Abstract
While the association between smoking and HPV infection, cervical cancer, and anal cancer has been well studied, evidence on the association between cigarette smoking and anal warts is limited. The purpose of this study was to investigate if cigarette smoking status influences the size of anal warts over time in HIV-infected women in a sample of 976 HIV-infected women from the Women’s Interagency HIV Study (WIHS). A linear mixed model was used to determine the effect of smoking on anal wart size. Even though women who were currently smokers had larger anal warts at baseline and slower growth rate of anal wart size after each visit than women who were not current smokers, there was no association between size of anal wart and current smoking status over time. Further studies on the role of smoking and interaction between smoking and other risk factors, however, should be explored.
Keywords: Anal warts, HIV infection, human papillomavirus, smoking
INTRODUCTION
The prevalence of genital warts among sexually active adults in the United States is estimated to be approximately 1%.1 HIV-infected persons are more likely to have genital warts than HIV-uninfected persons; they also have a greater risk for recurrence of warts.2,3 Specifically, anal warts pose a major problem for HIV-infected individuals and thus, should receive special attention for several reasons. They have not been studied separately from genital warts, and yet there are indications that anal warts might be more common than cervical warts in women.4 Furthermore, once infected with one type of HPV, patients are more likely to be infected with other HPV types (both low- and high-risk). In fact, some studies have recently shown that 20–50% of genital warts are co-infected with HPV high-risk types5,6. There is also evidence for a strong association between the presence of anal warts and the development of anal intraepithelial neoplasia (AIN), a precancerous lesion for anal cancer.7 Accordingly, Carter at et.7 reported that men with anal warts were 4.70 times (95% CI: 1.81–12.20) more likely to develop AIN than men without anal warts. Recently, from a Danish study of approximately 50,000 patients with genital warts, Blomberg et al.8 found that genital wart diagnosis is strongly associated with anal cancer (standardized incidence ratio: 12.5 and 7.8 for men and women, respectively). Finally, the serious economical9,10 and psychological11,12 burdens associated with having anal warts must also be considered. In 1997, the estimated cost of HPV burden was $3.8 billion (excluding HPV-related cervical cancer) or more than one third of the $10 billion spent annually on common STDs (excluding HIV) and related syndromes.9 Also different studies have reported decreased self-esteem and increased psychological distress, embarrassment, anger, shame, negative self-perception, anxiety, and relationship difficulties among patients with anogenital warts.11,12 It is therefore important to determine risk factors for the development, progression or regression of anal warts.
The role of smoking in cervical cancer was first reported by Naguib et al. in 1966.13 The concentration of nicotine was 45 times higher in cervical tissue than in serum of smoking women.14 Tobacco smoke is likely to contribute to carcinogenesis through its impact on immune function thereby altering the natural history of HPV infection and acting as a co-carcinogen in cervical tissue.15 Also, the relationships between cigarette smoking and HPV infection,16–18 as well as cervical and anal cancer19–30 have been explored and are well-documented. Evidence on the association between cigarette smoking and anogenital warts, in general, and anal warts, in particular, is limited and has only recently received attention in the literature. Accordingly, Feldman et al.31 reported that the incidence of genital warts was almost 3 times higher in smokers than non-smokers, both in HIV-infected women (13.3 vs. 5.0, respectively) and HIV-uninfected women (1.5 vs. 0.5, respectively). However, to date, there has been no study published on the association between cigarette smoking and anal warts.
Given the burden of disease and lack of understanding of risk factors for development of anal warts (i.e., smoking), we investigated whether cigarette smoking status is associated with the size of the largest anal wart in HIV-infected women over time using the public dataset obtained from the WIHS, an on-going cohort study of HIV-infected and HIV-uninfected women in the United States.
