Abstract
Although lipid disequilibrium has been documented for several types of cancer including colorectal cancer (CRC), it remains unknown whether lipid parameters are associated with the outcome of metastatic CRC (mCRC) patients. Here, we retrospectively examined the lipid profiles of 453 mCRC patients and investigated whether any of the lipid parameters correlated with the outcome of mCRC patients. Pretreatment serum lipids, including triglyceride, cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), were collected in 453 initially mCRC patients. The LDL-C to HDL-C ratio (LHR) was calculated and divided into the first, second, and third tertiles. Univariate and multivariate analyses were performed to evaluate the impact of lipids on overall survival (OS) and progression-free survival (PFS). Nearly two-fifths of the patients (41.3%) exhibited elevations in LDL-C while most patients (88.3%) showed normal HDL-C levels. Decreased HDL-C (P=0.542) and increased LDL-C (P=0.023) were prognostic factors for poor OS, while triglyceride (P=0.542) and cholesterol (P=0.215) were not. Multivariate analysis revealed that LDL-C (P=0.031) was an independent prognostic factor. Triglyceride, cholesterol, HDL-C, and LDL-C did not correlate with PFS. Among patients with elevations in LDL-C levels, patients in the third tertile of the LHR had a markedly shorter median OS compared to those in the first or second tertile (P=0.012). Thus, increased LDL-C level is an independent prognostic factor for poor prognosis in mCRC patients, and a high LHR predicts poor prognosis for initially mCRC patients with elevations in LDL-C.
Keywords: high low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, metastatic colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the most prevalent malignancies globally. While local CRC has a more favorable outcome with a 5-year survival rate of 90%, the presence of distant metastasis adversely impacts the survival of CRC patients with a 5-year survival of merely 12%1 despite the best currently available treatment modalities. Prevention, early diagnosis, and treatment can greatly reduce the incidence and mortality of metastatic CRC (mCRC) patients. It is also important to identify prognostic markers for mCRC for risk mitigation.
Cancer patients are considered to be in a constant state of malnutrition,2 likely due to hypermetabolism and cachexia. Lipid disequilibrium has been well documented in cancer patients.3 Saito et al4 followed up a total of 16,217 liver cancer patients over 25 years and found that low low-density lipoprotein cholesterol (LDL-C) levels were associated with elevated risk of liver cancer mortality and that this may be a predictive marker for liver-cancer-related death. In a prospective study of 244 breast cancer patients, Rodrigues et al5 found that the disease-free survival of breast cancer patients within the third LDL-C tertile were 12% higher than that of patients in the first tertile. It also has been found that CRC patients with distant metastases had markedly higher levels of total cholesterol (TC), LDL-C, and LDL-C to high-density lipoprotein cholesterol (HDL-C) ratio compared with patients without metastases, and the presence of metastases was positively associated with TC, LDL-C, and the LDL-C/HDL-C ratio (LHR).6 Liu et al7 retrospectively examined the fasting lipid profile of 968 patients undergoing curative resection for primary CRC and found that both LDL-C levels and LHR were independently associated with advanced N2 stage in male CRC patients and that the LHR could be a more effective biomarker for N2 stage CRC than LDL-C levels alone.
Although lipid disequilibrium has been documented for several types of cancer including CRC,3,8 it remains unknown whether lipid parameters are associated with the outcome of mCRC patients. In this study, we retrospectively examined the lipid profiles of 453 mCRC patients at a single tertiary care institution in the People’s Republic of China and investigated whether any of the lipid parameters correlated with the outcome of mCRC patients.
Patients and methods
Patients
Prior to use of the patients’ sera, written informed consent was obtained from each of the participants, and the experiment was approved by the Institute Research Ethics Committee of Cancer Center of Sun Yat-sen University, Guangzhou, People’s Republic of China. We retrospectively collected the clinicopathologic data of CRC patients who received medical treatment between January 1, 2005 and December 31, 2010 at Sun Yat-sen University Cancer Center. A patient was eligible for inclusion in the study: 1) if the patient had pathologically proven TNM stage IV CRC; 2) if the patient had completed at least three cycles of chemotherapy, including oxaliplatin- or irinotecan-based regimen; 3) if the patient had an Eastern Cooperative Oncology Group status score of 2 or less. A patient was excluded from the study: 1) if the patient had records of blood biochemical test before treatment; 2) if the patient did not have follow-up data; 3) if the patient had two or more primary tumors. The study protocol was approved by the local institutional review board at the authors’ affiliated institution.
