Abstract
The role of dopamine is extensively documented in weight regulation and food intake in both animal models and humans. Yet the role of dopamine has not been well studied in individual differences for food desirability. Genotype status of the dopamine-related catechol-O-methyltransferase (COMT) gene has been shown to influence dopamine levels, with greater COMT enzymatic activity in val/val individuals corresponding to greater degradation of dopamine. Decreased dopamine has been associated with poorer cognitive control and diminished goal-directed behavior in various behavioral paradigms. Additionally, dopaminergic-rich regions such as the frontal cortex and dorsal striatum have been shown to be important for supporting food-related decision-making. However, the role of dopamine, as assessed by COMT genotype status, in food desirability has not been fully explored. Therefore, we utilized an individual’s COMT genotype status (n=61) and investigated food desirability based on self-rated “healthy” and “unhealthy” food perceptions. Here we found val/val individuals (n=19) have greater desirability for self-rated “unhealthy” food items, but not self-rated “healthy” food items, as compared to val/met (n=24) and met/met (n=18) individuals (p<0.005). Utilizing an objective health measure for the food items, we also found val/val and val/met individuals have greater desirability for objectively defined “unhealthy” food items, as compared to met/met individuals (p<0.01). This work further substantiates a role of dopamine in food-related behaviors and more specifically in relationship to food desirability for “unhealthy” food items.
Keywords: Dopamine, COMT genotype, Feeding, Obesity
Introduction
Dopamine has been linked to general decision-making processes, with optimal dopamine signaling between the prefrontal cortex (PFC) and striatal regions supporting cognitive stability as well as flexibility during goal-directed behaviors [1–3]. Additionally, dopaminergic signaling is known to be involved in behavioral inhibition, with aberrations in inhibitory control corresponding to various impulsivity conditions [4].
The genotype status of the Val158Met polymorphism of the catechol-O-methyltransferase (COMT) gene has been shown to effect dopaminergic signaling [5], with the val/val variant associated with greater enzymatic activity in humans (i.e. higher degradation of dopamine) [6]). Behaviorally, genotype status of the val variant has been associated with decreased cognitive functions in humans [7–9], greater impulsive behavior [10], and higher novelty and reward seeking [11]. Although there are few studies examining the relationship between COMT and food-related behavior, val carriers have shown greater susceptibility for bulimia nervosa [12], prevalence for increased total body fat composition, larger waist circumference and are more susceptible to typically obesity-related type-2 diabetes [13]. In relation to food consumption, one report found val/val children had significantly greater intake of fatty foods (i.e. lipid dense foods) [14], while another study found that val/val in combination with the dopamine transporter (DAT1) 9+ carrier status corresponded with greater binge eating episodes [15]. However, to our knowledge, the relationship between COMT genotype status and food desirability in humans has yet to be explored.
The neurotransmitter dopamine has been widely studied in the context of reward-related behaviors, including consumption of palatable foods (for review see [16–18]), although not in the context of individual differences in food desirability. Additionally, decision-making regarding food preference has been shown to be supported by the ventromedial PFC (vmPFC) as well as dorsolateral PFC (DLPFC) [19]. Using a similar food desirability task, engagement of frontal cortex and dorsal striatal regions has also been shown with food desirability ratings, with sleep-deprived deactivation coinciding with increased desirability of highly palatable foods [20]. However, the role of dopamine-related genotypes on these neural and behavioral processes was not examined in these studies. Therefore, here we investigated the potential relationship between COMT genotype status – associated with dopamine levels - and individual differences in food desirability (i.e. deciding to want a food item more or less). Here we probed food desirability based on an individual’s perception of the “healthiness” of food items. We hypothesized that COMT genotype would correlate with food desirability based on health perception, with val/val individuals preferring the most “unhealthy” food items (typically indicative of highly palatable, rewarding foods), as compared to val/met and met/met individuals. We also investigated desirability based on an objective health rating (see Methods/Supplementary Table 1) and predicted that val/val individuals would have greater desirability for objectively rated “unhealthy” foods, as compared to val/met and met/met carriers.
Increased preference for high caloric yet nutritionally poor foods is one of the major contributors to weight gain and obesity. Therefore, if val/val COMT genotype status corresponded with greater food desirability, we also hypothesized that a significant relationship may exist between COMT and BMI, with val/val individuals having greater BMI, as a previous study has suggested [13].
Materials and Methods
Subjects
Seventy-three subjects were recruited and agreed to participate in the study. Subjects were screened by questionnaire for any history or current episode of drug abuse, eating disorders, major depression and anxiety disorders. Two subjects were excluded due to current self-reported depression/anxiety. Subjects were asked to eat a typical, but not too heavy meal an hour to two hours prior to the testing session. In order to encourage compliance with this request, testing sessions were scheduled after typical meal times (i.e. 9AM, 2PM and 7:30PM). Seven subjects were excluded due to large amounts of time passing from the time the last meal was eaten (as self-reported) until testing time (i.e. greater than 6 hours from the time the subject last ate, despite the request to eat approximately 1–2 hours before testing). Subjects were also asked if they were currently dieting or trying to lose weight. Two subjects were excluded because they reported currently trying to lose weight. One subject was excluded due to the DNA not able to be genotyped for the COMT single nucleotide polymorphism (SNP) (see Methods below). Sixty-one subjects were eligible for testing and had complete genotype analysis (34 female, age 18–33). Race/ethnicity was not recorded for all subjects. All gave written informed consent and were paid for participation according to institutional guidelines of the local ethics committee (University of California Berkeley Committee for the Protection of Human Participants and Lawrence Berkeley National Laboratory Institutional Review Board). Height was measured to the nearest quarter of an inch by the research team with a tape measure, and weight was measured on the same scale for every subject on the day of testing by the research team. Body mass index (BMI: ((weight in kilograms)/(height in meters)^2) was calculated for each individual. BMI ranged from 17.1–34.4, average 23.27 ± 3.19 (SD) with 3 obese, 9 overweight, 47 healthy-weight and 2 underweight subjects.
