Fragile X–associated tremor/ataxia syndrome (FXTAS) is a neurodegenerative disorder that usually begins in the early 60s1,2 and affects carriers of premutation expansion (55–200 CGG repeats) of the fragile × mental retardation 1 (FMR1) gene. It is caused by elevated levels of FMR1 messenger RNA, leading to toxicity. Clinical features include progressive cerebellar ataxia and intention tremor. Associated symptoms include autonomic dysfunction, peripheral neuropathy, and cognitive impairment2.
Report of a Case
To our knowledge, we report the youngest deceased patient with FXTAS yet known. He was a man in his mid-30s with 88 CGG repeats and 3.8 times the normal messenger RNA level. He was diagnosed as having possible FXTAS stage 2 (mild tremor and/or ataxia) in his early 30s.
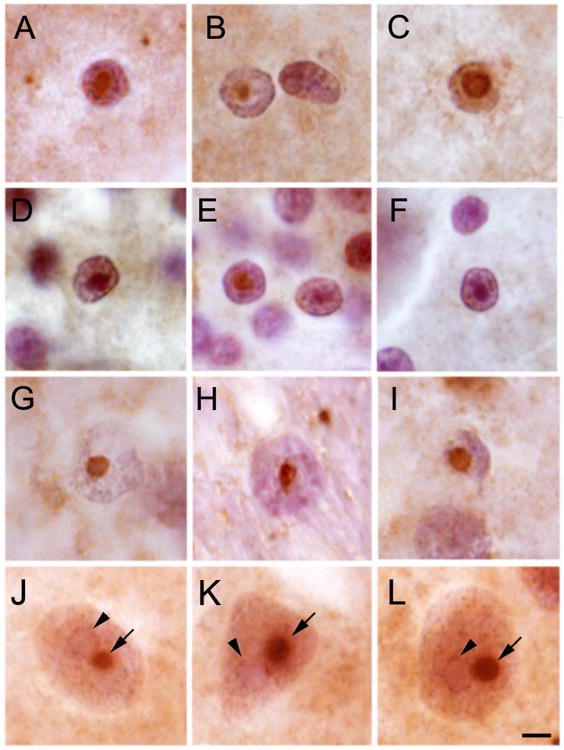
His medical history included Asperger syndrome, restless legs syndrome, irritable bowel syndrome, type 2 diabetes mellitus, obesity, depression/anxiety, migraines, hypertension, and hypothyroidism, all associated with the premutation2. He experienced handwriting problems, balance problems with frequent tripping and 2 falls in the previous year, and a slight postural tremor and an intention tremor in the head and right hand beginning in his late 20s. His deep tendon reflexes were 1+ in all extremities, but he jerked his whole body when each reflex was tested. His snout, jaw jerk, and palmomental and glabellar reflexes were positive, but his Babinski reflex was negative. His cognitive testing (Wechsler Adult Intelligence Scale III) included a score of 112 for verbal IQ, 98 for performance IQ, and 106 for full-scale IQ. However, his visual attention vigilance (Integrated-Visual and Auditory-Continuous Performance Test) score was 70 and his sustained visual attention score was 35. He used crack cocaine, methamphetamines, and alcohol (6 drinks per sitting once a week). He had 2 psychiatric hospitalizations, one for depression related to bipolar disorder in his late 20s and a second after a manic episode due to crack cocaine use, which led to 6 mild strokes in rapid succession in his late 20s. We propose that the early onset of FXTAS in this patient may have been related to substance abuse as has been previously reported.3-5 He died in an accident. The patient's mother, a woman in her early 60s with FXTAS, had an FMR1 CGG repeat of 70. The patient's magnetic resonance imaging demonstrated subtle white matter hyperintensities in the insula with increased perivascular spaces (Figure 1A). His brain presented with diffuse microvascular change (Figure 1B), perivascular clearing, hemosiderin accumulation, and subacute and ongoing hypoxic ischemic brain injury likely related to his substance abuse and strokes. There was a moderate loss of Purkinje cells, a mild-moderate loss of CA1 pyramidal cells, scattered microglia in the dentate gyrus (Figure 1C), focal loss of ependymal lining, and subependimal gliosis with diffuse white matter gliosis. Ubiquitin-positive nuclear inclusions were present within astrocytes in the cerebral cortex (Figure 2A-D), caudatum, cerebellum (Figure 2E and F), and hippocampus (Figure 2G-I). While inclusions in astrocytes were very large, in neurons, they were similar or smaller than the nucleolus. This suggests that the formation of inclusions in astrocytes may precede that in neurons. The biggest neuronal inclusions were present in the prefrontal cortex (Figure 2J-L). Inclusions were detected in 9.5% of cells in the prefrontal cortex (14.2% astrocytes; 4.8% neurons; 325 cells), 6.4% in CA1 (18% astrocytes; 9.6% neurons; 941 cells), 5.7% in caudatum (20% astrocytes; 6.4% neurons; 470 cells), and 5.3% in cerebellum (3.2% astrocytes; 6.0% neurons; 225 cells).
