Abstract
Context
Vapocoolant spray, commonly known as cold spray (CS), is a cryotherapy modality used in sports medicine, athletic training, and rehabilitation settings. Proposed physiologic effects of cryotherapy modalities include reductions in tissue blood flow, oxygenation, and cell metabolism in addition to attenuation of pain perception attributed to reduced superficial nerve conduction velocity.
Objective
To examine the effects of CS on subcutaneous and intramuscular blood flow and oxygenation on the thigh muscle using near-infrared spectroscopy, an optical method to monitor changes in tissue oxygenated (O2Hb), deoxygenated (HHb), and total (tHb) hemoglobin.
Design
Cross-sectional study.
Setting
Muscle Biophysics Laboratory.
Patients or Other Participants
Participants were 13 healthy adults (8 men, 5 women; age = 37.4 ± 6 years, body mass index = 27.4 ± 2.6, adipose tissue thickness = 7.2 ± 1.8 mm).
Intervention(s)
Conventional CS was applied to the vastus medialis muscles.
Main Outcome Measure(s)
Changes in chromophore concentrations of O2Hb, HHb, and tHb at superficial and deep layers were monitored for 5 minutes using a 2-channel near-infrared spectroscopy.
Results
Thirty seconds after CS application, we observed a decrease from baseline in O2Hb and tHb only in the superficial layer that was maintained for 3 minutes.
Conclusions
Application of CS induced a transient change in blood flow and oxygenation of the superficial tissues with no change in deeper tissues over the healthy vastus medialis muscle. The limited physiologic effect of CS on the superficial hemodynamics and oxygenation of limb muscles may limit the therapeutic benefit of this cryotherapy modality to a temporary analgesic effect, a hypothesis that warrants a clinical trial on traumatized muscles.
Key Words: cryotherapy, muscle injury, blood flow, analgesics
Key Points
Cold-spray (CS) application induced a transient change in hemodynamics and oxygenation of the superficial tissues with no change in the deeper tissues over the vastus medialis muscle.
The physiologic effect of CS constrained to superficial hemodynamics and oxygenation of limb muscles may limit the therapeutic benefit of this cryotherapy modality to a temporary analgesic effect.
Researchers should study the effects of CS on subcutaneous and intramuscular blood flow and oxygenation, as well as nerve conduction velocity, after acute musculoskeletal and sport injuries.
Cryotherapy is a common treatment for acute sport injuries and musculoskeletal conditions.1 It is an accessible, simple, and often inexpensive therapy with a long history in medicine. Proposed therapeutic effects of cryotherapy include (1) reduced local tissue perfusion, oxygen consumption, metabolism, and inflammation2–7; (2) attenuated pain perception attributed to reduced superficial nerve conduction velocity8,9; and (3) reduced muscle spasm.8
Vapocoolant spray, also known as cold spray (CS), is a cryotherapy modality used in sports medicine, athletic training, and rehabilitation settings. It is commonly used in some international settings8,10 and interest in this modality is growing in the United States. Evaporation of the volatile liquid of CS from the skin surface induces a rapid reduction in local temperature that results in temporary local anesthesia, possibly through desensitization of pain receptors or activation of ion channels involved in pain transmission.9,11–14 To date, most researchers11,15 have investigated the rapid and transient anesthesia of CS, but the potential for CS to alter local hemodynamics and oxygenation has not been examined.
Considering the proposed effects of cryotherapy, a better understanding of whether CS reduces tissue perfusion and oxygenation and its penetration depth to subcutaneous or deeper layers would facilitate clinical decision making about its use as a therapeutic modality. Use of CS as a therapeutic modality is of particular interest because it typically is applied for a much shorter duration than other modes of cryotherapy. Therefore, the purpose of our study was to examine the effects of CS of subcutaneous and intramuscular blood flow and oxygenation on the anteromedial thigh (over the vastus medialis [VM] muscle) in healthy individuals using near-infrared spectroscopy (NIRS). We hypothesized that NIRS monitoring before, during, and after CS application would demonstrate whether CS affects tissue hemodynamics and oxygenation in the subcutaneous and deeper tissue layers over the vastus medialis.
