Abstract
Human exposure to N,N-diethyl-m-toluamide (DEET) occurs because of the widespread use of DEET as an active ingredient in insect repellents. However, information on the extent of such exposure is rather limited. Therefore, we developed a fast on-line solid phase extraction–high performance liquid chromatography–isotope dilution tandem mass spectrometry (HPLC-MS/MS) method to measure in urine the concentrations of DEET and two of its oxidative metabolites: N,N-diethyl-3-(hydroxymethyl)benzamide and 3-(diethylcarbamoyl)benzoic acid (DCBA). To the best of our knowledge, this is the first HPLC-MS/MS method for the simultaneous quantification of DEET and its select metabolites in human urine. After enzymatic hydrolysis of the conjugated species in 0.1 mL of urine, the target analytes were retained and pre-concentrated on a monolithic column, separated from each other and from other urinary biomolecules on a reversed-phase analytical column, and detected by atmospheric pressure chemical ionization in positive ion mode. The limits of detection ranged from 0.1 ng mL−1 to 1.0 ng mL−1, depending on the analyte. Accuracy ranged between 90.4 and 104.9%, and precision ranged between 5.5 and 13.1% RSD, depending on the analyte and the concentration. We tested the usefulness of this method by analyzing 75 urine samples collected anonymously in the Southeastern United States in June 2012 from adults with no known exposure to DEET. Thirty eight samples (51%) tested positive for at least one of the analytes. We detected DCBA most frequently and at the highest concentrations. Our results suggest that this method can be used for the analysis of a large number of samples for epidemiological studies to assess human exposure to DEET.
Keywords: DEET; N,N-diethyl-m-toluamide; HPLC; Mass spectrometry; On-line SPE; Column switching
Graphical abstract
1. Introduction
N, N-Diethyl-m-toluamide (DEET) was developed in 1946 by the US Army [1], and has become the most effective and ubiquitous insect repellent in the United States. About one third of the US population uses DEET-containing products at least once per year [1]. There are over 225 insect repellents brands containing DEET at concentrations ranging from 4% to 100% [2], and human exposure to DEET is expected to occur. However, information on the concentrations of DEET or its metabolites in humans is rather limited [2].
While DEET is generally considered a safe insect repellent, documented cases of acute intoxication exist because DEET is often applied repeatedly directly onto the skin [3–5]. In animals, DEET has shown neurotoxicity [6–8], and these effects were amplified when DEET was combined with cholinesterase inhibitors, such as the insecticide permethrin. DEET applied in combination with permethrin and malathion shows neurobehavioral deficits in rats [9,10]. Furthermore, DEET can inhibit cholinesterase activity in insects and mammals [11]. Information on population exposure to DEET is needed to evaluate whether exposure to DEET may affect human health.
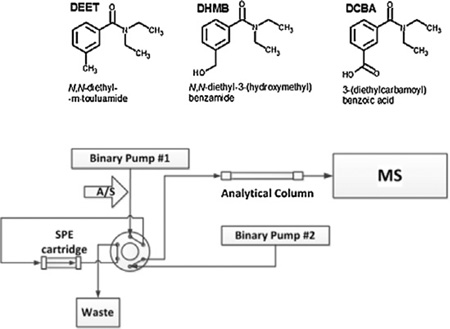
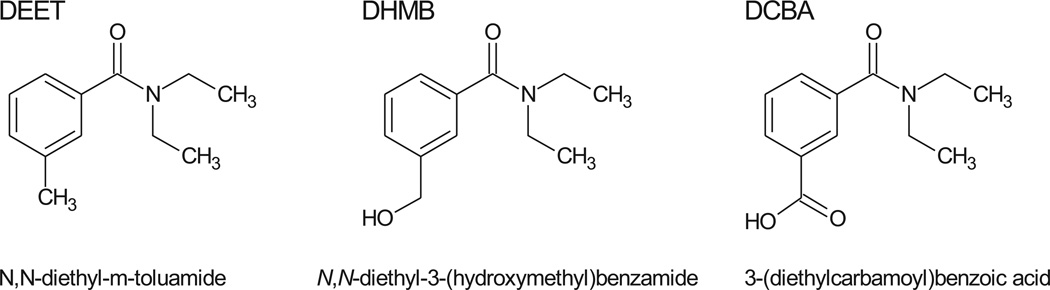
DEET undergoes Phase I metabolism by cytochrome P-450 enzymes [12,13]. Constantino and Iley proposed a pathway for the in vitro metabolism of DEET [14]. In a Phase I metabolism reaction, DEET can undergo a two-step N-dealkylation to form N-ethyl-3-methylbenzamide. In a Phase I oxidative pathway, the methyl group on the benzyl ring is oxidized first to form N, N-diethyl-3-hydroxymethylbenzamide (DHMB, Fig. 1). DHMB may go through a very short lived aldehyde intermediate to produce the ring-carboxylic acid final metabolite, 3-(diethylcarbamoyl)benzoic acid (DCBA). In addition, DEET and these metabolites may also undergo Phase II metabolism (e.g., glucuronidation) [15–17]. The elimination half-life of DEET is estimated to be in the range of a few hours [15,18,19]. Therefore, both DEET and its urinary metabolites can serve as biomarkers to assess recent exposure to DEET [15,16].
