Colorectal cancer is the third most common malignancy and the third leading cause of cancer-related death in the United States, with more than 40,000 rectal cancer cases diagnosed each year.1 Standard treatment for localized (ie, resectable) rectal cancer includes 5-fluorouracil (5-FU)-based chemoradiation therapy followed by surgery. Improved radiation technology including image-guided radiation therapy (RT) and brachytherapy (contact therapy) can allow for the delivery of higher doses of RT to the rectal tumor over a shorter time period. These treatments may result in improved outcomes; however, they require fiducial markers to allow better localization and targeting of the rectal tumor. In this retrospective study, we evaluated the role of gold fiducial markers in patients receiving neoadjuvant endorectal brachytherapy in patients with localized rectal tumors.
BACKGROUND
The 2 major goals of treatment of rectal cancer include complete (margin-negative) resection of the tumor and sphincter preservation.2,3 Neoadjuvant chemoradiation therapy results in downstaging in the majority of rectal tumors, and nearly 8% to 12% of patients achieve a pathologic complete response (pCR).2,4,5 Patients who achieve a pCR after neoadjuvant therapy have been shown to have improved disease-free and distant metastases-free survival rates.6 However, neoadjuvant chemoradiation therapy with conventional RT is typically associated with high rates of acute toxicity, which can lead to treatment breaks, decreased treatment efficacy, and delayed surgical resection.7
Recently, the use of a more localized form of RT called high-dose rate endorectal brachytherapy (HDR-EBT) has increased in popularity. HDR-EBT allows the delivery of high doses of radiation to the rectal tumor plus a margin over only 4 days instead of 6 weeks, as is the case with conventional RT. To ensure accurate dose delivery during HDR-EBT, radiographic markers, also called fiducials, are placed around the tumor to facilitate image guidance. Although there are no standardized techniques or formal guidelines for fiducial placement in rectal cancer patients, EUS-guided fiducial placement is often used in HDR-EBT planning because of its relatively noninvasive nature and its success in other types of cancer (eg, pancreas, prostate).8–12 Inaccurate fiducial placement may lead to poor fiducial visualization or fiducial migration during the delivery of image-guided HDR-EBT. As a result, the precise target cannot be delineated, and dose delivery to the target volume and/or surrounding normal tissues (bladder, bowel) may be altered, thereby reducing treatment efficacy and compromising the clinical outcome.2,3,13,14 Before the advent of fiducials, clips were placed near the tumor area; however, these clips were not compatible with magnetic resonance imaging (MRI) and led to difficulties when staging and simulating patients with MRI. This resulted in an increased interest and use of gold MRI-compatible fiducial markers.
To our knowledge, this is the first report to describe EUS-guided fiducial placement used in the management of rectal cancer with HDR-EBT. Here we present our experience with fiducial visibility and migration for HDR-EBT in a group of patients with localized rectal cancer.
METHODS
Patient selection
Data were collected and retrospectively analyzed for patients with localized resectable rectal adenocarcinoma T2N1-2 or T3N0-2 that was 12 cm or less from the anal verge. All patients underwent EUS-guided fiducial placement followed by HDR-EBT at Johns Hopkins Hospital from January 2010 to December 2013. This study was approved by the Johns Hopkins Hospital Institutional Review Board for Human Research.
Treatment intervention
After meeting eligibility requirements, patients were enrolled in the study. All patients underwent CT and MRI simulation, and treatment plans were fused by using the Oncentra brachytherapy planning system (Nucletron, Veenendaal, The Netherlands) (Supplemental Figure 1, available online at www.giejournal.org). After receiving 26 Gy (6.5 Gy × 4 fractions) of HDR-EBT, patients underwent total mesorectal excision with a lower anterior resection or an abdominoperineal resection 6 to 8 weeks later. After surgical resection, it was recommended that all patients receive adjuvant 5-FU and oxaliplatin chemotherapy.
Fiducial markers
Two fiducial markers were evaluated in this study: traditional fiducials (TFs) (Best Medical International, Inc, Springfield, Va, USA) (5 mm in length, 0.80 mm in diameter) and X-mark fiducials (XMFs) (ONC Solutions Inc, Acton, Mass, USA) (1, 2, or 3 cm in length, 0.85 mm in diameter) (Fig.1). All fiducials were preloaded onto a FNA needle and directly inserted into the tumor by using EUS guidance (Fig. 2). There was no specific methodology with regard to the choice of fiducials for this initial cohort of patients. To assist with delineation of the rectal tumor and HDR-EBT treatment planning, the compatibility of these fiducials with a 2 Tesla MRI was determined.
