Abstract
Aim:
Neonatal sepsis is an important cause of mortality and morbidity in newborns. The causative agents may be different in different units and may change in time. It was aimed to examine the microbiological agents leading to sepsis, clinical features and antibiotic resistances in babies with sepsis hospitalized in our unit in a two-year period.
Material and Methods:
The clinical features, microbiological and laboratory results, antibiotic resistance patterns and mortality rates of the newborns with sepsis followed up in our unit between 2010 and 2011 were examined in the patient record system.
Results:
351 babies diagnosed with sepsis among 3219 patients hospitalized in the neonatal intensive care unit were included in the study. The mean gestational age was found to be 30.1±4.1 weeks, the mean birth weight was found to be 1417.4±759.1 g and the mean hospitalization time was found to be 43.6±34.4 days. Blood cultures were found to be positive in 167 (47.6%) patients, urine cultures were found to be positive in 6 (7.1%) patients and cerebrospinal fluid cultures were found to be positive in 34 (9.6%) cases. Candida grew in 5 patients (2 patients with early-onset sepsis and 3 patients with late-onset sepsis). The most common cause of sepsis was found to be staphylococci (coagulase negative staphylococcus was found in 65 patients (51%) and Staphylococcus aureus was found in 38 patients (39%). 49.6% (n=63) of the gram positive bacteriae and 60% (n=21) of the gram negative bacteriae were resistant to antibiotics. Six (7.1%) of the patients who were infected with these bacteriae were lost. In total 24 babies were lost because of sepsis. The bacteriae which caused to mortality with the highest rate included E. coli, coagulase negative staphylocicci, S. aureus and Klebsiella. Low birth weight, mechanical ventilation and parenteral nutrition were found to be significant risk factors in terms of mortality.
Conclusions:
Staphylococci were found to be the most common agents in neonatal sepsis. Low birth weight, mechanical ventilation and parenteral nutrition are significant risk factors in terms of mortality.
Keywords: Infection, mortality, spesis, newborn
Introduction
Neonatal sepsis is an important cause of mortality and morbidity both in developed and developing countries (1). The gold standard in the diagnosis of sepsis is isolation of the pathogen in one or more blood cultures. However, it is not easy to grow the pathogenic microorganism in culture in all cases because of many reasons. Therefore, assistive diagnostic methods based on clinical and laboratory findings have been recommended in addition to blood culture for the diagnosis of neonatal sepsis (2, 3). The frequency of neonatal sepsis varies in different studies depending of the diagnostic methods used. Risk factors and the type of the microorganism causing sepsis and its resistance against antimicrobial agents are the most important factors which affect the prognosis. These factors may be different in each unit or may show variance in the same unit in time.
Accurate demonstration, monitoring and evaluation of these factors is important in terms of improving the prognosis and preventing mortality and sequelas.
In this study, we aimed to examine the babies who were born in our hospital between the years 2010 and 2011 and diagnosed with sepsis in our neonatal intensive care unit in terms of frequency, etiological agents, agent microorganisms and antibiotic resistance and to determine the effect of these factors on prognosis.
Material and Methods
The study was conducted with patients who were followed up in our neonatal intensive care unit and diagnosed with sepsis according to the clinical and laboratory findings. Blood and urine cultures were obtained from all patients and cerebrospinal fluid (CSF) samples were obtained if there was no contraindication. C-reactive protein (CRP) and procalcitonin were studied. Chest x-ray was performed in patients who had respiratory system symptoms. Presence of rupture of membranes 18 hours before delivery was considered premature rupture of membranes (PROM) (4). A platelet count below 150 000/mm3 was considered thrombocytopenia (5). In patients in whom antibotic treatment was initiated, treatment was continued until the culture results were obtained. Among the microorganisms grown in culture, the gram positive ones with meticillin resistance and the gram negative ones which were sensitive to only meropenem, sefaperazon-sulbactam and ciprofloxacin were considered to have multiple antibiotic resistance. The patients who died in seven days after the diagnosis of sepsis and had no other cause were considered as being died because of sepsis. The patients who were found to have any condition (renal failure, pneumothorax etc.) as the cause of death other than sepsis were classified in the group “mortality due to other causes”. Approval was obtained from the ethics committee of our hospital for the study (10.09.2012-17645).