Fig. 1.
Chemical structures of DEET and its oxidative metabolites.
Analytical separation of DEET and its metabolites has been reported using gas chromatography (GC) [16,17,20–22] and high performance liquid chromatography (HPLC) [12–16,18–20,23–30]. Separation by GC requires derivatization of the compounds [12]. By contrast, using HPLC avoids the derivatization step [24–27]. Detection of DEET and its metabolites has been achieved by UV [12,14,24–26,30] and mass spectrometry.[20,21,29,31]
Off-line solid phase extraction (SPE) has been used for pre-concentration of DEET [27,29,31]. However, off-line SPE involves considerable sample handling (e.g., evaporation and reconstitution of the SPE urine extract), and can be labor intensive. By contrast, online processing of biological matrices (e.g., on-line SPE) has several advantages over the conventional manual, off-line sample preparation processes: it generally uses a smaller amount of sample and reagents thus potentially reducing exposure to hazardous reagents and infectious materials; it can achieve lower limits of detection; and it minimizes possible systematic errors [32,33]. Therefore, we developed the first on-line SPE-HPLC-isotope dilution tandem mass spectrometry method with the adequate throughput, sensitivity, selectivity and precision to measure DEET and two of its oxidative metabolites using 100 µL of urine. We also evaluated the applicability of the method for the analysis of urine samples collected anonymously from adults with no known exposure to DEET.
2. Experimental
2.1. Reagents and chemicals
Methanol and acetonitrile, both of HPLC grade, were purchased from Thermo Fisher Scientific (Pittsburg, PA). Deionized water was purified with a Type I DI Reagent Grade Water Purification System, Model 2121BL (Aqua Solutions, Jasper GA). Glacial acetic acid, sodium acetate, 4-methylumbelliferyl glucuronide, and β-glucuronidase/sulfatase (Helix Pomatia, type H-1) were purchased from Sigma–Aldrich (St Louis, MO). 13C4-4-methylumbelliferone was obtained from Cambridge Isotope Laboratories Inc. (Andover, MA). DEET was purchased from Supelco (Sigma–Aldrich). DHMB, DCBA, their deuterium labeled analogs, and deuterated DEET were custom-synthesized by CanSyn (Toronto, Canada). All solvents used were analytical grade. Reagents, solvents and standard materials were used without further purification.
2.2. Human urine collection for method validation
To prepare quality control pools and for method validation, we anonymously collected urine samples in Atlanta, GA during 2010–2012 from a diverse group of male and female adult volunteers with no documented occupational exposure to DEET. CDC’s Human Subjects Institutional Review Board reviewed and approved the study protocol. A waiver of informed consent was requested under 45 CFR 46.116(d). We did not have access to any personal or demographic data.
2.3. Preparation of standard solutions and quality control (QC) materials
We prepared the initial stock solutions of analytical standards by dissolving measured amounts of the target analytes in acetonitrile. Similarly, we prepared the initial stock solutions of stable isotope-labeled internal standards in acetonitrile. We dissolved the deconjugation markers 4-methylumbelliferyl glucuronide and 13C4-4-methylumbelliferone in methanol. We stored all of these solutions at −20 °C.
We prepared ten calibration standard spiking solutions that contained DEET and its two oxidative metabolites in acetonitrile using the initial stock. Final concentrations in 100 µL urine of the ten calibration standards ranged from 0.1 to 50 ng mL−1 (DEET, DHMB) and 1 to 500 ng mL−1 (DCBA). We prepared the stable isotope-labeled internal standard and deconjugation markers spiking solution by diluting the corresponding stock solutions with water, so that a 30 µL spike would result in concentrations in the urine of 10 ng mL−1 (DEET, DHMB), 100 ng mL−1 (DCBA), and 600 ng mL−1 (4-methylumbelliferyl glucuronide, 13C4-4-methylumbelliferone).
We prepared urine pools by obtaining urine from adult anonymous donors and screening the individual samples for endogenous amounts of the target analytes. Individual urine samples with undetectable concentrations were combined to form a blank pool. The blank urine was stored at −20 °C. QC materials were prepared by spiking blank urine with native target compounds. The low-concentration QC (QCL) for DEET and DHMB was 3 ng mL−1; the high concentration QC (QCH) was 20 ng mL−1. The preliminary screening of the anonymously collected urine samples suggested that DCBA is present at higher concentrations than DEET or the other metabolite. Therefore, we set the DCBA QCL concentration at 30 ng mL−1 and the QCH concentration at 200 ng mL−1. The spiked QC materials were refrigerated, mixed for over 48 h, then aliquoted and stored at −20 °C until use.
