Abstract
We have developed a dual-modality CT/SPECT imaging system for small-animal imaging applications. The X-ray system comprises a commercially available micro-focus X-ray tube and a CCD-based X-ray camera. X-ray transmission measurements are performed based on cone-beam geometry. Individual projections are acquired by rotating the animal about a vertical axis in front of the CCD detector. A high-resolution CT image is obtained after reconstruction using an ordered subsets-expectation maximization (OS-EM) reconstruction algorithm. The SPECT system utilizes a compact semiconductor camera module previously developed in our group. The module is mounted perpendicular to the X-ray tube/CCD combination. It consists of a 64×64 pixellated CdZnTe detector and a parallel-hole tungsten collimator. The field of view is 1 square inch. Planar projections for SPECT reconstruction are obtained by rotating the animal in front of the detector. Gamma-ray and X-ray images are presented of phantoms and mice. Procedures for merging the anatomical and functional images are discussed.
Index Terms: Biomedical imaging, cadmium zinc telluride (CdZnTe), multimodality imaging, single-photon emission computed tomography, small-animal imaging
I. Introduction
Over the last several years, there has been increasing demand for small-animal imaging systems. This demand has been primarily due to recent advances in mouse genetics, where transgenic mouse models have been developed that can mimic human diseases. Nuclear medicine imaging systems offer a noninvasive alternative to autoradiography, which requires the sacrifice of the animal. By performing longitudinal studies on the same animal, the progress of a disease can be monitored and/or molecular changes due to gene expression can be detected. This information is particularly important in the area of gene therapy, where an imaging system can assess the success of vector delivery and obtain accurate time curves of gene expression. Several tomographic gamma-ray imaging systems have recently been developed based on scintillators [1]–[6] or semiconductor arrays [7], [8].
Functional images such as gamma-ray images can occasionally be difficult to interpret due to a lack of identifiable anatomical features. By combining images from an anatomical modality, such as X-ray CT, we can enhance our understanding of the image and obtain complementary information about the object that can assist in the localization of the uptake of the radiopharmaceutical. In recent years, a great interest has emerged in the development of dual-modality imaging systems. Several groups have recently reported on their progress in the development of such systems for small-animal imaging applications [9]–[16], [22]. Others are developing micro-CT systems to incorporate with their already developed gamma-camera systems [17], [18].
In this paper we will discuss the development of a compact dual-modality CT/SPECT system for imaging mice. The X-ray system consists of a commercially available micro-focus X-ray tube and a CCD-based X-ray camera. The SPECT system utilizes a compact semiconductor camera module previously developed in our group [19].
II. System Description
A. X-Ray System
Our CT system has three main components: an X-ray tube, a CCD camera and a mechanical shutter system.
The X-ray camera is a CCD/phosphor screen detector manufactured by Dalsa-MedOptics (AC series). It consists of a Kodak KAF-1001E series 1024 × 1024 pixel CCD array that has an active area of 24.5 mm × 24.5 mm. The CCD is coupled via a 2:1 fiberoptic taper to a gadolinium oxysulfide phosphor screen which increases the active area to 50 × 50 mm2. The CCD array is a silicon charge-coupled device based on a full-frame (FF) architecture and true two-phase (T2φ) technology for charge transfer [20]. The camera is cooled to −10°C via a TEC (thermo-electric cooler). The warm side of the TEC is air cooled using a fan housed inside the camera.
The X-ray tube employed is an Oxford Instruments XTF5000/75 with a 0.005 inch Be window. The same X-ray tube has been studied extensively by Paulus et al. [21] for their microCAT system. It has a stationary tungsten anode and a 130μm spot size. The electron gun assembly is packaged inside a stainless steel lead-lined tube that provides X-ray shielding to 0.25 mR/hr at 2 inches. Cooling oil is placed inside the tube to enhance heat dissipation. The tube is mounted inside an aluminum extrusion where a fan is placed on one end to circulate air and cool the tube. The temperature is monitored via a resistive temperature device (RTD) placed inside the tube housing and is always maintained below 45°C. The tube is powered by a Spellman Electronic Corporation power supply. X-rays can be generated in the range of 4–50 kVp with a maximum anode current of 1.5 mA. The tube is operated in a positive anode mode with the filament at ground. We positioned the X-ray tube with its axis perpendicular to the floor due to malfunction when operated in a horizontal orientation.