Biochemical determinations
Baseline serum triglyceride, cholesterol, LDL-C, and HDL-C were routinely determined using a Hitachi Automatic Analyzer 7600-020 (Hitachi, Tokyo, Japan) when patients first presented at the hospital. The LHR was calculated and divided into the first, second, and third tertiles.
Patient follow-up
Patients were followed up by telephone interview twice a year to learn whether they were alive or not. The primary endpoint of this study was overall survival (OS), defined as the time interval from diagnosis to death of any cause or the time of the last follow-up visit. The secondary endpoint was progression-free survival (PFS) of first-line chemotherapy, which was defined as the time interval from the first administration of chemotherapy to the date of disease progression or death.
Statistical analysis
Descriptive statistics were used to describe patient demographic and baseline characteristics. Normally distributed data were expressed as median ± standard deviation and analyzed using the SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Nonnormally distributed data were expressed as median. Kaplan–Meier method was used to calculate survival curves, and log-rank test was used to compare differences. We performed multivariate analysis using Cox proportional hazards model, and independent significance was tested by backward elimination of insignificant explanatory variables. A P-value #0.05 was considered as significant.
Results
Patient demographic and baseline characteristics
The study flowchart is shown in Figure 1. Five hundred sixty-six CRC patients were identified in the database during the review period. Sixty-eight patients failed to meet the eligibility criteria and were excluded from the study. Among them, three patients declined treatment; 29 patients underwent palliative primary tumor resection but received no chemotherapy; and 36 patients only completed one or two cycles of chemotherapy. Consequently, a total of 453 patients were included in the final analysis. The demographic and baseline characteristics of these patients are shown in Table 1. Their median age was 59 (range, 18–89) years, and the majority of these patients were aged at least 50 years (62.9%) and were male (65.3%). CRC was located in the right colon in 38.9% of the patients followed by the left colon (32.2%) and the rectum (28.9%). Carcinoembryonic antigen was 5 ng/mL or above in most cases (82.6%). The majority of the patients (60.7%) had metastasis to one organ, 30.0% had metastasis to two organs, and 9.3% had metastasis to at least three organs.
Figure 1.
The study flowchart.
Table 1.
Demographic and baseline characteristics of patients with metastatic colorectal cancer
| Variables | N (%) |
|---|---|
| Number of patients | 453 (100) |
| Age (years) | |
| >50 | 285 (62.9) |
| Sex | |
| Male | 296 (65.3) |
| Location | |
| Right | 176 (38.9) |
| Left | 146 (32.2) |
| Rectal | 131 (28.9) |
| Palliative primary tumor resection | |
| Yes | 325 (71.7) |
| Chemotherapy | |
| First-line | |
| Oxaliplatin-based regimen | 303 (66.9) |
| Irinotecan-based regimen | 150 (33.1) |
| Second-line | |
| Oxaliplatin-based regimen | 96 (21.2) |
| Irinotecan-based regimen | 219 (48.3) |
| None | 138 (30.5) |
| Number of metastatic organs | |
| 1 | 275 (60.7) |
| 2 | 136 (30.0) |
| ≥3 | 42 (9.3) |
| CEA (ng/mL) | |
| >5 | 374 (82.6) |
| BMI (kg/m2) | |
| >24 | 105 (23.2) |
| ≤24 | 348 (76.8) |
Abbreviations: CEA, carcinoembryonic antigen; BMI, body mass index.