METHODS
Participants
Participants were 13 adults (8 men, 5 women; age = 37.4 ± 6 years, body mass index = 27.4 ± 2.6, adipose tissue thickness beneath the NIRS probes = 7.2 ± 1.8 mm). Volunteers were included if they were adults, were healthy, had no history of lower limb abnormality or previous surgery, had no history of skin hypersensitivity, were nonsmokers, could provide informed consent, and had adipose tissue thickness of more than 5 mm and less than 15 mm at the site used for NIRS monitoring. All participants provided written informed consent, and the study was approved by the Clinical Research Ethics Board of the University of British Columbia.
Instrumentation
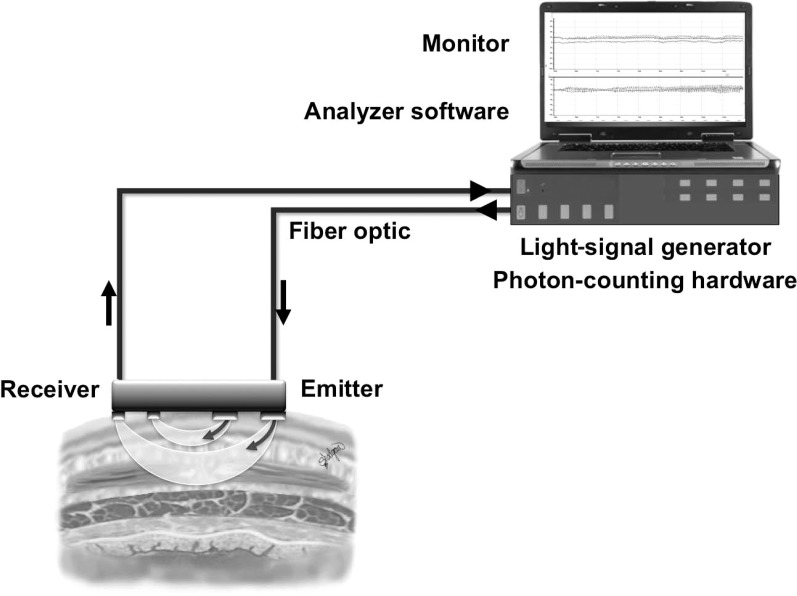
We used a continuous-wave NIRS device (OxyMon M III; Artinis Medical Systems, Elst, the Netherlands), which comprises a light-signal generator, fiberoptic bundles, an NIRS probe, and photon-counting hardware connected to a computer for converting raw optical data into chromophore concentrations.
The NIRS is a noninvasive optical technology that uses wavelengths of light in the near-infrared spectrum to monitor changes in tissue chromophore concentrations, including oxygenated and deoxygenated hemoglobin (O2Hb and HHb, respectively).16–18 This technology is applied widely as a research and clinical monitoring tool,19,20 and comprehensive reviews of its application, instrumentation, measurement methods, and limitations are available in the literature.13,14,16,21 By monitoring changes in the concentrations of local tissue O2Hb and HHb, NIRS can monitor the status of tissue oxygenation in real time.22,23 Furthermore, changes in total hemoglobin (tHb), the sum of O2Hb and HHb, can be calculated and offer a surrogate measure of local blood volume and, therefore, hemodynamics of the tissue of interest.16,23,24 The main components of a conventional NIRS system are shown in Figure 1.
Figure 1.
A conventional 2-channel near-infrared spectroscopy setup for tissue interrogation at 2 superficial and deep levels.