Socio-economic status was also assessed using the Barratt simplified measure of social status (BSMSS) [21].
Behavioral paradigm
Prior to testing, we measured hunger and fullness with a visual analog scale (VAS), as validated by previous studies [22]. Hunger and fullness VAS measures were also highly correlated in our sample (r=−0.54, p<0.001). Meal and serving sizes were verbally self-reported by subjects and recorded by the research team, and the calories of the meal eaten prior to testing were estimated by the resource www.caloriecount.com and calculated for each subject by our research team.
The task we used assessed desirability and has been shown to engage frontal-striatal and limbic regions, among others, as measured by fMRI [20]. Pictures of eighty food items ([23], also see Supplementary Table), were used in which subjects were asked to rate the items in separate blocks. Subjects were first asked to rate the degree to which they “desired” or “wanted” a serving size of each of the eighty food items presented (scale of 1 (strongly do not want) to 4 (strongly want)) with the question “On a scale from 1 to 4, rate how much you want each food item right now”. The food item would appear and the subject would have up to 4 seconds to respond. They rated all eighty food items before continuing to the subsequent “health” block (see below). For all subjects, responses were made for all 80 food items, except for 1 subject, for 1 food item (the first food presented, because the subject took longer than 4 seconds to respond). In an attempt to capture how much the subject actually wanted the food items presented, subjects were informed that they would receive a food item from the task at the end of testing based on their “desirability” ratings. The subjects also did not know that in the upcoming second block (described below) they would be asked to judge how healthy they found each of the same eighty food items.
In the second block, subjects rated how much they perceived the eighty food items as “healthy” or “unhealthy” (−3 for very “unhealthy” to 3 for very “healthy”). The same eighty food items that were presented in the desirability block were also presented in the health block in a randomized order for each block. The subjects were informed that their ratings of health would not affect the item they would receive at the end of the experiment based on their answers in the “desirability” block. We chose a 6-point rating scale for health values to allow for a wider range in measuring health perception, including a “neutral” rating corresponding to −1 and +1, whereas the 4-point scale of the desirability block would reflect only “wanted” or “unwanted” food items. In a third block, we also tested the taste perception of the food items – however, as the focus of this study was to assess desirability based on “health” perceptions, regardless of taste, the “taste” perception data was not used in this study. The total task lasted approximately 30 minutes.
The task was programmed in E-Prime Professional (Psychology Software Tool, Inc., Sharpsburg, PA, USA). Pictures consisted of a food item centered in the screen with a white foreground and surrounded by a black box (see Figure 1). The task was balanced with approximately equal numbers of food items based on letter grades (ranging from F-minus (very “unhealthy”) to A-plus (very “healthy”)), from the on-line resource www.caloriecount.com and nutritional information from the USDA food website http://ndb.nal.usda.gov/ndb/search/list. We also used these resources to obtain objective health scores to approximately balance the task with objectively-rated “healthy” (objective ratings of 1 to 3) and objectively-rated “unhealthy” (objective ratings of −1 to −3) foods. Food items were selected based on two previously published reports by our research group [20, 23]. Also see Supplementary Table for list of the 80 food items, average letter grade from website, nutritional information, averages and standard deviations of desired scores from subjects, as well as averages and standard deviations of self-rated health scores from subjects and derived objective health scores.
Figure 1.
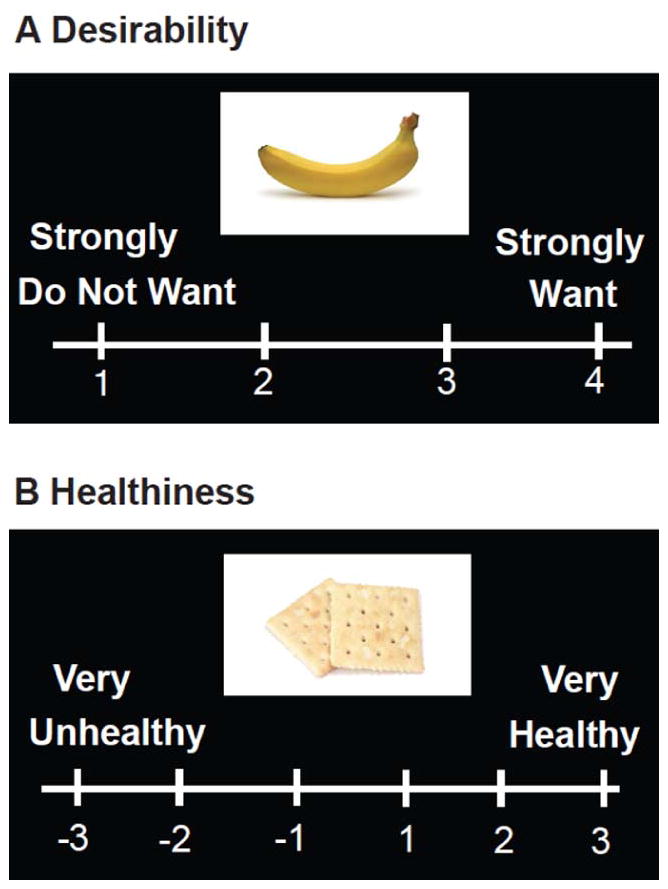
Behavioral Task. Subjects rated food items based on A) Desirability, rated on a scale of 1–4 and B) Self-rated “healthiness” of foods, rated on a scale of −3 (very “unhealthy”) to +3 (very “healthy”). Food items rated 3 or 4 in the desirability block were scored as “desired” while those rated 2 or 3 in the self-rated healthiness block were rated as self-rated “healthy” and −2 or −3 as self-rated “unhealthy”.
