Abstract
Background
GalNAc-T3 catalyzes initial glycosylation of mucin-type O-linked protein involved in proliferation, adhesion, and migration of tumor cells. This study was performed to explore the relationships of the expression of GalNAc-T3 in small peripheral lung adenocarcinoma, especially as an indicator of prognosis.
Materials and methods
A retrospective analysis of the patients with small peripheral lung lesions, including 106 adenocarcinoma and two precancerous lesions (atypical adenomatous hyperplasia and adenocarcinoma in situ) after complete surgical resection, was launched. Expression of GalNAc-T3 was examined using immunohistochemistry staining on primary tumor specimens, and the tumors were reclassified in light of the IASLC/ATS/ERS (International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society) adenocarcinoma classifications followed by grading and scoring. Moreover, reverse transcription polymerase chain reaction and Western blot were used to study the expression of GalNAc-T3 in vivo.
Results
The low expression of GalNAc-T3 was found in the cytoplasm of tumor cells in 56 of 108 patients (51.9%) and was associated with IASLC/ATS/ERS classification of high risk groups (P=0.007), high Sica score (P=0.036), poorly differentiated tumor (P=0.023), poor tumor-node-metastasis (TNM) stage (P=0.007), pleural invasion (P=0.007), and vascular invasion (P<0.001) by Pearson’s chi-squared test, but not with sex, age, smoking status, concentration of carcinoembryonic antigen, and lymph node metastasis. In logistic regression analysis, low GalNAc-T3 expression was only correlated with high-ranking TNM stage (odds ratio [OR] =8.975, 95% confidence interval [CI]: 1.797–44.661), vascular invasion (OR =5.668, 95% CI: 1.827–17.578), and the higher risk grade (low risk grade: OR =0.141, 95% CI: 0.027–0.719; moderate risk grade: OR =0.122, 95% CI: 0.017–40.871). The low expression of the GalNAc-T3 usually in adenocarcinoma cell lines was compared with normal bronchial epithelium cell line. Based on the univariate and multivariate analysis, poor TNM stage (P<0.001), pleural invasion (hazard ratio [HR]: 7.958, P=0.021), vascular invasion (HR: 2.403, P=0.040), and low GalNAc-T3 expression (HR: 3.317, P=0.016) were shown to be independently associated with an unfavorable prognosis. However, IASLC/ATS/ERS classification of risk groups and Sica score (P=0.034 and P=0.032, respectively) was correlated with overall survival on Kaplan–Meier method but not Cox regression model.
Conclusion
GalNAc-T3 expression was correlated with the IASLC/ATS/ERS classification and also associated with prognosis of patients with completely resected small (≤2 cm) peripheral lung adenocarcinoma.
Keywords: GalNAc-T3, classification, lung adenocarcinoma, prognosis
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide.1 Adenocarcinoma is the most common aggressive histopathologic type in nonsmoking lung cancer patients, and it mainly occurred in peripheral field of the lung.2,3 With the development of the advanced computed tomography (CT) screening and improved surgical techniques, adenocarcinoma has been detected frequently, especially small peripheral lung adenocarcinoma with diameter <2 cm in surrounding areas of the lobe. However, there exists a widely divergent prognosis in different lung adenocarcinoma subtypes.4–7
Introduction of the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) classification8 has recently been used as a new method for classifying different subtypes relying on the predominant morphological structure to provide essential references for individual prognosis.9,10 However, lung adenocarcinoma has the characteristics of early metastasis and recurrence, whereas 5-year survival rate is only approximately 70% even in the early lung cancer. GalNAc-T3 is a member of UDP-GalNAc: polypeptide N-acetyl-galactosaminyl transferases (GalNAc-transferase) which catalyzes initial glycosylation of mucin-type O-linked protein. The mucin-type O-linked protein has been considered to play a key role in determining the tumor characteristics of malignant biological behavior, and is also under investigation as a possible diagnostic marker for malignancies in which they are most commonly (high or low) expressed.11–13 GalNAc-T3 was restricted to cell lines derived from epithelial gland adenocarcinoma.14–16 The different expression of GalNAc-T3 may affect proliferation and invasion of malignant cells, as proven in several studies.17–21 It has already been evaluated as an expected prognostic marker in different cancer.15,22 However, to our current knowledge, there is no report about the GalNAc-T3 in subtypes of lung adenocarcinoma, especially in small peripheral lung adenocarcinoma.
The purpose of our study was to retrospectively detect GalNAc-T3 expression according to IASLC/ATS/ERS pulmonary adenocarcinoma classification in small peripheral lung adenocarcinoma by using immunohistochemical (IHC) staining and to evaluate the effect of the expression level of GalNAc-T3 and histologic subtypes on the patients’ overall survival. Furthermore, reverse transcription polymerase chain reaction (RT-PCR) and Western blot were used to explore the expression of GalNAc-T3 in human lung adenocarcinoma cell lines in vitro.
Materials and methods
Patients and follow-up
Primary focus specimens from 108 patients (median age 62 years: range from 40 to 81 years) with complete surgical resection of small peripheral lung nodule was consecutively obtained at the First Affiliated Hospital of Dalian Medical University between January 2009 and December 2010. The patients who received chemotherapy or radiotherapy prior to the operation were excluded. The seventh edition International Union Against Cancer/American Joint Committee on Cancer TNM classification23 was applied to all enrolled patients. The clinicopathological data, obtained by a retrospective chart review, are shown in Table 1.
Table 1.
