | 69.1±3.1 | 7.3±0.4** | ||||
N: Naïve, C: Conditioned, RMP: Resting membrane potential, SIF: spontaneous impulse frequency, S-EPSP: spontaneous EPSP
p<0.05,
p<0.01
Figure 6.
The amplitude of spontaneous EPSPs in naive preparations was significantly larger than that of the good performer preparations in B3 motoneuron. In addition to the larger amplitude, the EPSP frequency was also higher in naïve preparations.
Responses to CS in the identified neurons
The above-mentioned electrophysiological data were obtained prior to any presentation of the CS to the semi-intact preparation. That is, the observed spiking and synaptic activity was occurring spontaneously. We next examined if there were differences in the neuronal response elicited following the application of the CS to the lips of the semi-intact preparation in good performers vs. naïve preparations. Figure 7 shows the representative electrophysiological paired recordings from N1M and B3 in naïve and good performer preparations.
Figure 7.
Simultaneous recordings from N1M and B3 in response to CS presentation from a naïve A) and the good performer preparation B). The dotted line in each record represented the timing to start perfusion of 1 ml of 100 mM sucrose. In the naïve preparation, the sucrose application induced rhythmic fictive feeding activity in N1M and B3 in push-pull manner. However, such rhythmic fictive feeding activities did not induce by the presentation of CS in the good performers preparation.
In naïve preparations the presentation of the CS leads to fictive feeding. Thus for example, the naive preparation in Figure 7-A presentation of the CS leads to rhythmic alternating bursts of impulses in N1M and B3 in a ‘push-pull’ manner. These data are similar to the data presented by the Benjamin group in their examination of the underlying neuronal events of feeding in Lymnaea1. Thus, during the rasp phase of feeding N1M is excited while B3 is inhibited. Notice, however, that in the semi-intact preparation taken from a preparation exhibiting LTM that the presentation of the CS does not lead to rhythmic spiking activity in N1M or B3 (Fig. 7-B). Rather, tonic firing increases in N1M and does not become rhythmically excited, in fact it stays in a relatively inhibited state compared to what is observed in B3’s in naïve preparations. Finally, there was no apparent change in the firing frequency of CGC between naive and good performer preparations.
We conclude then that TAC alters the electrophysiological response of the CPG neuron, N1M and a motor neuron B3; but does not alter the activity of the modulatory neuron CGC when recordings are made in semi-intact preparations from naïve vs. good performers. The schematic wiring diagram was displayed after acquisition of learning that was the inhibitory connection from N1M to B3 was augmented in the good performers as shown in Figure 2-B.
CGC was inhibited by excitation of RPeD11
Depolarizing current (2 nA) was injected into RPeD11. This depolarization resulted in spiking activity on RPeD11 and this spiking activity caused an inhibitory post-synaptic potential (IPSP) of approximately 5 mV in CGC. Transient excitation of RPeD11 thus resulted in a cessation of the spontaneous regular spiking activity in CGC (Fig. 8). The inhibitory input from RPeD11 to the CGC disappeared when the CNS was perfused with a calcium free saline. Moreover, when a high divalent cation (high Mg2+/high Ca2+) containing saline was perfused over the CNS, the inhibitory input from RPeD11 to the CGC was still observed (data not shown). These data are consistent with the hypothesis that the information from RPeD11 to the CGC is mediated via a monosynaptic chemical synapse. In every instance tested (3/3) when we simultaneously recorded from RPeD11 and CGC, we observed that excitation of RPeD11 inhibited the activity of CGC. We also found that RPeD11 excitation also caused inhibition of activity in B3 (data not shown). We therefore conclude that after Lymnaea acquire TAC the CS induces less fictive feeding activity due to a crucial role played by RPeD11 in raising the ‘alert’ level in the snail.
Figure 8.
Simultaneous recordings from RPeD11 and CGC. Depolarizing current injection into RPeD11 caused an inhibitory post-synaptic potential in CGC of approximately 5 mV in amplitude. This inhibitory input was sufficient to inhibit the spontaneous firing of the CGC.
Discussion
We successfully employed a TAC training procedure on a cohort of naïve snails resulting in the formation of LTM. In this procedure a CS sucrose, which elicits feeding behavior in the snail, was paired with an US, a tactile stimulus to the head of the snail, which causes the whole-body withdrawal response and thus inhibition of feeding. Following 20 paired CS-US training trials, the CS in approximately 30% (8/26) of the conditioned snails (the so-called good performers) elicited significantly fewer bouts of feeding when presented 24 h later. Thus, these ‘good-performing’ snails formed long-term memory (LTM) and were able to recall that memory. We then made semi-intact preparations from these good performers and compared the spontaneous and CS-evoked electrophysiological properties of 3 neuronal types (a modulatory neuron, a CPG neuron, and a motor neuron) with those of the same 3 types of neurons in semi-intact preparations made from naïve snails.