B. Gamma-Ray System
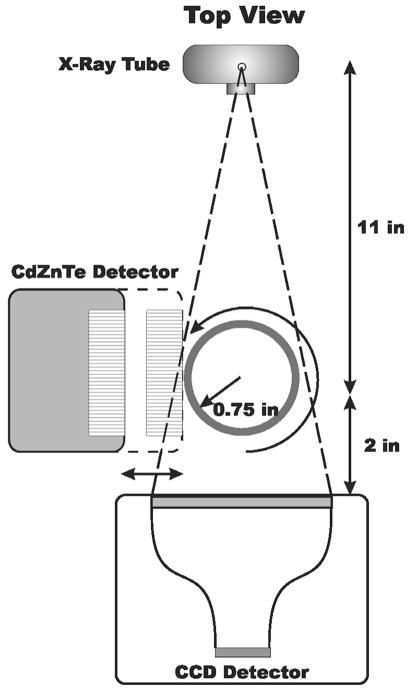
The gamma-ray imaging component of our system is a compact semiconductor camera module previously developed in our group [22]. Briefly, the module consists of a 2.5 cm × 2.5 cm × 0.20 cm slab of CdZnTe with a continuous gold electrode on the top and a 64 × 64 pixellated array of gold electrodes on the bottom that are electrically connected to a readout ASIC via indium-bump bonding. The detector pitch is 380 μm, with a pixel size of 330 μm. A 7-mm thick high-resolution parallel-hole collimator with a matching pitch is used as the imaging aperture. The detector-collimator assembly is mounted inside a tungsten-aluminum housing (shown in Fig. 1) and located perpendicular to the X-ray tube/CCD combination. Individual gamma-ray projections can be acquired by injecting the animal with a radio-pharmaceutical and vertically rotating it in front of the detector.
Fig. 1.

Photograph of the compact semiconductor module previously developed in our group [19].
C. System Housing
The dual-modality system resides inside a 79 cm × 48 cm × 46 cm aluminum housing that is laminated with 1.5 mm of lead. A photograph of the enclosure is shown in Fig. 2. The enclosure allows visual inspection of the animal during imaging through a CLEAR-Pb leaded plastic window. This window can be opened for immediate access to the animal. Two interlock micro-switches are placed in parallel to disable the power supply of the X-ray tube when either of the covers is open. Administration of isoflurane/oxygen gas anesthesia is provided to the animal through a properly shielded opening. A 4-inch ventilation outlet and a 3-inch ventilation inlet allow circulation of air. The scavenging of the anesthetic gas is performed by a charcoal canister which is connected to the air outlet using duct tubing. All system components are mounted on an optical breadboard using standard optical posts and mounts.
Fig. 2.
Photograph of the dual-modality system enclosure.
III. Imaging Methods
The X-ray tube, semiconductor array, and rotation stage are controlled using a National Instruments (PCI-6024E) general-purpose analog and digital I/O board and custom Labview software. An additional PCI board was provided by Dalsa-MedOptics for communication with the X-ray camera. A PC in Windows NT environment controls the system. X-ray and gamma-ray projections can be acquired by rotating objects in front of the two cameras using a Velmex B5990TS rotating stage.
The X-ray and gamma-ray cameras are mounted orthogonally to each other. A diagram of the geometry of the dual-modality system is shown in Fig. 3. The X-ray tube emits a cone-beam of X-rays. To minimize beam hardening artifacts, beam filtration is applied in front of the tube’s exit window with a 0.5 mm aluminum plate. X-ray projections are acquired in a step-and-shoot fashion by rotating the animal in front of the camera. The X-ray tube emits X-rays continuously while a mechanical shutter, triggered by the X-ray camera, allows exposure of the CCD only during acquisition and not during readout.
Fig. 3.