Procedures
Participants refrained from consuming caffeine and alcohol and from exercising for 12 hours before testing; measurements were performed during the afternoon. We assessed all participants in a seated position with the knee joint flexed to 90° and positioned a 2-channel NIRS probe over the right VM at a standardized placement, which was defined and marked at 10 cm proximal to the knee-joint line. Skinfold thickness at this point was measured using a skinfold caliper (Jamar 2058; Sammons Preston Rolyan, Bolingbrook, IL) before placement and fixation of a 2-channel NIRS probe. For measurement at the superficial and deep layers, interoptode distances were set at 20 and 55 mm, respectively, because the depth of penetration is equal to half the interoptode distance.25 The NIRS probe setup is illustrated in Figure 2. The probes were connected to a continuous-wave NIRS via fiberoptic cables, and changes in muscle O2Hb, HHb, and tHb were determined before, during, and after application of CS. After 2 minutes of baseline NIRS measurement, a CS (Cramer Cold Spray; Cramer Products Inc, Gardner, KS) was applied to the VM point under the NIRS probes. The baseline was defined by zeroing all NIRS values at the superficial and deep layers immediately before CS application. From a distance of 15 cm, we applied the CS to the skin surface at a 45° angle for 15 seconds. The NIRS monitoring continued for 5 minutes after CS application. Chromophore concentrations of O2Hb, HHb, and the summed tHb were monitored in real time, sampled at 10 Hz, filtered with a moving gaussian filter, and stored on a hard disk for further offline analysis using Oxysoft software (Artinis Medical Systems).
Figure 2.
Placement of the 2-channel near-infrared spectroscopy probe over the vastus medialis muscle.
Statistical Analysis
Mean changes in O2Hb, HHb, and tHb from baseline were calculated at 30-second intervals during the 5 minutes after CS application to the superficial and deep layers for each participant. For each depth of penetration, a 2-way repeated-measures analysis of variance was performed to examine changes in O2Hb, HHb, and tHb between baseline and the 10 time points (every 30 seconds during 5 minutes) after CS application. Data are presented as mean ± SD. The α level was set at .05 for all comparisons. All statistical analyses were performed with SPSS (version 17.0 for Mac OS; SPSS, Inc, Chicago, IL).
RESULTS
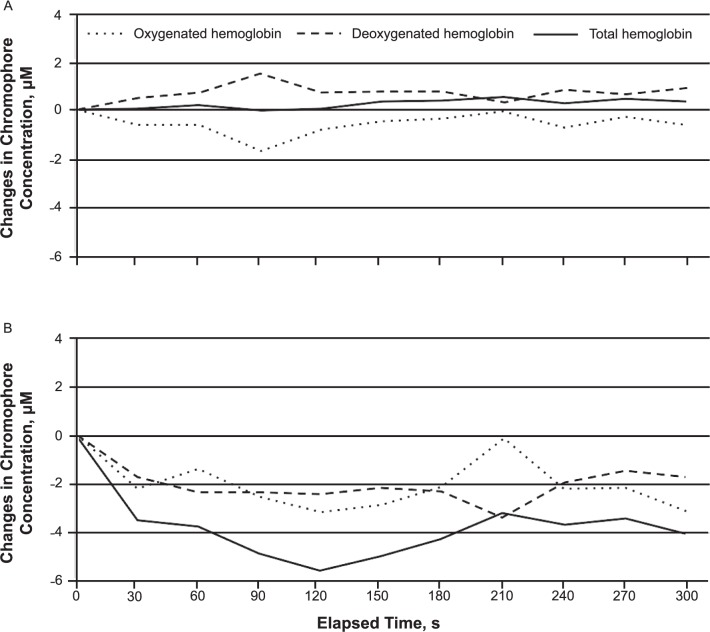
The mean values of tHb, O2Hb, and HHb at baseline and during the 5-minute period after CS application are provided in Figure 3. Thirty seconds after CS application, we observed a decrease in O2Hb and tHb only in the superficial layer (Figure 3).
Figure 3.