Taste avoidance conditioning in Lymnaea
Snails easily learn and form LTM following the TAC training. This has been demonstrated in many previous studies using slightly different USs and numbers of paired presentations11–14,17,34. As well, APC of the feeding response has also been frequently demonstrated35,36. In addition in most of studies involving either operant or classical conditioning there are always both poor performers (showing no long lasting memory) and good performers showing long lasting memory17,37,38. This topic of why some snails do not form LTM is not dwelt on but we might possibly learn more about the causal neuronal mechanisms underlying LTM by studying those that do not form LTM. Regarding the mechanisms of LTM formation, our recent studies indicated that a space training procedure where there is an interposition of a couple of hours between CS-US paired presentations effectively enhances the formation of LTM11,13. Depending on the criteria used the percentages of good vs. poor performers can vary greatly. Here we show that close to 30% (8/26) meet our criterion (number of bites evoked by the CS had to be less than 50% of initial response examined at 24 h latter post-test) to be classifiedas having formed LTM. In other studies, the percentage may be higher or lower, but that might only be indicative of the criteria used. In any case, what we show here is that in the majority of snails conditioned (20 paired presentations), the CS 24 h after training evoked statistically fewer bouts of feeding. In addition, it should be remembered that we were primarily interested to see if we could observe neural correlates of LTM in 3 neuronal types that are associated or needed to produce feeding behavior (see below). Thus, we were pleased that the training procedure used here produced TAC in a sufficient number of snails such that we could make semi-intact preparations to study the changes that may have occurred in the nervous system that mediate the LTM seen behaviorally.
Feeding related neural circuit in Lymnaea
The necessary neural circuit mediating feeding behavior, even in a ‘relatively simple model system’ such as Lymnaea is complicated. A ‘relatively simple’ overview of the neuronal feeding circuitry, based in large part on the work of the Benjamin group is presented in Figure 2B1,39. Briefly, the neural system can be parsed out in the following manner. Motor neurons (B1−B10) cause the muscles to contact and relax. Thus, the proper sequence of excitation and inhibition of agonistic and antagonistic muscles will repetitively produce the necessary movements of the radula to scrape, ingest food into the mouth, and swallow. The ‘proper’ firing sequence of the motor neurons is controlled by a group of interconnected interneurons (N1, N2, and N3) that form the CPG. The activity of the CPG circuit is modulated by another set of interneurons known as the modulatory neurons (CGC, SO, CV1, etc.). To a greater or lesser extent they drive the CPG faster or slower. Finally, and not that well worked out, are the sensory neurons (not identified) which supply the necessary sensory information concerning the ‘taste’ of the food and the necessary proprioceptive feedback. The sensory neurons input onto the modulatory and CPG neurons and may also input directly onto motor neurons. Here we decided to examine a modulatory neuron (CGC), a CPG interneuron (N1M; protraction phase) and a motor neuron (B3, a retraction-phase feeding motor neuron, known as B3 that receives synaptic input from all 3 types of CPG neurons) in conditioned (i.e. exhibiting LTM) vs. naïve semi-intact preparations as shown in Figure 2B.
The electrophysiological data that we obtained from the 3 neuronal types in semi-intact naïve preparations are similar to those published by the Benjamin group in their initial studies concerned with elucidating the neuronal circuit underlying feeding18,20,40,41. Both the spontaneous activity and the CS-evoked fictive feeding responses seen here was what we expected. For example, with the presentation of the CS in naïve preparations we observed maintained rhythmic fictive feeding in N1N and B3 with appropriate alternating bursts of activity in these two cells. In similar fashion, the CS in naïve preparations again elicited rhythmic bursts of activity in B3 with little or no change in tonic activity of the CGC. Typically, the CGC in intact snails during feeding fires tonically at an average firing rate of between 1–20 spikes/min based on fine-wire extracellular recordings 42.
