Abstract
Concerns exist regarding children’s exposure to bisphenol A (BPA) and other phenols because of the higher sensitivity, compared to adults, of children’s developing organs to endocrine disruptors. Several studies reported the urinary concentrations of these phenols in children, but data on levels of these compounds in children’s serum are limited. We present here the total (free plus conjugated) and free concentrations of BPA and seven other phenols in 24 pooled serum samples prepared from individual specimens collected from 936 children 3–11 years old who participated in the 2001–2002 National Health and Nutrition Examination Survey. We detected benzophenone-3, triclosan, 2,4-dichlorophenol, 2,5- dichlorophenol, and three parabens in at least 60% of the pools suggesting children’s exposure to these compounds or their precursors. Conjugated phenols were the major species. However, although many previous studies have shown widespread detection of BPA in children’s urine, we only detected total or free BPA in 3 and 2 pooled serum samples, respectively, at concentrations of 0.1–0.2 µg/L. The non-persistent nature of BPA and the phenols examined and the likely episodic nature of the exposures to these compounds (or their precursors) suggest that for general population biomonitoring of these non-persistent phenols, urine, not serum or plasma, is the preferred matrix.
Keywords: Bisphenol A, environmental phenols, prevalence, exposure, biomonitoring, children, NHANES
Introduction
People are exposed to bisphenol A (BPA) and other environmental phenols (or their precursors) through industrial pollution, pesticide use, food consumption, and use of personal-care and consumer products. BPA, one of the high production-volume chemicals, is used to manufacture polycarbonate plastic and epoxy resins, some dental sealants and composites, and thermal receipt paper (1, 2). Other phenols include chlorophenols, sunscreen agents like 2-hydroxy-4-methoxybenzophenone (benzophenone-3), and antimicrobial agents like triclosan and parabens. Chlorophenols, such as 2,4-dichlorophenol and 2,5- dichlorophenol, are the metabolites of certain herbicides and disinfectants (or fungicides) for industrial and indoor home use (3). Benzophenone-3 and triclosan are used extensively in personal care and consumer products (4). Methyl-, ethyl-, and propyl parabens are widely used in cosmetics, pharmaceuticals, and in food and beverage processing (5).
Because of the extensive use of these environmental phenols (or their precursors) in consumer and personal-care products, human exposure to these chemicals is widespread. The urinary concentrations of these compounds or their metabolites have been used as biomarkers of exposure. Specifically, data from the National Health and Nutrition Examination Survey (NHANES), conducted by the Centers for Disease Control and Prevention (CDC), have shown widespread exposure to select environmental phenols among the U.S. general population, including children of school age (6–10).
Concerns exist for children’s exposure to certain environmental phenols because of the higher sensitivity of children’s developing organs to exogenous hormones compared to adults. Information on urinary concentrations of these environmental phenols among children in the United States is readily available (6–14). However, although for non-persistent chemicals, normally urine is the preferred biomonitoring matrix (15), data on the circulating levels of these environmental phenols in children’s serum, which may be useful for risk asseessment, are scarce. To address this gap, measuring the serum concentrations in large populations of children is of scientific interest. However, biomonitoring is an expensive effort, in part due to the high cost of the analytical measurements. One alternative involves pooling of individual samples (16). In fact, a proper pooling design can allow the estimation of the prevalence of exposure of a given population (16). In this study, we report the free and total (free plus conjugated) concentrations of BPA, 2,4-dichlorophenol, 2,5-dichlorophenol, benzophenone-3, triclosan, and methyl-, ethyl-, and propyl parabens, in 24 serum pools, prepared from 936 individual samples collected from 3–11 years old children participating in 2001–2002 NHANES.
Materials and Methods
Preparation of the Pools
The sampling scheme for 2001–2002 NHANES was a complex multistage area probability design. The institutional review board of the National Center for Health Statistics (NCHS) of the CDC approved the study, and participation occurred only after obtaining informed consent (17). To prepare the pools, we randomly selected 936 individual samples of 1049 with at least 500 µL of serum which were collected from 2001–2002 NHANES participants 3–11 years of age, both sexes and, on the basis of self-reported data, belonging to three major racial/ethnic groups: non-Hispanic blacks, non-Hispanic whites, and Mexican Americans. These individual samples had been previously analyzed for cotinine, a marker of environmental tobacco smoke at the CDC’s National Center for Environmental Health. With assistance from NCHS’ statisticians, we categorized the 936 residual serum samples, stored in polypropylene cryovials, into 12 demographic groups, each representing a combination of age (3–5 years and 6–11 years), sex, and race/ethnicity (Table 1). A detailed description of the preparation of the pools, the available number of individual sera for each demographic group, and the actual number of individual specimens used to prepare each pool have been described before (18). For each demographic group, we prepared two pools in polypropylene cryovials that included randomly selected 21 (3–5 years of age) or 57 (6–11 years of age) individual samples. After preparation, the serum pools were stored at or below −20 °C until analysis. These pools, prepared in 2007, were analyzed for polyfluoroalkyl compounds (PFCs) (19) and other persistent organic pollutants (unpublished results) before we analyzed them for environmental phenols in 2012.
Table 1.
