Abstract
Metabolic syndrome is associated with higher rates of cardiovascular morbidity and mortality. Although significant disparities in the risks of metabolic syndrome by occupation type and sex are well documented, the factors associated with metabolic syndrome in low‐ to middle‐income countries remain unclear. These gaps in evidence identify the need for patterns of metabolic syndrome among hospital personnel of both sexes in Nigeria. A total of 256 hospital workers comprising 32.8% men were studied. The mean age of the participants was 42.03±9.4 years. Using International Diabetic Federation criteria, the prevalence of metabolic syndrome was 24.2%. Women were substantially and significantly more likely to be identified with metabolic syndrome compared with men (34.9% vs 2.4%, respectively; P=.0001). This study identified metabolic syndrome among health workers with over one third of women with metabolic syndrome compared with <10% of men. These results support the implementation of lifestyle modification programs for management of metabolic syndrome in the health care workplace.
Cardiovascular diseases account for 17.3 million deaths per year globally, and by 2030 this is expected to rise to 23 million.1 The factors associated with increased cardiovascular disease tend to cluster in peculiar biological traits such as increased blood pressure (BP), fasting blood glucose, triglycerides, low high‐density lipoprotein cholesterol, and abdominal obesity. The constellation of these factors is identified as metabolic syndrome (MetS) and is associated with an approximate doubling of the risk of cardiovascular morbidity and mortality.2 MetS is an increasingly important public health issue and is deserving of more clinical attention.
The worldwide prevalence of MetS varies from 13.6% to 46%,3, 4, 5, 6 depending on the diagnostic criteria used and the population.7 The prevalence of MetS in Nigeria has been documented in recent studies as ranging between 12.1% and 54.3%, highest in people with diabetes mellitus.5, 8, 9, 10 The prevalence of MetS has been found to be affected by factors such as age, ethnicity, sex, and occupation.11, 12, 13, 14, 15 Previous studies have reported differences between sex, with some reporting a higher incidence of MetS in men12, 13 and some reporting the opposite.3
Recent studies have also shown that the prevalence of MetS varies between occupational groups. Job description and duty time schedules have been shown to affect the risk of developing MetS.14 For mechanisms not clear, shift duty workers were more prone to metabolic disturbances.16, 17 Mismatching of circadian rhythm, social disturbances, and behavioral changes associated with shift duties have been suggested as an independent pathway model for developing MetS.17 Increasing incidence of coronary heart disease among night duty workers may be explained by a higher prevalence of MetS. MetS is an independent risk factor for coronary events.18 Studies performed in Spanish14 and US15 workers have corroborated this finding. While vast studies exist regarding the sex and occupational effect on MetS, the same cannot be said of developing countries, with rising trends in cardiovascular events. In Nigeria, for example, the factors associated with MetS remain unclear as few data exist on its prevalence. These gaps in evidence identify the need for patterns of MetS among hospital personnel of both sexes in Nigeria. Therefore, this current study aimed to evaluate the prevalence of MetS among Nigerian health personnel and determine the influence of occupation and sex on its prevalence.
Methods
A total of 256 core hospital workers comprising 172 (67.2%) women were recruited from the University College Hospital. The participants who consented to the study were randomly selected from five clinical departments of the hospital. Baseline clinical and demographic characteristics were obtained from the study participants using a structured questionnaire. Clinical examination for height, weight, hip and waist circumferences was performed. Height was measured in meters (without shoes) and weight in kilograms (with heavy clothing removed and 1 kg deducted for remaining garments). Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. We measured waist circumference on standing patients with a soft tape midway between the lowest rib and the iliac crest. Hip circumference was measured over the widest part of the gluteal region, and the waist‐to‐hip ratio was calculated as a measure of central obesity. Two BP recordings were obtained from the right arm of patients in a sitting position after 30 minutes of rest. Measurements were taken in 5‐minute intervals and the average of the two measurements was used in the analysis.
Laboratory assessment included obtaining venous blood samples in a fasted state for the determination of components of the lipid panel (total cholesterol, high‐density cholesterol, low‐density cholesterol, and triglycerides), and blood glucose. Serum glucose was measured using glucose oxidase method and lipid profile by enzymatic colorimetric method.