Conditioning effects on CGC, N1M, and B3 in Lymnaea
When we examine the data obtained from semi-intact preparations exhibiting LTM for TAC, we found a different pattern of cellular activity compared to the naïve preparations as far as N1M and B3 were concerned. The CS did not elicit rhythmic fictive feeding in N1M and B3. N1M became tonically more active (due to it being more depolarized-see Fig. 5) and B3 never reached the threshold of bursting activity it did in the naïve preparations. In the naïve preparations, B3 becomes rhythmically active while in the conditioned preparations it remained inhibited. However, what has to be pointed out is that CGC impulse activity was not different between conditioned vs. naïve preparations both as regards spontaneous activity or CS evoked activity. This was unexpected and we consider this a significant finding. Our hypothesis was that CGC activity would be less in conditioned vs. naive snails (see below). From these findings we concluded CGC did not directly participate in TAC rather the apparent conditioning effects appeared downstream in the neuronal circuit that drives feeding.
As regards spontaneous activity (i.e. before presentation of the CS) observed in the conditioned vs. the naïve preparations, the data can be best summarized as follows. N1M became more excitable, due to its RMP becoming significantly more depolarized in the conditioned vs. the naïve preparation. On the other hand, B3 was significantly more hyperpolarized in the conditioned vs. naïve preparations. Thus in conditioned preparations N1M was more easily excitable while B3 was less easy to excite. Finally, even though B3 was more hyperpolarized in the conditioned preparations compared to naïve preparations, the amplitude of spontaneously occurring EPSP’s to the neuron was significantly smaller. Again this would tend to make the neuron less excitable. Bear in mind also that since the driving force of the ions responsible for the EPSP should have increased because the cell was more hyperpolarized (i.e. the difference in the RMP and presumed reversal potential for the EPSP should be larger) the size of the EPSP became smaller. This indicates that some pre-synaptic inhibitory factors are at work here. In any case, since B3’s RMP is significantly more hyperpolarized and the EPSPs to it are significantly smaller, B3 will be much less likely to become active in the conditioned preparations. All of the observed electrophysiological differences in B3 and N1M between conditioned vs. naïve preparations are consistent with the fact that conditioned snails exhibited less feeding in the presence of the CS following TAC.
Our biggest surprise was that the ongoing activity we recorded from the CGCs was not different between conditioned vs. naïve preparations though the resting membrane level was significantly hyperpolarized in the conditioned preparations. Previously, it was demonstrated that in APC depolarization of the CGC occurred and was thought to be sufficient for the conditioned response to be triggered by the CS39,43,44. The CGCs become persistently depolarized after a single-APC trial without significant changes in the firing rate or shape of action potentials43. This finding was explained by a balanced increase in three identified conductance; INaP, ID and IHVA from voltage-clamp study in conjunction with computer simulation45. Here we obtained the opposite, a persistent hyperpolarization of the CGC membrane level occurred after TAC without significant changes in the spontaneous or CS-induced impulse activity. However, we did observe a tendency in a decrease in the spontaneous firing rate (see Fig. 4) but it was not statistically significant. Following appetitive conditioning the CS increases the likelihood of feeding; whereas in TAC the likelihood of feeding being elicited by the CS decreases. Since the CGC is thought to modulate feeding by increasing the serotoninergic drive to the CPG (the CGCs are serotoninergic) then it makes sense that if one wanted to increase the probability of feeding one should make the CGC more active. However, the CGC firing frequency is relatively slow, compared to other cells in the Lymnaea feeding network so that we might not expect too much of a change in its activity when feeding is not induced by the CS following TAC. A recent study indicated the molluscan insulin-related peptides (MIPs) are involved in TAC, especially the synaptic augmentation observed between CGC-B1 neurons when MIPs are activated46. Furthermore this synaptic modification was mediated post-synaptically in the B1 motor neuron47. These findings regarding changes in synaptic plasticity following TAC training occur downstream of CGCs in the feeding related circuit so it may not be too surprising that the impulse frequency in CGC stayed constant after acquisition of TAC. TAC training may have more of an impact on other modulatory neurons such as RPeD11. We believe this because when RPeD11 is activated by the tactile stimulus it mediates the whole snail withdrawal response. Incompatible behaviors such as aerial respiration and feeding are inhibited when the whole animal withdrawal response is activated.