Total concentrations (µg/L) of eight environmental phenols in pooled sera (two pools, P1 and P2, per demographic group) from 2001–2002 NHANES participants 3–11 years of agea,b
| Sex | Race (years) | Age | BPA | Benzophenone-3 | Triclosan | 2,4- Dichlorophenol |
2,5- Dichlorophenol |
Methyl paraben |
Ethyl paraben |
Propyl paraben |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P1 | P2 | P1 | P2 | P1 | P2 | P1 | P2 | P1 | P2 | P1 | P2 | P1 | P2 | ||||
| F | NHB | 3–5 | ND | ND | ND | 0.6 | 46.0 | 69.6 | 0.3 | 1.6 | 3.5 | 102.0 | 138.0 | 74.8 | 1.1 | 1.6 | 14.5 | 14.4 | |
| F | NHB | 6–11 | ND | ND | ND | 0.8 | 13.2 | 12.4 | 0.2 | 0.3 | 4.4 | 17.0 | 32.2 | 31.2 | 1.0 | 1.1 | 5.3 | 2.7 | |
| M | NHB | 3–5 | ND | ND | ND | ND | 8.2 | 40.0 | 0.5 | 0.9 | 27.4 | 43.1 | 91.3 | 15.3 | 0.1 | ND | 7.9 | 0.9 | |
| M | NHB | 6–11 | ND | 0.2 | ND | ND | 29.3 | 17.5 | 0.6 | 1.0 | 28.4 | 75.1 | 13.0 | 15.6 | 0.3 | ND | 2.1 | 1.5 | |
| F | MA | 3–5 | ND | ND | 1.6 | 8.9 | 7.2 | 2.1 | 0.4 | 0.4 | 4.7 | 5.0 | 13.0 | 19.2 | ND | 2.0 | 3.3 | 4.8 | |
| F | MA | 6–11 | ND | ND | 0.6 | 11.3 | 3.4 | 2.3 | 0.2 | 0.2 | 1.9 | 3.4 | 11.5 | 5.5 | ND | 0.2 | 1.1 | 0.6 | |
| M | MA | 3–5 | ND | 0.1 | 0.7 | ND | 3.2 | 2.8 | 0.3 | 0.2 | 7.8 | 2.8 | 12.6 | 15.9 | 0.3 | 0.8 | 2.5 | 2.1 | |
| M | MA | 6–11 | ND | ND | 0.6 | ND | 5.3 | 8.6 | 0.2 | 0.4 | 6.4 | 9.9 | 4.0 | 12.3 | 0.3 | ND | 0.9 | 2.4 | |
| F | NHW | 3–5 | ND | ND | 7.3 | ND | 3.5 | 3.8 | 0.2 | 0.2 | 0.3 | 0.3 | 23.4 | 55.6 | 0.3 | 0.6 | 1.4 | 2.3 | |
| F | NHW | 6–11 | ND | ND | 3.0 | 1.5 | 5.1 | 2.6 | 0.2 | 0.2 | 0.5 | 1.0 | 10.5 | 3.8 | ND | 0.3 | 1.6 | 0.5 | |
| M | NHW | 3–5 | ND | ND | 3.0 | 0.7 | 2.6 | 5.3 | 0.2 | 0.1 | 0.8 | 0.3 | 5.8 | 7.8 | ND | ND | 0.4 | 0.2 | |
| M | NHW | 6–11 | ND | 0.1 | 3.3 | 0.8 | 3.5 | 4.8 | 0.2 | 0.1 | 0.6 | 0.7 | 3.8 | 1.6 | 0.2 | ND | 0.3 | 0.2 | |
| LOD (µg/L) | 0.1 | 0.5 | 1.1 | 0.1 | 0.4 | 0.1 | 0.1 | 0.2 | |||||||||||
| Frequency of detectionc | 13 % | 63 % | 100 % | 100 % | 100 % | 100 % | 63 % | 100 % | |||||||||||
Female (F), male (M), non-Hispanic black (NHB), non-Hispanic white (NHW), Mexican American (MA).
ND: the concentrations were below the limit of detection (LOD).
Frequency of detection (%) was based on all 24 pools.
Laboratory Measurements
We measured the concentrations of BPA and environmental phenols using a modified on-line solid phase extraction coupled to isotope dilution-high performance liquid chromatography tandem mass spectrometry (on-line SPE-HPLC-MS/MS) method described previously (20). We built the on-line SPE-HPLC-MS/MS system from several Agilent 1100 modules (Agilent Technologies, Wilmington, DE, USA) coupled with an API 5500 Q Trap™ mass spectrometer (Applied Biosystems, Foster City, CA, USA) equipped with an atmospheric pressure chemical ionization interface. Briefly, to estimate the concentrations of free species, we added 50 µL of internal standard solution and 100 µL of serum into 850 µL of 0.1 M formic acid in a 1.5 mL conical bottom autosampler vial. To determine the total concentrations, we added 50 µL of internal standard, 50 µL of 4-methylumbelliferyl glucuronide/4-methylumbelliferyl sulfate/13C4-4-methylumbelliferone mixed standard (0.5 µg/mL), and 50 µL of H1 Helix Pomatia enzyme solution to 100 µL of serum in an autosampler vial. After gentle mixing, the sample was incubated at 37 °C for 4 hours, and then 750 µL of 0.1 M formic acid was added. We vortex-mixed and centrifuged all samples—with or without enzyme treatment—before the on-line SPE-HPLC-MS/MS analysis. To ensure data accuracy and precision, we analyzed two high- and two low-concentration quality control (QC) samples, along with two reagent blanks with the pooled serum samples. We prepared the QC materials by spiking calf serum (Gibco, Grand Island, NY, USA) with native target compounds as previously described (20). We prepared all of the samples using the same procedure as described above but replaced the serum with the same volume of standard stock solution (for the standards), QC serum (for the QCs), or HPLC grade H2O (for the reagent blanks). We calculated the limit of detection (LOD) as 3S0, where S0 is the standard deviation as the concentration approaches zero (21). The LODs were 0.1 µg/L (BPA, methyl paraben, ethyl paraben, 2,4-dichlorophenol), 0.2 µg/L (propyl paraben), 0.4 µg/L (2,5-dichlorophenol), 0.5 µg/L (benzophenone-3), and 1.1 µg/L (triclosan). We calculated the mean conjugate % as the ratio of concentrations of conjugated (i.e., total minus free) and total species for the pools with detectable total concentrations of the target analytes as described before (22).
Statistical Analysis
We performed the statistical analyses by using SAS software (SAS Institute, Cary, NC, version 9.1). For concentrations below the LOD, we used a value equal to the LOD divided by the square root of 2 (23). When a pooled sample consists of equal volumes of individual samples, the measured concentration of the pool should be equivalent to the arithmetic mean of the measured concentrations of the individual samples (18). However, because concentrations of environmental chemicals in individual samples from the U.S. general population tend to be log-normally distributed (10), the measured concentrations from the pools are comparable to the arithmetic mean of the log normal concentrations (18) which should be approximately Gaussian. Therefore, we used analysis of variance (ANOVA) methods modified by the heterogeneity of the variance as described before (19). Because the numbers of samples per pool differed by age group, we used separate ANOVA models for each age group (3–5 years vs 6–11 years). As we did before for the analyses of the pooled data for PFCs (19), in the initial ANOVA models, we included sex, race, and the interaction between sex and race. In the models, we assumed that the measured concentrations in the pools are independently and identically distributed. However, this distributional assumption is unlikely because the individual serum used to make the pools was obtained from a stratified multistage probability sample design. Therefore, to account for the potential underestimation of variance associated with our analyses, we also computed adjusted significance levels (Adj_ProbF) assuming design effects of 3.5 and 10 based on NCHS’s recommendations. Then, we compared our “unadjusted” statistical results with those obtained using these two conservative estimates of how large the design effect might be. We considered analyses to be statistically significant when p ≤ 0.05.