Statistical analysis
The descriptive analyses were expressed as mean and standard deviation for variables with a normal distribution. The numerical data of more than two groups with a non-homogeneous distribution were compared using Kruskal Wallis test. In paired comparison of the variables for which a difference was found, Mann Whitney U test was used and the significance was evaluated using Bonferroni correction. Crosstabs were prepared for the categorical variables and chi-square test was used in comparison. A p value of <0.05 was considered significant.
Results
Among 3 291 babies internalized in our unit between January 2010 and December 2011, a total of 351 patients (169 (48.1%) females and 182 (51.9%) males) diagnosed with sepsis were included in the study (Figure 1). The mean gestational age of the patients was found to be 30.1±4.1 weeks, the mean birth weight was found to be 1417.4±759.1 g and the mean hospitalization time was found to be 43.6±34.4 days. The gestational weeks, birth weights, status of parenteral nutrition and supportive ventilation treatment, procalcitonin levels, hemograms and total hospitalization times of the patients are shown in Table 1. Congenital anomaly was not found in any of the patients. PROM was found in 61 of the patients (17.3%), central catheter was found in 164 (46.7%), parenteral nutrition was found in 318 (90.6%), mechanical ventilation was found in 250 (71.2%), CPAP was found in 189 (53.8%), meningitis was found in 64 (18.2%) and ventilator-related pneumonia (VRP) was found in 42 (12%). A diagnosis of early sepsis was made in 142 patients (40.5%) and a diagnosis of late sepsis was made in 209 patients (59.5%). 7 (22.2%) babies were lost. 24 of these babies (30.8%) were lost because of sepsis and 54 (69.2%) were lost because of causes other than sepsis. The features and post-hoc analyses of the babies who died because of sepsis (n=24), who died because causes other than sepsis (n=54) and who were discharged (n=273) are given in Table 1.
Figure 1.
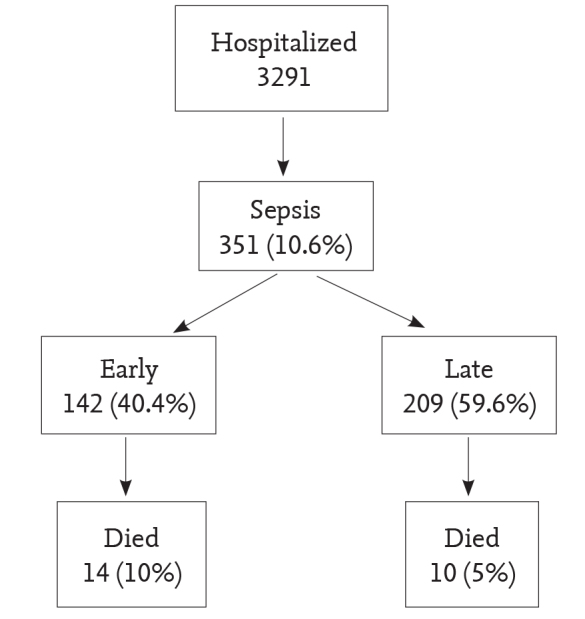
Frequency of sepsis and mortality rate. The mortality rate was higher in early sepsis compared to late sepsis. However, the difference was not statistically significant (p=0.064)
Table 1.