Data was extracted from E-Prime and MATLAB (2007a, The Mathworks, Natick, MA, USA) to obtain the number of food items that each individual 1) desired and self-rated “healthy” and 2) desired and self-rated unhealthy “ ” (Desired items rated as 3 or 4 in the desirability block; perceived “healthy” items rated as 2 or 3 and perceived “unhealthy” items rated as −2 or −3 in the “healthiness” block). Additionally, we utilized the objective health scores (see Methods above and Supplemental Table) to determine the number of 3) desired objectively-rated “unhealthy” and 4) desired and objectively-rated “healthy” food items. The ratio of number of items rated as “healthy” (score of 2 or 3) to number of items rates as “unhealthy” (score −2 or −3) was also computed for all subjects. The average want score and average health score for all 80 food items was also calculated for every subject. In addition, we compared subject’s self-rated health scores to the objective health scores for all 80 food items to gain a sense of how strongly health perception of foods related to an objective measure of health (i.e. higher correlation values would indicate closer health perception to an objective measure). The averages and standard deviations for want scores, health scores, and health perception scores were calculated for each genotype group.
Genotype Analysis
Using the Oragene DNA self-collection kit (DNA genotek Inc., Ottawa, Ontario, Canada), we collected saliva samples from each subject and the single nucleotide polymorphism (SNP) genotype for the COMT gene (rs 4680) [10] was determined by polymerase chain reaction (PCR) using primers designed specifically for the polymorphism (Creative Genomics, Port Jefferson Station, New York, USA and University of California Genetics Core Facility). This method of genotyping for the SNP genotype of the COMT gene from saliva is validated and supported by the University of Berkeley Genetics Core Facility. Of the 61 subjects eligible for the study with genotype information, 19 subjects (age 24±4.17, 12 female) were homozygous for the val/val SNP of the COMT gene, 24 subjects (age 22.6±3.55, 11 female) were heterozygous (val/met) and 18 subjects (age 22.6±3.5, 11 female) were homozygous met/met. This sample size is comparable or larger to that of previously published reports investigating the effects of COMT genotypes on behavior [8, 10, 15]. Our subject population also did not differ significantly from expected values as predicted by the Hardy-Weinberg equilibrium (χ2=1.188, p-value=0.552).
Statistical Analysis
Forward step-wise multiple regression analysis was used to investigate the relationship between the dependent variable (either the number of desired self-rated “unhealthy” food items or desired objectively-rated “unhealthy” food items) and the independent variables of COMT genotype status, BMI, age, sex, BSMSS, estimated number of calories eaten prior to testing, time elapsed since last meal and hunger and fullness VAS measures conducted in SPSS version 19 (IBM, Chicago, Ill., USA). Stepping-wise method criteria for inclusion in the model was set at a p<0.01 F-value (corrected for model selection, p<0.01, less than 0.05/sqrt(2*log(9))), and removal at p>0.05. R-values and adjusted R square values are reported, along with p-values. Follow-up one-way ANOVAs were conducted to test the relationships between COMT genotype status and food desirability, and COMT genotype and BMI in the program GraphPad Prism software (San Diego, CA, USA), with F-values and p-values reported. We also correlated food desirability with time the subject had last eaten, how long ago the subject ate, the time of day the testing session occurred, the estimated number of calories eaten at the last meal, and VAS hunger and fullness measures.
Pearson’s correlations were computed for every subject between self-rated health scores and objectively-rated health scores to give a sense of how closely an individual’s perception of health corresponded with an objective rating of health (i.e. a subject with a health perception score closest to 1 would have self-rated health scores highly comparable to objective health scores). A one-way ANOVA was conducted to test the relationship between COMT genotype status and health perception score (i.e. how strongly self-rated health scores correlated with objective health scores). Two-tailed unpaired (independent) t-tests were used for direct comparisons between genotype groups for desired self-rated “unhealthy” items, desired objectively-rated “unhealthy” items and overall average desirability score of all 80 food items.