Results
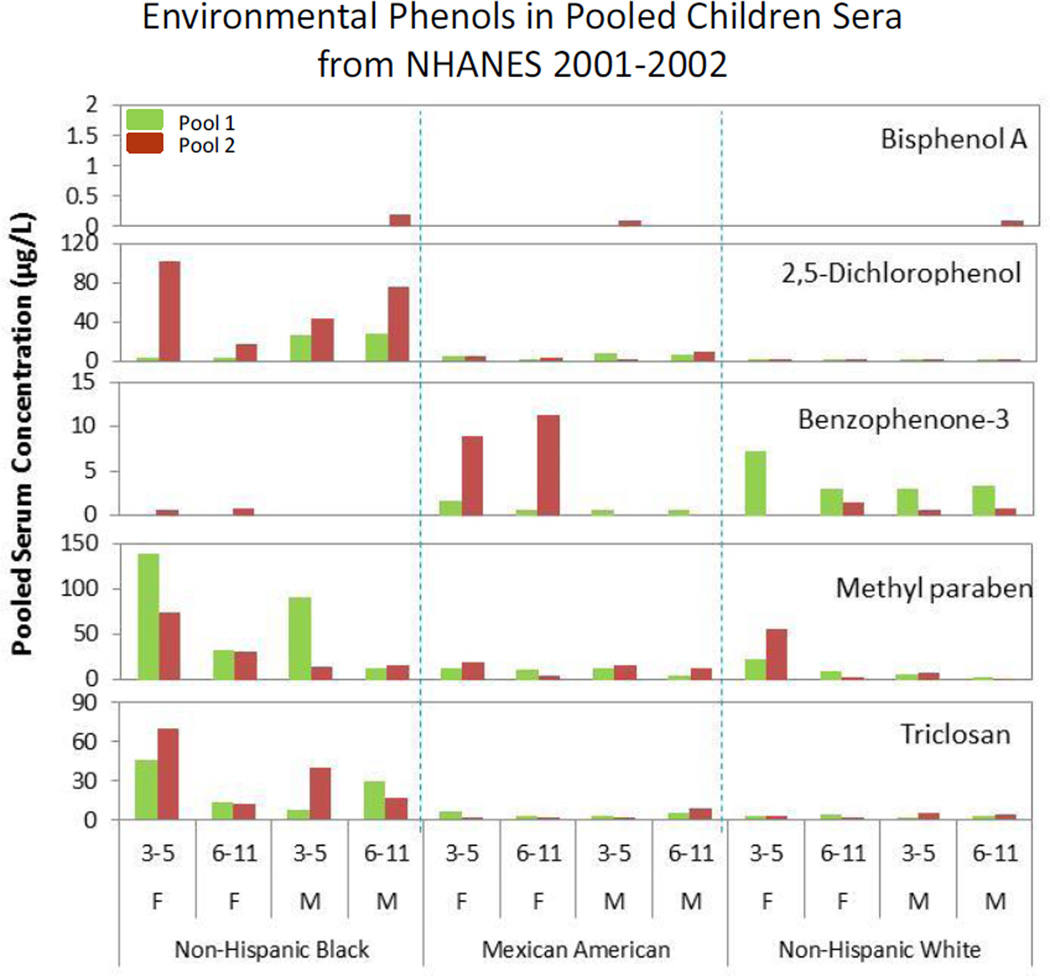
We detected triclosan, 2,4-dichlorophenol, 2,5-dichlorophenol, methyl paraben, and propyl paraben in all of the 24 pools analyzed, benzophenone-3 and ethyl paraben in 15 of the pools, and BPA in only three of them at total concentrations ranging from 138 µg/L (methyl paraben) in one of the pools to 0.1 µg/L (BPA) in two of the pools (Table 1).
We detected the free species of benzophenone-3, methyl paraben, ethyl paraben, propyl paraben, and BPA in some of the 24 serum pools, but we did not detect free species of triclosan, 2,4-dichlorophenol, and 2,5-dichlorophenol in any of the pools (Table 2). However, even though we detected the free species of parabens in all (methyl paraben) or some (ethyl- and propyl parabens) of the pools, the free concentration ranges (0.4 to 7.8 µg/L (methyl paraben); <0.1 to 0.3 µg/L (ethyl paraben); <0.2 to 0.9 µg/L (propyl paraben)) were much lower than the total concentration ranges (1.6 to 138 µg/L (methyl paraben); <0.1 to 2.0 µg/L (ethyl paraben); 0.2 to 14.5 µg/L (propyl paraben)) (Tables 1 and 2). The mean conjugate % and number of pools with detectable total concentrations were 33 % (BPA, 3 pools), 63 % (benzophenone-3, 15 pools), 88 % (methyl paraben, 24 pools), 98 % (propyl paraben, 24 pools), 99 % (ethyl paraben, 15 pools), and 100% (triclosan, 2,4-dichlorophenol, 2,5-dichlorophenol, 24 pools).
Table 2.
Free concentrations (µg/L) of select environmental phenols in pooled sera (two pools, P1 and P2, per demographic group) from 2001–2002 NHANES participants 3–11 years of agea, b
| Race | Sex | Age | BPA | Benzophenone-3 | Methyl paraben |
Ethyl paraben |
Propyl paraben |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (years) | P1 | P2 | P1 | P2 | P1 | P2 | P1 | P2 | P1 | P2 | ||
| NHB | F | 3–5 | NDc | ND | ND | ND | 2.4 | 7.8 | ND | ND | ND | 0.9 |
| NHB | F | 6–11 | ND | ND | ND | ND | 3.3 | 3.2 | ND | ND | ND | ND |
| NHB | M | 3–5 | ND | ND | ND | ND | 3.6 | 0.6 | ND | ND | 0.3 | ND |
| NHB | M | 6–11 | ND | 0.2 | ND | ND | 1.3 | 2.1 | ND | ND | ND | ND |
| MA | F | 3–5 | ND | ND | 0.9 | 6.2 | 2.4 | 3.6 | ND | 0.3 | 0.3 | 0.3 |
| MA | F | 6–11 | ND | ND | ND | 8.7 | 1.5 | 0.6 | ND | ND | ND | ND |
| MA | M | 3–5 | ND | ND | ND | ND | 4.0 | 1.8 | ND | ND | 0.3 | ND |
| MA | M | 6–11 | ND | ND | ND | ND | 0.4 | 2.0 | ND | ND | ND | ND |
| NHW | F | 3–5 | ND | ND | 3.9 | ND | 0.8 | 1.7 | ND | ND | ND | ND |
| NHW | F | 6–11 | ND | ND | 1.4 | 1.0 | 1.2 | 0.6 | ND | ND | ND | ND |
| NHW | M | 3–5 | ND | ND | 1.4 | ND | 0.8 | 0.3 | ND | ND | ND | ND |
| NHW | M | 6–11 | ND | 0.1 | 1.1 | ND | 0.4 | 0.4 | ND | ND | ND | ND |
| Frequency of detectiond | 8 % | 33 % | 100 % | 4 % | 25 % | |||||||
2,4-Dichlorophenol, 2,5-dichlorophenol, and triclosan were not listed here because no free species of these analytes were detected in any of the 24 pools.