Treatment characteristics of the patients
The majority of patients (71.7%) underwent palliative primary tumor resection (Table 1). The median number of chemotherapy cycles was 7 (range, 3–12). For first-line chemotherapy, two-thirds of the patients (66.9%) received oxaliplatin-based regimen while the remaining one-third (33.1%) received irinotecan-based regimen. For second-line chemotherapy, approximately half of the patients (48.3%) received irinotecan-based regimen and one-fifth of the patients (21.2%) received oxaliplatin-based regimen. Approximately one-third of the patients (30.5%) received no second-line chemotherapy.
The lipid profile of the patients
The distribution of HDL-C, LDL-C, cholesterol, and triglycerides is shown in Table 2. The majority of the patients (78.2%) had normal triglyceride levels while nearly one-fifth of the patients (21.9%) had increased serum triglyceride contents. Meanwhile, most patients (89.2%) had normal cholesterol contents with one in ten patients (10.8%) had increased cholesterol levels. Slightly less than half of the patients (47.7%) had normal LDL-C while nearly two-fifths of the patients (41.3%) exhibited elevations in LDL-C. Most patients (88.3%) showed normal HDL-C levels, with about one in ten patients (10.8%) exhibiting reduced HDL-C levels. The median LHR was 3.02 (range, 0.61–28.38). Approximately 151 (33.33%) patients fell within the third tertile (3.51–28.38), 151 (33.33%) patients fell within the second tertile (2.55–3.50), and 151 (33.33%) patients fell within the first tertile (0.61–2.55).
Table 2.
The distributions of triglyceride, cholesterol, LDL-C, HDL-C, and LHR in patients with metastatic colorectal cancer
| Variable | Normal referent value | Median | Mean ± SD | Patients with elevated levels (n, %) | Patients with normal levels (n, %) | Patients with decreased levels (n, %) |
|---|---|---|---|---|---|---|
| Triglyceride (mmol/L) | 0.2–1.7 | 1.25 | 1.35±0.39 | 99 (21.85) | 354 (78.15) | 0 (0) |
| Cholesterol (mmol/L) | 2.1–6.4 | 4.96 | 5.15±1.18 | 49 (10.82) | 404 (89.18) | 0 (0) |
| LDL-C (mmol/L) | 2.2–3.4 | 3.12 | 3.32±1.15 | 187 (41.28) | 216 (47.68) | 50 (11.04) |
| HDL-C (mmol/L) | 0.78–2.2 | 1.12 | 1.14±0.32 | 4 (0.89) | 400 (88.30) | 49 (10.81) |
| ALT (U/L) | 0–40 | 21.15 | 27.95±22.69 | 61 (13.47) | 392 (86.53) | 0 (0) |
| AST (U/L) | 0–45 | 20.4 | 43.17±87.76 | 91 (20.09) | 362 (79.91) | 0 (0) |
| LHR | 3.03 | 3.30±1.89 |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LHR, LDL-C to HDL-C ratio; SD, standard deviation; U/L, units per liter; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
OS and PFS
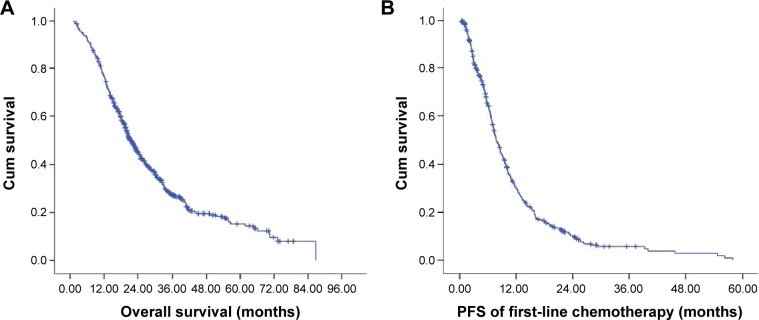
The median duration of follow-up time was 20 (range, 2–86) months. The Kaplan–Meier survival curve is shown in Figure 2. The median OS was 19.60 (range, 1.87–70.30) months and the median PFS was 6.47 (range, 1.57–58.03) months. One hundred thirty-eight (30.5%) patients were still alive at the last follow-up visit on September 30, 2014.
Figure 2.
The Kaplan–Meier curve for OS (A) and PFS (B) of the study patients.