2.4. Sample and standards preparation
Each analytical run included solvent and matrix blanks, analytical standards, QCH and QCL, and the study samples. To analyze study samples, first, we dispensed 30 µL of internal/deconjugation standard spiking solution to an autosampler vial containing a 250 µL silanized insert. Then, we added 100 µL of urine followed by 75 µL of enzyme solution. The enzyme solution, prepared immediately before every analytical run by dispersing β-glucuronidase/sulfatase in 0.1 M sodium acetate buffer, had an enzymatic activity of 0.5 modified Fishman units per µL of urine. After being gently mixed, the spiked urine was incubated at 37 °C for approximately 17 h, then vortex mixed before starting the SPE-HPLC process. We prepared QCs and blanks using this same procedure, but we replaced the sample urine with the same volume of QC materials, HPLC-grade H2O (reagent blank), or blank urine (matrix blank). To prepare the analytical standards, we followed the procedure above replacing the sample urine with 100 µL of blank pooled urine to which we added 10 µL of the calibration standard solution.
2.5. On-line SPE and analytical separation
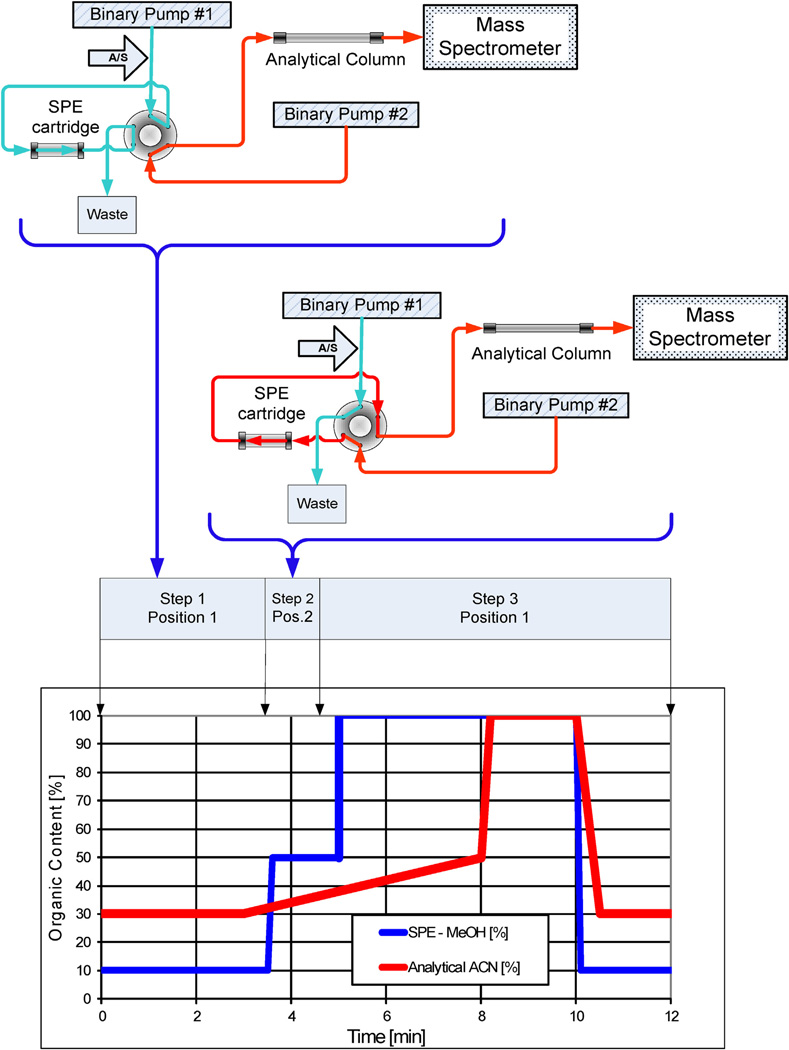
On-line SPE and analytical separation were performed using Agilent HPLC modules (Santa Clara, CA) as follows: two binary pumps, one 1200 series G1312A as the analytical pump and one 1100 series G1312A binary pump as the SPE pump; one G1329A temperature controlled autosampler connected to a G1330A type chiller; and one G1316A temperature controlled column compartment outfitted with a six port valve. The system flow diagram is depicted in Fig. 2. The SPE cartridge was a Chromolith Flash RP-18e monolithic column (25 × 4.6 mm) manufactured by EMD Chemicals (Gibbstown, NJ), a subsidiary of the Merck Group. The SPE pump sample-loading mobile phase was 10% methanol in 0.1% aqueous acetic acid. The HPLC analytical separation was performed on a Phenomenex (Torrance, CA) Prodigy 5u Phenyl-3 (PH-3) column (100 mm × 4.6 mm). HPLC mobile phases were 0.1% acetic acid in water and 100% acetonitrile.
Fig. 2.
On-line SPE system connections and timing diagram.