Female (F), male (M), non-Hispanic black (NHB), non-Hispanic white (NHW), Mexican American (MA).
ND: the concentrations were below the limits of detection of 0.1 µg/L (BPA, methyl-, and ethyl parabens), 0.2 µg/L (propyl paraben), and 0.5 µg/L (benzophenone-3).
Frequency of detection (%) was based on all 24 pools.
In the ANOVA final models adjusted by the heterogeneity of the variance, race was the only significant demographic factor for serum concentrations of triclosan and 2,5-dichlorophenol in both age groups (Table 3). Race was also the only significant demographic factor for the serum concentration of 2,4-dichlorophenol (for children aged 3–5 years) and methyl paraben (for children aged 6–11 years) (Table 3). Both race and sex were significant demographic factors for the propyl paraben serum concentration of children aged 3–5 years. However, after we adjusted the F value by the two different design effects of 3.5 and 10, race and sex were no longer significant. The estimated least-squares means (LSMs) and associated 95% confidence intervals (CIs) of triclosan, 2,4-dichlorophenol, 2,5-dichlorophenol, methyl paraben, and propyl paraben concentrations for these demographic groups are given in Table 4 (data adjusted by the design effect are not shown). Of note, the fairly large standard error term for 2,5-dichlorophenol among non-Hispanic black children (both age groups) resulted in wide 95% CIs with negative low bounds (Table 4).
Table 3.
Significance levels associated with statistically significant demographic variables from ANOVA after adjustment of the mean square error to account for an assumed design effect.
| Analyte | Age group (in years) |
Demographic variable in the final model |
Prob Fa | Adj Prob F (Deff=3.5) |
Adj Prob F (Deff=10) |
|---|---|---|---|---|---|
| triclosan | 3–5 | Race | 0.0489 | 0.34 | 0.66 |
| triclosan | 6–11 | Race | 0.0181 | 0.21 | 0.55 |
| 2,4-dichlorophenol | 3–5 | Race | 0.0019 | 0.06 | 0.3 |
| 2,5-dichlorophenol | 3–5 | Race | 0.0027 | 0.08 | 0.34 |
| 2,5-dichlorophenol | 6–11 | Race | 0.0278 | 0.26 | 0.6 |
| methyl paraben | 6–11 | Race | 0.0256 | 0.41 | 0.72 |
| propyl paraben | 3–5 | Race | 0.0014 | 0.04 | 0.25 |
| Propyl paraben | 3–5 | Sex | 0.0023 | 0.05 | 0.2 |
ProbF is the observed significance level (p value) from ANOVA adjusted by the heterogeneity of variance assuming the measured pool values are independently identically distributed, which is unlikely since the original sample data were obtained from a stratified multistage probability design. Therefore, adjusted significance levels (Adj_ProbF) were also computed assuming a design effect (deff) equal to 3.5 or 10, chosen as a conservative estimate of how large the design effect might be based on NCHS’ recommendations.
Table 4.
Least squares means (LSMs) estimates and their 95% confidence limits (µg/L).
| Analyte | Age (years) |
Racea (Sex) |
LSM | Standard Error |
95% Confidence Limits |
|
|---|---|---|---|---|---|---|
| triclosan | 3–5 | MA | 3.83 | 1.14 | 1.25 | 6.40 |
| 3–5 | NHB | 40.94 | 12.66 | 12.31 | 69.57 | |
| 3–5 | NHW | 3.79 | 0.55 | 2.54 | 5.04 | |
| 6–11 | MA | 4.90 | 1.38 | 1.77 | 8.02 | |
| 6–11 | NHB | 18.10 | 3.90 | 9.28 | 26.92 | |
| 6–11 | NHW | 4.00 | 0.58 | 2.68 | 5.32 | |
| 2,4-dichlorophenol | 3–5 | MA | 0.32 | 0.04 | 0.24 | 0.40 |
| 3–5 | NHB | 0.84 | 0.28 | 0.21 | 1.47 | |
| 3–5 | NHW | 0.15 | 0.01 | 0.14 | 0.17 | |
| 2,5-dichlorophenol | 3–5 | MA | 5.08 | 1.03 | 2.74 | 7.41 |
| 3–5 | NHB | 44.00 | 20.98 | −3.47 | 91.46 | |
| 3–5 | NHW | 0.40 | 0.12 | 0.13 | 0.68 | |
| 6–11 | MA | 5.39 | 1.76 | 1.42 | 9.36 | |
| 6–11 | NHB | 31.22 | 15.43 | −3.68 | 66.12 | |
| 6–11 | NHW | 0.72 | 0.11 | 0.46 | 0.97 | |
| Methyl paraben | 6–11 | MA | 8.34 | 2.09 | 3.62 | 13.06 |
| 6–11 | NHB | 23.00 | 5.06 | 11.56 | 34.44 | |
| 6–11 | NHW | 4.93 | 1.93 | 0.57 | 9.28 | |
| Propyl paraben | 3–5 | MA | 3.16 | 0.34 | 2.37 | 3.94 |
| 3–5 | NHB | 9.44 | 2.81 | 2.96 | 15.92 | |
| 3–5 | NHW | 1.08 | 0.22 | 0.57 | 1.59 | |
| 3–5 | (Female) | 5.38 | 0.96 | 3.15 | 7.60 | |
| 3–5 | (male) | 3.75 | 0.96 | 1.52 | 5.97 | |
Non-Hispanic black (NHB), non-Hispanic white (NHW), Mexican American (MA).
Discussion
The detection of total concentrations of triclosan, 2,4-dichlorophenol, 2,5-dichlorophenol, methyl paraben, and propyl paraben in all pools examined suggest exposure to these compounds among young children in the United States in the early 2000s. Of interest, NHANES urinary data for triclosan, methyl paraben, propyl paraben and the two dichlorophenols also suggest widespread exposure to these compounds or their precursors in the U.S. general population at least since 2003 (10). Together these findings suggest that, despite the non-persistent physicochemical characteristics (e.g., rapid metabolism in humans) and the likely episodic nature of the exposures to triclosan, methyl paraben, and propyl paraben or to the precursors of 2,4-dichlorophenol and 2,5-dichlorophenol, trace concentrations of these chemicals are not only detectable in urine of young children (6–14), but can also be detected in blood.
Even though the sensitivity of the analytical method (i.e., LOD) was very similar for all parabens (0.1–0.2 µg/L), we detected total concentrations of ethyl paraben in fewer of 2/3 of the serum pools but methyl paraben and propyl paraben in all of the pools (Table 1). Compared with methyl- and propyl parabens, the usage of ethyl paraben as an antimicrobial preservative in a variety of consumer products is much more limited (24). Interestingly, the NHANES 2005–2006 biomonitoring urinary data on parabens showed that ethyl paraben was detected in fewer of the participants 6 years of age and older examined (42.4%) than methyl paraben (99.1%) or propyl paraben (92.7%) and also at lower concentrations (8). The pooled serum data presented here also demonstrate that although we detected circulating levels of ethyl paraben in children’s serum, it was at a lower frequency of detection and lower concentrations than those of methyl- and propyl parabens (Table 1).
