To the Editor
Stroke is a leading cause of long-term disability. Over 78% of patients never achieve normal levels of function despite rehabilitation [1]. The persistence of functional impairment underscores the need for new neurorehabilitative treatments. Non-invasive brain stimulation (NIBS) is one such technique that can directly boost underlying plasticity to augment rehabilitative recovery [2]. However, despite a significant body of evidence that has accumulated over the past decade [3], no NIBS modality is clinically approved for stroke rehabilitation.
A crucial reason is that evidence regarding efficacy of NIBS is mixed [2], where reports cite a significant response to modest-to-no response. Further, while large-scale trials would be needed to help account for the factors generating inconsistency (e.g. variance in etiology, pathology and baseline disability), such trials are lacking in stroke. In particular, we note that clinical, randomized, sham-controlled studies in stroke are especially unable to enroll large enough samples, unlike non-rehabilitation neurological indications where NIBS is clinically approved. For example, clinical trials that have helped accomplish FDA approval for NIBS in depression or migraine have generally enrolled an average of >100 patients in each intervention group per clinical trial compared to the ~10–50 patients for stroke rehabilitation (Fig. 1). This observation is staggering considering that stroke is a leading cause of disability and that most NIBS studies have investigated the most common post-stroke impairment-deficit of the paretic upper limb [4].
Fig. 1.
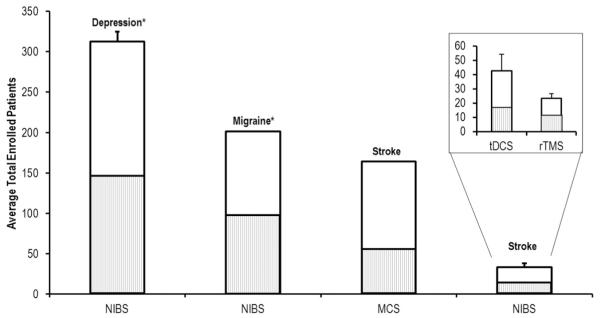
Literature review of total patient enrollment across clinical trials investigating non-invasive and invasive modalities of brain stimulation.
Even more alarming however, is that enrollment for NIBS is lower than that in recent trials of invasive epidural motor cortical stimulation (MCS) in stroke (Fig. 1) [5]. For example, phase I/II trials of MCS in stroke reported that ~31% were excluded, while exclusion rates for NIBS trials in stroke are on average from 65 to 95% [6,7]. This observation is surprising since NIBS is safer and simpler than invasive stimulation. Therefore, besides concerns for approval, this specific paradox raises serious ethical concerns regarding the clinical utility of NIBS in stroke. In particular, while we acknowledge that exclusion criteria may differ between studies, since safety risks vary based on the nature of stimulation modality and disease pathology, we question as to why such a drastic difference exists between exclusion rates across NIBS and MCS trials in stroke. And if there is a reason for the difference, if a possible solution may exist to address the paradox.
Average number of enrolled patients across trials of NIBS in depression, NIBS in migraine, and motor cortical stimulation (MCS)/NIBS in stroke. Etiologies with a star (*) denote applications that are currently FDA approved. Boxed inset denotes enrollment numbers for the most common modalities of NIBS in stroke rehabilitation, including repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS). Hashed gray inner bar denotes the average number of participants that were allocated to receive sham intervention for each application: Depression (n = 146; 46.7%), Migraine (n = 99; 49.2%), Stroke (MCS) (n = 60; 36.6%), Stroke (NIBS) (n = 14.8; 45.1%), tDCS (n = 17.3; 39.1%), rTMS (n = 13.1; 48.9%). Data were collected using intensive searching in PubMed and Google Scholar. While many studies were found (>100), only publications explicitly stating patient enrollment rates were included in analysis (n = 42) and ranged between years 2000 to 2014.
1. Improving enrollment for NIBS studies in stroke: learning from Mcs studies
One possible reason for such a discrepancy may be the inherent differences in exclusion criteria. Indeed, most of the common exclusion criteria for NIBS studies in stroke including recurrent strokes, ongoing use of neuroactive medications, history of a seizure, magnetic resonance imaging (MRI)-compatible or -incompatible metal in the head, pacemakers and concurrent rehabilitation, were not exclusionary in MCS trials. For example, in the phase III clinical trial for MCS in stroke, though epilepsy was a criterion for exclusion, patients could be enrolled if a seizure had occurred in the first month post-stroke. Further, in the current phase III Nexstim rTMS trial in stroke (NCT02089464), while epilepsy was also exclusionary, patients can be enrolled if a one-time seizure did not occur in the last 12 months prior to enrollment. In the case of NIBS, however, such a history would be exclusionary [5]. Based on these discrepancies, one possible solution would be to simply modify exclusion criteria for NIBS in a manner that is pertinent to stroke based on risks or exclusions set up for the MCS/rTMS study. In particular, considering exclusion criteria for the MCS study resulted in a relatively low rate of adverse events [5], it is probable that adoption of similar criteria for NIBS would pose the same level of risk, if not lower.