This on-line SPE method uses a three step process to isolate the target analytes from endogenous interfering urine components and to chromatographically resolve the urinary constituents by HPLC for detection by tandem mass spectrometry. In the first step (SPE loading), 100 µL of spiked urine was injected and deposited onto the Chromolith SPE cartridge by the SPE pump (binary pump #1, Fig. 2) using a flow rate of 1.0 mL min−1 of 10% methanol in 0.1% aqueous acetic acid for 1 min. Then, the cartridge was washed for 2.5 min with 10% methanol in 0.1% aqueous acetic acid at 4 mL min−1 (Table 1). DEET and its metabolites were retained on the SPE cartridge while other urinary components were not retained and directed to waste. During the SPE loading cycle, the analytical pump (binary pump #2, Fig. 2) was equilibrating the analytical column with 30% acetonitrile (mobile phase A = 0.1% acetic acid in water, B = 100% acetonitrile) at 1 mL min−1 and column temperature of 35 °C. Switching of the six port valve initiated step two (SPE eluting): the SPE pump was temporarily stopped, and the analytical pump was in line with the SPE cartridge and the analytical column. During the SPE eluting step, the target analytes were eluted from the SPE cartridge onto the analytical column with 30% acetonitrile at 1 mL min−1. Flow was directed for one minute to transfer DEET and its metabolites to the analytical column. Then, the six port valve switched position to initiate the next step. In step three (analytical chromatography), the SPE cartridge was bypassed and the flow from binary pump #2 was directed solely to the analytical column. By changing the mobile phase from 30% to 50% acetonitrile over 3 min, the target analytes were chromatographically resolved and the mass transitions detected by the mass spectrometer. During the analytical column elution, the SPE cartridge was washed with 100% methanol for 5 min at a flow rate of 4 mL min−1, and then equilibrated with 10% methanol in 0.1% aqueous acetic acid for 2 min to prepare for the next injection. Total run time for each sample was 12 min.
Table 1.
Valve and pumps overlay timing chart.a
| Valve | SPE pump (Binary Pump #1) | Analytical pump (Binary Pump #2) | ||||||
|---|---|---|---|---|---|---|---|---|
| Time (min) | Step | Valve Pos. | Time (min) | Methanol (%) | Flow Rate (mL min−1) | Time (min) | Acetonitrile (%) | Flow Rate (mL min−1) |
| 0 | 1 | 1 | 0 | 10 | 1.00 | 0 | 30.00 | 1.00 |
| 1.00 | 10 | 1.00 | ||||||
| 1.10 | 10 | 4.00 | ||||||
| 3.50 | 2 | 2 | 3.50 | 10 | 4.00 | |||
| 3.60 | 50 | 0 | ||||||
| 4.50 | 3 | 1 | ||||||
| 4.60 | 50 | 0 | ||||||
| 5.00 | 100 | 4.00 | ||||||
| 8.20 | 100.00 | 1.00 | ||||||
| 10.00 | 100 | 4.00 | 10.00 | 100.00 | 1.00 | |||
| 10.10 | 10 | 1.00 | ||||||
| 10.50 | 30.00 | 1.00 | ||||||
| 12.00 | 12.00 | 10 | 1.00 | 12.00 | 30.00 | 1.00 | ||
The aqueous mobile phase for the SPE and HPLC separation was 0.1% acetic acid in water.
The organic mobile phase was methanol (SPE) and acetonitrile (HPLC).
2.6. Mass spectrometry
We used a TSQ Quantum Ultra™ triple quadrupole mass spectrometer equipped with an atmospheric pressure chemical ionization (APCI) as ion source (Thermo Fisher Corporation, Waltham, MA). The APCI parameters were set as follows: discharge current (4.0 µA), sheath gas pressure (25 PSI), auxiliary gas flow (5 arbitrary units), vaporizer temperature (450 °C), and ion transfer tube temperature (250 °C). Nitrogen was used as both the auxiliary and sheath gas. Argon was used as collision gas (1.5 mTorr). No skimmer offset (declustering potential) was used. The mass spectrometer operated in positive polarity selected reaction monitoring (SRM) mode, with a scan time of 0.060 s. Table 2 shows the transitions and collision energies used for each analyte.
Table 2.
| Analyte | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy (V) | |
|---|---|---|---|---|
| DEET | Native 1 | 192 | 91 | 42 |
| Native 2 | 192 | 119 | 26 | |
| Internal Standard 1 | 202 | 91 | 42 | |
| Internal Standard 2 | 202 | 119 | 26 | |
| DHMB | Native 1 | 208 | 135 | 26 |
| Native 2 | 208 | 89 | 42 | |
| Internal Standard 1 | 218 | 135 | 26 | |
| Internal Standard 2 | 218 | 89 | 42 | |
| DCBA | Native 1 | 222 | 149 | 27 |
| Native 2 | 222 | 121 | 38 | |
| Internal Standard 1 | 232 | 149 | 27 | |
| Internal Standard 2 | 232 | 121 | 38 |
1 and 2 are the quantification and confirmation ions, respectively.
The internal standards are deuterium substituted compounds on the N-ethyl moiety.
The separation system and the mass spectrometry data acquisition were controlled by the Xcalibur software (Thermo Fisher Corporation). This software is used to control all components of the analytical system.
3. Results and discussion
DEET and its metabolites undergo phase II metabolism to form conjugates (e.g., glucuronides) [15,17]. These conjugates must be hydrolyzed to measure the concentration of the total (unconjugated plus conjugated) species of DEET and its metabolites. We chose to hydrolyze the conjugates enzymatically using β-glucuronidase/sulfatase as was previously done for DEET and other environmental chemicals [29,34,35]. To monitor the efficiency of the enzymatic hydrolysis and confirm that the enzyme functioned properly, we used the area ratio of 4-methylumbelliferone/13C4-4-methylumbelliferone for each sample [36–39].