Abbreviations: OS, overall survival; PFS, progression-free survival; Cum, cumulative.
LDL-C was an independent prognostic determinant of OS in mCRC patients
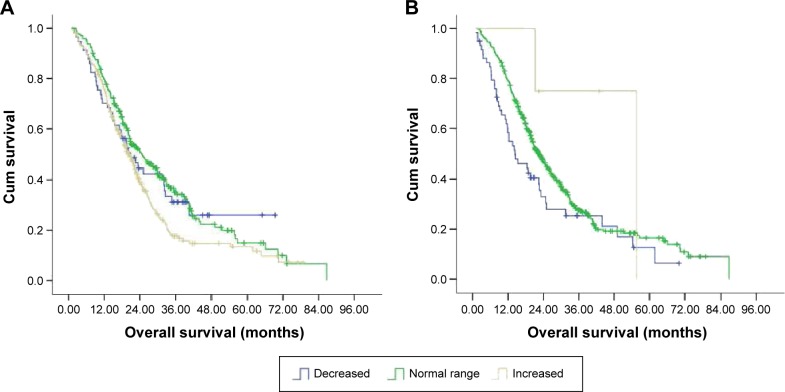
We were interested in and examined whether HDL-C, LDL-C, cholesterol, and triglyceride were prognostic determinants of mCRC patients. Our univariate analysis revealed that LDL-C (P=0.023) and HDL-C (P=0.035) were significant prognostic factors, while cholesterol (P=0.215) and triglyceride (P=0.542) were not. The median OS of patients with elevated LDL-C levels was 19.17 (range, 11.87–61.80) months while that of patients with normal LDL-C levels was 20.07 (range, 2.77–70.30) months (Figure 3A and Table 3). The median OS of patients with decreased LDL-C levels was 18.63 (range, 1.87–47.30) months. HDL-C was also a prognostic factor (P=0.035) while cholesterol (P=0.215) and triglycerides (P=0.542) were not (Table 3). The OS of patients with decreased and normal LDL-C was similar (P=0.652). In contrast, the median OS of patients with elevated HDL-C and those with normal or decreased HDL-C was 22.43 (range, 21.20–42.93) months and 16.28 (range, 1.87–70.30) months, respectively (P=0.006) (Figure 3B). Furthermore, multivariate analysis revealed that only LDL-C (P=0.031) was an independent predictor of outcome while HDL-C (P=0.337) was not (Table 4).
Figure 3.
The Kaplan–Meier curve for OS of the study patients stratified by LDL-C (A) and HDL-C (B).
Abbreviations: OS, overall survival; Cum, cumulative; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Table 3.
The OS and PFS of patients with metastatic colorectal cancer according to levels of triglyceride, cholesterol, LDL-C, and HDL-C (n=453)
| Variable | Patients (n) | Number of events | OS (months), median and range | P-value | PFS (months), median and range | P-value |
|---|---|---|---|---|---|---|
| Triglyceride | 0.542 | 0.984 | ||||
| Normal | 354 | 241 | 19.65 (1.87–70.30) | 6.70 (1.57–58.03) | ||
| Increased | 99 | 74 | 18.95 (2.87–54.60) | 9.34 (1.95–44.67) | ||
| Cholesterol | 0.215 | 0.127 | ||||
| Normal | 404 | 279 | 19.67 (2.70–70.30) | 7.67 (2.03–58.03) | ||
| Increased | 49 | 36 | 14.70 (1.87–55.03) | 6.46 (1.57–47.54) | ||
| LDL-C | 0.023 | 0.162 | ||||
| Decreased | 50 | 32 | 18.63 (1.87–47.30) | 5.56 (1.57–24.40) | ||
| Normal | 216 | 152 | 20.07 (2.77–70.30) | 7.28 (2.03–58.03) | ||
| Increased | 187 | 131 | 19.17 (11.87–61.80) | 8.16 (3.28–45.26) | ||
| HDL-C | 0.035 | 0.865 | ||||
| Decreased | 49 | 35 | 12.27 (1.87–61.80) | 5.52 (1.57–45.26) | ||
| Normal | 400 | 277 | 19.67 (1.87–70.30) | 7.85 (1.62–58.03) | ||
| Increased | 4 | 3 | 22.43 (21.20–42.93) | 6.35 (2.42–28.74) |
Abbreviations: OS, overall survival; PFS, progression-free survival; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Table 4.