Figure 1.
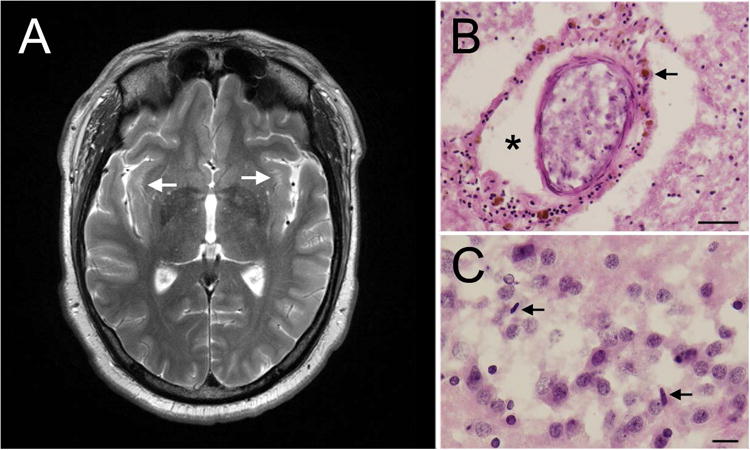
Imaging and pathology. A, Bilateral white matter hyperintensities in the insula. B, Intraparenchymal blood vessel with perivascular clearing (asterisk) and hemosiderin accumulates (arrowhead) (hematoxylin-eosin; scale bar= 100 μm). C, Scattered rod-shaped microglia seen in the dentate gyrus. Arrowheads point to microglial nuclei (hematoxylin-eosin; scale bar = 25 μm).
Figure 2.
Ubiquitin Inclusions. Ubiquitin inclusions (brown) in astrocytes within the prefrontal cortex (A-D), cerebellum (E-F), and hippocampus (G-I). J-L, Ubiquitin inclusions in neurons in the prefrontal cortex. The inclusion is labeled in brown (blue arrowheads) and the nucleolus in light purple (black arrowheads). Scale bar= 10 μm.
Discussion
Greco et al6 described the presence of inclusions in 11 cases of men with FXTAS aged 67 to 87 years. In our patient, the number of inclusions were in the low range when compared with those cases.
In summary, FXTAS is thought to be a disorder of aging in carriers of FMR1 premutation; however, this case documents that FXTAS can occur earlier in adult life, particularly if another disease process is occurring, such as substance abuse, that may exacerbate the pathological process of FXTAS5.
Acknowledgments
Dr. Hagerman has received funding from Novartis, Roche/Genentech, Alcobra, and Neuren for treatment trials in fragile X syndrome, autism, and Down syndrome. She has also consulted with Novartis and Roche/Genentech regarding treatment for fragile X syndrome.
Funding/Support: This work was supported by National Institutes for Health grants HD036071 (Dr. Hagerman), HD040661 (Dr. Hagerman), and MH094681 (Dr. Martínez-Cerdeño).
Role of the Funder/Sponsor: The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: No other disclosures were reported.
References
- 1.Leehey MA, Berry-Kravis E, Min SJ, et al. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord. 2007;22(2):203–206. doi: 10.1002/mds.21252. [DOI] [PubMed] [Google Scholar]
- 2.Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013;12(8):786–798. doi: 10.1016/S1474-4422(13)70125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorn MB, Mazzocco MM, Hagerman RJ. Behavioral and psychiatric disorders in adult male carriers of fragile X. J Am Acad Child Adolesc Psychiatry. 1994;33(2):256–264. doi: 10.1097/00004583-199402000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Kogan CS, Turk J, Hagerman RJ, Cornish KM. Impact of the fragile X mental retardation 1 (FMR1) gene premutation on neuropsychiatric functioning in adult males without fragile X-associated tremor/ataxia syndrome: a controlled study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):859–872. doi: 10.1002/ajmg.b.30685. [DOI] [PubMed] [Google Scholar]
- 5.Muzar Z, Lozano R, Schneider A, et al. Methadone use in a male with the FMR1 premutation and FXTAS. Am J Med Genet A. 2015;167(6):1354–1359. doi: 10.1002/ajmg.a.37030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greco CM, Berman RF, Martin RM, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129(pt 1):243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]