Figure 1.
A, Traditional fiducial. B, X-mark fiducial (Published with permission from X-mark, Onc Solutions).
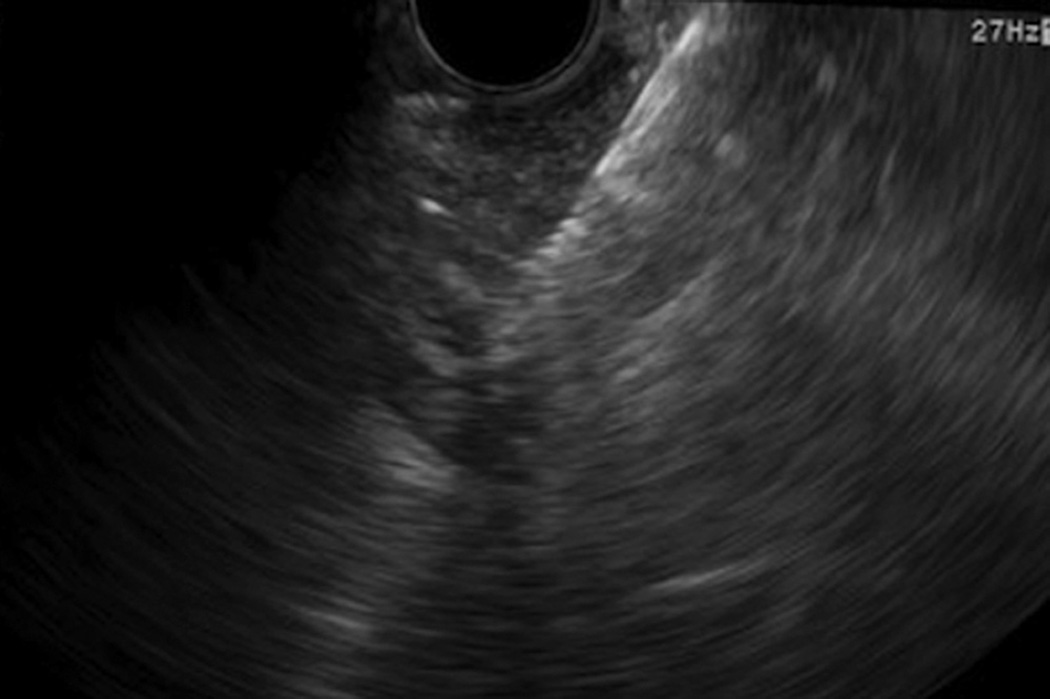
Figure 2.
EUS of a fiducial placed in the rectum.
Fiducial placement
All fiducials were placed under EUS guidance by 1 of 3 gastroenterologists. Before the procedure, the risks and benefits were discussed with the patient, and informed consent was obtained. The patient was then placed under propofol-based sedation and monitored closely under the care of the anesthesia team. Prophylactic antibiotics were routinely given before fiducial placement.
A digital rectal examination was performed. A curvilinear-array echoendoscope (FG36-UA; Pentax Medical Corp, Montvale, NJ or GF-UC140P-AL5; Olympus America, Center Valley, Pa, USA) was used to identify the margins of the tumor. Endoscopic stage was first determined (if necessary), then fiducials were backloaded into a 19-gauge FNA needle by using standard techniques; the needle tip was sealed with sterile bone wax to prevent unintentional loss of markers as the needle was advanced through the echoendoscope.8 Fiducials were then placed at the superior and inferior extents as well as in the center of the tumor. There were no specific guidelines for the number of fiducials placed per patient; however, in general, we asked the gastroenterologist to place a total of 3 fiducials. In some cases, more or fewer than 3 fiducials were placed because of technical or anatomic limitations. Specifically, it was slightly more difficult to place fiducials in tumors that were more proximal to the rectosigmoid junction. In addition, there may have been heterogeneity in the number of fiducials because of varying gastroenterologists.
The location of fiducial placement was confirmed with fluoroscopy, when available. After fiducial placement, the endoscope was withdrawn. Patients were observed for up to 2 hours after the procedure for any acute adverse events. Postendoscopic care included an oral prophylactic antibiotic regimen for 5 days.10 Ideally, fiducials were placed 1 to 2 days before the planned simulation for HDR-EBT.