The features of the patients by discharge status and post-hoc analyses
| Non-sepsis related mortality n=54 | Sepsis-related mortality n=24 | Discharged with recovery n=273 | p1 | p2 | p3 | p | |
|---|---|---|---|---|---|---|---|
| Gestational week | 26.5 (24.1–31) | 28.3 (26.3–32.2) | 30 (27.6–33.2) | 0.19 | <.001* | 0.19 | <.001 |
| Birth weight (g) | 810 (693–1420) | 1045 (647–1680) | 1 290 (975–1760) | 0.56 | <.001* | 0.03 | <.001 |
| TPN duration (days) | 17.5 (10.5–30) | 5.5 (4–7) | 12 (7–18) | <.001* | <.001* | <.001* | <.001 |
| MV days | 18 (9–33) | 5 (4–7) | 6 (3–12) | <.001* | <.001* | 0.34 | <.001 |
| CPAP (days) | 5 (3–7) | 3 (0–4) | 5 (3–8) | 0.12 | 0.84 | 0.09 | 0.24 |
| CRP (mg/dL) | 1.5 (0.3–3.6) | 1.1 (0.4–3) | 0.7 (0.3–3.2) | 0.74 | 0.26 | 0.45 | 0.44 |
| Procalcitonin (ng/dL) | 3.2 (0.7–20.3) | 5.7 (1.7–30.8) | 1.9 (0.7–11) | 0.38 | 0.1 | 0.023 | 0.03 |
| White blood cell count (mm3) | 12 850 (7 100–21 400) | 5 700 (3 900–10 400) | 9 300 (5 500–15 100) | 0.004* | 0.04 | 0.017 | 0.01 |
| Platelets (mm3) | 126 000 (70 000–193 000) | 120 000 (43 000–149 500) | 156 000 (98 000–228 000) | 0.16 | 0.067 | 0.002* | 0.003 |
| Absolute neutrophil count (mm3) | 3 300 (1 600–6 000) | 800 (500–2500) | 2 000 (900–4750) | 0.002* | 0.038 | 0.01* | 0.003 |
| Duration of hospitalization (days) | 27 (15–52) | 5 (4.5–7) | 41 (24–63) | <.001* | 0.002* | <.001* | <.001 |
p: Comparison of the three groups (Kruskal Wallis)
p1: Comparison of the non-sepsis related mortality group with the sepsis-related mortality group
p2: Comparison of the non-sepsis related mortality group with the group discharged
p3: Comparison of the sepsis-related mortality group with the group discharged
p1, p2 and p3: Mann Whitney U test, Bonferroni correction were used, p values maked “*” are significant (p<0.0166)
CRP: C-reactive protein; CPAP: Continuous Positive Airway Pressure; MV: mechanical ventilation; TPN: total parenteral nutrition
Growth was positive in blood in 167 patients (47.6%), in urine in 6 patients (6.5%) and in CSF in 34 patients. The microorganisms grown are shown in Table 2. Fourty nine point six percent (n=63) of the gram positive bacteriae and 60% (n=21) of the gram negative bacteriae were resistant to antibiotics. Six percent (7.1%) of the patients infected with these bacteriae were lost. Candida was grown in blood in five patients (two early and three late sepsis). The microorganisms grown in cases of early and late sepsis are shown in Table 3. The mortality rates by microorganims are shown in Table 4.
Table 2.
Distribution of the mocroorganisms grown in sepsis (n=163)
| Gram positive | Gram negative | ||
|---|---|---|---|
| n | n (%) | ||
| CNS | 65 (51) | Klebsiella | 13 (37.1) |
| 25 early, 40 late sepsis | 4 early, 9 late sepsis | ||
| S. aureus | 38 (29.5) | E. coli | 12 (34.2) |
| 18 early, 20 late sepsis | 4 early, 8 late sepsis | ||
| Group | 21 (16.4) | Enterobacter | 8 (22.8) |
| B Strep | 9 early, 12 late sepsis | 2 early, 6 late sepsis | |
| Group | 4 (3.1) | Serratia | 1 (2.8) |
| D Strep | 2 early, 2 late sepsis | Late sepsis | |
| Acinetobacter | 1 (2.8) | ||
| Late sepsis | |||
| Total | 128 (100) | Total | 35 (100) |
CNS: Coagulase negative staphylococcus
Table 3.