Results
Homozygous val/val COMT genotype corresponds with greater desirability for self-defined “unhealthy” foods
We tested the hypothesis that a relationship exists between desirability for self-rated “unhealthy” food items and COMT genotype, as well as age, sex, BMI, BSMSS, time elapsed between last meal and testing session, estimated calories consumed before testing session and VAS measures of fullness or hunger. Using step-wise multiple regression analysis, we found that COMT genotype had a significant relationship with the number of desired self-rated “unhealthy” items (R=0.443, Adjusted R square = 0.183, COMT genotype included variable in the model, t=−4.127, p<0.005). No other factor (age, sex, BSMSS, time elapsed between last meal and testing session, estimated calories consumed before testing session or VAS measures of fullness or hunger) was entered into the model as significant (t<2.64, p>0.01).
A follow-up one-way ANOVA between COMT genotype and desired self-rated “unhealthy” items also indicated that val/val individuals desired the most self-rated “unhealthy” food items (F(2,58) = 7.149, p =0.0017, Figure 2). Follow-up unpaired t-tests between groups showed that val/val individuals significantly desired more self-rated “unhealthy” food items than both val/met and met/met groups (t=2.072, p=0.045; val/val: 14.63±6.45 vs. val/met: 9.96±7.97, and t=4.45, p<0.0001; val/val: 14.63±6.45 vs. met/met: 6.38±4.61). Heterozygous val/met individuals did not significantly desire more self-rated “unhealthy” foods than met/met individuals (t=1.695, p=0.098; val/met: 9.96±7.97 vs. met/met: 6.38±4.61).
Figure 2.
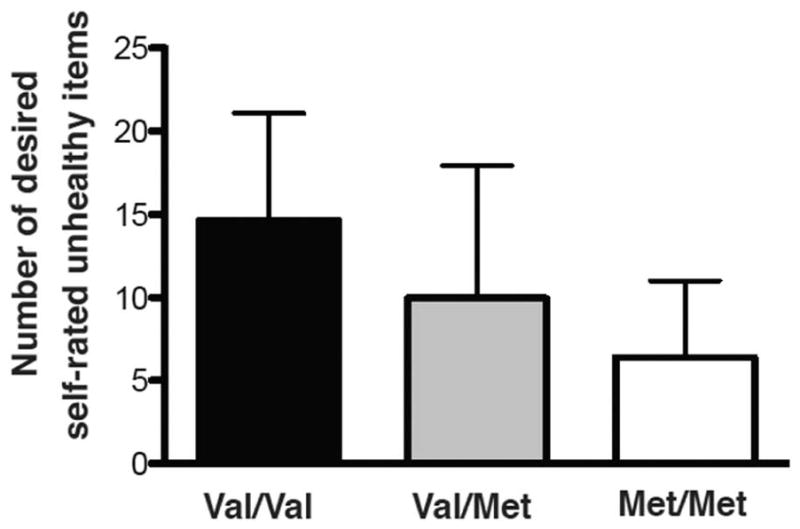
COMT genotype and self-rated unhealthy items. COMT genotype was associated with number of desired self-rated “unhealthy” food items (F(2,58) = 7.149, p=0.0017, n=61, average for each group shown + standard deviation).
Homozygous val/val COMT genotype corresponds with greater desirability for objectively-rated “unhealthy” foods
Using an objective measure of “unhealthiness” (see Methods and Supplemental Table), we investigated the relationship between desired objectively-rated “unhealthy” foods and COMT genotype status. Using step-wise multiple regression analysis, we found that only COMT genotype showed a significant relationship with the number of desired objectively-rated “unhealthy” items (R=0.473, Adjusted R square = 0.211, COMT genotype included variable in the model, t=−4.125, p<0.001). No other factor (age, sex, BMI, BSMSS, time elapsed between last meal and testing session, estimated calories consumed before testing session or VAS measures of fullness or hunger) was entered into the model as significant (t<1.82, p>0.01).
A one-way ANOVA confirmed the relationship between COMT genotype and desired objectively-rated “unhealthy” foods (F(2,58)=8.591, p<0.001, Figure 3). Follow-up two-way unpaired t-tests showed significant differences between val/val and met/met, with val/val individuals desiring the most objectively-rated “unhealthy” foods (t=4.66, p<0.0001, val/val: 16.36±6.64 vs. met/met: 7.94±3.93), and val/met and met/met (t=2.754, p<0.01, val/met: 13.31±7.2 vs. met/met: 7.94±3.93) with val/met desiring more objectively-rated “unhealthy” foods than met/met. No significant difference was found between val/val and val/met groups (t=1.52, p=0.14, val/val: 16.36±6.64 vs. val/met: 13.31±7.2).
Figure 3.