3.1. Selection of the on-line SPE sorbent
First, we tested an Oasis HLB column (20 × 5 mm, 5 µm particle size) because this particular sorbent had been used before for the off-line SPE cleanup of urine to be analyzed for DEET [29]. However, we discovered that, under our experimental settings, the chemistry of this particular sorbent exhibited too strong an affinity for the toluamide derivatives which manifested itself in a gradually worsening carryover problem. The compound most affected was DEET as it is the most hydrophobic of the three determined in the present method.
Next, we evaluated the aforementioned Chromolith RP-18e. The affinity of the C18 sorbent of this column for the target analytes is weaker than that of the nitrogen-containing heterocyclic rings of the HLB sorbent. Of interest, the Chromolith RP-18e column is designed primarily for analytical separations instead of pre-concentration [40]. However, for the present application, the monolithic nature and large primary pore sizes (130 Å) of this type of sorbent allowed for the high speed washing of the cartridge without detrimental loss of the target analytes.
To determine the maximum volume and optimal composition of the wash mobile phase, we mapped an elution profile. In this experiment, the Chromolith column was connected as a regular HPLC column. The mobile phases used were mixtures of methanol – water, with 20–100% organic content. The analyte retention times at 1 mL min−1 flow rate were recorded and plotted against the methanol concentration. We found that when using the Chromolith RP-18e cartridge, mobile phase concentrations with methanol content below 20% resulted in relatively long retention times (>5 min @ 1 mL min−1 flow rate) of the target analytes. We chose to use 10% methanol as the loading and washing SPE mobile phase composition because it provided the optimal balance between organic content and allowable wash volume.
3.2. Selection of the analytical column
The reversed phase columns that we tested (e.g., C-18, alkylphenyl) worked acceptably well; the alkyl-phenyl chemistry-based columns retained the target analytes longer than the C-18 columns (data not shown). We selected the Prodigy Phenyl-3 (PH-3) column mainly for two reasons. First, it gave the best peak shapes with the least amount of tailing, although the differences among the various columns tested were rather small. Second, this particular column provided the smallest back pressure among the 100 mm columns. This is an important consideration while performing on-line SPE because at step #2, when analytes elute from the SPE cartridge to the analytical column, the analytical system pressure increases as a result of the SPE cartridge and the analytical column being in line.
3.3. Matrix effects
Urine composition varies considerably from person to person and depending on the time of collection. Both the complexity and variability in the composition of the urine can be manifested in matrix effects, such as selective signal suppression. To minimize matrix effects, we used deuterium-labeled internal standards. However, even with the use of isotope labeled materials, matrix effects may exist. To estimate matrix effects, we compared the slopes of five calibration curves obtained from standards spiked in urine and in deionized water. The mean slope ± standard deviation in urine vs. water, respectively, were 0.140 ± 0.005 vs. 0.132 ± 0.006 (DEET), 0.100 ± 0.018 vs. 0.067 ± 0.007 (DHMB), and 0.158 ± 0.005 vs. 0.241 ± 0.015 (DCBA). Because the slopes were considerably different for DHMB and DCBA, we chose to use urine-based calibration curves for quantification,
3.4. Recoveries
We modified our SPE-HPLC system to measure recoveries at two concentrations: 1 ng mL−1 and 100 ng mL−1. We used the divert valve on the mass spectrometer to set up a secondary injector loop. The injector was outfitted with a 100 µL loop and this loop was pre filled with a solution containing the same concentration of analytes as the spiked urine in the autosampler tray. First, 100 µL of the spiked urine was injected and the on-line SPE was followed by the analytical separation. Exactly one minute after the transfer (step #2) the divert valve/secondary injector was programmed to switch and inject the preloaded sample. This process yielded double peak chromatograms. One set of peaks represented 100% recoveries, as these came from the divert valve injector. The other set of peaks represented the analytes passing through the SPE system. Recoveries, calculated as area ratios of the two peaks, are shown in Table 3. We achieved excellent SPE recoveries (~95%) at 100 ng mL−1 for all analytes. At 1 ng mL−1, the recoveries were also very good (~95–106%) except for DCBA (~62%) because it is the analyte with the poorest sensitivity of the three measured. Nonetheless, these values are comparable to the 73–88% recoveries reported in the literature [26,27].
Table 3.