Novel findings obtained from this study
Previously it was shown that a poly- synaptic inhibitory input from the CGCs to the central pattern generator inter-neuron N1M was enhanced by a TAC training procedure16. In a more recent paper34, utilizing an optical recording technique from the area N1M is typically located, the authors reported a reduction in the impulse generation in response to electrical stimulation at median lip nerve, which was assumed to simulate as CS presentation, in TAC conditioned snails compared with naïve preparations. These data are opposite to what we have found in this study. In addition, Kojima et al. (2001) using the same optical recording methods found no change in the activity patterns in the area where B3 is located on electrical stimulation of the median lip nerve in conditioned vs. naïve preparations. Possible reasons for these discrepancies in data are that the earlier studies used a different US (KCl to the lips) and fewer CS-US presentations than we employed here and of course the recording techniques. We used intracellular recording techniques while they used an externally applied optical dye to the area of interest. Moreover we used the same CS here (sucrose application) while they employed electrical stimulation to the lips to mimic the CS. Recent study by Ito et al. (2012) suggested the two possibilities for modulation of the synaptic influence due to the conditioning from CGC to N1M; one is excitatory influence from CGC to N3t, and the other is inhibitory connection from N3t to N1M48. They concluded the taste aversion training facilitated the synaptic connection from N3t to N1M thus to more suppressive effect on the N1M activity. These findings were contrast to our present findings that N1M became more depolarized thus to influence the excitatory effect on B3 after TAC conditioning. These discrepancies may arise from the following different experimental conditions; Ito’s model was described under much simpler situation to remove inputs and outputs as simple as possible, furthermore they assumed the activity of CPG neurons observed at B3 was via N3t indirectly, while our observations shown in this study were made from the intact preparation with active surrounding tissues.
With the APC procedure there was simultaneously an enhanced excitatory response that increases N1M activity and reduces the tonic inhibitory synaptic modulation of the N3t. If the tonic inhibition, which originates from N3t, is reduced by conditioning then it would make the feeding CPG and the CPG-driven motoneurons such as B3 more easily activated by the CS49. With the TAC procedure we may have to consider an excitation of RPeD11 in response to the presentation of US. This excitation of RPeD11 then inhibits CGC. Inhibition of the CGCs results in more excitation to N3t. Thus, N3t exerts less tonic inhibition which will induce the depolarization and more vigorous firing N1M. The end result then is that the motor neuron B3 is effectively inhibited when the CS is presented (see Fig. 2B). These changes in the CGCs and N1M as a result of the TAC procedure are in marked contrast to the changes seen in with the APC procedure43,49,50. Involvement of RPeD11 in TAC remained for the future study.
The role of RPeD11 in TAC
We hypothesize that changes in activities of right pedal dorsal 11 (RPeD11) may be a key component in the bringing about the inhibition of feeding to the presentation of the CS following TAC training. We know that this neuron plays a key role in mediating the withdrawal behavior evoked by the various types of USs, such as KCl, mechanical tapping and electric shock. We have recently shown that this interneuron plays a crucial role in raising the ‘alert’ level in the snail to avoid noxious stimuli. For example, following predator detection the activity level of this neuron is increased so that it responds to a greater extent to a shadow stimulus51. Moreover, activity in this neuron increases in response to traumatic tactile stimuli as well as to noxious chemical stimuli27,28,30. All of these stimuli cause the snail to withdraw itself partially or fully into its shell. Thus, activity in RPeD11 causes incompatible behaviors such as aerial respiration and feeding to be inhibited52. With TAC the CS gained the capability to elicit the behavior initially evoked by the US. Thus, we hypothesize that the CS following TAC now recruits RPeD11 activity rather than eliciting the onset of feeding activity. Thus we presume that RPeD11 activity ultimately causes the N1M neuron to more depolarized and thus more excitable in that it is more tonically active and no longer becomes rhythmic with the CS presentation. As shown in Figure 8 brief excitation of RPeD11 causes direct inhibition of CGC activity. This finding is consistent with our hypothesis that in Lymnaea RPeD11 is the key interneuron to suppress the feeding circuit in response to noxious stimuli, such as KCl, quinidine sulfate, electrical shock and strong tactile stimuli. In this study we observed that the resting membrane potential in the CGCs of good performer’s was significantly hyperpolarized, although the impulse activity stayed constant. This observation is consistent with hypothesis that the elevated alert signal due to the avoidance conditioning originates from RPeD11 and causes a persistent inhibition of the CGC.
To confirm the crucial role of RPeD11 involving in TAC, we recently demonstrated that snails could be conditioned with paired presentation of the CS (sucrose) to the lip and direct current injection to depolarize RPeD11 as the US in an in-vitro semi-intact preparation comprising of a mouth, CGC and buccal ganglion29. Future experiments will direct test the phenomenon of the persistent conditioning-induced depolarization of one of the modulatory neuron, CV1 observed following appetitive conditioning53 was identical to the findings obtained in this study in one of CPGs, N1M.