Multivariate analysis of LDL-C and HDL-C as prognostic determinants of OS patients with metastatic colorectal cancer
| Variable | B | P-value | Exp(B) | 95% CI for exp(B) |
|---|---|---|---|---|
| LDL-C | −0.238 | 0.031 | 0.788 | 0.635–0.978 |
| HDL-C | 0.683 | 0.337 | 1.980 | 0.491–7.987 |
Abbreviations: OS, overall survival; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CI, confidence interval.
HDL-C, LDL-C, cholesterol, or triglyceride shows no correlation with PFS
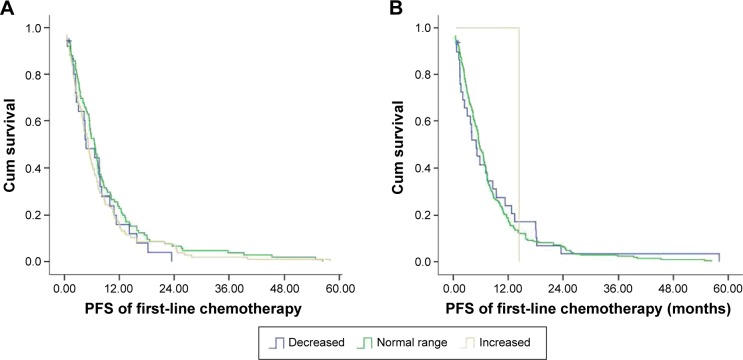
We further evaluated whether HDL-C, LDL-C, cholesterol, and triglyceride correlated with PFS of mCRC patients (Table 3). The median PFS of patients with elevated LDL-C levels was 8.16 (range, 3.28–45.26) months while that of patients with normal LDL-C levels was 7.28 (range, 2.03–58.03) months (Figure 4A and Table 3). The median PFS of patients with decreased LDL-C levels was 5.56 (range, 1.57–24.40) months. The median PFS of patients with elevated HDL-C and those with normal or decreased HDL-C was 7.85 (range, 1.62–58.03) months and 5.52 (range, 1.57–45.26) months, respectively (P=0.865) (Figure 4B). None of the parameters including HDL-C, LDL-C, cholesterol, and triglyceride correlated with PFS.
Figure 4.
The Kaplan–Meier curve for PFS of the study patients stratified by LDL-C (A) and HDL-C (B).
Abbreviations: PFS, progression-free survival; Cum, cumulative; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
The LHR is a novel prognostic factor
Our findings indicated that LDL-C negatively correlated with OS while HDL-C positively correlated with OS. We speculated that the LHR could be a more discriminative prognostic predictor. The median OS of mCRC patients in the second or third tertile of the LHR was 16.70 (range, 2.53–66.73) months, which was significantly shorter than that of patients in the first tertile of the LHR (P<0.001) (median, 21.67 months; range, 1.87–70.30 months). To evaluate whether LHR was superior to LDL-C, we stratified patients according to their LDL-C levels. Among patients with elevations in LDL-C levels, patients in the third tertile of the LHR had a markedly shorter median OS (16.73 months; range, 11.87–60.47 months) compared to those in the first (22.77 months; range, 13.73–61.8 months) or second tertile (21.03 months; range, 12.67–61.0 months) (P=0.012; Figure 5 and Table 5). Although patients in the third tertile who had no elevations in LDL-C showed a lower median OS (15.62 months; range, 2.53–49.07 months), no statistically significant difference was observed in OS between these patients and patients in the first (21.17 months; range, 1.87–70.30 months) or second tertile (19.97 months; range, 2.50–66.73 months) of the LHR (P=0.073).
Figure 5.