Patient characteristics and univariate analysis of overall survival
| Characteristics | Cases, N (%) | 5-year OS rate, % | P-value |
|---|---|---|---|
| Overall | 108 | 62.8 | |
| Age, years | 0.933 | ||
| <62 | 53 (49.1) | 60.8 | |
| ≥62 | 55 (50.9) | 65 | |
| Sex | 0.032 | ||
| Male | 48 (44.4) | 53.3 | |
| Female | 60 (55.6) | 72.7 | |
| Smoking status | 0.727 | ||
| Smoker | 37 (34.3) | 61.4 | |
| Never smoked | 71 (65.7) | 65.3 | |
| Concentration of CEA (μg/L) | 0.682 | ||
| <5 | 89 (82.4) | 62.4 | |
| ≥5 | 19 (17.6) | 66.6 | |
| Operating type | 0.059 | ||
| Lobectomy | 17 (15.7) | 49.1 | |
| Partial resection | 91 (84.3) | 66.5 | |
| IASLC/ATS/ERS classification risk group | 0.034 | ||
| Low risk | 13 (12.0) | 88.9 | |
| AAH | 1 (0.9) | ||
| AIS | 1 (0.9) | ||
| MIA | 1 (0.9) | ||
| Lepidic | 10 (9.3) | ||
| Moderate risk | 78 (71.3) | 64 | |
| Papillary | 30 (27.8) | ||
| Acinar | 47 (43.5) | ||
| High risk | 20 (16.7) | 36.3 | |
| Micropapillary | 4 (3.70) | ||
| Solid | 12 (11.1) | ||
| Mucinous | 2 (1.9) | ||
| Sica score | 0.032 | ||
| 0 or 2 or 3 | 12 (11.1) | 90.9 | |
| 4 | 52 (48.1) | 68.4 | |
| 5 or 6 | 44 (40.7) | 50.2 | |
| Differentiation | 0.679 | ||
| Well | 51 (47.2) | 72.9 | |
| Moderate | 47 (43.5) | 57.4 | |
| Poor | 10 (9.3) | 47.4 | |
| Pleural invasion | <0.001 | ||
| Absent | 94 (87) | 70.5 | |
| Present | 14 (13) | 18.4 | |
| Vascular invasion | <0.001 | ||
| Absent | 80 (74.1) | 70 | |
| Present | 28 (25.9) | 43.4 | |
| Lymph node metastasis | <0.001 | ||
| With | 7 (6.5) | 67.6 | |
| Without | 101 (93.5) | 38.1 | |
| TNM stage | <0.001 | ||
| 0 or I | 92 | 72.1 | |
| 0 | 2 (1.8) | 100 | |
| IA | 61 (56.5) | 82.3 | |
| IB | 29 (26.9) | 51.1 | |
| II | 13 | 20.5 | |
| IIA | 10 (9.3) | 45.0 | |
| IIB | 3 (2.8) | 33.3 | |
| IIIA | 3 (2.8) | 0 | |
| GalNAc-T3 expression | <0.001 | ||
| Low | 56 (51.9) | 45 | |
| High | 52 (48.1) | 82.3 |
Abbreviations: CEA, carcino-embryonic antigen; AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; OS, overall survival; IASLC, International Association for the Study of Lung Cancer; ATS, the American Thoracic Society; ERS, European Respiratory Society; TNM, tumor-node-metastasis.
Patients were followed every 3 months within the 1st year and at approximately 5- to 6-month interval thereafter. All methods of patients’ physical examination, analysis of blood chemistry, carcinoembryonic antigen (CEA) assay, and chest, upper abdominal, brain CT scan data collection and analyses were approved. Follow-up ranged from 3 to 60 months after the primary operation (median follow-up time: 31.5 months, the end follow-up time at January 2013), and this study was approved by the Medical Ethical Committees of the First Affiliated Hospital of Dalian Medical University.
Histopathology evaluation
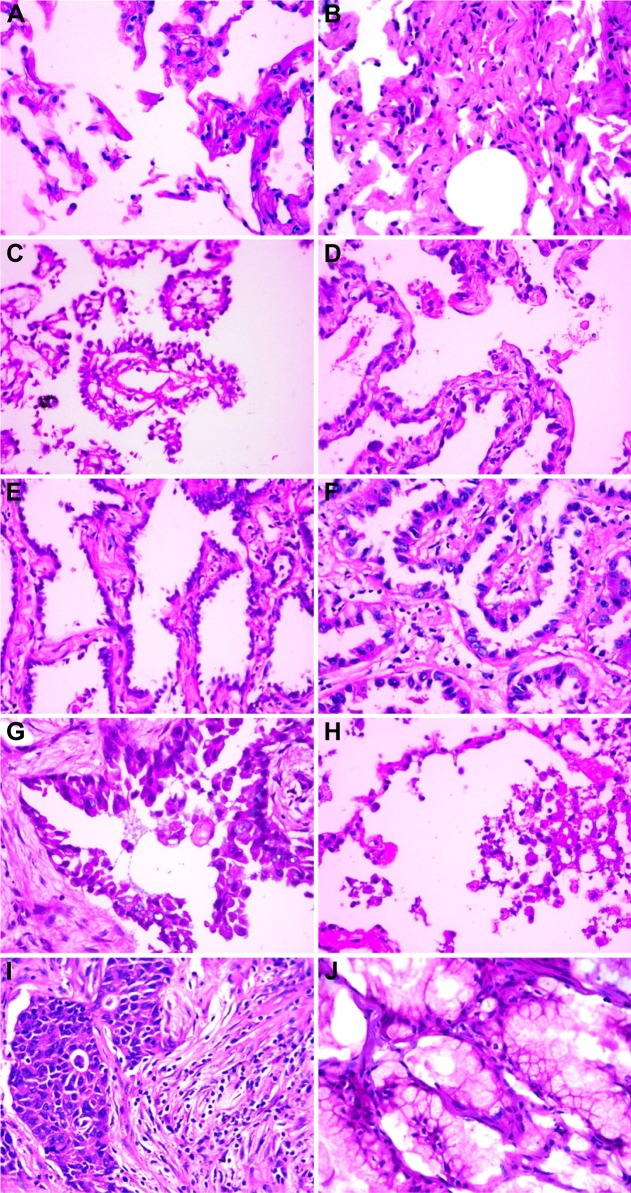
All resected specimens were obtained from 108 samples of primary lesions, and then these specimens were fixed with formalin. The paraffin-embedded, serial 3 μm sections were stained with hematoxylin and eosin. Histologic classification of each slide was independently performed by two pulmonary pathologists and was based on IASLC/ATS/ERS international multidisciplinary classification of lung adenocarcinoma,8 and each tumor was recorded by the predominant and the secondary patterns for each histologic component. The new classification included: 1) atypical adenomatous hyperplasia (AAH), 2) adenocarcinoma in situ (AIS), 3) minimally invasive adenocarcinoma (MIA), 4) acinar (Aci), 5) papillary (Pap), 6) micropapillary (MP), 7) solid (Sol), and 8) mucinous (Mc), which are shown in Figure 1. Each specimen was graded according to the pattern-based grading system as proposed by Ambrosini-Spaltro et al24 and Sica et al25 and the primary and secondary patterns were also establishing as well as the final score.