On-line SPE recoveries of DEET and its oxidative metabolites.
| Analyte | Concentration (ng mL−1) | Recovery (%) | RSD of Recovery (%) |
|---|---|---|---|
| DEET | 1 | 95.4 | 4.8 |
| 100 | 94.5 | 4.7 | |
| DHMB | 1 | 106.0 | 6.4 |
| 100 | 95.5 | 7.8 | |
| DCBA | 1 | 61.6 | 10.5 |
| 100 | 95.4 | 7.3 |
3.5. Analytical sensitivity
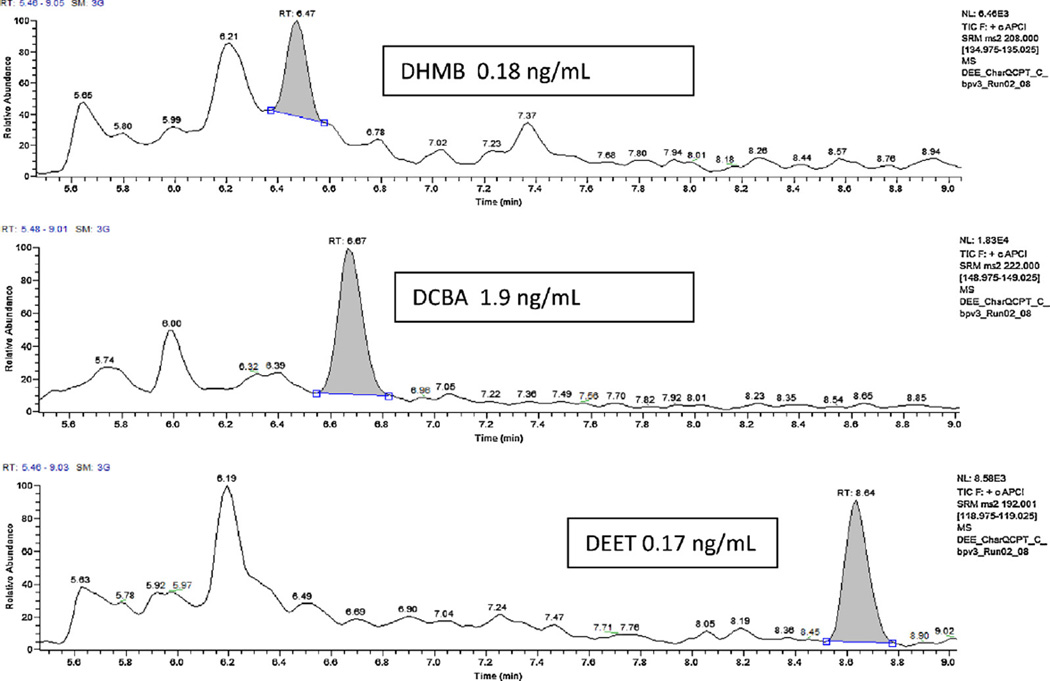
We determined the limits of detection (LODs) by repeated measurements (n = 20) of low-concentration standards and by plotting the standard deviation of the measured concentration versus the calibrators concentration. We determined S0, the expected standard deviation of the concentration measurements at zero concentration, by extrapolating the standard deviation values to zero concentration. We calculated the LOD as 3 times S0 [41]. The LODs (Table 4) were 0.1 ng mL−1 for DEET and DHMB. For DCBA, the LOD was 1 ng mL−1, which is still adequate for biomonitoring purposes because, of the three compounds measured, DCBA is found at the highest concentrations. Therefore, analytical sensitivity is likely to be less critical for DCBA than it would be for the other analytes. Fig. 3 shows typical chromatograms for all analytes at concentrations close to the LODs.
Table 4.
Limits of detection, precision and accuracy of concentration measurements.
| Analyte | LOD (ng mL−1) |
Accuracy (%)a | Precision (%)b | |||
| 0.4 ng mL−1 | 3 ng mL−1 | 45 ng mL−1 | QCL 3 ng mL−1 | QCH 20 ng mL−1 | ||
| DEET | 0.1 | 99.7 | 101.6 | 99.3 | 10.2 | 5.5 |
| DHMB | 0.1 | 101.0 | 99.9 | 99.4 | 9.8 | 8.6 |
| Analyte | LOD (ng mL−1) |
Accuracy (%)a | Precision (%)b | |||
| 4 ng mL−1 | 31 ng mL−1 | 500 ng mL−1 | QCL 30 ng mL−1 | QCH 200 ng mL−1 | ||
| DCBA | 1 | 93.8 | 90.4 | 104.9 | 12.4 | 13.1 |
n = 20.
n = 29, two instruments used by two analysts over one month.
Fig. 3.
Chromatograms of DEET and its two oxidative metabolites.
3.6. Precision and accuracy
We determined precision by calculating the coefficients of variation (CV) using 29 QC low and QC High samples (Table 4). Two instruments were used by two analysts over the course of one month. CVs ranged from 5.5 to 14.1%. We calculated accuracy at three concentrations from twenty different measurements. Accuracy, expressed as a percentage of the expected value, was good (90.4–104.9%) for the three analytes (Table 4). These precision and accuracy data are within the ranges observed in other mass-spectrometry based methods for measuring DEET [14,16,18,20,21,29,31,42].
3.7. Application example
We tested the effectiveness of the method by analyzing 75 urine samples collected anonymously in June 2012 from Atlanta adult residents with no known occupational exposure to DEET. Out of the 75 samples, 38 tested positive for at least one of the analytes (Table 5). The most prevalent analyte was DCBA, which was detected in 36 of the samples even though its LOD is the highest among the target analytes examined. DCBA was also the compound detected at the highest concentrations. These preliminary results suggest that these DEET oxidative metabolites are potential biomarkers of DEET exposure. However, because we only tested 75 samples, our findings are only applicable to this convenience population and should be replicated in future studies with larger population sample sizes.