Comparison of the late preterm and term babies in terms of outcome relation
| Growht status | Sepsis type | Total | |
|---|---|---|---|
|
| |||
| Early | Late | ||
| No growth | 76 (53.5%) | 107 (51.2%) | 183 (52.1%) |
| Gram (+) | 54 (38%) | 74 (35.4%) | 128 (36.5%) |
| Gram (−) | 10 (7%) | 25 (12%) | 35 (10%) |
| Candida | 2 (1.4%) | 3 (1.4%) | 5 (1.4%) |
| Total | 142 (100%) | 209 (100%) | 351 (100%) |
Table 4.
Mortality rates by the microorganism growth
| Died n (%) | |
|---|---|
| E. coli | 5 (41.7) |
| Coagulase negative staphylociccus | 16 (24.6) |
| S. aureus | 9 (23.7) |
| Klebsiella | 3 (23.1) |
| Group B streptococcus | 1 (4.8) |
| Gram + bacteriae | 26 (20.3) |
| Gram − bacteriae | 12 (34.3) |
| Candida | 1 (20) |
Discussion
In studies conducted in Western countries, the most commonly isolated agent in early-onset sepsis is group B streptococci (GBS). This is followed by gram (−) bacilli and staphylococci (6). In contrast, the most commonly isolated agents in early-onset sepsis in our country include gram (−) bacilli and staphylococci (7). In patients with a diagnosis of late-onset sepsis, the most commonly isolated agents include staphylococci (primarily coagulase negative staphylococci (CNS), secondarily S.aureus). This is followed by gram (−) bacilli. The least frequently isolated agents include GBSs (8). In the study of Gürsu (7), gram (−) bacilli, staphylococci and candida were isolated with the rates of 52.9%, 41.1% 5.9% respectively in the cases with early onset sepsis. Staphylococci were isolated with a rate of 75% and gram (−) bacilli were isolated with a rate of 16.6% in cases of late onset sepsis. In the study of Topuz (9), staphylococci, gram (−) bacilli, GBS and streptococcus spp. were isolated in order of frequency in cases of early onset sepsis and staphylococci and gram (−) bacilli were isolated in order of frequency in cases of late early onset sepsis. Candida albicans was isolated only in one patient with late onset sepsis. In the study of Payaslı (10), the most commonly isolated microorganism in blood culture was CNS (17.5%). This was followed by Klebsiella oxitoca with a rate of 10% and Klebsiella penumoniae with a rate of 5% and GBS were grown only in one patient (2.5%). GBS which are the most common agents in early sepsis in developed countries, were found to be in the third rank in our study similar to the studies conducted in our country, whereas staphylococci were in the first rank in our unit in both early and late sepsis.
In premature newborns, the risk of sepsis and sepsis-related mortality increase as the birth weight decreases (11). In our study, it was found that the mortality rate was significantly high below 1000 g of birth weight. The rate of discharge is 63,7% below 1000 g, while it increases to 85% above 1000 g. In the study conducted by Vesikari et al. (12), low birth weight was found to be correlated with mortality rate in neonatal sepsis and mortality related with sepsis was found to be 67% in babies with a birth weight below 1 500 g, 28% in babies born with a birth weight of 1 500–2 500 g and 10% in babies born with a birth weight above 2 500 g. In the study of Gürsu (7), the mortality rate was found to be 24.3% in preterm newborns and 16.1% in term newborns. The mortality rate was found to be 50% in newborns with sepsis with a birth weight of <1 500 g, 23.8% in the ones with a birth weight of 1 500–2 500 g and 18.2% in the ones with a birth weight of >2 500 g. In the patients in the sepsis group born with a birth weight of ≥2 500 g after the 38th gestational week, the birth weight and gestational week were found to be significantly lower compared to the control group. In the study of Topuz (9), the mean gestational age was found to be 31.17±3.86 weeks, the mean birth weight was found to be 1619.76±695.99 g in the sepsis group and no significant difference was found between the sepsis and control groups in terms of prematurity and low birth weight. In the study conducted by Yancey et al. (13), prematurity was found to be correlated with neonatal sepsis. In our study, the birth weight was found to be lower in the patients who died because of sepsis compared to the patients who were discharged. In these patients, the gestational week was also younger, though not statistically significantly. This suggests that low birth weight in neonatal sepsis is an important risk factor in terms of mortality rate.