Figure 1.
Images of normal and predominant growth patterns.
Notes: (A) Normal alveolar tissue, (B) atypical adenomatous hyperplasia, (C) adenocarcinoma in situ, (D) minimally invasive adenocarcinoma, (E) lepidic, (F) acinar, (G) papillary, (H) micropapillary, (I) solid, and (J) mucinous predominant adenocarcinoma.
Immunohistochemistry staining
GalNAc-T3 protein was stained by a streptavidin–biotin–peroxidase complex method,16 and IHC staining was done according to our previously published research:22 sections were briefly incubated with xylene, rehydrated with graded ethanol solutions, incubated with methyl alcohol containing 3% hydrogen peroxide and immersed in a citrate buffer for antigen retrieval, then incubated with polyclonal GalNAc-T3 antibody (diluted 1:2,000 in phosphate-buffered saline containing 2% bovine serum albumin), which was obtained from Dr T Oyama at Department of Surgery II University of Occupational and Environmental Health School of Medicine, for 90 minutes at room temperature by using Vectastain ABC-HRP Kits (Vector Laboratories, Inc, Burlingame, CA, USA).
Immunostaining was evaluated by two pulmonary pathologists (Dr Guan and Dr Li) using a blind protocol design. The cells with stained cytoplasm or membrane were considered to be positive for GalNAc-T3 expression. The final overall appraised score GalNAc-T3 expression of each specimen was conducted as intensity expression (negative: 0 points; weak: 1 point; moderate: 2 points; and strong: 3 points) multiplying stained cell numbers (positive cells as ≤25% of the cells: 1 point; 26–50% of the cells: 2 points; 51–75% of the cells: 3 points; >75% of the cells: 4 points). When the sample scored ≥6 points, we defined it was high expression, otherwise it was low expression. The negative and positive controls of GalNAc-T3 expression were the same as those used by Nomoto et al16 in breast carcinoma and our previous work.22
Cell lines and cell culture
Human lung adenocarcinoma cell lines (A549, H322, Hcc827, and H1299) and normal human embryonic lung fibroblasts HLF cell line were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in either Dulbecco’s Modified Eagle’s Medium (HyClone, Thermo Scientific, Waltham, MA, USA) or DMEM (HyClone, Thermo Scientific) supplemented with 10% fetal bovine serum and maintained at 37°C in a humidified atmosphere containing 5% CO2.
RT-PCR
Total RNA was prepared from cultured cells using Trizol Reagent (TaKaRa Bio., Shiga, Japan) according to the manufacturers’ instructions. Reverse transcription was carried out with TIAN Script II RT Kit (Tiangen, Beijing, People’s Republic of China). The PCR primer sequences were listed as follows: for GaINAC-T3 (sense: 5′ TGTTTCTATGGTTGGCTAG 3′, antisense: 5′ CACGG TTATGGTTACTTCC 3′) and β -actin (sense: 5′ AGCCTC GCCTTTGC-CGA 3′, antisense: 5′ CTGGTGCCTGGG GCG 3′). The samples were denatured at 94°C for 5 minutes, and 30 cycles of PCR amplification were performed with the following conditions: denature 94°C for 30 seconds, anneal 55°C–60°C for 15–30 seconds, and extend 65°C–70°C for 1 minute, followed by finally maintaining the reaction at 4°C after cycling. The PCR products were run on a 1% agarose gel and visualized by ethidium bromide staining under UV light.
Western blot analysis
The different cells were collected at optimum times during cell culture in a microcentrifuge tube. Total protein was collected as followed: lyse the cells by pipetting 1 mL complete Cell Extraction Buffer (Invitrogen, Waltham, MA, USA) with Protease Inhibitor Cocktail (Sigma, 100X, St Louis, MO, USA) and phenylmethanesulfonyl fluoride (final concentration 1 mM) per 108 cells on the ice, then clarify the lysates by centrifugation at 14,000 rpm at 4°C for 20 minutes. Afterward, collect total protein, which is present in the supernatant for analysis, and store at −80°C.