Benzophenone-3 is a commonly used sunscreen agent in a variety of cosmetic products (4). Because of its extensive usage, we detected benzophenone-3 in the urine of 96.8% of NHANES 2003–2004 participants 6 years of age and older with a geometric mean of 22.2 µg/g creatinine (7). We also detected benzophenone-3 in these children’s pooled sera from all demographic groups except non-Hispanic blacks. The frequent detection and the magnitude of the detected serum concentrations (mean: 0.5–5.9 µg/L) are in agreement with widespread exposure of some children to this non-persistent sunscreen agent. Interestingly, based on urinary concentrations of benzophenone-3 in NHANES participants (7), exposure to this sunscreen agent appeared to be higher in females than in males and in non-Hispanic whites compared to Mexican Americans and non-Hispanic blacks. The racial and sex differences observed in these NHANES serum pools are in good agreement with those reported before from the urinary benzophenone-3 concentrations of individual NHANES participants (7, 10).
Among the phenols examined, in recent years, BPA has attracted attention worldwide from regulatory agencies, researchers and the general public. Scientific debate continues about the existence of low-dose effects of BPA in animal studies and their potential implications for human health (1, 2, 25–27). Because of the extensive use of BPA in consumer products, exposure to BPA, assessed from the urinary concentrations of total BPA, among the general population in the United States and abroad is widespread (9, 28–31). However, despite the relatively low LOD for BPA (0.1 µg/L), we only detected total BPA in 3 of the 24 (13 %) NHANES 2001–2002 serum pools with the highest concentration being 0.2 µg/L, compared with a detection of over 90% and median concentrations ~ 3 µg/L in urine samples from NHANES 2003–2004 (9).
These NHANES 2001–2002 serum results are in agreement with a previous study from our laboratory in which we detected BPA in only 1 out of 15 adults’ serum samples (32), and with two recent reports, from Germany (29) and the United States (33). In samples from the German environment specimen bank collected between 1995 and 2009, the detection frequency of total BPA was 12% in plasma (N =60) and >96% in 24-h urine (N = 600) (29). Furthermore, the total BPA concentrations in plasma were much lower than in the urine with maxima of 0.4 µg/L vs 34.5 µg/L, respectively (29). In serial samples collected approximately hourly over a 24-h period from 20 adults after ingestion of BPA-containing meals, total BPA was detected in only 17% of 320 serum samples but in 74% of 385 urine samples (33). Furthermore, the total BPA serum concentrations were on average 42 times lower than in urine, although the mean BPA daily exposure (0.27 µg/kg) in this group of adults was higher than the 95th percentile of the aggregate exposure (from all routes) in the general U.S. adult population obtained from NHANES biomonitoring urinary data (33, 34). Our results, however, do not agree with several other studies in which total and free BPA concentrations in blood, plasma, and serum were detected more frequently and at higher concentrations (35) than we did in these children’s NHANES serum pools. Some of these other studies relied on detection by enzyme-linked immunosorbent assays, which are not specific enough and therefore inadequate for the quantitative determination of BPA in biological samples (27), and/or involved specific adult populations (e.g., pregnant women at delivery, patients undergoing medical treatment in hospital settings) with relatively small sample sizes (35–38). We speculate that the relatively high detection frequency of BPA in samples collected in hospitals might be related to the exposures to BPA from the medical interventions (39–41). Of note, because pooling provides an average estimate (in this case concentration) of all individual samples used to make the pools (16), the fact that most NHANES pools had undetectable concentrations of BPA suggests that a large proportion of the individual samples had BPA concentrations close to or below the LOD. Therefore, we can’t rule out that the LOD of our multi-analyte method (0.1 µg/L) is inadequate for the detection of BPA in blood and that by analyzing pools made from individual samples with BPA concentrations that appear to be largely non-detectable or around the LOD, we may have underestimated the frequency of detection of BPA compared to using the individual samples. Nonetheless, our main findings of lower average concentrations of BPA in serum than in urine would not change even if we had used a more sensitive method or individual samples instead of pools. Taken together, these results suggest that blood or its products (serum/plasma) is an unfavorable matrix for the biomonitoring of BPA exposure in the general population (29).
The phenols evaluated in this study or, in the case of the dichlorophenols, their parent compounds, rapidly metabolize in the body after exposure through Phase I and/or Phase II biotransformations before excretion in the urine (5, 42–45). Both conjugated and free species have been used as valid biomarkers of exposure to the parent compounds (6, 9, 46). Because conjugation (i.e., Phase II biotransformation) is considered to be a detoxification process (47, 48), the presence of the unconjugated (i.e., free) species in circulation can be taken to reflect exposure to the biologically active form of the compounds (35, 49–51). Of note, even young children soon after birth appear to have some capacity to metabolize non-persistent compounds to their corresponding conjugates, as suggested from studies on exposure to BPA, triclosan, benzophenone-3, methyl- and propyl-parabens and phthalates in premature infants (39, 52). Therefore, we also measured the free serum concentrations of BPA and the other phenols in these pooled serum samples (Table 2).
To accurately measure the concentrations of the free species, one has to ensure the integrity (i.e., stability of the biomarkers in the matrix, absence of noticeable external contamination) of the biological sample during its handling and storage. Previous data suggest that the conjugated species of several phenols in serum are much more stable than in urine (53, 54), even under extreme conditions (e.g., stored at 37 °C for up to 30 days after collection) (53); such conditions are unlikely to have been experienced by the samples used for the present study. Moreover, for six of the phenols examined (no data exist for BPA and benzophenone-3), conjugates appear to be the main species in serum (53). In good agreement with these findings, we mainly detected the conjugates of triclosan, 2,4-dichlorophenol, 2,5-dichlorophenol, and methyl-, ethyl-, and propyl parabens in the children’s pooled sera (mean conjugate % ranged from 88 % to 100 %). By contrast, the mean conjugate % was lower for BPA (33 %) and benzophenone-3 (63 %) than for the other six phenols. Furthermore, the concentrations of free and total benzophenone-3 were similar in some pools (e.g., Mexican American females 3–5 years of age: 8.9 µg/L vs 6.2 µg/L). Similarly, in two of the three pools with detectable total BPA, the total and free concentrations were the same (0.1 and 0.2 µg/L) but rather low at or slightly above the LOD. The potential for trace level external contamination exists when the measured compound (biomarker) is the actual compound present in the products/environment (27, 29, 55–57). We speculate that the relatively high percentage of free benzophenone-3 and BPA in some of these children pools might be due to the random contamination with BPA and/or benzophenone-3 of the serum during its collection, processing, and/or during the pooling procedure. Contamination with triclosan and the parabens may also have occurred, but its impact might not be as evident as for BPA and benzophenone-3 because the serum concentrations of triclosan and the methyl- and propyl parabens in the pools were higher than those of BPA and benzophenone-3. Nonetheless, even if contamination with triclosan and parabens occurred, the ranges of serum concentrations of these compounds was much lower than the reported urinary concentrations in the general US population (10), thus supporting that serum is not an adequate matrix for assessing exposure to these compounds.