Changes in A, deep, and B, superficial near-infrared spectroscopy chromophore concentrations after cold-spray application. Changes in superficial deoxygenated hemoglobin and deep total hemoglobin, oxygenated hemoglobin, and deoxygenated hemoglobin were not different at any time after cold-spray application.
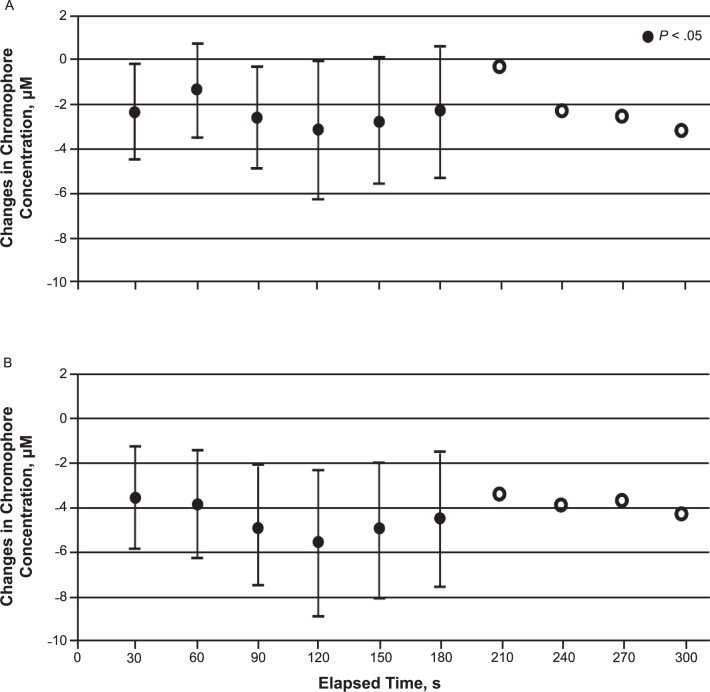
Superficial O2Hb decreased consistently for 3 minutes after CS application; the lowest value compared with the baseline value occurred at the second minute (F1,12 = −3.35, P = .02). Similarly, superficial tHb also decreased at all time points during the first 3 minutes, with the lowest value seen at the second minute after CS application (F1,12 = −5.56, P = .007; Figure 4). In contrast, values for superficial HHb and deep tHb, O2Hb, and HHb were not different at any time after CS during the 5-minute period application compared with baseline values (Figure 3).
Figure 4.
Changes in A, superficial tissue oxygenated hemoglobin, and B, total hemoglobin concentrations after cold-spray application. Superficial oxygenated hemoglobin and total hemoglobin showed a consistent decrease for 3 minutes after cold-spray application.
DISCUSSION
Our findings demonstrated that CS application produced only a transient change in hemodynamics and oxygenation of the skin and superficial tissues (approximate depth = 10 mm) and no change in deeper tissues over the VM in adults with no underlying pathologic condition. The absence of changes in deep tissue hemodynamics and oxygenation after CS application in healthy people raises some question about the scope of its therapeutic benefit for managing acute soft tissue trauma. Whereas CS application provided local anesthesia,9,11–14 it did not change local perfusion in the deeper layers. Thus, its effect on inflammatory changes in deeper tissues might be limited after injury and requires further investigation.
Cooling the skin to innocuous or noxious temperatures is detected by cutaneous afferent neurons, Aδ mechanoheat nociceptors, and C-fiber polymodal nociceptors.12 The interaction between activation of these receptors and inflammation is not entirely understood.12 Schaser et al7 reported that skin cooling for 6 hours diminished soft tissue injury and inflammation of the left tibial compartment in a rat model.7 However, evidence of short-term skin cooling directly affecting inflammation is lacking.26 Thus, the effectiveness of cryotherapy based on measurements of skin surface temperature27,28 needs to be interpreted with caution, especially given that surface skin temperature is an unreliable indicator of deep tissue temperature.27 In view of the limited evidence that links short-term skin cooling to inflammation, the spectrum of therapeutic effects of CS might be very focused on temporary pain relief.