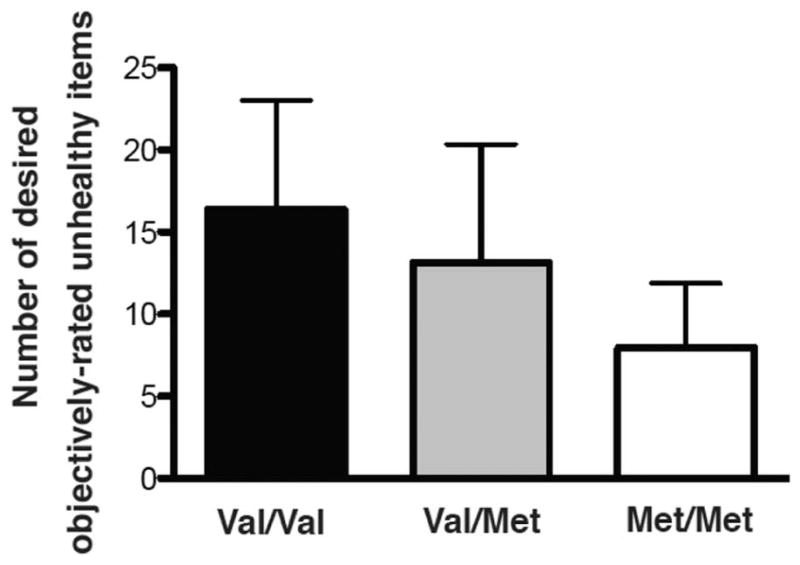
COMT genotype and objectively-rated unhealthy items. COMT genotype was associated with number of desired objectively-rated “unhealthy” food items (F(2,58) = 8.591, p<0.0017, n=61, average for each group shown + standard deviation).
Homozygous val/val COMT genotype corresponds with greater overall desirability of food
One-way ANOVA also showed an effect of genotype on overall desirability scores (i.e. desirability scores for all foods regardless of health ratings) between groups (F(2,58)=5.295, p=0.0077, Figure 4). Follow-up t-tests showed significant differences for overall average desirability scores between val/val and met/met (t=3.243, p<0.01, val/val: 2.61±0.44 vs. met/met: 2.18±0.42) and between val/met and met/met (t=1.981, p=0.0459, val/met: 2.43±0.36 vs. met/met: 2.18±0.42). No significant difference was found between val/val and val/met (t=1.4, p=0.14, val/val: 2.61±0.44 vs. val/met: 2.43±0.36).
Figure 4.
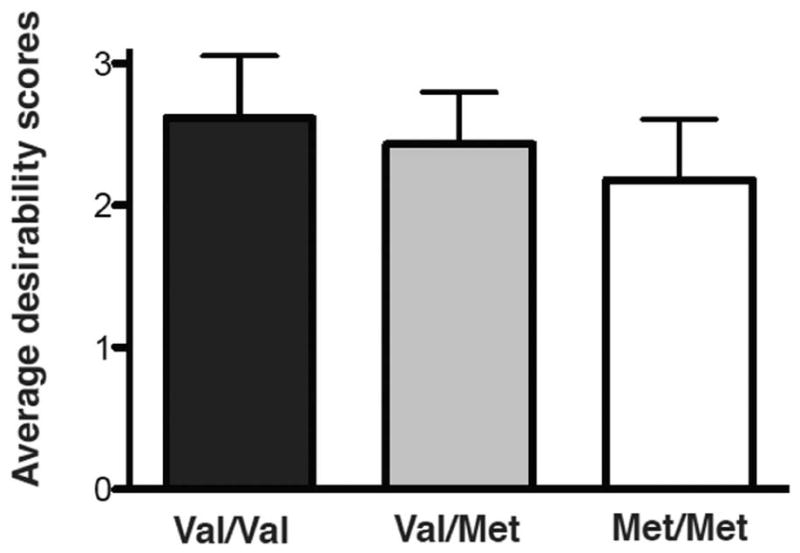
COMT genotype and overall desirability. COMT genotype was associated with overall desirability of food, regardless of health ratings (F(2,58) = 5.295, p=0.0077, n=61, average for each group shown + standard deviation).
COMT genotype does not correspond to differences in desirability for “healthy” foods
We investigated the potential relationship between desired self-rated “healthy” food items and COMT genotype status, age, sex, BMI, BSMSS, time elapsed between last meal and testing session, estimated calories consumed before testing session and VAS measures of fullness or hunger. However, no variable was entered into the model as significant.
We also tested the potential relationship between desired objectively-rated “healthy” food items and COMT genotype status, age, sex, BMI, BSMSS, time elapsed between last meal and testing session, estimated calories consumed before testing session and VAS measures of fullness or hunger. However, no variable was entered into the model as significant.
COMT genotype is not associated with differences in health perception
The ratio of perceived “healthy”-to-”unhealthy” items was not associated with COMT genotype (F(2,58)=0.5967, p=0.554), indicating COMT genotype was not associated with differences in the perception of healthiness or unhealthiness of the food items. Moreover, as we designed the experiment with an approximate equal number of objective “healthy” and “unhealthy” food items (see Methods), the average health rating of all foods by the subjects was −0.016 ± 1.83, as compared to the average objective health rating of −0.163 ± 2.346. Additionally, there was no effect of genotype on average health score ratings of all foods (F(2,58)=0.22, p=0.80, val/val:0.51±0.41; val/met:0.003±0.42; met/met:−0.04±0.45).
Furthermore, we calculated the correlation value between a subject’s self-rated health score and the objective health rating for all food items to gain a sense of how strong health perception of foods related to an objective measure of health (i.e. higher correlation values would indicate closer health perception to an objective measure). For all subjects, the correlation between the average self-rated health scores for all 80 foods and the objective health scores for all 80 foods was highly significant (r=0.851, p<0.0001, see Methods and Supplementary Table for additional information). However, there was no relationship between genotypes and health perception scores (F(2, 58)=0.24, p=0.786, met/met:0.721±0.084; val/met:0.732±0.041; val/val: 0.733±0.053).