Visibility and migration scoring
Scored by a radiologist blinded to the type of fiducials placed, fiducial visibility and migration were primarily measured by using CT and fluoroscopy. Visibility of the fiducial was scored on CT imaging with a previously used subjective scoring system: 0 = not visible, 1 = barely visible, 2 = clearly visible (Figs. 3A and B).11 Migration of fiducials, calculated by measuring interfiducial distance, was measured on CT imaging by using 3-dimensional multiplanar reconstruction of fiducial location. Calculations were performed based on CT scans at the time of EUS-guided fiducial placement and at the end of treatment. In addition, fluoroscopy images were obtained on every on-treatment day to accurately position the applicator based on fiducial location and to measure the number of fiducials.
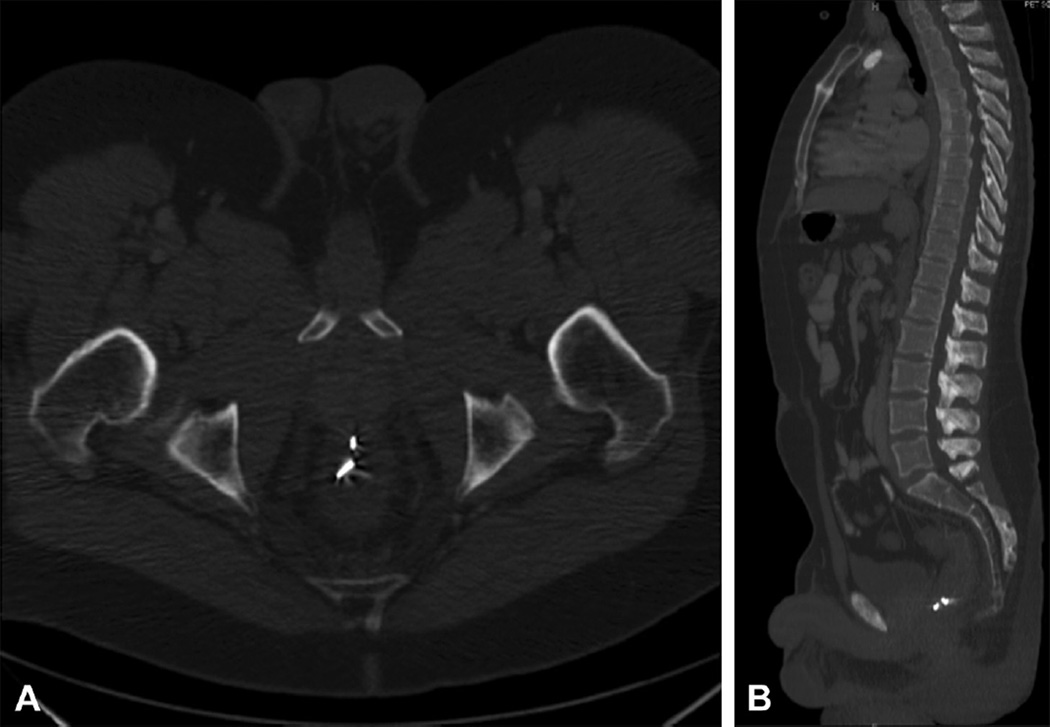
Figure 3.
A, Axial CT image with X-Mark fiducials. B, Sagittal CT image with X-mark fiducials.
Statistical analysis
Statistical analysis was performed by using SPSS software, version 21 (SPPS, Inc, Chicago, Ill). Descriptive statistics along with the Pearson χ2 test and Mann-Whitney U test were performed to compare the TF and XMF groups. The median and interquartile ranges (IQR) are given in all cases in which the Mann-Whitney U test was used.
RESULTS
Patient demographic characteristics
A total of 11 patients underwent EUS-guided placement of fiducials for localized rectal cancer. The median age of patients in our cohort was 50 years (IQR, 46–62). The median size of the rectal mass before treatment was 3.8 cm (IQR, 2.9–4.6). Patient demographic and fiducial characteristics are further described in Tables 1 and 2.
TABLE 1.
Patient demographic and fiducial characteristics
| TFs | XMFs | |
|---|---|---|
| No. of patients | 3 | 8 |
| Median age, y | 61 | 48 |
| Technical difficulty reported | ||
| Immediate adverse events | 0 | 0 |
| Late adverse events | 0 | 0 |
| Median migration, cm | 0.1 | 0.45 |
TFs, Traditional fiducials; XMFs, X-mark fiducials.
TABLE 2.