Numerous researchers have used NIRS to examine blood flow and oxygen uptake in inflamed soft tissues29–32 and in muscle during exercise.23,33,34 In addition, the reliability of NIRS for determining local tissue blood flow and oxidative metabolism by measuring oxygen consumption and variations in hemoglobin level has also been established.16,35 Although not used previously, its well-established validity and noninvasive monitoring capability will provide great utility to examine the dimension of local physiologic responses in addition to skin temperature to establish a greater depth of evidence for cryotherapy modalities.
Our study suggested that in a group of adults without localized or relevant systemic pathologic conditions, CS application produced only a transient physiologic response in the oxygenation and blood flow of the skin and superficial tissues. The absence of changes in deep tissue oxygenation and hemodynamics after CS application might indicate insignificant effects on the inflammatory consequences of acute soft tissue trauma. However, this requires further study. Until the benefits of CS on inflammation are demonstrated, professionals using CS should be aware of its sole influence on temporary analgesia.9,11–14
One limitation of our study was the small sample size. We did not perform a power analysis due to the lack of previous data. Another limitation was the lack of temperature measurements of the superficial and deep layers of the quadriceps muscles. In addition, all participants were healthy individuals without injury to the site of measurement, and consequently, it is inappropriate to extend these findings to a population with acute injury. Indeed, researchers have long known that in the inflammatory phase of an acute injury, chemical mediators change local capillary permeability,36 which in turn affects tissue hemodynamics.
CONCLUSIONS
Our study demonstrated that CS application induced a transient change in the hemodynamics and oxygenation of the superficial tissues with no change in the deeper tissues over the VM. The physiologic effect of CS constrained to superficial hemodynamics and oxygenation of limb muscles may limit the therapeutic benefit of this cryotherapy modality to a temporary analgesic effect, a hypothesis that warrants a clinical trial on traumatized muscles. We recommend that future researchers study the effects of CS on subcutaneous and intramuscular blood flow and oxygenation, as well as nerve conduction velocity, after acute musculoskeletal and sport injuries, because these are the prevalent conditions for which cryotherapy modalities frequently are used to facilitate a quick return to participation.
REFERENCES
- 1.Knight KL, Brucker JB, Stoneman PD, Rubley MD. Muscle injury management with cryotherapy. Athl Ther Today. 2000;5(4):26–30. [Google Scholar]
- 2.Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251–261. doi: 10.1177/0363546503260757. [DOI] [PubMed] [Google Scholar]
- 3.Ho SS, Illgen RL, Meyer R, Torok PJ, Cooper MD, Reider B. Comparison of various icing times in decreasing bone metabolism and blood flow in the knee. Am J Sports Med. 1995;23(1):74–76. doi: 10.1177/036354659502300112. [DOI] [PubMed] [Google Scholar]
- 4.Hubbard TJ, Denegar CR. Does cryotherapy improve outcomes with soft tissue injury? J Athl Train. 2004;39(3):278–279. [PMC free article] [PubMed] [Google Scholar]
- 5.Karunakara RG, Lephart SM, Pincivero DM. Changes in forearm blood flow during single and intermittent cold application. J Orthop Sports Phys Ther. 1999;29(3):177–180. doi: 10.2519/jospt.1999.29.3.177. [DOI] [PubMed] [Google Scholar]