Conclusion
Previously we established the TAC procedure in Lymnaea stagnalis with sucrose application as the CS and tactile stimulus to the head as the US. Backward conditioning (i.e. US-CS pairing) with the tactile stimulus to presented to the head first and sucrose application second did not modulate feeding behavior. Thus the TAC procedure we employed here demonstrates the CS-US temporal specificity and that this is a true example of associative learning and subsequent memory formation. In the present study we analyzed and compared both the spontaneous and CS-induced activity of three different types of neurons involved in the modulation and mediation of feeding behavior in semi-intact preparations made from snails exhibiting LTM (i.e. good performers) following TAC and from naïve snails. TAC training significantly altered both the spontaneous and CS induced activity in a feeding CPG neuron (N1M) and a motor neuron (B3); but no significant change in activity (spontaneous or CS-induced) was observed in a feeding modulatory neuron (CGC). The observed changes in the CPG neuron and the motor neuron were all consistent with the behavioral data in that the changes in neuronal activity seen made it less likely that feeding behavior would be initiated following training. That no difference in the CGC activity was observed between conditioned vs. naïve preparations suggest to us that this neuron may be more involved with turning feeding behavior on rather than suppressing it.
Acknowledgments
Authors express sincerely thanks Prof. T. Horikoshi of Tokai University for valuable discussion and critical comments. Part of this study was supported by the Research Promotion Program of Graduated School of Bioscience, Tokai University.
Abbreviations:
- TAC
taste avoidance conditioning;
- APC
appetitive conditioning;
- CS
conditional stimulus;
- US
unconditional stimulus;
- CGC
cerebral giant cell;
- N1M
N1 medial neuron;
- B3
B3 motor neuron;
- CPG
central pattern generator;
- SO
slow oscillator neuron;
- CV1
cerebral ventral 1 neuron;
- RPeD11
right pedal dorsal 11 neuron;
- ANOVA
analysis of variance;
- RMP
resting membrane potential;
- LTM
long-term memory
- EPSP
excitatory post-synaptic potential;
- STM
short-term memory;
- SE
standard error
Footnotes
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Conceived research program: MS. Conceived and designed the experiments: HS, MS. Performed the experiments: HS, ST. Analyzed the data: HS, ST, MS. Wrote the paper: HS, KL, MS.
References
- 1.Benjamin PR, Staras K, Kemenes G. A systems approach to the cellular analysis of associative learning in the pond snail Lymnaea. Learn Mem. 2000;7:124–131. doi: 10.1101/lm.7.3.124. [DOI] [PubMed] [Google Scholar]
- 2.Jones N, Kemenes G, Benjamin PR. Selective expression of electrical correlates of differential appetitive classical conditioning in a feeding network. J Neurophysiol. 2001;85:89–97. doi: 10.1152/jn.2001.85.1.89. [DOI] [PubMed] [Google Scholar]
- 3.Staras K, Kemenes G, Benjamin PR. Pattern-generating role for motoneurons in a rhythmically active neuronal network. J Neurosci. 1998;18:3669–3688. doi: 10.1523/JNEUROSCI.18-10-03669.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staras K, Kemenes G, Benjamin PR. Cellular traces of behavioral classical conditioning can be recorded at several specific sites in a simple nervous system. J Neurosci. 1999;19:347–357. doi: 10.1523/JNEUROSCI.19-01-00347.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staras K, Kemenes G, Benjamin PR. Electrophysiological and behavioral analysis of lip touch as a component of the food stimulus in the snail Lymnaea. J Neurophysiol. 1999;81:1261–1273. doi: 10.1152/jn.1999.81.3.1261. [DOI] [PubMed] [Google Scholar]
- 6.Ito E, Kobayashi S, Kojima S, Sadamoto H, Hatakeyama D. Associative learning in the pond snail Lymnaea stagnalis. Zool Sci. 1999;16:711–723. [Google Scholar]