In neonatal sepsis, the mortality rate is higher in early-onset sepsis compared to late-onset sepsis (1). In the study of Gürsu (7), the mortality rate in neonatal sepsis was found to be 20% and the mortality rate was found to be 21.4% in the patients with early-onset sepsis and 18.8% in the patients with late-onset sepsis, but the difference was not significant. In our study, the mortality rate was found to be higher in the patients with early-onset sepsis compared to the patients with late-onset sepsis.
While the probability of sepsis is about 1% in the newborns whose mothers have (+) PROM, this rate increases to 4–6% in preterm newbors in presence of PROM (14). In the study of Gürsu (7), no statistical significance was found between the sepsis group and control group in terms of PROM (5). Umbilical catheterization for longer than 5 days, MV for longer than five days, NEC, a birth weight of 2 500 g and lower, use of nasogastric tube, total parenteral nutrition (TPN) and being referred from another hospital were found to be correlated with neonatal sepsis (15). In our study, PROM was positive in 17.4% of the patients, central catheter was inserted in 46.7%, TPB was administered in 90.6%, MV was used in 71.2% and CPAP was used in 53.8%. These rates were similar to the rates found in other studies.
Mechanical ventilation and parenteral nutrition are the main risk factors for late-onset sepsis. Hence, the study conducted by deSouza Rugolo et al. (16) found that use of central venous catheter, mechanical ventilation, parenteral nutriton and the time of first feeding were independent risk factors for late-onset sepsis in very low birth weight babies. In our study, it was demonstrated that the durations of mechanical ventilation and parenteral nutrition were significantly long and the duration of CPAP was not significant in the patients who died because of sepsis. On the other hand, Hornik et al. (11) reported that use of mechanical ventilatione on the first day was a risk factor for early sepsis, but not for late sepsis. In the same study, gestational week, male gender, Apgar score in the 5th minute and prenatal steroid and antibiotic usages were reported to be risk factors for early and late sepsis.
In the diagnosis of sepsis, neutropenia is more significant than increased white blood cell count and indicates poor prognosis. In our study, the neutrophil count was found to be lower in the babies who were lost because of sepsis compared to the other babies.
In the study of Topuz (9), the platelet count was found to be significantly low in the study group and it was concluded that the platelet count may be significant in the diagnosis of sepsis (7). In the study of Payaslı (10), thrombocytopenia was found in 9 (22.5%) of 40 patients in the sepsis group. In our study, thrombocytopenia (<150 000/mm3) was found in 47.6% of the patients which indicated the significance of thrombocytopenia in sepsis. This finding supported the findings of Topuz (9).
C-reactive protein (CRP) is an acute phase reactant which is used very frequently in the diagnosis of neonatal sepsis (17, 18). CRP release starts 4–12 hours after the onset of the infectious event, peaks in the 24th–60th hour and its amount decreases when infection regresses (19, 20). In this study, no correlation was found between CRP and prognosis.