MetS was defined according to International Diabetic Federation (IDF) criteria by the presence of waist circumference ≥94 cm in men and ≥80 cm in women and any two of the following characteristics: triglycerides ≥150 mg/dL; high‐density lipoprotein cholesterol <40 mg/dL in men and <50 mg/dL in women; BP ≥130/85 mm Hg; and fasting glucose ≥100 mg/dL. Ethical approval was obtained from the institutional research ethical review committee, and informed consent was sought from participants before embarking on the study.
Data Analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS Inc, Chicago, IL) version 15. Results were expressed as either mean±standard deviation or proportions (percentages). Comparisons for statistical significance was by independent Student t test for continuous variables or chi‐square analysis for categorical variables.
Results
Table 1 shows the baseline characteristics of the study population. The mean age of participants was 43.0±9.38 years and the overall prevalence of MetS was 24.2%. Women were older and heavier with wider waist circumference and had higher mean diastolic BP, fasting plasma glucose, and low‐density lipoprotein cholesterol compared with their male counterparts. Compared with men, women had a higher frequency of MetS (34.9% vs 2.4%, P<.0001). As shown in Table 2, participants with MetS were older and heavier with higher mean systolic BP and fasting plasma glucose when compared with patients without MetS. Table 3 shows that women with MetS had significant traditional cardiovascular risk factors compared with women without MetS. As shown in the Figure, nurses were found to have greater frequency of MetS when compared with other participants (40.2% vs 4.8%, P<.0001). Age and sex were shown as the independent correlates of MetS (Table 4.
Table 1.
General Characteristics of the Study Population
| Men (n=84) | Women (n=172) | Total (N=256) | P Value | |
|---|---|---|---|---|
| Age, y | 36.8±7.60 | 45.8±8.76 | 43.0±9.38 | <.0001 |
| Weight, kg | 73.6±12.57 | 73.5±13.22 | 73.5±12.98 | .954 |
| Height, cm | 171±8.59 | 161.3±7.80 | 164.55±9.26 | <.0001 |
| Body mass index, kg/m2 | 25.2±4.03 | 28.4±5.29 | 27.3±5.13 | <.0001 |
| Hip circumference, cm | 98.2±8.59 | 106.5±11.01 | 103.8±10.98 | <.0001 |
| Waist circumference, cm | 86.3±9.85 | 91.8±10.66 | 90.0±10.70 | <.0001 |
| Waist‐hip ratio | 0.89±0.08 | 0.87±0.08 | 0.87±0.08 | .040 |
| Systolic blood pressure, mm Hg | 120.1±14.69 | 116.5±13.73 | 117.7±14.13 | .063 |
| Diastolic blood pressure, mm Hg | 80.4±10.53 | 75.7±10.53 | 77.2±10.73 | <.0001 |
| Mean arterial blood pressure, mm Hg | 91.8±10.01 | 87.8±9.53 | 90.7±9.97 | .142 |
| Fasting plasma glucose, mg/dL | 103.8±19.37 | 97±13.41 | 98.4±15.09 | .031 |
| Total cholesterol, mg/dL | 176.3±18.16 | 172.8±17.37 | 173.6±17.55 | .264 |
| Triglycerides, mg/dL | 96.3±10.59 | 98.4±14.19 | 97.9±13.51 | .272 |
| Low‐density lipoprotein cholesterol, mg/dL | 98.2±9.55 | 104.6±11.53 | 103.2±11.42 | <.0001 |
| High‐density lipoprotein cholesterol, mg/dL | 42.8±3.53 | 43.4±7.17 | 43.3±6.56 | .417 |
| Prevalence of metabolic syndrome, % | ||||
| Metabolic syndrome | 2 (2.4) | 60 (34.9) | 62 (24.2) | <.0001 |
| No metabolic syndrome | 82 (97.6) | 112 (65.1) | 194 (75.8) | |
Table 2.