- 7.Ito E, Kojima S, Lukowiak K, Sakakibara M. From likes to dislikes: conditioned taste aversion in the great pond snail (Lymnaea stagnalis) Can J Zool. 2013;91:405–412. [Google Scholar]
- 8.Kawai R, Sunada H, Horikoshi T, Sakakibara M. Conditioned taste aversion with sucrose and tactile stimuli in the pond snail Lymnaea stagnalis. Neurobiol Learn Mem. 2004;82:164–168. doi: 10.1016/j.nlm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Kojima S, Yamanaka M, Fujito Y, Ito E. Differential neuroethological effects of aversive and appetitive reinforcing stimuli on associative learning in Lymnaea stagnalis. Zool Sci. 1996;13:803–812. [Google Scholar]
- 10.Sakakibara M. Cellular and molecular aspect of short-term and long-term memory from molluscan system. In: Yen C-T, Onozuka M, editors. Novel Trends in Brain Science. Springer; Tokyo: 2008. pp. 131–148. [Google Scholar]
- 11.Takahashi T, Takigami S, Sunada H, Lukowiak K, Sakakibara M. Critical Period of Memory Enhancement during Taste Avoidance Conditioning in Lymnaea stagnalis. PLoS ONE. 2013;8:e75276. doi: 10.1371/journal.pone.0075276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takigami S, Sunada H, Lukowiak K, Sakakibara M. High voltage with little current as an unconditional stimulus for taste avoidance conditioning in Lymnaea stagnalis. Neurosci Lett. 2013;555:149–153. doi: 10.1016/j.neulet.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 13.Takigami S, Sunada H, Lukowiak K, Sakakibara M. Spaced taste avoidance conditioning in Lymnaea. Neurobiol Learn Mem. 2014;107:79–86. doi: 10.1016/j.nlm.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Takigami S, Sunada H, Lukowiak K, Kuzirian A, Alkon DL, Sakakibara M. Protein kinase C mediates memory consolidation of taste avoidance conditioning in Lymnae stagnalis. Neurobiol Lern Mem. 2014;111:9–18. doi: 10.1016/j.nlm.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Lukowiak K. Operant Conditioning in Lymnaea: evidence for Intermediate- and Long-term Memory. Learn Mem. 2000;7:140–150. doi: 10.1101/lm.7.3.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kojima S, Nakamura H, Nagayama S, Fujito Y, Ito E. Enhancement of an inhibitory input to the feeding central pattern generator in Lymnaea stagnalis during conditioned taste-aversion learning. Neurosci Lett. 1997;230:179–182. doi: 10.1016/s0304-3940(97)00507-7. [DOI] [PubMed] [Google Scholar]
- 17.Sugai R, Azami S, Shiga H, Watanabe T, Sadamoto H, Kobayashi S, Hatakeyama D, Fujito Y, Lukowiak K, Ito E. One-trial conditioned taste aversion in Lymnaea: good and poor performers in long-term memory acquisition. J Exp Biol. 2007;210:1225–1237. doi: 10.1242/jeb.02735. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin PR, Rose RM. Central generation of bursting in the feeding system of the snail Lymnaea stagnalis. J Exp Biol. 1979;80:93–118. doi: 10.1242/jeb.80.1.93. [DOI] [PubMed] [Google Scholar]
- 19.Elliott CJ, Benjamin PR. Interactions of the slow oscillator interneuron with feeding pattern-generating interneurons inLymnaea stagnalis. J Neurophysiol. 1985;54:1412–1421. doi: 10.1152/jn.1985.54.6.1412. [DOI] [PubMed] [Google Scholar]
- 20.Elliott CJ, Benjamin PR. Interactions of pattern-generating interneurons controlling feeding in Lymnaea stagnalis. J Neurophysiol. 1985;54:1396–1411. doi: 10.1152/jn.1985.54.6.1396. [DOI] [PubMed] [Google Scholar]
- 21.Rose RM, Benjamin PR. The relationship of the central motor pattern to the feeding cycle ofLymnaea stagnalis. J Exp Biol. 1979;80:137–163. doi: 10.1242/jeb.80.1.137. [DOI] [PubMed] [Google Scholar]
- 22.Vehovszky A, Elliott CJ. Activation and reconfiguration of fictive feeding by the octopamine-containing modulatory OC interneurons in the snail Lymnaea. J Neurophysiol. 2001;86:792–808. doi: 10.1152/jn.2001.86.2.792. [DOI] [PubMed] [Google Scholar]
- 23.Rose RM, Benjamin PR. Interneuronal control of feeding in the pond snail Lymnaea stagnalis. II. The interneuronal mechanism generating feeding cycles. J Exp Biol. 1981;92:203–228. [Google Scholar]