Diagram illustrating the geometry of the dual-modality system.
X-ray projections are typically collected prior to gamma-ray images. Dark current subtraction and flat field division are applied to each transmission projection. Therefore, the total time period needed for a 1 s acquisition X-ray projection is approximately 5 s. This time period includes a 4 s overhead for applying the image corrections, saving the data to disk, and rotating the stage for the next projection. Each raw X-ray projection suffers from distortion due to the fiberoptic taper that couples the CCD to the gadolinium oxysulfide phosphor screen. Distortion correction is applied using a third-degree polynomial procedure, based on a correction map provided by the camera manufacturer.
All the images are transferred to a DEC Alpha workstation for reconstruction. A CT image is reconstructed using an ordered subsets-expectation maximization (OS-EM) reconstruction algorithm. We use a maximum likelihood-expectation maximization (ML-EM) iterative reconstruction algorithm for obtaining a SPECT image. The algorithm corrects for the effects of collimator blur but not attenuation and scatter. Flood correction and median filtering are also applied to each of the gamma-ray projections to correct for inhomogeneities of the detector.
A. Phantom Imaging
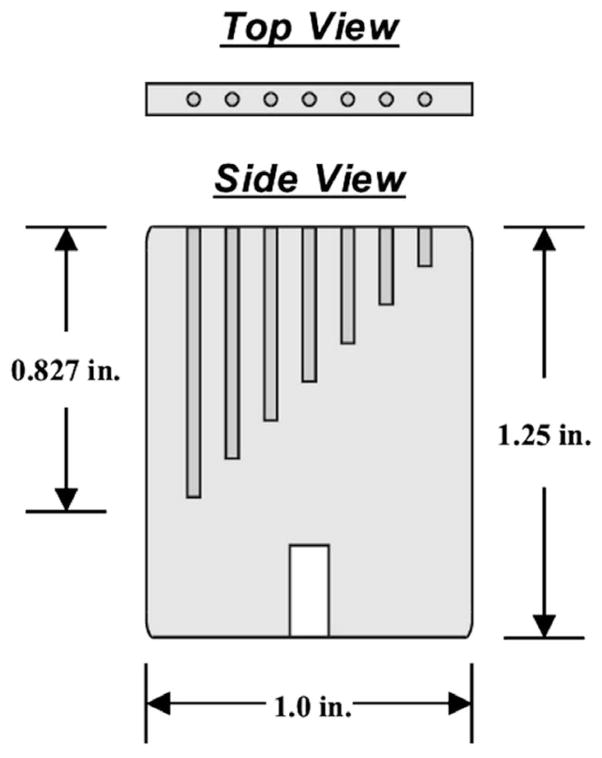
An acrylic line phantom was constructed for imaging with both modalities. A diagram of this phantom is shown in Fig. 4. The phantom consists of seven 1-mm diameter holes of different depths that are filled with Tc-99 m aqueous solution for SPECT imaging. The center-to-center distance between adjacent holes is 3 mm. A simultaneous X-ray CT of the same phantom can also be acquired by adding a contrast medium to the solution. The phantom images allow the calibration of the X-ray and gamma-ray systems for the co-registration of the images obtained from the two modalities.
Fig. 4.
Diagram of the acrylic phantom constructed for imaging with both modalities. The phantom can be filled with a mixture of 99mTc aqueous solution and X-ray contrast medium to allow simultaneous SPECT and CT imaging. Use of the phantom allows for the calibration of the X-ray and gamma-ray systems for the co-registration of the images obtained from the two modalities.
The line phantom was filled with a solution that combined approximately 2 mCi of 99mTc-pertechnetate and Omnipaque, an iodine-based X-ray contrast medium. We acquired 360 transmission projections at 1° increments, with acquisition time of 1 s per projection and the tube operating at a voltage of 32 kVp and an anode current of 0.4 mA. Immediately following the CT completion, we acquired 90 gamma-ray projections, with 60 s acquisition per projection.