Table 5.
Mean concentrations (ng mL−1) and frequency of detection of DEET and two oxidative metabolites in 75 urine samples form anonymous adult volunteers.a
| Analyte | ||||
|---|---|---|---|---|
| DEET | DHMB | DCBA | ||
| Number of samples with detectable concentrations | 9 | 12 | 36 | |
| Detection frequency | 12% | 16% | 48% | |
| Mean | (ng mL−1) | 4.4 | 10.2 | 1103 |
| Std Deviation of the mean | (ng mL−1) | 6.1 | 7.0 | 2120 |
Limit of detection (LOD) = 0.1 ng mL−1 (DEET, DHMB) and 1.0 ng mL−1 (DCBA).
To calculate the mean and standard deviation, we only used concentrations >LOD.
4. Conclusions
We developed an on-line SPE–HPLC–isotope dilution tandem mass spectrometry method for the measurement of DEET and two of its oxidative metabolites in urine. Sensitivity, precision and accuracy were satisfactory for biomonitoring purposes. Two major advantages of this method are its speed and automation. Furthermore, the method requires a small amount of urine (100 µL) and minimal sample pretreatment. Potential applications may include studies of geographical and seasonal distribution of DEET exposure, occupational exposure, and investigations to study the metabolism of DEET. Our preliminary data also suggest that human exposure to DEET may be assessed by measuring the total concentrations of DEET and its metabolites in urine. Nonetheless, additional considerations, such as adequate collection protocols, handling and storage of the samples, and data on the temporal stability of the analytes in urine, are needed to demonstrate the utility of these measures for exposure and risk assessment purposes.
Highlights.
A fast assay to quantify the concentrations of N,N-Diethyl-m-Toluamide and two urinary metabolites was developed
It uses online SPE, reversed phase HPLC and tandem mass spectrometry
The method is precise and accurate with limits of detection ≤1 ng mL−1
Footnotes
Disclaimer
The use of trade names is for identification only and does not constitute endorsement by the US Department of Health and Human Services or the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.U.S.E.P.A. Pesticides: Topical & Chemical Fact Sheets, The Insect Repellent DEET. United States: US EPA; 2007. [Google Scholar]
- 2.C.D.C. Centers for Disease Control and Prevention, Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, United States: 2009. [Google Scholar]
- 3.Snyder JW, Poe RO, Stubbins JF, Garrettson LK. J. Toxicol. –Clin. Toxicol. 1986;24:429. doi: 10.3109/15563658608992605. [DOI] [PubMed] [Google Scholar]
- 4.Chaney LA, Rockhold RW, Wineman RW, Hume AS. Toxicol. Sci. 1999;49:306. doi: 10.1093/toxsci/49.2.306. [DOI] [PubMed] [Google Scholar]
- 5.Sudakin DL, Trevathan WR. J. Toxicol. – Clin. Toxicol. 2003;41:831. doi: 10.1081/clt-120025348. [DOI] [PubMed] [Google Scholar]
- 6.Abou-Donia MB, Wilmarth KR, Abdel-Rahman AA, Jensen KF, Oehme FW, Kurt TL. Fundam. Appl. Toxicol. 1996;34:201. doi: 10.1006/faat.1996.0190. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Donia MB, Wilmarth KR, Jensen KF, Oehme FW, Kurt TL, Toxicol J. Environ. Health. 1996;48:35. doi: 10.1080/009841096161456. [DOI] [PubMed] [Google Scholar]
- 8.McCain WC, Lee R, Johnson MS, Whaley JE, Ferguson JW, Beall P, Leach G. J. Toxicol. Environ. Health. 1997;50:113. doi: 10.1080/009841097160528. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Rahman A, Dechkovskaia AM, Goldstein LB, Bullman SH, Khan W, El-Masry EM, Abou-Donia MB. J. Toxicol. Environ. Health. Part A. 2004;67:331. doi: 10.1080/15287390490273569. [DOI] [PubMed] [Google Scholar]
- 10.Abou-Donia MB, Goldstein LB, Jones KH, Abdel-Rahman AA, Damodaran TV, Dechkovskaia AM, Bullman SL, Amir BE, Khan WA. Toxicol. Sci. 2001;60:305. doi: 10.1093/toxsci/60.2.305. [DOI] [PubMed] [Google Scholar]
- 11.Corbel V, Stankiewicz M, Pennetier C, Fournier D, Stojan J, Girard E, Dimitrov M, Molgo J, Hougard JM, Lapied B. BMC Biology. 2009;7:47. doi: 10.1186/1741-7007-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor WG, Hall TW, Vedres DD. Drug Metab. Dispos. 1993;21:133. [PubMed] [Google Scholar]