The concentration of total cell proteinlysate was quantified by BCA protein assay kit (Thermo Fisher Scientific). Then, 30 g of protein lysate were separated by 6%–12% sodium dodecyl sulfate–polyacrylamide minigels and transferred to a polyvinylidene difluoride membrane. The membrane was incubated with the GalNAc-T3 antibodies diluted 1:4,000 in phosphate-buffered saline containing 2% bovine serum albumin overnight, and then developed with enhanced chemiluminescence system.
Statistical analysis
The association between GalNAc-T3 expression and categorical variables were compared by Pearson’s chi-squared test. For the correlation analysis of low GaINAC-T3 expression and clinicopathologic factors, logistic regression was constructed by backward selection of multiple variables. Student’s t-test was used to compare the values of the test and control samples in vitro. Survival curves were calculated using the Kaplan–Meier method. The log-rank test was used to analyze overall survival time between different clinicopathological factors in small peripheral lung adenocarcinoma. Multivariate analysis was performed using the Cox regression model. Data were analyzed by the SPSS 20 software (SPSS Inc, Chicago, IL, USA). Values of P<0.05 were considered statistically significant.
Results
Clinicopathologic characteristics
The clinicopathologic characteristics of the patients are summarized in Table 1. Of the 106 patients with complete surgical resection of small peripheral lung adenocarcinoma and two precancerous lesions (AAH and AIS), 48 (44.4%) were male and 60 (55.6%) were female, with a median age of 62 years (range: 40–81 years). Among 108 patients, 37 (34.3%) were former or current smoker and 71 patients had never smoked. The preoperative CEA of 19 patients exceeded 5 μg/L. The surgical procedure employed in 17 patients (15.7%) was lobectomy, and in 91 patients (84.3%) partial resection was performed.
Reclassification of the 108 specimens resulted in a low-risk group (1 AAH [0.9%, Figure 1B], 1 AIS [0.9%, Figure 1C], 1 MIA [0.9%, Figure 1D], and Lep [9.3%, Figure 1E]), a moderate risk group (47 Aci [43.5%, Figure 1F] and 30 Pap [27.8%, Figure 1G]), and a high-risk group (4 MP [3.7%, Figure 1H], 12 Sol [11.1%, Figure 1I], 2 Mc [1.9%, Figure 1J]). The percentage of the three scored groups from the Sica score25 such as the 0, 2, or 3 point group, 4 point group, and the 5 or 6 point group in 108 patients was 12 (11.1%), 52 (48.1%), and 44 (40.7%), respectively.
Tumors were classified as well, moderately, and poorly differentiated in 51 (47.2%), 57 (43.5%), and ten (9.3%) of the cases, respectively. Pleural, vascular, and lymphatic invasions were observed in 14 (13%), 28 (25.9%), and seven (6.5%) of the cases, respectively. The pathologic stage was 0 in one patient (1.8%), IA in 62 patients (56.5%), IB in 29 patients (26.9%), IIA in ten patients (9.3%), IIB in three patients (2.8%), and IIIA in three patients (2.8%).
Correlation between GalNAc-T3 expression and clinicopathologic factors
All 108 specimens were nearly evenly divided between the GalNAc-T3 expression (56 low expression [51.9%] and 52 high expression [48.1%]). In tumor cells, the GalNAc-T3 IHC staining was usually seen in cytoplasm or membranes, and representative examples of IHC stains for GalNAc-T3 are shown in Figure 2 corresponding to the acinar predominant growth pattern.
Figure 2.
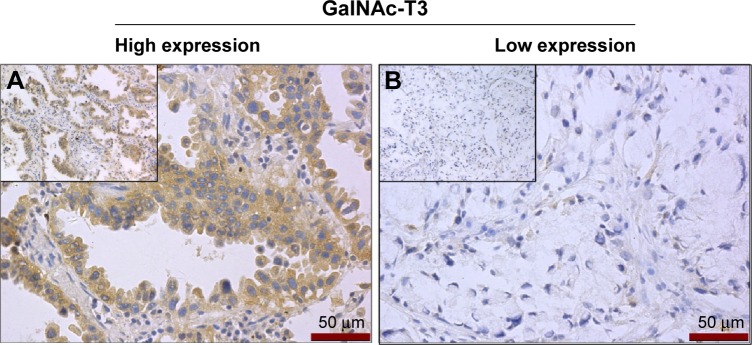
GalNAc-T3 IHC detection in adenocarcinoma.
Notes: The high and low expression of GalNAc-T3 was shown in (A) and (B), respectively (insert magnification 400×). High GalNAc-T3 expressing tumor cells had deep brown cytoplasm staining but low GalNAc-T3 expressing tumor cells without stained cytoplasm. The IHC staining corresponds to the acinar predominant growth pattern shown in Figure 1E.
Abbreviation: IHC, immunohistochemistry.
The expression of GalNAc-T3 was significantly associated with IASLC/ATS/ERS classification of risk groups, Sica score, differentiation, pleural invasion, vascular invasion, and tumor-node-metastasis (TNM) stage (P=0.007, P=0.036, P=0.023, P=0.007, P<0.001, and P=0.007, respectively) are shown in Table 2, but not sex, age, smoking status, CEA, and lymph node metastasis. Using logistic regression analysis, low GaINAC-T3 expression was correlated with a high-risk grade according to the IASLC/ATS/ERS classification, accompanying vascular invasion, and high-ranking TNM stage (excluding other confounding factors like Sica score or differentiation). These values are shown in Table 3. In addition, we detected that patients with lower GalNAc-T3 expression may have high-ranking TNM stage (odds ratio [OR] =8.975, 95% confidence interval [CI]: 1.797–44.661), vascular invasion (OR =5.668, 95% CI: 1.827–17.578), and the higher risk grade (low risk grade: OR =0.141, 95% CI: 0.027–0.719; moderate risk grade: OR =0.122, 95% CI: 0.017–40.871). Furthermore, the low expression of GalNAc-T3 in the micropapillary predominant pattern (highest risk group) was even up to 100%.