Characteristics of Patients With and Without Metabolic Syndrome
| Metabolic Syndrome (n=62) | No Metabolic Syndrome (n=194) | P Value | |
|---|---|---|---|
| Age, y | 48.74±8.25 | 41.05±8.96 | .0001 |
| Weight, cm | 79.21±13.17 | 71.73±12.43 | .0001 |
| Body mass index, kg/dL | 30.65±5.69 | 26.26±4.48 | .0001 |
| Height, cm | 160.85±8.32 | 165.70±9.26 | .0001 |
| Hip circumference, cm | 110.90±9.76 | 101.51±10.39 | .0001 |
| Waist circumference, cm | 96.33±9.24 | 87.94±10.35 | .0001 |
| Waist‐hip ratio | 0.87±0.06 | 0.87±0.09 | .929 |
| Systolic blood pressure, mm Hg | 120.95±13.00 | 116.72±14.35 | .046 |
| Diastolic blood pressure, mm Hg | 79.05±10.1 | 76.69±10.9 | .143 |
| Fasting plasma glucose, mg/dL | 108.8±12.2 | 94.0±14.0 | .000 |
| Plasma creatinine, mg/dL | 0. 94±0.16 | 0.95±0.19 | .767 |
| Plasma urea, mg/dL | 25.76±5.35 | 25.88±5.60 | .888 |
| Total blood cholesterol, mg/dL | 172.84±14.0 | 173.87±18.9 | .700 |
| Triglycerides, mg/dL | 95.39±13.45 | 99.04±13.44 | .076 |
| Low‐density lipoprotein cholesterol, mg/dL | 105.26±10.6 | 102.36±11.7 | .094 |
| Highdensity lipoprotein cholesterol, mg/dL | 42.48±3.33 | 43.67±7.51 | .235 |
| Occupation, % | |||
| Physicians | 91 (96.8) | 3 (3.2) | .0001 |
| Nursing staff | 76 (59.8) | 51 (40.2) | |
| Others | 27 (77.1) | 8 (22.9) | |
Table 3.
Characteristics of Study Women With Metabolic Syndrome
| Metabolic Syndrome (n=60) | No Metabolic Syndrome (n=112) | P Value | |
|---|---|---|---|
| Age, y | 48.77±8.0 | 44.18±8.8 | .001 |
| Weight, kg | 78.62±12.3 | 70.78±12.8 | .000 |
| Body mass index, kg/m2 | 30.55±5.58 | 27.25±4.78 | .000 |
| Height, cm | 160.53±8.27 | 161.74±7.54 | .340 |
| Hip circumference, cm | 110.78±9.89 | 104.25±10.94 | .000 |
| Waist circumference, cm | 95.89±8.72 | 89.55±11.0 | .000 |
| Waist‐hip ratio | 0.87±0.06 | 0.86±0.09 | .530 |
| Systolic blood pressure, mm Hg | 120.80±12.9 | 114.30±13.7 | .004 |
| Diastolic blood pressure, mm Hg | 78.84±10.1 | 74.07±10.4 | .006 |
| Fasting plasma glucose, mg/dL | 108.47±12.3 | 90.25±8.7 | .000 |
| Plasma creatinine, mg/dL | 0.94±0.16 | 0.97±0.18 | .307 |
| Plasma urea, mg/dL | 25.78±5.43 | 26. 06±5.72 | .763 |
| Total blood cholesterol, mg/dL | 172.65±14.2 | 172.93±19.0 | .921 |
| Triglycerides, mg/dL | 95.12±13.3 | 100.31±14.4 | .024 |
| Low‐density lipoprotein cholesterol, mg/dL | 105.7±10.4 | 103.9±12.2 | .354 |
| High‐density lipoprotein cholesterol, mg/dL | 42.43±3.24 | 44.04±8.63 | .168 |
Figure 1.
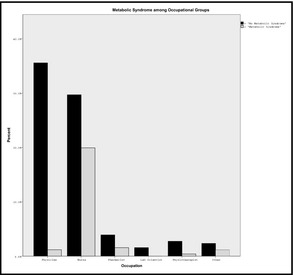
Metabolic syndrome among occupational groups.
Table 4.
Independent Correlates of Metabolic Syndrome in the Study Population
| Odds Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|
| Age, y | 1.083 | 1.04–1.13 | .000 |
| Women | 27.599 | 2.98–255.6 | .003 |
| Family history of diabetes | 1.236 | 0.52–2.91 | .628 |
| Family history of hypertension | 1.222 | 0.59–2.51 | .585 |
| Cigarette smoking | 0.303 | 0.06–1.60 | .160 |
| Alcohol consumption | 2.299 | 0.56–9.52 | .251 |
Nagelkerke R 2=0.297, P<.0001.
Discussion
The current findings show that the overall prevalence of MetS was 24.2%, with women having excess prevalence of MetS (34.9% vs 2.4%). While participants with MetS were older, with higher mean systolic BP and fasting plasma glucose, women with MetS had greater cardiovascular risk compared with women without MetS.