Fiducial and procedure characteristics
| Patient | Age at diagnosis, y |
Sex | Largest tumor diameter, cm |
Type of fiducial |
No. of fiducials |
No. of fiducials detached |
Average migration per patient, cm |
Technical difficulties |
|---|---|---|---|---|---|---|---|---|
| 1 | 42 | F | 6.0 | XMF | 3 | 2 | 0.30 | None |
| 2 | 44 | F | 4.6 | XMF | 3 | 2 | 1.10 | None |
| 3 | 62 | M | 3.0 | TF | 4 | 2 | 0 | None |
| 4 | 61 | M | 3.0 | TF | 4 | 0 | 0.10 | None |
| 5 | 55 | F | 2.5 | XMF | 3 | 1 | 0.50 | None |
| 6 | 50 | M | 3.8 | TF | 6 | 0 | 0.10 | None |
| 7 | 88 | F | 2.8 | XMF | 3 | 0 | 0.45 | None |
| 8 | 72 | F | 4.6 | XMF | 3 | 0 | 0.15 | None |
| 9 | 35 | M | 2.4 | XMF | 3 | 1 | 1.10 | None |
| 10 | 49 | F | 5.7 | XMF | 3 | 1 | 0.10 | None |
| 11 | 48 | F | 3.8 | XMF | 4 | 1 | 1.20 | None |
F, Female; XMF, X-mark fiducials; M, male; TF, traditional fiducial.
Fiducial characteristics
Table 2 illustrates the differences between the 2 fiducial groups. The mean number of fiducials placed per patient was 3.63 (standard deviation, 1.03; range 3.0–60) by using a 19-gauge needle. Of the 11 patients who received fiducials, 3 received TFs and 8 received XMFs. All fiducials, regardless of type, were clearly visible, with a visibility score of 2. The mean number of fiducials that detached from the implanted site before surgery for patients with TFs was 0.667 compared with 0.875 in patients with XMFs (P = .744). The median migration distance for XMFs, as measured by interfiduciary distance on CT, was significantly larger compared with TFs (0.45 cm vs 0.10 cm, respectively; P = .049).
Surgical outcomes
All patients were alive and free of progression (local or distant) at last follow-up (median, 8.1 months). All 11 patients underwent successful resection at a median time of 7.56 weeks from the end of RT (IQR, 6.5–7.8). Rates of margin positivity and lymphovascular invasion were 0%. Five patients (45%) had positive lymph nodes at resection (median, 3). Three patients (27%) were found to have a pCR.
DISCUSSION
The current standard of care for localized rectal cancer involves neoadjuvant 5-FU–based chemoradiation followed by surgical resection.2–4,13,14 Conventional chemoradiation regimens involving 5-FU–based external beam radiation therapy have been shown to improve local control; however, pCR rates are typically low.2 In addition, approximately 30% of patients will experience grade 2 or higher acute toxicity. In an attempt to decrease toxicity and improve pCR rates, recent strategies of local therapy have focused on the delivery of more localized high-dose RT to the rectal tumor and adjacent mesorectum.
HDR-EBT is an attractive local therapy technique because of its ability to deliver high-dose, highly conformal endoluminal RT in a short period of time (4 days). The higher total dose and rapid dose fall-off associated with HDR-EBT are suggested to be particularly effective in maximizing clinical outcome while minimizing treatment-related toxicity to surrounding organs at risk.2,3,13,14
Delivery of HDR-EBT to rectal tumors via the endorectal applicator requires a high degree of accuracy; therefore, EUS-guided placement of fiducials allows better visualization of the tumor with fluoroscopy and more accurate positioning of the endorectal applicator. This is significant because even a minor deviation from the treatment plan can cause considerable changes in the radiation dose distribution to the tumor and adjacent normal tissue.
Our study describes the placement of 2 types of fiducial markers (TFs and XMFs) and evaluates their visibility and migration and the subsequent effects on HDR-EBT delivery. To our knowledge, no other fiducial comparison studies have been performed in rectal cancer patients to date. Of note, we found that both types of fiducials created a void on MRI that could assist with treatment planning. Assessment of fiducials in other disease sites, especially the liver and prostate, has shown low rates of significant spontaneous migration.15 Based on our results, it appears that shorter fiducials, such as TFs, migrate significantly shorter distances compared with longer fiducials. However, medical literature on fiducials in pancreatic cancer has reported opposite results, with TFs having increased migration rates compared with non-TFs.11
Of note, these differences in the degree of migration may be attributed to the heterogeneity of tumor types and locations. Newer fiducial types (ie, Gold Anchor, Naslund Medical Inc, Huddinge, Sweden) expand after implantation and may therefore migrate less.8,9,12 However, neither TFs nor XMFs are capable of expanding after implantation in the tumor, which may contribute to increased migration seen in both fiducial types. Similar to studies on pancreas and liver fiducial placement,15 there were no adverse events or technical difficulties when placing EUS-guided fiducials in our cohort.