B. Planar Animal Imaging
A 20-g mouse was injected intradermally with 100 μCi of 99mTc-Sulfur Colloid in each foot. Immediately following the injection, the animal was anesthetized using a Ketamine/Xylazine mixture and placed inside an acrylic holder. A planar gamma-ray image of the pelvic area of the mouse was acquired for ten minutes. The animal was rotated 90° about the vertical axis and a 1-s X-ray projection was obtained of the same area.
C. Tomographic Animal Imaging
A 30-g mouse was injected with 8.6 mCi of 99mTc-MDP. Two hours later, the animal was euthanised with an overdose of pentobarbital and placed inside an animal holder. We collected 90 gamma-ray projection images over 360°, with 60 s acquisition per projection. The projection images correspond to the thorax and skull area of the mouse. After the SPECT image sequence was completed we began the acquisition of X-ray projections. The X-ray tube was operated at a voltage of 32 kVp and an anode current of 0.4 mA. We collected 360 projections with 1 s acquisition per projection.
IV. Results
A. Phantom Imaging
Reconstructed CT, SPECT, and combined slices of the line phantom are shown in Fig. 5. Different colormaps are used for the representation of the two different data sets. From the CT slice, we can see that an air-bubble was formed in the middle of the longest hole which corresponds to a lack of activity in the SPECT image. Overall, there is good agreement between the SPECT and CT images.
Fig. 5.
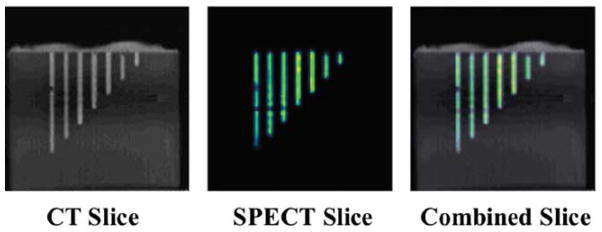
Reconstructed CT, SPECT, and combined slices of the acrylic phantom shown in Fig. 3. The combined slice indicated that there is a good agreement between the SPECT and CT images.
B. Planar Animal Imaging
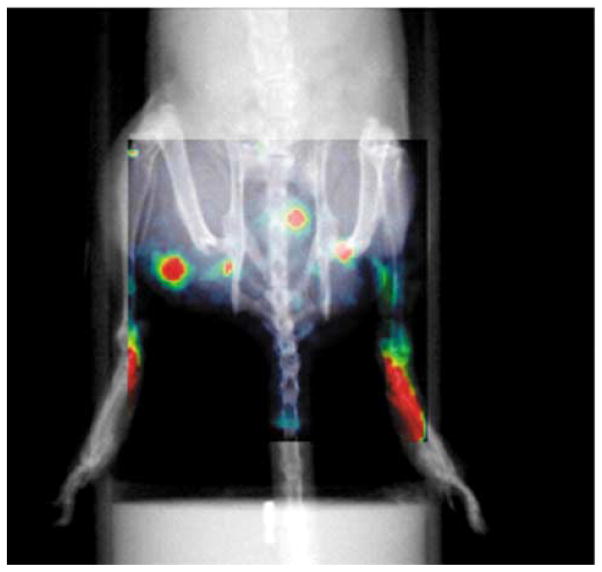
In Fig. 6 we present as uper position of the X-ray and gamma-ray images. The injection sites, lymphatic channels, and several nodes can be identified from the gamma-ray image. The superposition of the X-ray image facilitates the identification of the femoral and retroperitoneal nodes in both hind limbs.
Fig. 6.
Superposition of planar X-ray and gamma-ray images of the pelvic area of a mouse. The mouse was injected intradermally with 100 μCi of 99mTc-sulfur colloid in each foot. The injection sites, lymphatic channels, and several nodes can be identified.