Compared with other definition criteria, studies that used the IDF criteria tend to find a higher prevalence of MetS in their study population. Despite this observation, IDF criteria being made ethnic‐specific has been found to be useful in any population.19 Also pending the availability of more specific data, the Europid waist circumference cutoff, which was implored in this study, has been advocated for the sub‐Sahara African population.20 The prevalence of MetS in the general population ranges between 17% and 25%.21 In our study, the frequency of MetS compared with that of a previous study by Ijeh and colleagues22 in Nigeria, who reported a prevalence rate of 30.7%, was at variant with the study by Ulasi and colleagues,23 who reported a lower prevalence of 15.9% in a Nigerian community. The lower value they observed may be accounted for by the fact that the location of the study was in both rural and semi‐urban areas compared with our study, which was located in an urban setting. The prevalence of MetS in an urban setting would be expected to be higher because of the influence of western lifestyle and diet. The prevalence of Mets in developed countries is much higher because of these same reasons. The influence of affluence and intake of atherogenic diets predispose to abdominal obesity, which is a mainspring for MetS. Barrios and colleagues in Spain,24 Ford and colleagues7 in the United States, and Yassein and colleagues25 in Jordan reported prevalence rates of 52%, 62.9%, and 52%, respectively.
In the current study, the participants who had MetS were older and had higher BMI, mean systolic BP, and fasting plasma glucose. These findings confirm the constellation nature of metabolic risk factors in MetS. A constant finding in the prevalence of MetS is age dependence, with various studies showing a linear association between age increase and incidence of MetS.19, 26, 27, 28 The present work confirms this thought by demonstrating a significant linear association between age and MetS; the odds of a participant having MetS increased by 8% for every 1‐year increase in age. This is probably because aging is associated with the development of insulin resistance, hormonal alterations, and increase in visceral adipose tissue fat––all of which are important in the development of MetS.29 The finding of higher body mass in the participants with MetS may not be surprising since it has been shown that overweight and obesity are associated with insulin resistance and MetS.26 Obesity contributes to hypertension, high serum cholesterol, low high‐density lipoprotein, and hyperglycemia. Excess adipose tissue releases several products such as nonesterified fatty acids, cytokines, and adiponectin that exacerbate these metabolic risk factors.26 Although anthropometric measures of adiposity for epidemiological studies were obtained in this current work, these may not be as accurate correlates of MetS when compared with viscera and subcutaneous fat.30, 31, 32 To strengthen these observations, studies have shown that reduction in the viscera fat by increased physical exercise or surgical removal reduces the major components of MetS, thereby preventing untoward cardiovascular morbidity and mortality.33, 34, 35
The female participants had a higher prevalence of MetS (34.9%) compared with the male participants (2.4%). Our finding is consistent with similar findings of studies performed in Nigeria by Osuji and colleagues (23.6% vs 7.6).36 This similarity is a call for concern since their study participants were known hypertensive patients compared with the current study population who were health personnel and appeared healthy. Several studies performed in other parts of the world have also reported a female preponderance in the prevalence of MetS.25, 37, 38, 39 Additional concern in the current study finding is the excess prevalence of MetS in women, who were shown to have 30 times the risk of MetS when compared with their male counterparts. The reason may be the result of a higher prevalence of obesity in women, which may be caused by sociocultural factors that favor a more sedentary lifestyle for women in this part of the world. There is an urgent need for sex‐focused intervention programs in the study group to prevent epidemics of cardiovascular morbidity and mortality in the community.
Occupation types have been shown to affect the frequency of MetS.14, 15 In this study, nurses had the highest prevalence of MetS among all the occupational groups that were studied. This finding may be a result of the influence of sex on prevalence rate because the nursing profession in Nigeria is dominated by women. Age could also have contributed to this since the nurses were older in comparison with those in the other occupational groups. In the current study, there was a preponderance for shift duties in all participants, although more defined for nurses, which may also explain the higher frequency of MetS among nurses.
Study Limitations
While the relatively small sample size is representative of the hospital community studied, it is a limitation to the generalization of the findings for the entire Nigeria population. The study's cross‐sectional design makes the causality effect inferences difficult. Furthermore, assessment of general adiposity was done in our study. It would have been remarkable to explore the association of visceral fat and biomarkers of inflammations with MetS; however, some studies40 showed a strong relationship between waist circumference and visceral fat and more so that the current findings have identified the burden of MetS. There is a need for a prospective and larger population study where all direct correlates of MetS will be considered.