Because of relatively recent use of HDR-EBT in localized rectal cancer in the United States, our cohort of patients was very small and from a single institution. Furthermore, we did not adjust for other possible causes that might have led to fiducial migration, including decreased tumor size as a result of RT and measurement accuracy. Our low numbers preclude our ability to make any firm conclusions based on the data presented.
In conclusion, TFs and XMFs both had good visibility. Although TFs appeared to have increased stability with regard to migration rates, no firm conclusions can be made because of our small sample size. However, this information is important as our multicenter prospective study evaluating HDR-EBT is currently open (NCT02017704). In addition, our findings suggest that gold fiducials are compatible with MRI, and these markers may be used to target rectal tumors for additional treatments that require millimeter accuracy such as stereotactic body RT. We are actively evaluating other fiducials that may be better visualized on MRI.
Supplementary Material
Abbreviations
- 5-FU
5-fluorouracil
- HDR-EBT
high-dose rate endorectal brachytherapy
- IQR
interquartile range
- MRI
magnetic resonance imaging
- pCR
pathologic complete response
- RT
radiation therapy
- TF
traditional fiducial
- XMF
X-mark fiducial
Footnotes
DISCLOSURE: All authors disclosed no financial relationships relevant to this publication.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Smith JA, Wild AT, Singhi A, et al. Clinicopathologic comparison of high-dose-rate endorectal brachytherapy versus conventional chemoradiotherapy in the neoadjuvant setting for resectable stages II and III low rectal cancer. Int J Surg Oncol. 2012;2012:406568. doi: 10.1155/2012/406568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffe SE, Shridhar R, Biagioli MC. Radiation therapy for rectal cancer: current status and future directions. Cancer Control. 2010;17:25–34. doi: 10.1177/107327481001700104. [DOI] [PubMed] [Google Scholar]
- 4.Sauer R, Fietkau R, Wittekind C, et al. Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis. 2003;5:406–415. doi: 10.1046/j.1463-1318.2003.00509.x. [DOI] [PubMed] [Google Scholar]
- 5.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 6.Fietkau R, Barten M, Klautke G, et al. Postoperative chemotherapy may not be necessary for patients with ypN0-category after neoadjuvant chemoradiotherapy of rectal cancer. Dis Colon Rectum. 2006;49:1284–1292. doi: 10.1007/s10350-006-0570-x. [DOI] [PubMed] [Google Scholar]
- 7.Rodel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 8.Owens DJ, Savides TJ. EUS placement of metal fiducials by using a backloaded technique with bone wax seal. Gastrointest Endosc. 2009;69:972–973. doi: 10.1016/j.gie.2008.05.052. [DOI] [PubMed] [Google Scholar]
- 9.Sanders MK, Moser AJ, Khalid A, et al. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc. 2010;71:1178–1184. doi: 10.1016/j.gie.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Kothary N, Heit JJ, Louie JD, et al. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J Vasc Interv Radiol. 2009;20:235–239. doi: 10.1016/j.jvir.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Khashab MA, Kim KJ, Tryggestad EJ, et al. Comparative analysis of traditional and coiled fiducials implanted during EUS for pancreatic cancer patients receiving stereotactic body radiation therapy. Gastrointest Endosc. 2012;76:962–971. doi: 10.1016/j.gie.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiMaio CJ, Nagula S, Goodman KA, et al. EUS-guided fiducial placement for image-guided radiation therapy in GI malignancies by using a 22-gauge needle (with videos) Gastrointest Endosc. 2010;71:1204–1210. doi: 10.1016/j.gie.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Chuong MD, Fernandez DC, Shridhar R, et al. High-dose-rate endorectal brachytherapy for locally advanced rectal cancer in previously irradiated patients. Brachytherapy. 2013;12:457–462. doi: 10.1016/j.brachy.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Vuong T, Belliveau PJ, Michel RP, et al. Conformal preoperative endorectal brachytherapy treatment for locally advanced rectal cancer: early results of a phase I/II study. Dis Colon Rectum. 2002;45:1486–1493. doi: 10.1007/s10350-004-6455-y. discussion 1493-5. [DOI] [PubMed] [Google Scholar]
- 15.Choi JH, Seo DW, Park do H, et al. Fiducial placement for stereotactic body radiation therapy under only endoscopic ultrasonography guidance in pancreatic and hepatic malignancy: practical feasibility and safety. Gut Liver. 2014;8:88–93. doi: 10.5009/gnl.2014.8.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.