It has been demonstrated that bacterial endotoxin (lipopolysaccharide) is the most strong stimulus which provides procalcitonin production (21). Viral diseases, autoimmune diseases, oncologic diseases and local and limited infections do not cause an increase in procalcitonin level. Therefore, procalcitonin is used mostly to differentiate bacterial and non-bacterial diseases. In addition, serum procalcitonin levels have been found to be increased in sepsis, bacteriemia, meningitis and fungus infections which cause severe systemic infection (22). A persistently increased or increasing procalcitonin level indicates that the disease activity continues and the prognosis will be poor. A decreasing procalcitonin level indicates that treatment is effective and the prognosis may be good (21). In our study, the procalcitonin levels of the babies who were lost because of sepsis were found to be higher compared to the ones who were discharged in accordance with these data.
In conclusion, it was found that newborns with sepsis had low birth weight and low gestational age. Early onset sepsis was correlated with mortality to a greater extent compared to late onset sepsis. Coagulase negative staphylococci were the leading agents among the agents causing sepsis. It was found that procalcitonin was efficient in indicating the prognosis in neonatal sepsis. In addition, thrombocytopenia and neutropenia were found to be correlated with poor prognosis. It was demonstrated that mechanical ventilation and parenteral nutrition were significant risk factors for mortality related with sepsis.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study.
Informed Consent: Written informed consent was not obtained from patients due to the retrospective nature of this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - T.G., F.O.; Design - E.E.T., T.G., F.O.; Supervision - T.G., F.O.; Data Collection and/or Processing - E.E.T.; Analysis and/or Interpretation - E.E.T., T.G., F.O.; Literature Review - E.E.T., T.G.; Writer - T.G., F.O.; Critical Review - F.O.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ovalı F. Neonatoloji. 2. baskı. İstanbul: Nobel Tıp Kitabevleri; 2007. Bakteriyel enfeksiyonlar. İçinde: Dağoğlu T, Ovalı F, (yazarlar) pp. 765–810. [Google Scholar]
- 2.Chiesa C, Panero A, Osborn JF, Simonetti AF, Pacifico L. Diagnosis of neonatal sepsis: a clinical and laboratory challenge. Clin Chem. 2004;50:279–87. doi: 10.1373/clinchem.2003.025171. http://dx.doi.org/10.1373/clinchem.2003.025171. [DOI] [PubMed] [Google Scholar]
- 3.Ovalı F. İçinde: Ovalı F. Yenidoğan enfeksiyonları. İstanbul: İstanbul medikal yayıncılık; 2006. Bakteryel enfeksiyonlar; pp. 109–249. [Google Scholar]
- 4.Blumenfeld YJ, Lee HC, Gould JB, Langen ES, Jafari A, El-Sayed YY. The effect of preterm premature rupture of membranes on neonatal mortality rates. Obstet Gynecol. 2010;116:1281–6. doi: 10.1097/AOG.0b013e3181fe3d28. http://dx.doi.org/10.1097/AOG.0b013e3181fe3d28. [DOI] [PubMed] [Google Scholar]
- 5.Roberts I, Stanworth S, Murray NA. Trombocytopenia in the neonate. Blood Rev. 2008;22:173–86. doi: 10.1016/j.blre.2008.03.004. http://dx.doi.org/10.1016/j.blre.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Gerdes JS. Diagnosis and management of bacterial infections in the neonate. Pediatr Clin North Am. 2004;51:939–59. doi: 10.1016/j.pcl.2004.03.009. http://dx.doi.org/10.1016/j.pcl.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Gürsu HA. 2005. Yenidoğan sepsisi tanısında Serum Amiloid A (SAA)’nın önemi ve CRP ile karşılaştırılması. Uzmanlık tezi. İstanbul, Dr. Lütfi Kırdar Kartal Eğitim ve Araştırma Hastanesi I. Çocuk Sağlığı ve Hastalıkları Kliniği, [Google Scholar]