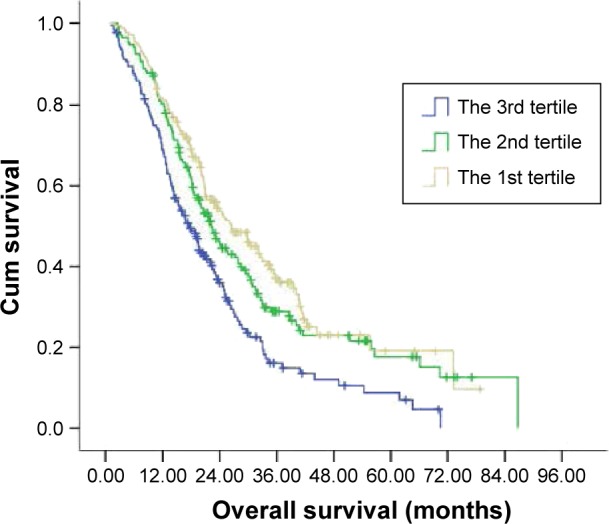
The Kaplan–Meier curve for OS of the study patients stratified by the LHR.
Abbreviations: OS, overall survival; Cum, cumulative; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LHR, LDL-C to HDL-C ratio.
Table 5.
The OS of patients with metastatic colorectal cancer according to the LHR
| T1 | T2 | T3 | P-value | |
|---|---|---|---|---|
| Patients with elevations in LDL-C | 0.01 | |||
| Patients (n) | 13 | 55 | 119 | |
| Number of events | 8 | 22 | 96 | |
| Median | 22.77 | 21.03 | 16.73 | |
| Range | 13.73–61.8 | 12.67–61.0 | 11.87–60.47 | |
| Patients with no elevations in LDL-C | 0.07 | |||
| Patients (n) | 138 | 96 | 32 | |
| Number of events | 90 | 76 | 23 | |
| Median | 21.17 | 19.97 | 15.62 | |
| Range | 1.87–70.30 | 2.50–66.73 | 2.53–49.07 |
Abbreviations: OS, overall survival; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LHR, LDL-C to HDL-C ratio; T1, T2, and T3 represent the first, second, and third tertiles of LDL-C contents, respectively.
Discussion
Lipid disequilibrium has been documented for several types of cancer, including CRC.3,8 In this paper, we found that 41.28% of mCRC patients showed elevations in LDL-C. Our univariate analysis revealed that baseline LDL-C and HDL-C were prognostic determinants of mCRC, and our multivariate analysis found that LDL-C was an independent prognostic predictor of mCRC. Furthermore, the LHR could also yield prognostic information, with a high LHR predicting an adverse outcome for patients with elevations in LDL-C.
Cholesterol is a structural component of the cell membrane and is localized in membrane microdomains that assemble the signal transduction machinery and associate to proteins implicated in key cellular signaling pathways that are closely associated with malignant transformation.9 LDL-C and HDL-C are lipoproteins responsible for cholesterol transportation. However, there has been scant knowledge available about LDL-C in cancer patients. This study was the first to examine the correlation between LDL-C, HDL-C, and the LHR and the OS of mCRC patients. Our findings are consistent with those reported by others.6 Notarnicola et al6 found that metastases in CRC patients were positively associated with LDL-C and the LHR. Liu et al7 also found that both LDL-C levels and the LHR were independently associated with advanced N2 stage in male CRC patients.
Although the relation between lipids and cancer has been known for years, most previous studies have focused on the influence of lipids on the incidence of tumor among healthy subjects.10,11 It was reported that people with low LDL-C level were more likely to develop a tumor.10,11 It was reported that most cancer patients had low LDL-C and cholesterol levels.8 Cancer patients are in poor nutritional status as a result of cachexia and poor appetite due to chemotherapy and other treatment modalities. Cancer cells could also take up and degrade a large amount of LDL-C in order to compose biomembrane, required for the division of malignant cells.12 Previous studies involved patients with various cancer types, including breast cancer, gastric cancer, and lung cancer.8 However, patients with different types of cancer might have different LDL-C profiles. For example, breast cancer patients had elevated LDL-C levels13 whereas gastric and lung cancer patients8 had decreased LDL-C levels. In this paper, we focused on mCRC patients, and found that 41.28% of these patients had increased LDL-C, which was an adverse prognostic factor for mCRC patients. We saw increased rather than decreased LDL-C levels in these patients which may be due to the fact that we focused on initially mCRC patients. Notarnicola et al6 reported that LDL-C levels may increase as CRC develops from the early stage to the advanced stage. Several other studies also support our result that increased LDL-C was a predictor for poor prognosis. Montel et al14 and Song et al15 reported that cancer cells expressed LDL-receptor-related protein, which could facilitate the growth and invasion of cancer cells. Another study reported16 that oxidized LDL could stimulate the proliferation of ovarian carcinoma cells. These results could help explain the unfavorable influence of LDL-C on OS observed in our study.