Table 2.
Relations between the level of GalNAC-T3 expression and clinicopathologic characteristics in 108 lung adenocarcinoma patients
| Variable | GalNAC-T3 expression
|
P-value | |
|---|---|---|---|
| Low (%) | High | ||
| Overall | 56 | 52 | |
| Age, years | 0.853 | ||
| <62 | 27 (50.9) | 26 | |
| ≥62 | 29 (52.7) | 26 | |
| Sex | 0.06 | ||
| Male | 32 (66.7) | 16 | |
| Female | 24 (40) | 36 | |
| Smoking status | 0.051 | ||
| Smoker | 32 (45.1) | 39 | |
| Never smoked | 24 (64.9) | 13 | |
| Concentration of CEA (μg/L) | 0.277 | ||
| <5 | 44 (49.4) | 45 | |
| ≥5 | 12 (63.2) | 7 | |
| IASLC/ATS/ERS classification risk group | |||
| Low risk grade | 5 (38.5) | 8 | 0.007 |
| AAH | 0 (100) | 1 | |
| AIS | 0 (100) | 1 | |
| MIA | 0 (100) | 1 | |
| Lepidic | 5 (50) | 5 | |
| Moderate risk grade | 37 (46.8) | 42 | |
| Papillary | 17 (56.7) | 13 | |
| Acinar | 20 (42.6) | 27 | |
| High risk grade | 14 (87.5) | 2 | |
| Micropapillary | 4 (100) | 0 | |
| Solid | 9 (75) | 3 | |
| Mucinous | 1 (50) | 1 | |
| Sica score | 0.036 | ||
| 0 or 2 or 3 | 6 (50) | 6 | |
| 4 | 22 (42.3) | 30 | |
| 5 or 6 | 28 (63.6) | 16 | |
| Differentiation | 0.023 | ||
| Well | 20 (39.2) | 31 | |
| Moderate | 28 (59.6) | 19 | |
| Poor | 8 (80) | 2 | |
| Pleural invasion | 0.007 | ||
| Absent | 44 (41.2) | 50 | |
| Present | 12 (82.1) | 2 | |
| Vascular invasion | <0.001 | ||
| Absent | 33 (40) | 47 | |
| Present | 23 (85.7) | 5 | |
| Lymph node metastasis | 0.064 | ||
| With | 6 (85.7) | 1 | |
| Without | 50 (49.5) | 51 | |
| TNM | 0.007 | ||
| Stage I and 0 | 42 (45.7) | 50 | |
| Stage II | 11 (84.6) | 2 | |
| Stage III | 3 (100) | 0 | |
Abbreviations: CEA, carcino-embryonic antigen; AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IASLC, International Association for the Study of Lung Cancer; ATS, the American Thoracic Society; ERS, European Respiratory Society; TNM, tumor-node-metastasis.
Table 3.
Logistic regression analyses on patients with low GalNAC-T3 expression and clinical pathologic characteristics
| Variable | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| IASLC/ATS/ERS classification risk group | |||
| High risk grade | 1 | ||
| Moderate risk grade | 0.122 | 0.017–0.871 | 0.018 |
| Low risk grade | 0.141 | 0.027–0.719 | 0.036 |
| Sica score | |||
| 0 or 2 or 3 | 1 | ||
| 4 | 0.286 | 0.016–4.981 | 0.391 |
| 5 or 6 | 0.227 | 0.011–5.597 | 0.334 |
| Differentiation | |||
| Poor | 1 | ||
| Moderate | 1.407 | 0.182–10.891 | 0.744 |
| Well | 0.699 | 0.093–5.266 | 0.010 |
| Pleural invasion | |||
| Absent | 1 | ||
| Present | 172.128 | 0.012–1,291.175 | 0.94 |
| Vascular invasion | |||
| Absent | 1 | ||
| Present | 5.668 | 1.827–17.578 | 0.003 |
| TNM stages | |||
| Stage I and 0 | 1 | ||
| Stage II and III | 8.957 | 1.797–44.661 | 0.007 |
Abbreviations: IASLC, International Association for the Study of Lung Cancer; ATS, the American Thoracic Society; ERS, European Respiratory Society; CI, confidence interval; TNM, tumor-node-metastasis.
Low expression of GalNAc-T3 in human lung adenocarcinoma cell lines
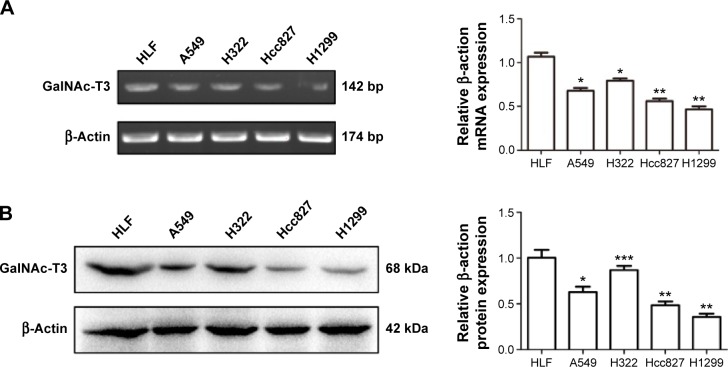
To study the expression of GalNAc-T3 in different lung adenocarcinoma cell lines and normal bronchial epithelium cell line, we extracted related total mRNA and proteins and analyzed them by RT-PCR and Western blot. The mRNA expression of GalNAc-T3 was lower in A549, H322, Hcc827, and H1299 adenocarcinoma cell lines as compared with HLF normal epithelium cell line (Figure 3A). Moreover, the A549, Hcc827, and H1299 cells had low GalNAc-T3 protein expression than HLF cells, except H322 cells (Figure 3B). We found that almost all lung adenocarcinoma cell lines had low expression of GalNAc-T3, while normal epithelium cell lines had high expression.