COMT genotype and BMI are not significantly related
One-way ANOVA found no significant relationship between BMI and COMT genotype (F(2,58) = 0.478, p =0.62, met/met: 22.75±3.23; val/met: 23.725±3.19; val/val: 23.2±3.25).
Potential confounds do not influence food desirability
No significant correlations were found for the time the subject had last eaten (desired self-rated “unhealthy” foods: r=−0.16, p=0.218; desired objective-rated “unhealthy” foods: r: −0.12, p=0.357), how long ago the subject ate (desired self-rated “unhealthy” foods: r=0.03, p=0.819; desired objective-rated “unhealthy” foods: r: −0.109, p=0.404), the time of day the testing session occurred (desired self-rated “unhealthy” foods: r=−0.22, p=0.088; desired objective-rated “unhealthy” foods: r: −0.18, p=0.165) or the number of calories eaten at the last meal with either desired self-rated “unhealthy” or desired objectively-rated “unhealthy” foods (desired self-rated “unhealthy” foods: r=0.026, p=0.839; desired objective-rated “unhealthy” foods: r: −0.009, p=0.942). We also found no relationship between VAS hunger and fullness measures with either desired self-rated “unhealthy” or desired objectively-rated “unhealthy” foods (hunger with desired self-rated “unhealthy” foods: r=−0.008, p=0.995l, desired objectively-rated “unhealthy” foods: r=0.107, p=0.464; fullness with desired self-rated “unhealthy” foods: r=0.027, p = 0.856; fullness with desired objectively-rated “unhealthy” foods: r=−0.077, p=0.599). Subjects’ VAS measures of hunger and fullness were significantly correlated with each other (r=−0.545, p<0.001). There was no effect of genotype on estimated number of calories consumed prior to testing (F(2,58)=0.3165, p=0.73, met/met:362±177; val/met:416±238; val/val: 395±215).
Discussion
We tested the hypothesis that a significant relationship exists between COMT genotype status and food desirability (i.e., deciding to want a food item more or less, knowing that one decision was randomly selected and implemented at the end of the study). We found a significant relationship between COMT genotype status and desirability for 1) self-rated “unhealthy” foods (Figure 2) and 2) objectively-rated “unhealthy” foods (Figure 3), with val/val individuals desiring the greatest number of “unhealthy” food items as compared to met/met individuals. COMT genotype status was also associated with enhanced overall desirability for food items (Figure 4). This effect was driven by the above-mentioned effects on desirability of “unhealthy” foods, as there was no significant relationship between desirability of “healthy” foods (self or objectively-rated) and COMT genotype status. Furthermore, we found no relationship between BMI and COMT genotype status.
Given that COMT enzymatic activity is responsible for approximately 60% of dopaminergic turnover in the PFC, as compared to 15% in the striatum [24], and inhibition of the COMT enzyme has been shown to specifically increase PFC dopamine release [25], it has been hypothesized that the val/val COMT genotype corresponds to lower endogenous dopaminergic concentration particularly in the PFC [26]. We therefore hypothesize val/val individuals may have weaker sustained PFC dopaminergic neural activity when desiring “unhealthy” foods. The DLPFC (IFG/BA9) has been shown to have greater activity on ‘self-controlled’ food preference trials in which “liked-but-unhealthy” food items were not desired [19]). Our results suggest that lower levels of post-synaptic dopamine in the DLPFC (i.e. presumably greater COMT activity in val/val individuals) may underlie these behavioral as well as neural findings. Additionally, val/val in comparison to val/met individuals showed decreased DLPFC activity in response to anticipation of food stimuli (although desirability of food was not investigated in this study [27]). Sleep deprivation has also been shown to increase food desirability for highly palatable foods, coinciding with decreased activation of frontal regions, including the middle frontal gyrus [20]. We postulate that decreased activation in the DLPFC may also occur in val/val individuals when desiring “unhealthy” food items, reflecting decreased inhibitory control in food desirability. Future fMRI studies investigating the influence of COMT genotype on food desirability would further substantiate this hypothesis, as well as elucidate additional neural structures interacting with the DLPFC that may contribute to these behavioral differences.
In comparison to PFC dopamine, val/val individuals are also hypothesized to have lower tonic striatal dopamine, corresponding with greater phasic bursting in response to rewarding stimuli [26]. For example, greater striatal dopamine release has been shown in response to cigarette smoking in individuals with the val/val COMT genotype [28]. We hypothesize that individuals with the val/val COMT genotype may also have greater striatal dopamine release in response to palatable “unhealthy” foods, corresponding with greater sensitivity to the rewarding properties of these food items. However, one study has shown that val/val COMT individuals actually have decreased activation in the striatum in response to reward anticipation, as well as decreased OFC activation in response to reward receipt, and it is therefore hypothesized that reward saliency is actually lower in individuals with the val/val COMT genotype [29]. Furthermore, sleep deprivation was also shown to decrease OFC and dorsal striatum fMRI activation, corresponding with increased food desirability [20]. Associations between COMT Val158Met genotype and measures of reward experience have been found in daily life events, with the val/val COMT genotype status associated with diminished reward experience [30]. Therefore, it may also be possible that the val/val COMT genotype corresponds with less reward derived from consuming palatable “unhealthy” foods and therefore a predisposition towards overconsumption of these foods as a compensation mechanism to experience the same amount of pleasure.