Our final models suggested that race was the only significant demographic factor for serum concentrations of triclosan (for both age groups), 2,5-dichlorophenol (for both age groups), 2,4-dichlorophenol (for ages 3–5 years), and methyl paraben (for ages 6–11 years) (Table 3). Both race and sex were significant demographic factors for serum concentrations of propyl paraben for 3–5 year old children. The LSMs concentrations of triclosan and 2,5-dichlorophenol (for both age groups), 2,4-dichlorophenol (for ages 3–5 years), methyl paraben (for ages 6–11 years), and propyl paraben (for aged 3–5 years) in non-Hispanic blacks were significantly higher than for the other ethnic groups (Table 4). Of interest, compared with the other ethnics groups, non-Hispanic blacks also have the highest geometric mean urinary concentrations of parabens among the general U.S. population (8). The reason for the observed differences by race in serum concentrations (and presumably exposures) of environmental phenols (or their precursors) among children is unclear, but we speculate that lifestyle choices by race may play a role. Nevertheless, several factors must be taken into consideration when interpreting these modeling results. By pooling across design cells, we cannot assure that estimates based on the pooled samples are unbiased. Besides, the ANOVA LSMs represent positively biased estimates of the central values of the 12 demographic groups (6 for children aged 3–5 years and 6 for children aged 6–11 years). This additional positive bias, which we made no attempt to correct, arises from the inherent bias associated with the measured concentrations of the pools (18). Among the different ethnic groups, we observed the biggest standard errors among non-Hispanic blacks, which likely contributed to the wider 95% CIs for this ethnic group compared to others. After exposure, environmental phenols, like any other non-persistent compounds, have short half-lives (~6–12 hours). Therefore, concentrations of these compounds in spot samples (urine or serum/plasma) primarily reflect exposure(s) which occurred within a relatively short period preceding sample collection (58) and there is considerable temporal variability of concentrations in spot samples (59, 60). The variability of serum concentrations from spot sampling plus the higher concentrations of some phenols in non-Hispanic blacks may have contributed to the higher standard errors for this particular ethnic group.
In summary, although the degree of the exposure and the dominant exposure pathway(s) to environmental compounds likely depend on the age and associated lifestyle of each person, we found that exposure of 3–11 year old children to triclosan, benzophenone-3, methyl-, ethyl-, and propyl parabens, and the precursors of 2,4-dichlorophenol and 2,5-dichlorophenol, was prevalent in the United States in the early 2000s based on the detection of these phenols in at least more than half of the NHANES 2001–2002 serum pools. A few recent epidemiologic studies have suggested that prenatal and childhood exposure to some of these phenols (or their precursors) may be associated with altered neurodevelopment, obesity, and precocious puberty (11, 26, 61–64). Therefore, our findings confirming that these chemicals not only are present in U.S. children’s urine (6–14), but also could circulate in blood warrant additional research to further evaluate the potential effects on human health upon exposure to these compounds (or their precursors).
Conjugated species of six of these chemicals were the major species in serum, as they are in urine. On the other hand, even though BPA has been widely detected in U.S. children’s urine (9–14), total BPA in these serum pools was only detected in 3 of the 24 pools and at concentrations at or slightly above 0.1 µg/L. We detected BPA and the seven other environmental phenols at much lower concentrations and frequencies in serum than those reported in urine. These findings support that urine, not serum or plasma, is a more appropriate matrix for biomonitoring of these compounds in the general population.
Despite the fact that we used 936 individual serum samples to make the pools, our results are based on the analyses of a limited number of pools. Furthermore, the pools only include samples from children 3–11 years of age; additional studies should include even younger children. Nonetheless, the widespread exposure to some of these environmental phenols among these children highlights the need for continuing efforts to identify sources of human exposure to these chemicals, especially among young children, and to study the environmental distribution of these compounds.
Figure.
Acknowledgments
We thank the National Center for Health Statistics for conducting the sample collection for the National Health and Nutrition Examination Survey, and Dr. Andreas Sjodin for providing the children serum pools used for the present study.
List of abbreviations
- ANOVA
Analysis of variance
- BPA
Bisphenol A
- CDC
Centers for Disease Control and Prevention
- CI
Confidence interval
- LOD
Limit of detection
- LSMs
Least-squares means
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- NHB
non-Hispanic black
- NHW
non-Hispanic white
- MA
Mexican American
- On-line SPE-HPLC-MS/MS
On-line solid phase extraction high performance liquid chromatography tandem mass spectrometry
- PFC
Polyfluoroalkyl compounds
- QC
Quality control
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
The authors declare that they have no competing financial interests.
Reference List
- 1.European Union. Updated European Risk Assessment Report 4,4'-Isopropylidenediphenol (Bisphenol-A). Environment Addendum of February 2008 (to be read in conjunction with published EU RAR of BPA, 2003 for full details) [accessed 20 September 2012];European Union. 2008 Available: http://publications.jrc.ec.europa.eu/repository/bitstream/111111111/15069/1/lbna24589enn.pdf. [Google Scholar]
- 2.National Toxicology Program. [accessed 3 September 2012];NTP Brief on Bisphenol A [CAS NO. 80 – 05 – 07] 2008 Available: http://ntp.niehs.nih.gov/ntp/ohat/bisphenol/bisphenol.pdf.
- 3.IPCS. Chlorophenols. International Programme on Chemical Safety (IPCS); 1989. [accessed 12 July 2012]. Environmental Health Criteria Monographs (EHCs) Available: http://www.inchem.org/documents/ehc/ehc/ehc093.htm. [Google Scholar]
- 4.Household products database. Available at http://hpd.nlm.nih.gov/index.htm.