Conclusions
This study revealed a high prevalence of MetS among female health workers. This calls for deployment of policies to support the implementation of lifestyle modification programs for prevention, control, and management of MetS in the health care workplace. In extrapolating, the prevalence of MetS in non–health workers/general population may be higher.
Disclosure
None.
J Clin Hypertens (Greenwich). 2015;17:880–884. DOI: 10.1111/jch.12595. © 2015 Wiley Periodicals, Inc.
References
- 1. Mathers CD, Loncar D. Updated Projections of Global Mortality and Burden of Disease, 2002–2030: Data Sources, Methods and Results. Geneva: World Health Organization; 2005. [Google Scholar]
- 2. Adeoye AM, Adebiyi A, Falase A. PT270 Excess metabolic and cardiovascukar risks among females health workers compared with males. Glob Heart. 2014;9:e219. [Google Scholar]
- 3. He Y, Jiang B, Wang J, et al. Prevalence of the metabolic syndrome and its relation to cardiovascular disease in an elderly Chinese population. J Am Coll Cardiol. 2006;47:1588–1594. [DOI] [PubMed] [Google Scholar]
- 4. Salaroli LB, Barbosa GC, Mill JG, Molina MC. Prevalence of metabolic syndrome in population‐based study, Vitoria, ES‐Brazil. Arq Bras Endocrinol Metabol. 2007;51:1143–1152. [DOI] [PubMed] [Google Scholar]
- 5. Adegoke OA, Adedoyin RA, Balogun MO, et al. Prevalence of metabolic syndrome in a rural community in Nigeria. Metab Syndr Relat Disord. 2010;8:59–62. [DOI] [PubMed] [Google Scholar]
- 6. Siminialayi I, Emem‐Chioma P, Odia O. Prevalence of metabolic syndrome in urban and suburban Rivers State, Nigeria: International Diabetes Federation and Adult Treatment Panel III definitions. Niger Postgrad Med J. 2010;17:147–153. [PubMed] [Google Scholar]
- 7. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. [DOI] [PubMed] [Google Scholar]
- 8. Sani MU, Wahab KW, Yusuf BO, et al. Modifiable cardiovascular risk factors among apparently healthy adult Nigerian population‐a cross sectional study. BMC Res Notes. 2010;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alebiosu C, Odusan O, Familoni O, Jaiyesimi A. Cardiovascular risk factors in type 2 diabetic Nigerians with clinical diabetic nephropathy. Cardiovasc J S Afr. 2003;15:124–128. [PubMed] [Google Scholar]
- 10. Isezuo SA. Is high density lipoprotein cholesterol useful in diagnosis of metabolic syndrome in native Africans with type 2 diabetes? Ethn Dis. 2004;15:6–10. [PubMed] [Google Scholar]
- 11. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 12. Fezeu L, Balkau B, Kengne A‐P, et al. Metabolic syndrome in a sub‐Saharan African setting: central obesity may be the key determinant. Atherosclerosis. 2007;193:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahonen T, Saltevo J, Laakso M, et al. Gender differences relating to metabolic syndrome and proinflammation in Finnish subjects with elevated blood pressure. Mediators Inflamm. 2009;2009:959281 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sánchez‐Chaparro M‐A, Calvo‐Bonacho E, González‐Quintela A, et al. Occupation‐related differences in the prevalence of metabolic syndrome. Diabetes Care. 2008;31:1884–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davila EP, Florez H, Fleming LE, et al. Prevalence of the metabolic syndrome among US workers. Diabetes Care. 2010;33:2390–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27 485 people. Occup Environ Med. 2001;58:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knutsson A, Bøggild H. Shiftwork and cardiovascular disease: review of disease mechanisms. Rev Environ Health. 2000;15:359–372. [DOI] [PubMed] [Google Scholar]
- 18. Haffner SM. Epidemiology of insulin resistance and its relation to coronary artery disease. Am J Cardiol. 1999;84:11–14. [DOI] [PubMed] [Google Scholar]
- 19. Hildrum B, Mykletun A, Hole T, et al. Age‐specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: the Norwegian HUNT 2 study. BMC Public Health. 2007;7:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alberti G, Zimmet P, Shaw J, Grundy SM. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels: International Diabetes Federation; 2006:1–23. [Google Scholar]