- 24.Straub VA, Staras K, Kemenes G, Benjamin PR. Endogenous and network properties of Lymnaea feeding central pattern generator interneurons. J Neurophysiol. 2002;88:1569–1583. doi: 10.1152/jn.2002.88.4.1569. [DOI] [PubMed] [Google Scholar]
- 25.Yeoman MS, Brierley MJ, Benjamin PR. Central pattern generator interneurons are targets for the modulatory serotonergic cerebral giant cells in the feeding system of Lymnaea. J Neurophysiol. 1996;75:11–25. doi: 10.1152/jn.1996.75.1.11. [DOI] [PubMed] [Google Scholar]
- 26.McCrohan CR. Initiation of feeding motor output by an identified interneurons in the snail Lymnaea stagnalis. J Exp Biol. 1984;113:351–366. [Google Scholar]
- 27.Pankey S, Sunada H, Horikoshi T, Sakakibara M. Cyclic nucleotide-gated channels are involved in phototransduction of dermal photoreceptors in Lymnaea stagnalis. J Comp Physiol B. 2010;180:1205–1211. doi: 10.1007/s00360-010-0490-x. [DOI] [PubMed] [Google Scholar]
- 28.Sunada H, Horikoshi T, Lukowiak K, Sakakibara M. Increase in excitability of RPeD11 results in memory enhancement of juvenile and adult of Lymnaea stagnalis by predator-induced stress. Neurobiol Learn Mem. 2010;94:269–277. doi: 10.1016/j.nlm.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Sunada H, Lukowiak K, Sakakibara M. In vitro aversion conditioning in Lymnaea. Short communication. Acta Biol Hung. 2012;63(Suppl 2):190–193. doi: 10.1556/ABiol.63.2012.Suppl.2.24. [DOI] [PubMed] [Google Scholar]
- 30.Sunada H, Sakaguchi T, Horikoshi T, Lukowiak K, Sakakibara M. The shadow-withdrawal response, dermal photoreceptors and their input to a higher order interneuron, RPeD11 in the pond snail Lymnaea stagnalis. J Exp Biol. 2010;213:3409–3415. doi: 10.1242/jeb.043521. [DOI] [PubMed] [Google Scholar]
- 31.Ono M, Kawai R, Horikoshi T, Yasuoka T, Sakakibara M. Associative Learning Acquisition and Retention Depends on Developmental Stage in Lymnaea stagnalis. Neurobiol Learn Mem. 2002;78:53–64. doi: 10.1006/nlme.2001.4066. [DOI] [PubMed] [Google Scholar]
- 32.Mita K, Okuta A, Okada R, Hatakeyama D, Otsuka E, Yamagishi M, Morikawa M, Naganuma Y, Fujito Y, Dyakonova V, Lukowiak K, Ito E. What are the elements of motivation for acquisition of conditioned taste aversion? Neurobiol Learn Mem. 2014;107:1–12. doi: 10.1016/j.nlm.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Alania M, Sakharov DA, Elliott CJ. Multilevel inhibition of feeding by a peptidergic pleural interneuron in the mollusc Lymnaea stagnalis. J Comp Physiol A, Neuroethol Sens Neural Behav Physiol. 2004;190:379–390. doi: 10.1007/s00359-004-0503-x. [DOI] [PubMed] [Google Scholar]
- 34.Kojima S, Hosono T, Fujito Y, Ito E. Optical detection of neuromodulatory effects of conditioned taste aversion in the pond snail Lymnaea stagnalis. J Neurobiol. 2001;49:118–128. doi: 10.1002/neu.1069. [DOI] [PubMed] [Google Scholar]
- 35.Kemenes G, Benjamin PR. Goal-tracking behavior in the pond snail Lymnaea stagnalis. Behav Neural Biol. 1989;52:260–270. doi: 10.1016/s0163-1047(89)90383-x. [DOI] [PubMed] [Google Scholar]
- 36.Kemenes G, Benjamin PR. Appetitive learning in snails shows characteristics of conditioning in vertebrates. Brain Res. 1989;489:163–166. doi: 10.1016/0006-8993(89)90019-x. [DOI] [PubMed] [Google Scholar]
- 37.Sangha S, McComb C, Lukowiak K. Forgetting and the extension of memory in Lymnaea. J Exp Biol. 2003;206:71–77. doi: 10.1242/jeb.00061. [DOI] [PubMed] [Google Scholar]
- 38.Spencer GE, Syed NI, Lukowiak K. Neural changes after operant conditioning of the aerial respiratory behavior in Lymnaea stagnalis. J Neurosci. 1999;19:1836–1843. doi: 10.1523/JNEUROSCI.19-05-01836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Straub VA, Benjamin PR. Extrinsic modulation and motor pattern generation in a feeding network: a cellular study. J Neurosci. 2001;21:1767–1778. doi: 10.1523/JNEUROSCI.21-05-01767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brierley MJ, Staras K, Benjamin PR. Behavioral function of glutamatergic interneurons in the feeding system of Lymnaea: plateauing properties and synaptic connections with motor neurons. J Neurophysiol. 1997;78:3386–3395. doi: 10.1152/jn.1997.78.6.3386. [DOI] [PubMed] [Google Scholar]