- 6.Puntel GO, Carvalho NR, Dobrachinski F, et al. Cryotherapy reduces skeletal muscle damage after ischemia/reperfusion in rats. J Anat. 2013;222(2):223–230. doi: 10.1111/joa.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaser KD, Disch AC, Stover JF, Lauffer A, Bail HJ, Mittlmeier T. Prolonged superficial local cryotherapy attenuates microcirculatory impairment, regional inflammation, and muscle necrosis after closed soft tissue injury in rats. Am J Sports Med. 2007;35(1):93–102. doi: 10.1177/0363546506294569. [DOI] [PubMed] [Google Scholar]
- 8.Meeusen R, Lievens P. The use of cryotherapy in sports injuries. Sports Med. 1986;3(6):398–414. doi: 10.2165/00007256-198603060-00002. [DOI] [PubMed] [Google Scholar]
- 9.Nadler S, Weingand K, Kruse R. The physiologic basis and clinical applications of cryotherapy and thermotherapy for the pain practitioner. Pain Physician. 2004;7(3):395–399. [PubMed] [Google Scholar]
- 10.Swenson C, Swärd L, Karlsson J. Cryotherapy in sports medicine. Scand J Med Sci Sports. 1996;6(4):193–200. doi: 10.1111/j.1600-0838.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 11.Farion KJ, Splinter KL, Newhook K, Gaboury I, Splinter WM. The effect of vapocoolant spray on pain due to intravenous cannulation in children: a randomized controlled trial. CMAJ. 2008;179(1):31–36. doi: 10.1503/cmaj.070874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleetwood-Walker SM, Proudfoot CW, Garry EM, Allchorne A, Vinuela-Fernandez I, Mitchell R. Cold comfort pharm. Trends Pharmacol Sci. 2007;28(12):621–628. doi: 10.1016/j.tips.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Waterhouse MR, Liu DR, Wang VJ. Cryotherapeutic topical analgesics for pediatric intravenous catheter placement: ice versus vapocoolant spray. Pediatr Emerg Care. 2013;29(1):8–12. doi: 10.1097/PEC.0b013e31827b214b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon WY, Chung SP, Lee HS, Park YS. Analgesic pretreatment for antibiotic skin test: vapocoolant spray vs ice cube. Am J Emerg Med. 2008;26(1):59–61. doi: 10.1016/j.ajem.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Hijazi R, Taylor D, Richardson J. Effect of topical alkane vapocoolant spray on pain with intravenous cannulation in patients in emergency departments: randomized double blind placebo controlled trial. BMJ. 2009;338:b215. doi: 10.1136/bmj.b215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boushel R, Langberg H, Olesen J, et al. Regional blood flow during exercise in humans measured by near-infrared spectroscopy and indocyanine green. J Appl Physiol. 2000;89(5):1868–1878. doi: 10.1152/jappl.2000.89.5.1868. [DOI] [PubMed] [Google Scholar]
- 17.Delpy DT, Cope M, van der Zee P, Arridge S, Wray S, Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol. 1988;33(12):1433–1442. doi: 10.1088/0031-9155/33/12/008. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29(4):463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 19.Gagnon RE, Macnab AJ. Near infrared spectroscopy (NIRS) in the clinical setting: an adjunct to monitoring during diagnosis and treatment. Spectroscopy. 2005;19(5–6):221–233. [Google Scholar]
- 20.Shadgan B, Reid WD, Gharakhanlou R, Stothers L, MacNab AJ. Wireless near-infrared spectroscopy of skeletal muscle oxygenation and hemodynamics during exercise and ischemia. Spectroscopy. 2009;23(5–6):233–241. [Google Scholar]
- 21.Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bulow J, Kjaer M. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports. 2001;11(4):213–222. doi: 10.1034/j.1600-0838.2001.110404.x. [DOI] [PubMed] [Google Scholar]