- 21. Yadav D, Mahajan S, Subramanian SK, et al. Prevalence of metabolic syndrome in type 2 diabetes mellitus using NCEP‐ATPIII, IDF and WHO definition and its agreement in Gwalior Chambal region of Central India. Glob J Health Sci. 2013;5:p142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ijeh II, Okorie U, Ejike C. Obesity, metabolic syndrome and BMI‐metabolic‐risk sub‐phenotypes: a study of an adult Nigerian population. J Med Med Sci. 2010;1:254–260. [Google Scholar]
- 23. Ulasi II, Ijoma CK, Onodugo OD. A community‐based study of hypertension and cardio‐metabolic syndrome in semi‐urban and rural communities in Nigeria. BMC Health Serv Res. 2010;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barrios V, Escobar C, Calderón A, et al. Prevalence of the metabolic syndrome in patients with hypertension treated in general practice in Spain: an assessment of blood pressure and low‐density lipoprotein cholesterol control and accuracy of diagnosis. J Cardiometab Syndr. 2007;2:9–15. [DOI] [PubMed] [Google Scholar]
- 25. Yasein N, Ahmad M, Matrook F, et al. Metabolic syndrome in patients with hypertension attending a family practice clinic in Jordan. EMHJ. 2010;16:375–380. [PubMed] [Google Scholar]
- 26. Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation. 2004;109:433–438. [DOI] [PubMed] [Google Scholar]
- 27. Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States. Natl Health Stat Report. 2009;13:1–8. [PubMed] [Google Scholar]
- 28. Gupta R, Deedwania PC, Gupta A, et al. Prevalence of metabolic syndrome in an Indian urban population. Int J Cardiol. 2004;97:257–261. [DOI] [PubMed] [Google Scholar]
- 29. Boden G, Chen X, DeSantis RA, Kendrick Z. Effects of age and body fat insulin resistance in healthy men. Diabetes Care. 1993;16:728–733. [DOI] [PubMed] [Google Scholar]
- 30. Pascot A, Despres J, Lemieux I, et al. Contribution of visceral obesity to the deterioration of the metabolic risk profile in men with impaired glucose tolerance. Diabetologia. 2000;43:1126–1135. [DOI] [PubMed] [Google Scholar]
- 31. Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–435. [DOI] [PubMed] [Google Scholar]
- 32. Sandeep S, Gokulakrishnan K, Velmurugan K, et al. Visceral and subcutaneous abdominal fat in relation to insulin resistance and metabolic syndrome in non‐diabetic south Indians. Indian J Med Res. 2010;131:629–635. [PubMed] [Google Scholar]
- 33. Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:76–88. [DOI] [PubMed] [Google Scholar]
- 34. Gabriely I, Ma XH, Yang XM, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging an adipokine‐mediated process? Diabetes. 2002;51:2951–2958. [DOI] [PubMed] [Google Scholar]
- 35. Brochu M, Tchernof A, Turner AN, et al. Is there a threshold of visceral fat loss that improves the metabolic profile in obese postmenopausal women? Metabolism. 2003;52:599–604. [DOI] [PubMed] [Google Scholar]
- 36. Osuji CU, Omejua EG. Prevalence and characteristics of the metabolic syndrome among newly diagnosed hypertensive patients. Indian J Endocrinol Metab. 2012;16(suppl 1):S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kelishadi R, Derakhshan R, Sabet B, et al. The metabolic syndrome in hypertensive and normotensive subjects: the Isfahan Healthy Heart Programme. Ann Acad Med Singapore. 2005;34:243–249. [PubMed] [Google Scholar]
- 38. Sorkhou E, Al‐Qallaf B, Al‐Namash H, et al. Prevalence of metabolic syndrome among hypertensive patients attending a primary care clinic in Kuwait. Med Princ Pract. 2003;13:39–42. [DOI] [PubMed] [Google Scholar]
- 39. Kelliny C, William J, Riesen W, et al. Metabolic syndrome according to different definitions in a rapidly developing country of the African region. Cardiovasc Diabetol. 2008;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carr DB, Utzschneider KM, Hull RL, et al. Intra‐abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–2094. [DOI] [PubMed] [Google Scholar]