In our study, although both LDL-C and HDL-C were prognostic factors, only LDL-C was found to be independent. The exact reason was unknown. Tumor cells need plenty of lipids to maintain division. Both increased LDL-C and decreased HDL-C reflected that lipids were more likely to be transported into the blood rather than stored in the liver. We speculated that both increased LDL-C and decreased HDL-C indicated that tumor had resulted in altered lipid profile, in a way favoring the growth and division of tumor cells. The reason why only LDL-C was independent might be that tumor cells express LDL-C receptor and could directly uptake and degrade LDL-C.3 To fully consider the implications of both LDL-C and HDL-C, we also studied the prognostic value of the LHR, and found that the LHR could provide further information based on LDL-C. Thus, the LHR may be a promising prognostic factor in mCRC patients, especially those with elevations in LDL-C.
Since both preclinical and clinical data support that LDL-C plays a role in cancer patients, one important issue is whether bringing down LDL-C is of therapeutic value for mCRC patients. Although the impact of statin, a commonly used agent used to reduce lipid, on the incidence of cancer had been extensively studied,11,17–19 it was reported that statin was correlated with a significant reduction in CRC risk.20,21 To the best of our knowledge, no study had evaluated the therapeutic value of statin in mCRC patients. Further studies are urgently needed to evaluate the therapeutic effect of statins in mCRC patients.
There were several limitations in this study. First, the retrospective nature restricted its power. Second, it is not clear that whether the increased LDL-C was caused by CRC. In other words, there were two possible situations: 1) cancers which gained the ability to adjust lipid profile yield poor prognosis and 2) patients who had increased LDL-C yield poor prognosis when they developed CRC. Third, increased LDL-C itself was a risk factor. There was one possibility that there was no interaction between LDL-C and cancer, and the unfavorable prognosis was caused by risks resultant from increased LDL-C. Further prospective studies are expected to answer those questions.
In conclusion, we found that increased LDL-C level was a prognostic factor for poor prognosis in mCRC patients. Further prospective and multiple center studies are required, and the therapeutic value of statin in mCRC patients is also worth being explored.
Acknowledgments
The authors thank the patients and the colleagues at the Sun Yat-sen University Cancer Center who participated in this study. The abstract of this paper was presented at the 2014 World Congress as an oral presentation with interim findings. The abstract was published in Asia-Pacific Journal of Clinical Oncology titled as “A high LDL-C to HDL-C ratio predicts poor prognosis for initially metastatic colorectal cancer patients with elevations in LDL-C”. This study was supported by grants from the Natural Science Foundation of Guangdong, China (2015A030313010), Science and Technology Program of Guangzhou, China (1563000305) and National Natural Science Foundation of China (81272641and 81572409).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hu T, Yao Y, Yu S, et al. Clinicopathologic significance of CXCR4 and Nrf2 in colorectal cancer. J Biomed Res. 2013;27(4):283–290. doi: 10.7555/JBR.27.20130069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rose G, Blackburn H, Keys A, et al. Colon cancer and blood-cholesterol. Lancet. 1974;1(7850):181–183. doi: 10.1016/s0140-6736(74)92492-1. [DOI] [PubMed] [Google Scholar]
- 3.Vitols S, Gahrton G, Bjorkholm M, Peterson C. Hypocholesterolaemia in malignancy due to elevated low-density-lipoprotein-receptor activity in tumour cells: evidence from studies in patients with leukaemia. Lancet. 1985;2(8465):1150–1154. doi: 10.1016/s0140-6736(85)92679-0. [DOI] [PubMed] [Google Scholar]