Figure 3.
Human lung adenocarcinoma cell lines (A549, H322, Hcc827, and H1299) and normal human embryonic lung fibroblasts HLF cell line were cultured in complete growth media, the total mRNA and protein were extracted and tested by RT-PCR and Western blot.
Notes: (A) and (B) were the result of the mRNA and protein expression of GalNAc-T3 respectively and the histogram was used as quantification of GalNAc-T3 expression in different cell lines. The expression of GalNAc-T3 was calculated relative to the β-actin expression. The data were repeated three times and are presented as the mean ± SD (*P<0.05, **P≤0.001, and ***P>0.005).
Abbreviations: RT-PCR, reverse transcription polymerase chain reaction; SD, standard deviation.
Survival analyses
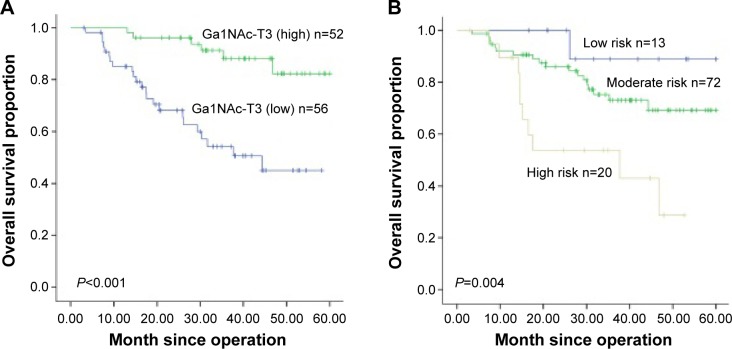
The 5-year overall survival and clinicopathologic characteristics of 108 patients with small peripheral pulmonary lumps was calculated by the Kaplan–Meier method and is shown in Table 1. The log-rank test indicated that sex (P=0.032), IASLC/ATS/ERS classification of risk group (P=0.034), Sica score (P=0.032), pleural invasion (P<0.001), vascular invasion (P<0.001), lymph node metastasis (P<0.001), GalNAc-T3 expression (P<0.001), and TNM stage (P<0.001) were significantly associated with survival outcome. The small peripheral lung adenocarcinoma patients with low GalNAc-T3 expression had a poorer prognosis than those with high GalNAc-T3 expression (5-year overall survival, 45% vs 82.3%), and this curve is shown in Figure 4A. Moreover, patients with higher IASLC/ATS/ERS classification grade had poorer prognosis shown in Figure 4B. Further, a multivariate Cox proportional hazards model analysis (Table 4) of the small peripheral lung adenocarcinoma patients indicated that vascular invasion (hazard ratio [HR]: 2.403, P=0.040), low GalNAc-T3 expression (HR: 3.317, P=0.016), TNM (P<0.001), and pleural invasion (HR: 7.958, P=0.021) were significant independent factors for predicting poor prognosis, but age, sex, smoking status, concentration of CEA, operating type, IASLC/ATS/ERS classification risk group, Sica score, differentiation, and lymph node metastasis were not.
Figure 4.
Survival curves by GalNAc-T3 expression and different grades of predominant growth patterns in adenocarcinoma.
Notes: (A) The overall 5-year survival rates in the patients with low GalNAc-T3 expression and high GalNAc-T3 expression were 25.4% and 72.1%, respectively (P<0.001). (B) Survival curves of low, moderate, and high risk grade of predominant growth patterns were 88.9%, 69.1%, and 28.6%, respectively (P=0.04).
Table 4.
Multivariate analysis of overall survival
| Variable | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| Vascular invasion | |||
| Absent | 1 | ||
| Present | 2.403 | 1.043–5.538 | 0.040 |
| Pleural invasion | |||
| Absent | 1 | ||
| Present | 7.958 | 1.374–46.079 | 0.021 |
| TNM | |||
| Stage I and 0 | 1 | ||
| Stage II | 19.409 | 4.228–314.333 | ,0.001 |
| Stage III | 36.454 | 3.810–98.873 | 0<001 |
| GalNAc-T3 expression | |||
| High | 1 | ||
| Low | 3.317 | 1.246–8.833 | 0.016 |
Abbreviations: CI, confidence interval; TNM, tumor-node-metastasis.
Discussion
We studied the expression of GalNAc-T3 and its association with clinicopathologic characteristics in patients with small peripheral lung adenocarcinoma, referring to the new classification of lung adenocarcinoma. Furthermore, we studied the expression of GalNAc-T3 in vitro and detected high-risk factors affecting survival of patients with small peripheral lung adenocarcinoma in order to provide a reference for individualized treatment.