C. Tomographic Animal Imaging
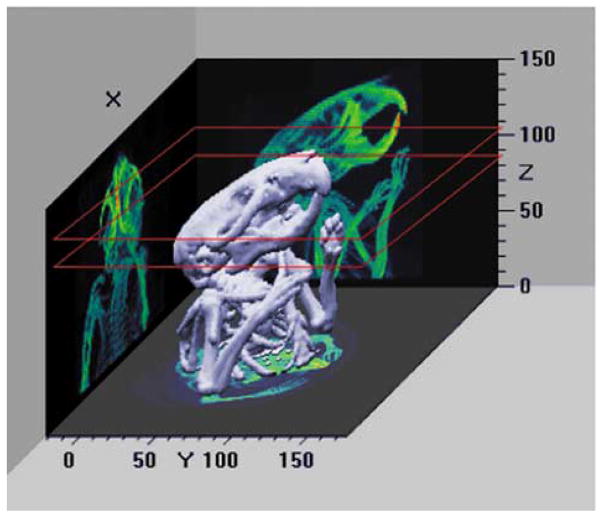
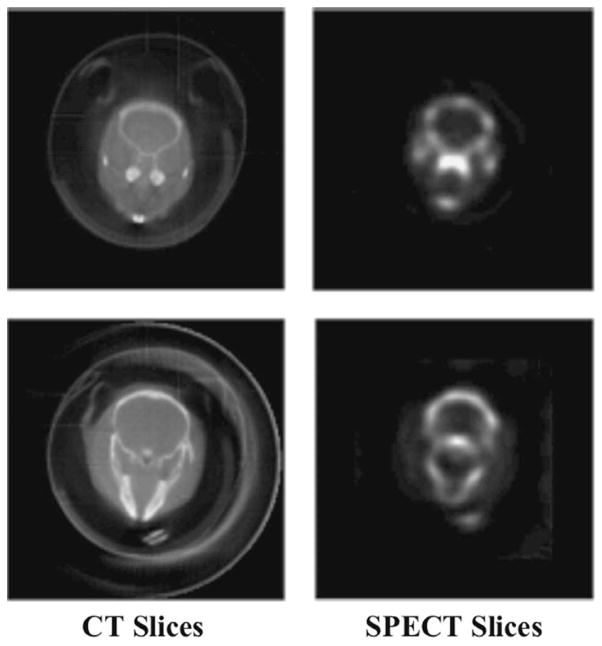
In Fig. 7 we display a three-dimensional representation of the CT data set of the skull. CT and SPECT slices corresponding to the same skull location are shown in Fig. 8. There is good agreement between the X-ray images and the corresponding uptake of the radiotracer in the bone.
Fig. 7.
Three-dimensional representation of a reconstructed CT data set of the skull of a mouse. The overlayed red squares indicate the location of the representative slices shown below.
Fig. 8.
Corresponding CT and SPECT reconstructed slices of the skull. The mouse was injected with 8.6 mCi of 99mTc-MDP two hours prior to imaging.
V. Conclusion
The full potential of functional imaging can be realized only when areas of tracer uptake can be unambiguously assigned to organs or other anatomical features. Since well chosen radiotracers may have excellent uptake in the target, for example a tumor, but rapidly clear from surrounding tissues, SPECT images sometimes contain few recognizable anatomical features. The addition of independently measured, but co-registered anatomical information overcomes this limitation. The CT/SPECT system described above provides high-quality images from both modalities and will be a useful instrument for supporting biomedical research in a variety of fields.
Acknowledgments
This work was supported by the National Institutes of Health under Grants R24 CA83148 (Southwest Animal Imaging Resource) and P41 RR14304 (Center for Gamma-Ray Imaging). The work of T. E. Peterson, Ph.D., was supported by a Career Award at the Scientific Interface from the Burroughs Wellcome Fund.
The authors would like to thank Z. Liu, C. Howison, G. Stevenson, and B. Skovan for their assistance with the animals. The lymphatic images were acquired with collaboration of Dr. M. H. Witte and the assistance of M. J. Bernas and B. M. Kriederman.
Contributor Information
George A. Kastis, Department of Radiology, Division of Nuclear Medicine, University of Arizona, Tucson, AZ 85724 USA.
Lars R. Furenlid, Email: furen@radiology.arizona.edu, Department of Radiology, Division of Nuclear Medicine, University of Arizona, Tucson, AZ 85724 USA, and also with the Optical Sciences Center, University of Arizona, Tucson, AZ 85721 USA.