- 5.Soni MG, Carabin IG, Burdock GA. Safety assessment of esters of p-hydroxybenzoic acid (parabens) Food Chem. Toxicol. 2005;43:985–1015. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of Triclosan in the US population: 2003–2004. Environ. Health Perspect. 2008;116:303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calafat AM, Wong L-Y, Ye X, Reidy JA, Needham LL. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003–2004. Environ. Health Perspect. 2008;116:893–897. doi: 10.1289/ehp.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calafat AM, Ye XY, Wong LY, Bishop AM, Needham LL. Urinary Concentrations of Four Parabens in the US Population: NHANES 2005–2006. Environ. Health Perspect. 2010;118:679–685. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calafat AM, Ye XY, Wong LY, Reidy JA, Needham LL. Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC. Updated Tables, February 2012. Atlanta, GA: Centers for Disease Control and Prevention; National Center for Environmental Health; Division of Laboratory Sciences; 2012. [accessed 1 February 2012]. Fourth National Report on Human Exposure to Environmental Chemicals. Available: http://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Feb2012.pdf. [Google Scholar]
- 11.Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye XY, Dietrich KN, Lanphear BP. Impact of Early-Life Bisphenol A Exposure on Behavior and Executive Function in Children. Pediatrics. 2011;128:873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan MK, Jones PA, Calafat AM, Ye XY, Croghan CW, Chuang JC, Wilson NK, Clifton MS, Figueroa Z, Sheldon LS. Assessing the Quantitative Relationships between Preschool Children's Exposures to Bisphenol A by Route and Urinary Biomonitoring. Environ. Sci. Technol. 2011;45:5309–5316. doi: 10.1021/es200537u. [DOI] [PubMed] [Google Scholar]
- 13.Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ. Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, Godbold J, Biro F, Kushi LH, Pfeiffer CM, Calafat AM. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ. Health Perspect. 2007;115:116–121. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Needham LL, Sexton K. Assessing children's exposure to hazardous environmental chemicals: an overview of selected research challenges and complexities. J. Expos. Anal. Environ. Epidemiol. 2000;10:611–629. doi: 10.1038/sj.jea.7500142. [DOI] [PubMed] [Google Scholar]
- 16.Caudill SP. Characterizing populations of individuals using pooled samples. J. Expos. Sci. Environ. Epidemiol. 2010;20:29–37. doi: 10.1038/jes.2008.72. [DOI] [PubMed] [Google Scholar]
- 17.CDC. National Health and Nutrition Examination Survey. [accessed 11 August 2008];National Center for Health Statistics. 2003 Available: http://www.cdc.gov/nchs/nhanes.htm.
- 18.Caudill SP, Turner WE, Patterson DG. Geometric mean estimation from pooled samples. Chemosphere. 2007;69:371–380. doi: 10.1016/j.chemosphere.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 19.Kato K, Calafat AM, Wong LY, Wanigatunga AA, Caudill SP, Needham LL. Polyfluoroalkyl Compounds in Pooled Sera from Children Participating in the National Health and Nutrition Examination Survey 2001–2002. Environ. Sci. Technol. 2009;43:2641–2647. doi: 10.1021/es803156p. [DOI] [PubMed] [Google Scholar]
- 20.Ye X, Tao LJ, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method for measuring environmental phenols and parabens in serum. Talanta. 2008;76:865–871. doi: 10.1016/j.talanta.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 21.Taylor JK. Quality Assurance of Chemical Measurements. Chelsea, MI: Lewis Publishers; 1987. [Google Scholar]
- 22.Ye XY, Bishop AM, Reidy JA, Needham LL, Calafat AM. Temporal stability of the conjugated species of bisphenol A, parabens, and other environmental phenols in human urine. J. Expos. Sci. Environ. Epidemiol. 2007;17:567–572. doi: 10.1038/sj.jes.7500566. [DOI] [PubMed] [Google Scholar]
- 23.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990;5:46–51. [Google Scholar]
- 24.Elder RL. Final report on the safety assessment of methylparaben, ethylparaben, propylparaben and butylparaben. J. Am. Coll. Toxicol. 1984;3:147–209. [Google Scholar]
- 25.vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapin RE, Adams J, Boekelheide K, Gray LE, Hayward SW, Lees PSJ, McIntyre BS, Portier KM, Schnorr TM, Selevan SG, Vandenbergh JG, Woskie SR. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Research Part B-Developmental and Reproductive Toxicology. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- 27.WHO. Joint FAO/WHO Expert Meeting to Review Toxicological and Health Aspects of Bisphenol A: Summary Report including Report of Stakeholder Meeting on Bisphenol A. [21 July 2012];2010
- 28.Health Canada. Results of the Canadian Health Measures Survey Cycle 1 (2007–2009) Ottawa: Ontario; 2010. [accessed 16 August 2012]. Report on Human Biomonitoring of Environmental Chemicals in Canada. Available: http://www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/chms-ecms/index-eng.php. [Google Scholar]
- 29.Koch HM, Kolossa-Gehring M, Schroter-Kermani C, Angerer J, Bruning T. Bisphenol A in 24?h urine and plasma samples of the German Environmental Specimen Bank from 1995 to 2009: A retrospective exposure evaluation. J Expos Sci Environ Epidemiol. 2012 May 23; doi: 10.1038/jes.2012.39. in press [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30.Volkel W, Kiranoglu M, Fromme H. Determination of free and total bisphenol A in human urine to assess daily uptake as a basis for a valid risk assessment. Tox. Lett. 2008;179:155–162. doi: 10.1016/j.toxlet.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Yang MH, Kim SY, Lee SM, Chang SS, Kawamoto T, Jang JY, Ahn YO. Biological monitoring of bisphenol A in a Korean population. Arch. Environ. Contam. Toxicol. 2003;44:546–551. doi: 10.1007/s00244-002-2124-0. [DOI] [PubMed] [Google Scholar]
- 32.Ye X, Tao LJ, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method for measuring environmental phenols and parabens in serum. Talanta. 2008;76:865–871. doi: 10.1016/j.talanta.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 33.Teeguarden JG, Calafat AM, Ye XY, Doerge DR, Churchwell MI, Gunawan R, Graham MK. Twenty-Four Hour Human Urine and Serum Profiles of Bisphenol A during High-Dietary Exposure. Toxicol. Sci. 2011;123:48–57. doi: 10.1093/toxsci/kfr160. [DOI] [PubMed] [Google Scholar]
- 34.Lakind JS, Naiman DQ. Daily intake of bisphenol A and potential sources of exposure: 2005–2006 National Health and Nutrition Examination Survey. J. Expos. Sci. Environ. Epidemiol. 2011;21:272–279. doi: 10.1038/jes.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJR, Schoenfelder G. Urinary, Circulating, and Tissue Biomonitoring Studies Indicate Widespread Exposure to Bisphenol A. Environ. Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang M, Ryu JH, Jeon R, Kang D, Yoo KY. Effects of bisphenol A on breast cancer and its risk factors. Arch. Toxicol. 2009;83:281–285. doi: 10.1007/s00204-008-0364-0. [DOI] [PubMed] [Google Scholar]