Also, we cannot discount that low enrollment may have been the result of general exclusion of patients with more serious motor deficits (<30 Fugl–Meyer) [5]. While including a wide range of stroke patients with more severe deficits may reduce homogeneity, such inclusion would be beneficial in expanding enrollment that could allow for the study of subsets of patients with differing levels of impairment. For example, even within the clinical trial that led to approval of NIBS in migraine, only 40% of patients reported significant effects; however subset analyses revealed that patients on migraine preventative drugs had a select advantage to treatment [8]. Thus, while broader inclusion of patients could be advantageous, it is important to note that an appropriate sample size would still be required to fully arrive at conclusions following a subset analysis.
Higher recruitment rates in MCS trials in stroke also emerges from the inherent advantages offered by the use of multiple study sites across departments of neurology, rehabilitation and physical therapy. This is similar to the structure of DBS studies in the investigation of movement disorders, where the trials were conducted in close proximity to or within the departments of neurosurgery [9]. Thus, patients are potentially easily identified as candidates through clinical practice. In the same vein, one could envision that enrollment for NIBS studies could be boosted if it were to be delivered as a part of a clinical program, for instance, as an off-label procedure in outpatient settings. Further, open-label use, similar to naturalistic rTMS studies in pain [10], could have enhanced confidence of other health care providers, patients and payors in the utility of new modalities. In the end, encouraging funding agencies and medical device companies to sponsor investigator-led multi-site studies with NIBS could promote cooperative recruiting on the same scale as for the MCS study funded by one such agency.
Outside of study design, the influence of a general lack of a streamlined process for patient enrollment also cannot be overlooked. Few studies report databases or search terms utilized to identify potential candidates. Further, we know of no databases thus far that are available internationally to pool recruitment efforts. Therefore, in order to pool larger and/or create more homogeneous samples, it may be advantageous to pool candidates in approved national/international databases. For example, federal government led initiatives, such as the National Institutes of Health StrokeNet, could help investigators exploit recruitment infrastructures at sites across the country. In addition, researchers could access current databases such as the ‘Get With The Guidelines’ (GWTG) tPA registry of the American Heart Association or clinicaltrials.gov to identify patients with select criteria.
2. Moving forward
Given that evidence regarding efficacy of NIBS has been mixed, it is difficult to predict whether or not NIBS will prove as a clinically viable approach for augmenting recovery after stroke. However, by building from the MCS trials and increasing study enrollment, we would have the ability to:
-
–
Improve statistical power to determine efficacy
-
–
Allow investigators to plan large-scale phase II and phase III studies required for FDA approval
-
–
Understand which heterogeneous characteristics in stroke create mixed evidence of efficacy
-
–
Test generalizability of NIBS approaches using subset analyses across patients with wide-ranging damage and disability
-
–
Study clinical, physiologic, structural and lesion-related biomarkers across a wide variety of patients that will help understand characteristics of ‘responders’ to NIBS, and stratify patients for future studies.
Even though current research has suggested mixed efficacy of NIBS, we can be optimistic about the future for NIBS in stroke rehabilitation. Disciplines studying depression and migraine, first of all, have laid the groundwork explaining efforts in recruitment that would be required for FDA approval of NIBS. Further, recent trials of MCS and Nextstim rTMS in stroke have helped clarify the study criteria that would be needed to recruit larger samples to feasibly conduct a phase III clinical trial. By building off of these recent efforts, we would be able to conduct large scale clinical studies necessary to generate better evidence for therapeutic efficacy of NIBS in stroke.
References
- [1].Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics — 2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat. Rev. Neurol. 2014;10:597–608. doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- [3].Hummel FC, Celnik P, Pascual-Leone A, Fregni F, Byblow WD, Buetefisch CM, et al. Controversy: noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul. 2008;1:370–382. doi: 10.1016/j.brs.2008.09.003. [DOI] [PubMed] [Google Scholar]
- [4].Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001;32:1279–1284. doi: 10.1161/01.str.32.6.1279. [DOI] [PubMed] [Google Scholar]
- [5].Levy RM, Harvey RL, Kissela BM, Winstein CJ, Lutsep HL, Parrish TB, et al. Epidural electrical stimulation for stroke rehabilitation: results of the prospective, multicenter, randomized, single-blinded Everest trial. Neurorehabilitation and Neural Repair. 2015 doi: 10.1177/1545968315575613. [DOI] [PubMed] [Google Scholar]
- [6].Elkins JS, Khatabi T, Fung L, Rootenberg J, Johnston SC. Recruiting subjects for acute stroke trials: a meta-analysis. Stroke. 2006;37:123–128. doi: 10.1161/01.STR.0000195149.44390.aa. [DOI] [PubMed] [Google Scholar]
- [7].Anjos SM, Cohen LG, Sterr A, de Andrade KN, Conforto AB. Translational neurorehabilitation research in the third world: what barriers to trial participation can teach us. Stroke. 2014;45:1495–1497. doi: 10.1161/STROKEAHA.113.003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9:373–380. doi: 10.1016/S1474-4422(10)70054-5. [DOI] [PubMed] [Google Scholar]
- [9].Plow EB, Machado A. Invasive neurostimulation in stroke rehabilitation. Neurotherapeutics. 2014;11:572–582. doi: 10.1007/s13311-013-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hodaj H, Alibeu JP, Payen JF, Lefaucheur JP. Treatment of chronic facial pain including cluster headache by repetitive transcranial magnetic stimulation of the motor cortex with maintenance sessions: a naturalistic study. Brain Stimulation. 2015;8(4):801–807. doi: 10.1016/j.brs.2015.01.416. [DOI] [PubMed] [Google Scholar]