Donald W. Wilson, Email: dwwilson@radiology.arizona.edu, Department of Radiology, Division of Nuclear Medicine, University of Arizona, Tucson, AZ 85724 USA
Todd E. Peterson, Email: todd.e.peterson@vanderbilt.edu, Institute of Imaging Science, Vanderbilt University, Nashville, TN 37232 USA.
H. Bradford Barber, Email: bradb@radiology.arizona.edu, Department of Radiology, Division of Nuclear Medicine, University of Arizona, Tucson, AZ 85724 USA, and also with the Optical Sciences Center, University of Arizona, Tucson, AZ 85721 USA.
Harrison H. Barrett, Email: barrett@radi-ology.arizona.edu, Department of Radiology, Division of Nuclear Medicine, University of Arizona, Tucson, AZ 85724 USA, and also with the Optical Sciences Center, University of Arizona, Tucson, AZ 85721 USA.
References
- 1.Cherry SR, Shao Y, Silverman RW, Meadors K, Siegel S, Chatziioannou A, Young JW, Jones W, Moyers JC, Newport D, Boutefnouchet A, Farquhar TH, Andreaco M, Paulus MJ, Binkley DM, Nutt R, Phelps ME. MicroPET: a high resolution PET scanner for imaging small animals. IEEE Trans Nucl Sci. 1997 Jun;44:1161–1166. [Google Scholar]
- 2.Kastis GK, Barber HB, Barrett HH, Gifford HC, Pang IW, Patton DD, Sain JD, Stevenson G, Wilson DW. High resolution SPECT imager for three-dimensional imaging of small animals. J Nucl Med. 1998 May;39(5):9P. [Google Scholar]
- 3.Jeavons AP, Chandler RA, Dettmar CAR. A 3D HIDAC-PET camera with sub-millimeter resolution for imaging small animals. IEEE Trans Nucl Sci. 1999 Jun;46:468–473. [Google Scholar]
- 4.Schramm N, Wirrwar A, Sonnenberg F, Halling H. Compact high resolution detector for small animal SPECT. IEEE Trans Nucl Sci. 2000 Jun;47:1163–1167. [Google Scholar]
- 5.MacDonald LR, Patt BE, Iwanczyk JS, Tsui BMW, Wang Y, Frey EC, Wessell DE, Acton PD, Kung HF. Pinhole SPECT of mice using the LumaGEM gamma camera. IEEE Trans Nucl Sci. 2001 Jun;48(pt 2):830–836. [Google Scholar]
- 6.Furenlid LR, Wilson DW, Chen Y, Kim HK, Pietraski PJ, Crawford MJ, Barrett HH. FASTSPECT II: a second-generation high-resolution dynamic SPECT imager. Proc. 2002 IEEE Nuclear Science Symp./Medical Imaging Conf; Norfolk, VA. Nov. 2002; pp. 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kastis GA, Wu MC, Balzer SJ, Wilson DW, Furenlid L, Stevenson G, Barrett HH, Barber HB, Woolfenden JM, Kelly P, Appleby M. Tomographic small-animal imaging using a high-resolution semiconductor camera. IEEE Trans Nucl Sci. 2002 Feb;49(pt 1):172–175. doi: 10.1109/TNS.2002.998747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson TE, Kim H, Crawford MJ, Gershman BM, Hunter WCJ, Barber HB, Furenlid LR, Wilson DW, Woolfenden JM, Barrett HH. SemiSPECT: a small-animal imaging system based on eight CdZnTe pixel detectors. Proc. 2002 IEEE Nuclear Science Symp./Medical Imaging Conf; Norfolk, VA. Nov. 2002; pp. 1844–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams MB, Galbis-Reig V, Goode AR, Simoni PU, Majewski S, Weisenberger AG, Wojcik R, Phillips W, Stanton M. Multimodality imaging of small animals. RSNA Electron J. 1999 Aug;3 [Google Scholar]