- 4.Saito N, Sairenchi T, Irie F, et al. Low serum LDL cholesterol levels are associated with elevated mortality from liver cancer in Japan: the Ibaraki Prefectural health study. Tohoku J Exp Med. 2013;229(3):203–211. doi: 10.1620/tjem.229.203. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues Dos Santos C, Fonseca I, Dias S, Mendes de Almeida JC. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Cancer. 2014;14:132. doi: 10.1186/1471-2407-14-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Notarnicola M, Altomare DF, Correale M, et al. Serum lipid profile in colorectal cancer patients with and without synchronous distant metastases. Oncology. 2005;68(4–6):371–374. doi: 10.1159/000086977. [DOI] [PubMed] [Google Scholar]
- 7.Liu YL, Qian HX, Qin L, Zhou XJ, Zhang B. Serum LDL-C and LDL-C/HDL-C ratio are positively correlated to lymph node stages in males with colorectal cancer. Hepatogastroenterology. 2011;58(106):383–387. [PubMed] [Google Scholar]
- 8.Muntoni S, Atzori L, Mereu R, et al. Serum lipoproteins and cancer. Nutr Metab Cardiovasc Dis. 2009;19(3):218–225. doi: 10.1016/j.numecd.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327(5961):46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs D, Blackburn H, Higgins M, et al. Report of the conference on low blood cholesterol: mortality associations. Circulation. 1992;86(3):1046–1060. doi: 10.1161/01.cir.86.3.1046. [DOI] [PubMed] [Google Scholar]
- 11.Emberson JR, Kearney PM, Blackwell L, et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7(1):e29849. doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mack JT, Townsend DM, Beljanski V, Tew KD. The ABCA2 transporter: intracellular roles in trafficking and metabolism of LDL-derived cholesterol and sterol-related compounds. Curr Drug Metab. 2007;8(1):47–57. doi: 10.2174/138920007779315044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laisupasin P, Thompat W, Sukarayodhin S, Sornprom A, Sudjaroen Y. Comparison of serum lipid profiles between normal controls and breast cancer patients. J Lab Physicians. 2013;5(1):38–41. doi: 10.4103/0974-2727.115934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montel V, Gaultier A, Lester RD, Campana WM, Gonias SL. The low-density lipoprotein receptor-related protein regulates cancer cell survival and metastasis development. Cancer Res. 2007;67(20):9817–9824. doi: 10.1158/0008-5472.CAN-07-0683. [DOI] [PubMed] [Google Scholar]
- 15.Song H, Li Y, Lee J, Schwartz AL, Bu G. Low-density lipoprotein receptor-related protein 1 promotes cancer cell migration and invasion by inducing the expression of matrix metalloproteinases 2 and 9. Cancer Res. 2009;69(3):879–886. doi: 10.1158/0008-5472.CAN-08-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li AJ, Elmore RG, Chen IY, Karlan BY. Serum low-density lipopro-tein levels correlate with survival in advanced stage epithelial ovarian cancers. Gynecol Oncol. 2010;116(1):78–81. doi: 10.1016/j.ygyno.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: a systematic review and meta-analysis. Eur J Cancer. 2008;44(15):2122–2132. doi: 10.1016/j.ejca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295(1):74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 19.Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Statins and cancer risk: a literature-based meta-analysis and meta-regression analysis of 35 randomized controlled trials. J Clin Oncol. 2006;24(30):4808–4817. doi: 10.1200/JCO.2006.06.3560. [DOI] [PubMed] [Google Scholar]
- 20.Poynter JN, Gruber SB, Higgins PD, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352(21):2184–2192. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 21.Broughton T, Sington J, Beales IL. Statin use is associated with a reduced incidence of colorectal cancer: a colonoscopy-controlled case-control study. BMC Gastroenterol. 2012;12:36. doi: 10.1186/1471-230X-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]