- 41.Yeoman MS, Vehovszky A, Kemenes G, Elliott CJ, Benjamin PR. Novel interneuron having hybrid modulatory- central pattern generator properties in the feeding system of the snail Lymnaea stagnalis. J Neurophysiol. 1995;73:112–124. doi: 10.1152/jn.1995.73.1.112. [DOI] [PubMed] [Google Scholar]
- 42.Yeoman MS, Pieneman AW, Ferguson GP, Ter Maat A, Benjamin PR. Modulatory role for the serotonergic cerebral giant cells in the feeding system of the snail, Lymnaea. I. Fine wire recording in the intact animal and pharmacology. J Neurophysiol. 1994;72:1357–1371. doi: 10.1152/jn.1994.72.3.1357. [DOI] [PubMed] [Google Scholar]
- 43.Kemenes I, Straub VA, Nikitin ES, Staras K, O’Shea M, Kemenes G, Benjamin PR. Role of delayed nonsynaptic neuronal plasticity in long-term associative memory. Curr Biol. 2006;16:1269–1279. doi: 10.1016/j.cub.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 44.Nikitin ES, Vavoulis DV, Kemenes I, Marra V, Pirger Z, Michel M, Feng J, O’Shea M, Benjamin PR, Kemenes G. Persistent sodium current is a nonsynaptic substrate for long-term associative memory. Curr Biol. 2008;18:1221–1226. doi: 10.1016/j.cub.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 45.Vavoulis DV, Nikitin ES, Kemenes I, Marra V, Feng J, Benjamin PR, Kemenes G. Balanced plasticity and stability of the electrical properties of a molluscan modulatory interneuron after classical conditioning: a computational study. Front Behav Neurosci. 2010;4:19. doi: 10.3389/fnbeh.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murakami J, Okada R, Sadamoto H, Kobayashi S, Mita K, Sakamoto Y, Yamagishi M, Hatakeyama D, Otsuka E, Okuta A, Sunada H, Takigami S, Sakakibara M, Fujito Y, Awaji M, Moriyama S, Lukowiak K, Ito E. Involvement of insulin-like peptide in long-term synaptic plasticity and long-term memory of the pond snail Lymnaea stagnalis. J Neurosci. 2013;33:371–383. doi: 10.1523/JNEUROSCI.0679-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murakami J, Okada R, Fujito Y, Sakakibara M, Lukowiak K, Ito E. Paired pulse ratio analysis of insulin-induced synaptic plasticity in the snail brain. J Exp Biol. 2013;216:1771–1773. doi: 10.1242/jeb.083469. [DOI] [PubMed] [Google Scholar]
- 48.Ito E, Otsuka E, Hama N, Aonuma H, Okada R, Hatakeyama D, Fujito Y, Kobayashi S. Memory trace in feeding neural circuitry underlying conditioned taste aversion in Lymnaea. PLoS ONE. 2012;7:e43151. doi: 10.1371/journal.pone.0043151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marra V, Kemenes I, Vavoulis D, Feng J, O’Shea M, Benjamin PR. Role of tonic inhibition in associative reward conditioning in lymnaea. Front Behav Neurosci. 2010;4 doi: 10.3389/fnbeh.2010.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Staras K, Kemenes I, Benjamin PR, Kemenes G. Loss of self-inhibition is a cellular mechanism for episodic rhythmic behavior. Curr Biol. 2003;13:116–124. doi: 10.1016/s0960-9822(02)01435-5. [DOI] [PubMed] [Google Scholar]
- 51.Sunada H, Lukowiak K, Sakakibara M. Repetitive noxious stimulus altered the shadow-induced withdrawal behavior in Lymnaea. Acta Biol Hung. 2012;63(Suppl 2):179–189. doi: 10.1556/ABiol.63.2012.Suppl.2.23. [DOI] [PubMed] [Google Scholar]
- 52.Inoue T, Takasaki M, Lukowiak K, Syed NI. Identification of a putative mechanosensory neuron in Lymnaea: characterization of its synaptic and functional connections with the whole-body withdrawal interneuron. J Neurophysiol. 1996;76:3230–3238. doi: 10.1152/jn.1996.76.5.3230. [DOI] [PubMed] [Google Scholar]
- 53.Jones NG, Kemenes I, Kemenes G, Benjamin PR. A persistent cellular change in a single modulatory neuron contributes to associative long-term memory. Curr Biol. 2003;13:1064–1069. doi: 10.1016/s0960-9822(03)00380-4. [DOI] [PubMed] [Google Scholar]