In comparison to food desirability, COMT genotype has been shown to influence an individual’s choice between an immediate, smaller monetary reward versus a delayed, larger reward. In this paradigm, individuals with the val/val COMT genotype preferred immediate, smaller rewards, with greater activation in the OFC corresponding to longer delayed, yet larger monetary gains [10]. Moreover, the COMT enzyme inhibitor tolcapone, which has been shown to selectively increase dopamine in the PFC [25], decreased “now” versus “later” choices, with the drug most effective in highly impulsive individuals [31]. We hypothesize that food desirability may in some ways be analogous to delayed discounting, in that choosing an “unhealthy” food item corresponds with choosing an immediate reward over longer-term health benefits. Although we did not investigate impulsivity measures, impulsiveness has been shown to correlate with food overconsumption and food-“addictive-like” characteristics [32]. Findings implicating COMT genotype and impulsivity, along with our findings for greater desirability for “unhealthy” foods, may suggest a potential therapeutic use for tolcapone in individuals with overconsumption eating disorders, particularly in individuals with the val/val COMT genotype. COMT inhibition with tolcapone treatment has been shown to modulate dopamine in expectation as well as consumption of food in animal models [33]. Although tolcapone as a treatment option for compulsive over-eating warrants further investigation, previous studies have shown therapeutic effects of tolcapone with extreme shop-lifting [34], as well as pathological gambling, in which individuals with val/val COMT genotype status benefited the most, corresponding with drug-induced neural changes of the fronto-parietal network [35].
Notably, there were several limitations in our study. Although our sample population included overweight and obese individuals, the average body mass index (BMI = 23.27 kg/m^2) was indicative of a healthy-weight population. This limitation perhaps contributed to a lack of significance between COMT genotype and BMI. The sample size we used (n=61) was comparable to, if not larger than, other studies investigating the relationships between COMT and behaviors. However, our study nonetheless would benefit from replication with a larger sample size with more variability in BMI. Additionally, BMI is a relatively crude measurement and does not account for differences in body mass composition. As a previous study found that val/val individuals have greater fat composition [13], additional anthropometric measurements such as lean versus fat mass and hip-to-waist ratio measurements would add potential insight to future studies.
To limit potential confounding factors of hunger, we also tested the subjects after their mealtimes and asked them to eat a typical meal prior to testing. While we found that hunger and fullness measures did not correlate with desirability, the relationship between COMT genotype status and food desirability may be exacerbated in subjects who are hungry. Although we used additional factors in our multiple regression analysis such as age, sex, socioeconomic status, estimated number of calories eaten prior to testing and time elapsed since last meal, admittedly, there are many other factors that may have contributed to our effect that we may not have been able to assess. Additional confounds due to unknown environmental factors as well as false-positive associations due to a biased selection may have taken place. Ideally, replication of these results with a larger sample size and greater variability in age and BMI would further confirm our findings. In addition, future studies could use other decision-making paradigms, involving direct choices between more and less healthy food items, to substantiate the current results.
Additionally, here we only investigated the influence of COMT genotype status to assess the potential role of dopamine in food desirability. However, the relationship between other dopaminergic-related genes such as the D2-receptor associated Taq1A polymorphism (rs1800497) and food stimuli is well-studied, with individual differences in striatal fMRI activation to visual food stimuli as well as food intake dependent on A1+ gene status (for review see [17]). Moreover, multi-allelic composition of dopamine-related genes has been shown to predict neural activations in an additive manner as compared to a single gene status [27, 29]. Thus, it would be of interest to investigate differences in genotype status not only of the COMT gene, but also of dopaminergic genes typically related to striatal dopamine (i.e. the TaqIA A1+ status or differences in variable number of tandem repeats (VNTR) in the dopamine transporter (DAT1) gene [36]). Future studies employing positron emission tomography (PET) with ligands that are suitable to measure PFC dopamine D1 receptor binding (i.e. [11C]NNC112 or [11C]SCH23390) could also provide more direct measures of PFC dopamine processing in relation to food choice.
One important feature of our task was that subjects determined the health properties of the food items independently of rating their desirability for the food items. Moreover, they rated desirability for the foods first, without knowledge that they would also later be asked to rate their opinions of “healthiness” of the foods. Future studies may benefit from counterbalancing the health and desirability block order, to ensure items rated as desired are not over-rated as “healthy”. However, in our current study, utilizing an objective-rating of healthiness confirmed our result that COMT genotype status and desirability of “unhealthy” foods were significantly related.
Although there were no differences between genotype groups for “healthy”-to-”unhealthy” food ratios or health perception scores, desirability for self-rated “unhealthy” items was significantly different between genotype groups. This highlights the finding that in spite of the knowledge or perception that foods were “unhealthy”, individuals with the val/val COMT genotype still “wanted” these foods more than the other two groups, perhaps suggesting that desirability for “unhealthy” foods outweighs potential negative health consequences such as weight gain or increased risk for future diseases such as type-2 diabetes or heart disease. It would be interesting to investigate how directing attention to the health status of food items would influence desirability, as it has been previously shown that attention to health attributes of food can modulate behavior as well as PFC activity [37]. This might be a particular effective behavioral intervention strategy for individuals depending on COMT genotype, in which those with val/val genotype status may show greater benefit in mitigating desirability for “unhealthy” foods.