- 8.Pierce JR, Merenstein GB, Stocker JT. Immediate postmortem cultures in an intensive care nursery. Pediatr Infect Dis. 1984;3:510–3. doi: 10.1097/00006454-198411000-00005. http://dx.doi.org/10.1097/00006454-198411000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Topuz S. Yenidoğan sepsisinin tanı ve izleminde C-Reaktif Protein ile prokalsitonin değerlerinin karşılaştırılması. Nobel Medicus. 2012;8:6–72. [Google Scholar]
- 10.Payaslı MÖ. Uzmanlık tezi. İstanbul: Haseki Eğitim ve Araştırma Hastanesi Çocuk Sağlığı ve Hastalıkları Kliniği; 2007. Neonatal sepsisli hastalarda polimorfonük-leer lökosit elastaz düzeylerinin değerlendirilmesi. [Google Scholar]
- 11.Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very low birth weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2014;88:S69–74. doi: 10.1016/S0378-3782(12)70019-1. http://dx.doi.org/10.1016/S0378-3782(12)70019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vesikari T, Janas M, Grönroos P, et al. Neonatal septicaemia. Arch Dis Child. 1985;60:542–6. doi: 10.1136/adc.60.6.542. http://dx.doi.org/10.1136/adc.60.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yancey MK, Duff P, Kubilis P, Clark P, Frentzen BH. Risk factors for neonatal sepsis. Obstet Gynecol. 1996;87:188–94. doi: 10.1016/0029-7844(95)00402-5. http://dx.doi.org/10.1016/0029-7844(95)00402-5. [DOI] [PubMed] [Google Scholar]
- 14.St Geme JW, Jr, Murray DL, Carter J, et al. Perinatal bacterial infection after prolonged rupture of amniotic membranes: an analysis of risk and management. J Pediatr. 1984;104:608–13. doi: 10.1016/s0022-3476(84)80562-4. http://dx.doi.org/10.1016/S0022-3476(84)80562-4. [DOI] [PubMed] [Google Scholar]
- 15.Padula MA, Dewan ML, Shah SS, et al. Risk factors associated with laboratory-corfirmed blood stream infections in a tertiary neonatal intensive careunit. Pediatr Infect Dis J. 2014;33:1027–32. doi: 10.1097/INF.0000000000000386. http://dx.doi.org/10.1097/INF.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 16.deSouza Rugolo LM, Bentin MR, Mussi-Pinhata M, et al. Late onset sepsis in very low birth weight infants: A Brasilian Neonatal Research Network Study. J Trop Pediatr. 2014;60:415–21. doi: 10.1093/tropej/fmu038. http://dx.doi.org/10.1093/tropej/fmu038. [DOI] [PubMed] [Google Scholar]
- 17.Ballou SP, Kushner I. C-reactive protein and the acute phase response. Adv Intern Med. 1992;37:313–36. [PubMed] [Google Scholar]
- 18.Pizzini C, Mussap M, Plebani M, Fanos V. C-reactive protein and serum amyloid A protein in neonatal infections. Scand J Infect Dis. 2000;32:229–35. doi: 10.1080/00365540050165848. http://dx.doi.org/10.1080/00365540050165848. [DOI] [PubMed] [Google Scholar]
- 19.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. http://dx.doi.org/10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 20.Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C reactive protein in early onset neonatal sepsis. Current insights and new tasks. Neonatology. 2012;102:25–32. doi: 10.1159/000336629. http://dx.doi.org/10.1159/000336629. [DOI] [PubMed] [Google Scholar]
- 21.Hatherill M, Tibby SM, Sykes K, Turner C, Murdoch IA. Diagnostic markers of infection: comparison of procalcitonin with C reactive protein and leucocyte count. Arch Dis Child. 1999;81:417–21. doi: 10.1136/adc.81.5.417. http://dx.doi.org/10.1136/adc.81.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrol ED, Thomson AP, Hart CA. Procalcitonin as a marker of sepsis. Int J Antimicrob Agents. 2002;20:1–9. doi: 10.1016/s0924-8579(02)00047-x. http://dx.doi.org/10.1016/S0924-8579(02)00047-X. [DOI] [PubMed] [Google Scholar]