GalNAc-T3, a member of the GalNAc-transferases family which transfers an N-acetyl galactosamine to the hydroxyl group of a serine or threonine residue in the first step of O-linked oligosaccharide biosynthesis,16 is involved in the occurrence and development of tumors, but its mechanism was not studied at present and needs further investigation. The expression of GalNAc-T3 is restricted to cell lines derived from gland adenocarcinoma. It has been reported to be involved in cell proliferation and cytoskeletal remodeling in different types of tumors.17–19,26,27 To our knowledge, only Gu et al22 (our study) and Dosaka-Akita et al15 reported the different expression of GalNAc-T3 in lung cancer, but conflicting conclusions were reached in lung adenocarcinoma. At present, the small (≤2 cm) peripheral lung adenocarcinoma was detected frequently following with the application of low-dose spiral CT or high-resolution CT screening. In our previous study,22 only 49 patients were found with small (≤2 cm) peripheral lung adenocarcinoma from original data, the restriction of number of patients may cause the statistical error. In order to define the expression of GalNAc-T3 and its prognostic value, we additionally collected 108 patients with small (≤2 cm) peripheral lung adenocarcinoma in the current study and found that, using IHC staining, 48.1% of tumor specimens showed high expression of GalNAc-T3 and 52.1% showed low expression. Low expression of GalNAc-T3 correlated with unfavorable overall survival and was a significant independent factor for predicting poor prognosis in patients with small peripheral lung adenocarcinoma, yet it was a good prognosis indicator in other type of carcinomas, including ovarian,26 renal,18 and thyroid.17 One of the reasons for this discrepancy maybe due to the tissue specificity or different cutoff values to judge low or high expression of GalNAc-T3. Dysregulated expression of GalNAc-T3 may possess tissue specificity in different types of cancer. Abnormal GalNAc-T3 expression changes glycosylation of membrane proteins which mediate tumor cell migration under special circumstances. For instance, high expression of GalNAc-T3 glycosylated vWF, a glycoprotein whose function is to bind adhesion molecule in the blood, subsequently assisted cancer cells in adhering to the vascular endothelium. On the contrary, high GalNAc-T3 expression should have increased the adhesion between cells and prevented tumor cells metastasizing from the primary tumor mass to distant positions. In addition, batch slides, the traditional IHC staining process, was used in our study. Although it is time-consuming, it completely reflects the expression of the whole-mount tissue slides compared with tissue microarrays reported in other researches.
In 108 patients with small peripheral lung nidus after complete surgical resection, low expression of GalNAc-T3 is significantly associated with high-ranking TNM stage (OR =8.975, 95% CI: 1.797–44.661), vascular invasion (OR =5.668, 95% CI: 1.827–17.578), the pathological risk grade (low risk grade: OR =0.141, 95% CI: 0.027–0.719; moderate risk grade: OR =0.122, 95% CI: 0.017–40.871). However, it showed no significant correlation with lymph node metastasis. This may be because most of cases we selected were early-stage lung adenocarcinoma, only seven (6.5%) patients had lymph node metastasis, and this resulted in no significant statistical difference. We discovered that the proportion of low GalNAc-T3 expression in micropapillary predominant pattern was 100%, and this was followed by solid (75%) and papillary (56.7%) predominant patterns. The results above suggest that Gal-NAc-T3 may play an important role in affecting the prognosis of patients with small peripheral lung adenocarcinoma, and tumors with low expression of GalNAc-T3 may indicate malignant biological behavior. The prognostic value of the IASLC/ATS/ERS classification of lung adenocarcinoma8 has been popularized. Sica et al25 delimited the new subtypes of lung adenocarcinoma risk grades and scores based on the prognosis and heterogeneity of pulmonary adenocarcinoma. We detected that low expression of GalNAc-T3 was significantly associated with IASLC/ATS/ERS classification of risk groups and Sica score using his method. IASLC/ATS/ERS classification of risk groups (P=0.034) and Sica score (P=0.032) were significantly associated with survival outcome based on univariate analyses; however, the two factors were not found to be significantly correlated with an increased risk of 5-year overall survival based on multivariate analysis. This may be due to the small study population, but it could not highlight the value of IASLC/ATS/ERS classification in clinical applications,28 and further research should be launched.
Conclusion
In conclusion, vascular invasion, low GalNAc-T3 expression, TNM stage, and pleural invasion were significantly independent factors for predicting poor prognosis of patients with small peripheral lung adenocarcinoma through our study, and GalNAc-T3 expression is found to be correlated with the IASLC/ATS/ERS classification of risk groups and Sica score.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81173453) and Municipal Science and Technology Program of Dalian, People’s Republic of China (2012E15SF141). We also appreciate Dr Guo Wei from Dalian Medical University Institute of Cancer Stem Cell for writing assistance.