Supplementary Material
Highlights.
Val/Val COMT genotype status is associated with greater overall food desirability.
Val/Val COMT genotype status is associated with “unhealthy” food desirability.
No relationship was found between COMT genotype status and body mass index.
Acknowledgments
We would like to acknowledge NIH (DA20600 and F32DA276840) and Tanita Healthy Weight Community Trust for generous funding of this work.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69(12):e113–25. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grace AA, et al. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–7. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Mehta MA, Riedel WJ. Dopaminergic enhancement of cognitive function. Curr Pharm Des. 2006;12(20):2487–500. doi: 10.2174/138161206777698891. [DOI] [PubMed] [Google Scholar]
- 4.Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Gogos JA, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95(17):9991–6. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lachman HM, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Egan MF, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs E, D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. J Neurosci. 2011;31(14):5286–93. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60(2):141–51. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Boettiger CA, et al. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27(52):14383–91. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai SJ, et al. Association study of catechol-O-methyltransferase gene and dopamine D4 receptor gene polymorphisms and personality traits in healthy young chinese females. Neuropsychobiology. 2004;50(2):153–6. doi: 10.1159/000079107. [DOI] [PubMed] [Google Scholar]
- 12.Mikolajczyk E, Grzywacz A, Samochowiec J. The association of catechol-O-methyltransferase genotype with the phenotype of women with eating disorders. Brain Res. 2010;1307:142–8. doi: 10.1016/j.brainres.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Kring SI, et al. Polymorphisms of serotonin receptor 2A and 2C genes and COMT in relation to obesity and type 2 diabetes. PLoS One. 2009;4(8):e6696. doi: 10.1371/journal.pone.0006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galvao AC, et al. Association of MAOA and COMT gene polymorphisms with palatable food intake in children. J Nutr Biochem. 2012;23(3):272–7. doi: 10.1016/j.jnutbio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Hersrud SL, Stoltenberg SF. Epistatic interaction between COMT and DAT1 genes on eating behavior: a pilot study. Eat Behav. 2009;10(2):131–3. doi: 10.1016/j.eatbeh.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97(5):537–50. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burger KS, Stice E. Variability in reward responsivity and obesity: evidence from brain imaging studies. Curr Drug Abuse Rev. 2011;4(3):182–9. doi: 10.2174/1874473711104030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15(1):37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 20.Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun. 2013;4:2259. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barratt W. The Barratt Simplified Measure of Social Status (BSMSS) 2012 Available from: http://socialclassoncampus.blogspot.com/2012/06/barratt-simplified-measure-of-social.html.
- 22.Parker BA, et al. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur J Clin Nutr. 2004;58(2):212–8. doi: 10.1038/sj.ejcn.1601768. [DOI] [PubMed] [Google Scholar]
- 23.Wallace DL, et al. Dorsal striatal dopamine, food preference and health perception in humans. PLoS One. 2014;9(5):e96319. doi: 10.1371/journal.pone.0096319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51(4):593–628. [PubMed] [Google Scholar]
- 25.Tunbridge EM, et al. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24(23):5331–5. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilder RM, et al. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–61. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- 27.Stice E, et al. Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. J Neurosci. 2012;32(29):10093–100. doi: 10.1523/JNEUROSCI.1506-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brody AL, et al. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry. 2006;63(7):808–16. doi: 10.1001/archpsyc.63.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dreher JC, et al. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci U S A. 2009;106(2):617–22. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wichers M, et al. The catechol-O-methyl transferase Val158Met polymorphism and experience of reward in the flow of daily life. Neuropsychopharmacology. 2008;33(13):3030–6. doi: 10.1038/sj.npp.1301520. [DOI] [PubMed] [Google Scholar]
- 31.Kayser AS, et al. Dopamine, corticostriatal connectivity, and intertemporal choice. J Neurosci. 2012;32(27):9402–9. doi: 10.1523/JNEUROSCI.1180-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy CM, Stojek MK, MacKillop J. Interrelationships among impulsive personality traits, food addiction, and Body Mass Index. Appetite. 2014;73:45–50. doi: 10.1016/j.appet.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lapish CC, et al. Tolcapone enhances food-evoked dopamine efflux and executive memory processes mediated by the rat prefrontal cortex. Psychopharmacology (Berl) 2009;202(1–3):521–30. doi: 10.1007/s00213-008-1342-1. [DOI] [PubMed] [Google Scholar]
- 34.Grant JE. Kleptomania treated with tolcapone, a catechol-O-methyl-transferase (COMT) inhibitor. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):295–6. doi: 10.1016/j.pnpbp.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Grant JE, et al. A proof of concept study of tolcapone for pathological gambling: relationships with COMT genotype and brain activation. Eur Neuropsychopharmacol. 2013;23(11):1587–96. doi: 10.1016/j.euroneuro.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Aarts E, et al. Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neuropsychopharmacology. 2010;35(9):1943–51. doi: 10.1038/npp.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J Neurosci. 2011;31(30):11077–87. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