- 13.Usmani KA, Rose RL, Goldstein JA, Taylor WG, Brimfield AA, Hodgson E. Drug Metab. Dispos. 2002;30:289. doi: 10.1124/dmd.30.3.289. [DOI] [PubMed] [Google Scholar]
- 14.Constantino L, Iley J. Xenobiotica. 1999;29:409. doi: 10.1080/004982599238588. [DOI] [PubMed] [Google Scholar]
- 15.Selim S, Hartnagel RE, Osimitz TG, Gabriel KL, Schoenig GP. Fundam. Appl. Toxicol. 1995;25:95. doi: 10.1006/faat.1995.1043. [DOI] [PubMed] [Google Scholar]
- 16.Moody RP, Benoit FM, Riedel D, Ritter L. J. Toxicol. Environ. Health. 1989;26:137. doi: 10.1080/15287398909531240. [DOI] [PubMed] [Google Scholar]
- 17.Wu A, Pearson ML, Shekoski DL, Soto RJ, Stewart RD. J. High Resolut. Chromatogr. Chromatogr. Commun. 1979;2:558. HRC CC. [Google Scholar]
- 18.Schoenig GP, Hartnagel RE, Osimitz TG, Llanso S. Drug Metab. Dispos. 1996;24:156. [PubMed] [Google Scholar]
- 19.Yeung JM, Taylor WG. Drug Metab. Dispos. 1988;16:600. [PubMed] [Google Scholar]
- 20.Cherstniakova SA, Garcia GE, Strong J, Bi D, Weitz J, Roy MJ, Cantilena LR. J. Anal. Toxicol. 2006;30:21. doi: 10.1093/jat/30.1.21. [DOI] [PubMed] [Google Scholar]
- 21.Taylor WG. Drug Metab. Dispos. 1986;14:532. [PubMed] [Google Scholar]
- 22.Taylor WG, Spooner RW. J. Agric. Food Chem. 1990;38:1422. [Google Scholar]
- 23.Smallwood AW, Debord KE, Lowry LK. J. Anal. Toxicol. 1992;16:10. doi: 10.1093/jat/16.1.10. [DOI] [PubMed] [Google Scholar]
- 24.Qiu HC, Jun HW. J. Pharm. Biomed. Anal. 1996;15:241. doi: 10.1016/0731-7085(96)01828-6. [DOI] [PubMed] [Google Scholar]
- 25.Abu-Qare AW, Abou-Donia MB. J. Chromatogr. B. 2000;749:171. doi: 10.1016/s0378-4347(00)00407-2. [DOI] [PubMed] [Google Scholar]
- 26.Abu-Qare AW, Abou-Donia MB. J. Pharm. Biomed. Anal. 2001;26:291. doi: 10.1016/s0731-7085(01)00407-1. [DOI] [PubMed] [Google Scholar]
- 27.Abu-Qare AW, Abou-Donia MB. Fresenius J. Anal. Chem. 2001;370:403. doi: 10.1007/s002160100780. [DOI] [PubMed] [Google Scholar]
- 28.Cherstniakova S, Garcia G, Strong J, Helbling N, Bi D, Roy M, Cantilena LR. Clin. Pharmacol. Ther. 2003;73:P27. [Google Scholar]
- 29.Olsson AO, Baker SE, Nguyen JV, Romanoff LC, Udunka SO, Walker RD, Flemmen KL, Barr DB. Anal. Chem. 2004;76:2453. doi: 10.1021/ac0355404. [DOI] [PubMed] [Google Scholar]
- 30.Kasichayanula S, House JD, Wang T, Gu XC. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2005;822:271. doi: 10.1016/j.jchromb.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Quednow K, Puttmann W. Environ. Sci. Pollut. Res. Int. 2009;16:630. doi: 10.1007/s11356-009-0169-6. [DOI] [PubMed] [Google Scholar]
- 32.Kuklenyik Z, Calafat AM, Barr JR, Pirkle JL. J. Separ. Sci. 2011;34:3606. doi: 10.1002/jssc.201100562. [DOI] [PubMed] [Google Scholar]
- 33.Kuklenyik Z, Ye X, Needham LL, Calafat AM. J. Chromatogr. Sci. 2009;47:12. doi: 10.1093/chromsci/47.1.12. [DOI] [PubMed] [Google Scholar]
- 34.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Anal. Bioanal. Chem. 2005;383:638. doi: 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
- 35.Ye X, Wong LY, Jia LT, Needham LL, Calafat AM. Environ. Int. 2009;35:1160. doi: 10.1016/j.envint.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, Ye X, Calafat AM. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012;881–882:27. doi: 10.1016/j.jchromb.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 37.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Anal. Chem. 2005;77:5407. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 38.Silva MJ, Preau JL, Jr, Needham LL, Calafat AM. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;873:180. doi: 10.1016/j.jchromb.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 39.Ye X, Kuklenyik Z, Bishop AM, Needham LL, Calafat AM. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006;844:53. doi: 10.1016/j.jchromb.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 40.Ye X, Tao LJ, Needham LL, Calafat AM. Talanta. 2008;76:865. doi: 10.1016/j.talanta.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 41.Taylor JK. Quality Assurance of Chemical Measurements. Chelsea, MI: Lewis Publishers; 1987. [Google Scholar]
- 42.Knepper TP. Water Sci. Technol. 2004;50:301. [PubMed] [Google Scholar]