Footnotes
Author contributions
All authors contributed to the design of the study and the preparation and critical revision of the manuscript, and agree to be accountable for all aspects of the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Levin B, editors. World Cancer Report 2008. Lyon, France: IARC Press; 2008. [Google Scholar]
- 3.Travis WD. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Vol. 7. Lyon, France: IARC Press; 2004. [Google Scholar]
- 4.Rudomina DE, Lin O, Moreira AL. Cytologic diagnosis of pulmonary adenocarcinoma with micropapillary pattern: does it correlate with the histologic findings? Diagn Cytopathol. 2009;37(5):333–339. doi: 10.1002/dc.21011. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi N, Toyooka S, Ichimura K, et al. Non-BAC component but not epidermal growth factor receptor gene mutation is associated with poor outcomes in small adenocarcinoma of the lung. J Thorac Oncol. 2008;3(7):704–710. doi: 10.1097/JTO.0b013e31817c6080. [DOI] [PubMed] [Google Scholar]
- 6.Kamiya K, Hayashi Y, Douguchi J, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocar-cinoma. Mod Pathol. 2008;21(8):992–1001. doi: 10.1038/modpathol.2008.79. [DOI] [PubMed] [Google Scholar]
- 7.Tomita M, Ayabe T, Chosa E, Kawagoe K, Nakamura K. Epidermal growth factor receptor mutations in Japanese men with lung adenocarcinomas. Asian Pac J Cancer Prev. 2014;15(24):10627–10630. doi: 10.7314/apjcp.2014.15.24.10627. [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30(13):1438–1446. doi: 10.1200/JCO.2011.37.2185. [DOI] [PubMed] [Google Scholar]
- 10.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24(5):653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 11.Mendonca-Previato L, Penha L, Garcez TC, Jones C, Previato JO. Addition of alpha-O-GlcNAc to threonine residues define the post-translational modification of mucin-like molecules in Trypanosoma cruzi. Glycoconj J. 2013;30(7):659–666. doi: 10.1007/s10719-013-9469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22(6):736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty S, Bonthu N, Swanson BJ, Batra SK. Role of mucins in the skin during benign and malignant conditions. Cancer Lett. 2011;301(2):127–141. doi: 10.1016/j.canlet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibao K, Izumi H, Nakayama Y, et al. Expression of UDP-N-acetyl-alpha-D-galactosamine-polypeptide galNAc N-acetylgalactosaminyl transferase-3 in relation to differentiation and prognosis in patients with colorectal carcinoma. Cancer. 2002;94(7):1939–1946. doi: 10.1002/cncr.10423. [DOI] [PubMed] [Google Scholar]
- 15.Dosaka-Akita H, Kinoshita I, Yamazaki K, et al. N-acetylgalactosaminyl transferase-3 is a potential new marker for non-small cell lung cancers. Br J Cancer. 2002;87(7):751–755. doi: 10.1038/sj.bjc.6600536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nomoto M, Izumi H, Ise T, et al. Structural basis for the regulation of UDP-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyl transferase-3 gene expression in adenocarcinoma cells. Cancer Res. 1999;59(24):6214–6222. [PubMed] [Google Scholar]
- 17.Mochizuki Y, Ito K, Izumi H, Kohno K, Amano J. Expression of polypeptide N-acetylgalactosaminyl transferase-3 and its association with clinicopathological factors in thyroid carcinomas. Thyroid. 2013;23(12):1553–1560. doi: 10.1089/thy.2012.0613. [DOI] [PubMed] [Google Scholar]
- 18.Kitada S, Yamada S, Kuma A, et al. Polypeptide N-acetylgalactosaminyl transferase 3 independently predicts high-grade tumours and poor prognosis in patients with renal cell carcinomas. Br J Cancer. 2013;109(2):472–481. doi: 10.1038/bjc.2013.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taniuchi K, Cerny RL, Tanouchi A, et al. Overexpression of GalNAc-transferase GalNAc-T3 promotes pancreatic cancer cell growth. Oncogene. 2011;30(49):4843–4854. doi: 10.1038/onc.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato K, Takeuchi H, Kanoh A, et al. Loss of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase 3 and reduced O-glycosylation in colon carcinoma cells selected for hepatic metastasis. Glycoconj J. 2010;27(2):267–276. doi: 10.1007/s10719-009-9275-4. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa M, Kitayama J, Kohno K, Nagawa H. The expression pattern of UDP-N-acetyl-alpha-D-galactosamine-polypeptide N-acetyl-galactosaminyl transferase-3 in squamous cell carcinoma of the esophagus. Pathobiology. 2005;72(3):139–145. doi: 10.1159/000084117. [DOI] [PubMed] [Google Scholar]
- 22.Gu C, Oyama T, Osaki T, et al. Low expression of polypeptide GalNAc N-acetylgalactosaminyl transferase-3 in lung adenocarcinoma: impact on poor prognosis and early recurrence. Br J Cancer. 2004;90(2):436–442. doi: 10.1038/sj.bjc.6601531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. New York, NY: John Wiley & Sons; 2011. [Google Scholar]
- 24.Ambrosini-Spaltro A, Ruiu A, Seebacher C, et al. Impact of the IASLC/ATS/ERS classification in pN0 pulmonary adenocarcinomas: a study with radiological-pathological comparisons and survival analyses. Pathol Res Pract. 2014;210(1):40–46. doi: 10.1016/j.prp.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocar-cinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol. 2010;34(8):1155–1162. doi: 10.1097/PAS.0b013e3181e4ee32. [DOI] [PubMed] [Google Scholar]
- 26.Wang ZQ, Bachvarova M, Morin C, et al. Role of the polypeptide N-acetylgalactosaminyltransferase 3 in ovarian cancer progression: possible implications in abnormal mucin O-glycosylation. Oncotarget. 2014;5(2):544–560. doi: 10.18632/oncotarget.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshimura Y, Nudelman AS, Levery SB, et al. Elucidation of the sugar recognition ability of the lectin domain of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase 3 by using unnatural glycopeptide substrates. Glycobiology. 2012;22(3):429–438. doi: 10.1093/glycob/cwr159. [DOI] [PubMed] [Google Scholar]
- 28.Yim J, Zhu L-C, Chiriboga L, Watson HN, Goldberg JD, Moreira AL. Histologic features are important prognostic indicators in early stages lung adenocarcinomas. Mod Pathol. 2007;20(2):233–241. doi: 10.1038/modpathol.3800734. [DOI] [PubMed] [Google Scholar]