- 37.Kaddar N, Bendridi N, Harthe C, de Ravel MR, Bienvenu AL, Cuilleron CY, Mappus E, Pugeat M, Dechaud H. Development of a radioimmunoassay for the measurement of Bisphenol A in biological samples. Anal. Chim. Acta. 2009;645:1–4. doi: 10.1016/j.aca.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 38.Kuroda N, Kinoshita Y, Sun Y, Wada M, Kishikawa N, Nakashima K, Makino T, Nakazawa H. Measurement of bisphenol A levels in human blood serum and ascitic fluid by HPLC using a fluorescent labeling reagent. J. Pharm. Biomed. Anal. 2003;30:1743–1749. doi: 10.1016/s0731-7085(02)00516-2. [DOI] [PubMed] [Google Scholar]
- 39.Calafat AM, Weuve J, Ye XY, Jia LT, Hu H, Ringer S, Huttner K, Hauser R. Exposure to Bisphenol A and Other Phenols in Neonatal Intensive Care Unit Premature Infants. Environ. Health Perspect. 2009;117:639–644. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calafat AM, Needham LL. Factors affecting the evaluation of biomonitoring data for human exposure assessment. Int. J. Androl. 2008;31:139–143. doi: 10.1111/j.1365-2605.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- 41.Vandentorren S, Zeman F, Morin L, Sarter H, Bidondo ML, Oleko A, Leridon H. Bisphenol-A and phthalates contamination of urine samples by catheters in the Elfe pilot study: Implications for large-scale biomonitoring studies. Environ. Res. 2011;111:761–764. doi: 10.1016/j.envres.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 42.Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem. Res. Toxicol. 2002;15:1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- 43.Kadry AM, Okereke CS, Abdelrahman MS, Friedman MA, Davis RA. Pharmacokinetics of Benzophenone-3 After Oral-Exposure in Male-Rats. J. Appl. Toxicol. 1995;15:97–102. doi: 10.1002/jat.2550150207. [DOI] [PubMed] [Google Scholar]
- 44.Okereke CS, Kadry AM, Abdelrahman MS, Davis RA, Friedman MA. Metabolism of Benzophenone-3 in Rats. Drug Metab Dispos. 1993;21:788–791. [PubMed] [Google Scholar]
- 45.ATSDR. Draft for Public Comment. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2004. [accessed 29 March 2005]. Toxicological Profile for Dichlorobenzenes. Available: http://www.atsdr.cdc.gov/toxprofiles/tp10.pdf. [Google Scholar]
- 46.Ye XY, Kuklenyik Z, Needham LL, Calafat AM. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2005;383:638–644. doi: 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
- 47.Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem. Res. Toxicol. 2001;14:149–157. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- 48.Snyder RW, Maness SC, Gaido KW, Welsch F, Sumner SCJ, Fennell TR. Metabolism and disposition of bisphenol A in female rats. Toxicol. App. Pharmacol. 2000;168:225–234. doi: 10.1006/taap.2000.9051. [DOI] [PubMed] [Google Scholar]
- 49.Oishi S. Effects of propyl paraben on the male reproductive system. Food Chem. Toxicol. 2002;40:1807–1813. doi: 10.1016/s0278-6915(02)00204-1. [DOI] [PubMed] [Google Scholar]
- 50.Routledge EJ, Parker J, Odum J, Ashby J, Sumpter JP. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol. Appl. Pharmacol. 1998;153:12–19. doi: 10.1006/taap.1998.8544. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki T, Kitamura S, Khota R, Sugihara K, Fujimoto N, Ohta S. Estrogenic and antiandrogenic activities of 17 benzophenone derivatives used as UV stabilizers and sunscreens. Toxicol. Appl. Pharmacol. 2005;203:9–17. doi: 10.1016/j.taap.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Calafat AM, Needham LL, Silva MJ, Lambert G. Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics. 2004;113:e429–e434. doi: 10.1542/peds.113.5.e429. [DOI] [PubMed] [Google Scholar]
- 53.Ye XY, Wong LY, Jia LT, Needham LL, Calafat AM. Stability of the conjugated species of environmental phenols and parabens in human serum. Env. Int. 2009;35:1160–1163. doi: 10.1016/j.envint.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 54.Waechter J, Domoradzki J, Thornton C, Markham D. Factors affecting the accuracy of bisphenol a and bisphenol A-monoglucuronide estimates in mammalian tissues and urine samples. Toxicol. Mech. Method. 2007;17:13–24. doi: 10.1080/15376510600803581. [DOI] [PubMed] [Google Scholar]
- 55.Calafat AM, Needham LL. What Additional Factors Beyond State-of-the-Art Analytical Methods Are Needed for Optimal Generation and Interpretation of Biomonitoring Data? Environ. Health Perspect. 2009;117:1481–1485. doi: 10.1289/ehp.0901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Markham DA, Waechter JM, Wimber M, Rao N, Connolly P, Chuang JC, Hentges S, Shiotsuka RN, Dimond S, Chappelle AH. Development of a Method for the Determination of Bisphenol A at Trace Concentrations in Human Blood and Urine and Elucidation of Factors Influencing Method Accuracy and Sensitivity. J. Anal. Toxicol. 2010;34:293–303. doi: 10.1093/jat/34.6.293. [DOI] [PubMed] [Google Scholar]
- 57.Volkel W, Bittner N, Dekant W. Quantitation of bisphenol A and bisphenol A glucuronide in biological samples by high performance liquid chromatography-tandem mass spectrometry. Drug Metabol. Dispos. 2005;33:1748–1757. doi: 10.1124/dmd.105.005454. [DOI] [PubMed] [Google Scholar]
- 58.Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:2063–2078. doi: 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye XY, Wong LY, Bishop AM, Calafat AM. Variability of Urinary Concentrations of Bisphenol A in Spot Samples, First Morning Voids, and 24-Hour Collections. Environ. Health Perspect. 2011;119:983–988. doi: 10.1289/ehp.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meeker JD, Yang T, Ye XY, Calafat AM, Hauser R. Urinary Concentrations of Parabens and Serum Hormone Levels, Semen Quality Parameters, and Sperm DNA Damage. Environ. Health Perspect. 2011;119:252–257. doi: 10.1289/ehp.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environ. Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolff MS, Teitelbaum SL, Pinney SM, Windham G, Liao L, Biro F, Kushi LH, Erdmann C, Hiatt RA, Rybak ME, Calafat AM. BCERC Project 2 collaborators Investigation of Relationships between Urinary Biomarkers of Phytoestrogens, Phthalates, and Phenols and Pubertal Stages in Girls. Environ. Health Perspect. 2010;118:1039–1046. doi: 10.1289/ehp.0901690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Braun JM, Hauser R. Bisphenol A and children's health. Current Opinion in Pediatrics. 2011;23:233–239. doi: 10.1097/MOP.0b013e3283445675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ, Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT. Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 2007;24:131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]