- 10.Iwata K, Wu MC, Hasegawa BH. Design of combined X-ray CT and SPECT systems for small animals. Proc. Conf. Rec. 1999 IEEE Nuclear Science Symp./Medical Imaging Conf; Seattle, WA. Oct. 1999; pp. 1608–1612. [Google Scholar]
- 11.Williams MB, Zhang G, More MJ, Goode AR, Majewski S, Wojcik R, Kross B, Popov V, Weisenberger AG, Stanton M, Phillips W, Stewart A, McCauley T, Wu T, DiBella EVR. Integrated CT-SPECT system for small animal imaging. Proc SPIE—Penetrating Radiat Syst Applicat II. 2000 Dec;4142:265–274. [Google Scholar]
- 12.Iwata K, Soo-Il Kwon S-I, Hasegawa BH, Bennett PR, Cirignano L, Shah KS. Description of a prototype combined CT-SPECT system with a single CdZnTe detector. Proc. Conf. Rec. 2000 IEEE Nuclear Science Symp./Medical Imaging Conf; France. Oct. 2000; pp. 16/1–16/5. [Google Scholar]
- 13.Weisenberger AG, Wojcik R, Bradley EL, Brewer P, Majewski S, Qian J, Ranck A, Saha MS, Smith K, Smith MF, Welsh RE. SPECT-CT system for small animal imaging. Proc. Conf. Rec. 2001 IEEE Nuclear Science Symp./Medical Imaging Conf; San Diego, CA. Oct. 2001; pp. 1840–1844. [Google Scholar]
- 14.Iwata K, MacDonald LR, Hwang AB, Wu MC, Patt BE, Iwanczyk JS, Hasegawa BH. CT-SPECT for small animal imaging with A-SPECT. J Nucl Med. 2002 May;43(5):10P–11P. [Google Scholar]
- 15.Iwata K, MacDonald LR, Li J, Williams SP, Sakdinawat AE, Hwang AB, Wu MC, Patt BE, Iwanczyk JS, Hasegawa BH. Dual isotope imaging with a dedicated small animal CT-SPECT system. Molecular Imaging Biol. 2002 Jul-Aug;4(4):S21. [Google Scholar]
- 16.Khodaverdi M, Pauly F, Weber S, Schroder G, Ziemons K, Sievering R, Halling H. Preliminary studies of a micro-CT for a combined small animal PET/CT scanner. Proc. Conf. Rec. 2001 IEEE Nuclear Science Symp./Medical Imaging Conf; San Diego, CA. Oct. 2001; pp. 1605–1606. [Google Scholar]
- 17.Song X, Frey EC, Tsui BMW. Development and evaluation of a microCT system for small animal imaging. Proc. Conf. Rec. 2001 IEEE Nuclear Science Symp./Medical Imaging Conf; San Diego, CA. Oct. 2001; pp. 1600–1604. [Google Scholar]
- 18.Balzer SJ. MS thesis, Opt Sci Ctr. Univ. Arizona; Tucson, AZ: 2002. A Portable Gamma-Ray Imager for Small Animal Studies. [Google Scholar]
- 19.KAF-1001E Performance Specification. Rochester, NY: Eastman Kodak/Image Sensor Solutions; Feb, 2001. [Google Scholar]
- 20.Paulus MJ, Gleason SS, Kennel SJ, Hunsicker PR, Johnson DK. High resolution X-ray computed tomography: an emerging tool for small animal cancer research. Neoplasia. 2000 Jan-Apr;2(1–2):62–70. doi: 10.1038/sj.neo.7900069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matherson KJ, Barber HB, Barrett HH, Eskin JD, Dereniak EL, Marks DG, Woolfenden JM, Young ET, Augustine FL. Progress in the development of large-area modular 64×64 CdZnTe imaging arrays for nuclear medicine. IEEE Trans Nucl Sci. 1998 Jun;45:354–358. [Google Scholar]
- 22.Goertzen AL, Meadors K, Silverman RW, Cherry SR. Simultaneous PET and X-ray CT imaging of the mouse. Molecular Imaging Biol. 2002 Jul-Aug;4(4):20. [Google Scholar]