- 22.Van Beekvelt MC, Van Engelen BG, Wevers RA, Colier WN. In vivo quantitative near-infrared spectroscopy in skeletal muscle during incremental isometric handgrip exercise. Clin Physiol Funct Imaging. 2002;22(3):210–217. doi: 10.1046/j.1475-097x.2002.00420.x. [DOI] [PubMed] [Google Scholar]
- 23.Van Beekvelt MC, Shoemaker JK, Tschakovsky ME, Hopman MT, Hughson RL. Blood flow and muscle oxygen uptake at the onset and end of moderate and heavy dynamic forearm exercise. Am J Physiol Regul Integr Comp Physiol. 2001;280(6):R1741–R1747. doi: 10.1152/ajpregu.2001.280.6.R1741. [DOI] [PubMed] [Google Scholar]
- 24.Van Beekvelt MC, Colier WN, Wevers RA, Van Engelen BG. Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle. J Appl Physiol. 2001;90(2):511–519. doi: 10.1152/jappl.2001.90.2.511. [DOI] [PubMed] [Google Scholar]
- 25.Homma S, Eda H, Ogasawara S, Kagaya A. Near-infrared estimation of O2 supply and consumption in forearm muscles working at varying intensity. J Appl Physiol. 1996;80(4):1279–1284. doi: 10.1152/jappl.1996.80.4.1279. [DOI] [PubMed] [Google Scholar]
- 26.MacAuley DC. Ice therapy: how good is the evidence? Int J Sports Med. 2001;22(5):379–384. doi: 10.1055/s-2001-15656. [DOI] [PubMed] [Google Scholar]
- 27.Jutte L, Merrick M, Ingersoll C, Edwards JE. The relationship between intramuscular temperature, skin temperature, and adipose thickness during cryotherapy and rewarming. Arch Phys Med Rehabil. 2001;82(6):845–850. doi: 10.1053/apmr.2001.23195. [DOI] [PubMed] [Google Scholar]
- 28.Kanlayanaphotporn R, Janwantanakul P. Comparison of skin surface temperature during the application of various cryotherapy modalities. Arch Phys Med Rehabil. 2005;86(7):1411–1415. doi: 10.1016/j.apmr.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 29.Ardeshirpour Y, Gandjbakhche AH, Najafizadeh L. Biophotonics techniques for structural and functional imaging, in vivo. Anal Cell Pathol (Amst) 2012;35(5–6):317–337. doi: 10.3233/ACP-2012-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu KZ, Duarte PM, Santos VR, et al. Assessment of tissue oxygenation of periodontal inflammation in smokers using optical spectroscopy. J Clin Periodontol. 2014;41(4):340–347. doi: 10.1111/jcpe.12225. [DOI] [PubMed] [Google Scholar]
- 31.Pichler G, Pocivalnik M, Riedl R, et al. C reactive protein: impact on peripheral tissue oxygenation and perfusion in neonates. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):F444–F448. doi: 10.1136/archdischild-2011-300578. [DOI] [PubMed] [Google Scholar]
- 32.Polglase GR, Miller SL, Barton SK, et al. Initiation of resuscitation with high tidal volumes causes cerebral hemodynamic disturbance, brain inflammation and injury in preterm lambs. PLoS One. 2012;7(6):e39535. doi: 10.1371/journal.pone.0039535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalliokoski K, Knuuti J, Nuutila P. Relationship between muscle blood flow and oxygen uptake during exercise in endurance-trained and untrained men. J Appl Physiol. 2005;98(1):380–383. doi: 10.1152/japplphysiol.01306.2003. [DOI] [PubMed] [Google Scholar]
- 34.Moalla W, Elloumi M, Chamari K, et al. Training effects on peripheral muscle oxygenation and performance in children with congenital heart diseases. Appl Physiol Nutr Metab. 2012;37(4):621–630. doi: 10.1139/h2012-036. [DOI] [PubMed] [Google Scholar]
- 35.Quaresima V, Lepanto R, Ferrari M. The use of near infrared spectroscopy in sports medicine. J Sports Med Phys Fitness. 2003;43(1):1–13. [PubMed] [Google Scholar]
- 36.Page P. Pathophysiology of acute exercise-induced muscular injury: clinical implications. J Athl Train. 1995;30(1):29–34. [PMC free article] [PubMed] [Google Scholar]