METHODS
Study population
Data used for the current analysis were obtained from the public dataset (release 09) of WIHS. WIHS is an on-going prospective study of HIV-infected and uninfected women from six locations in the US: Bronx/Manhattan, NY; Brooklyn, NY; Washington DC; Los Angeles/Southern California/Hawaii; San Francisco/Bay Area, CA; and Chicago, IL. Details on the WIHS study were described previously.32,33 Briefly, WIHS recruited study participants through two enrollment phases: 1994–1995 and 2001–2002. The first enrollment phase was between October 1994 and November 1995. Initially, 2,059 HIV-infected women and 569 HIV-uninfected women were recruited from both clinic-based and population-based sources. Inclusion criteria in the first enrollment phase were: 1) being at least 13 years of age; 2) giving informed consent; 3) being tested for HIV; 4) ability to complete the interview in either English or Spanish; 5) ability to travel to and from the clinic site to participate in a baseline visit; and 6) giving blood for laboratory testing. During the second enrollment phase between October 2001 and September 2002, 1,144 women were recruited. Besides the above criteria, participants who were recruited in the second enrollment also met the following criteria: 1) documented results of an HIV ELISA and confirmatory Western blot for HIV-infection or documented HIV-negative status (within 30 days before recruitment); 2) no history of clinical AIDS-related conditions (confirmed by medical record abstraction); 3) documented laboratory testing results of HIV RNA levels and CD4 counts surrounding the HAART period for those enrolled as HAART exposed; and 4) consent to give specimens (31). During the first enrollment phase, frequency matching (age, ethnicity, education level, injection drug use since 1978, and total number of sex partners since 1980) was employed to ensure the comparability between HIV-infected and HIV-uninfected groups.
The WIHS study protocols included a baseline visit and follow-up visits every 6 months, conducted by trained interviewers and examiners. Information obtained during interview included general medical history, obstetric and gynecologic history, HAART use, alcohol and cigarette use, and sexual behaviors. Medical examination, gynecologic examination, and medical record abstraction were conducted during baseline and follow-up visits. Medical examination included height/weight/vital signs, lymph nodes, and abdomen. Gynecologic examination included external genitalia, internal vagina and cervix, cervical-vaginal lavage, bimanual and rectal exam, and colposcopy, biopsy, and dysplasia treatment if necessary. Medical record abstraction included development of cancer, infectious diseases or opportunistic infections, and any biopsies, surgeries or hospitalization as well as medications received.32,33
Details on specimens and laboratory techniques were previously reported by Barkan et al.32 and Bacon et al.33 Key clinical information collected in follow-up visits was CD4 and CD8 cell count, HIV sero-status among HIV-uninfected women, and Pap smear outcomes.34
Variables of interest and measurement
Outcome variable
The outcome variable for the current analysis was size of the largest anal wart present at the given visit. For this purpose, only those who had at least one anal wart during the course of follow-up were included in the analysis. During the gynecologic examination, a trained examiner identified the presence of anal warts, and then measured the length and width (in millimeters) of the largest wart. The size (area) of the anal wart was calculated by multiplying the width and length of the reported largest anal wart. The wart was identified as an anal wart if it presented in one of the following locations: “anus upper left”, “anus lower left”, “anus upper right”, “anus lower right”, “perineum left”, and “perineum right”. We assumed that the largest wart is an anal wart if there were multiple warts reported.
Independent variable
The independent variable for this analysis was current smoking status. This was obtained as “Yes”/“No” answer to the following question at baseline and each subsequent visit: “Do you currently smoke cigarettes?”
Other variables
CD4+ cell count (<200, 200–500, and >500 cells/mm3) and HIV viral load (<4,000, 4,000–20,000, 20,001–100,000, and >100,000 copies/mL) were analyzed descriptively, as they are important variables in the WIHS study. Potentially confounding variables included in the current analysis were: race/ethnicity (African-American, Caucasian, and others), number of sex partners in the past six months (0 and ≥1 sex partners), education level (less than high school education, high school education or GED, some college, and college graduate or graduate school), annual household income (≤$6,000, $6,001–$12,000, $12,001–$24,000, and ≥24,001), marital status (married or living with partner; widowed, separated or divorced; and never married), enrollment phase (enrollment phase 1 and enrollment phase 2), and HAART use (“Yes”/“No” if on HAART at that particular visit).
Blood was drawn at each visit for determination of HIV status, CD4+ cell count and HIV viral load. Laboratories certified by the AIDS Clinical Trial Groups measured CD4+ cell count level using an established flow cytometry technique.32 Serum HIV viral load was measured using the nucleic acid sequence-based amplification assay (NASBA) by Organon Teknika (Oklahoma City, OK). HIV viral load tests were conducted at the National Institute of Allergy and Infectious Diseases, AIDS Program, Virology Assurance HIV RNA Proficiency Program.32 A person was considered on HAART if she met one of the following criteria: 1) Two or more nucleoside reverse transcriptase inhibitors (NRTIs) in combination with at least one protease inhibitor (PI) or one non-nucleoside reverse transcriptase inhibitors (NNRTI); 2) One NRTI in combination with at least one PI and at least one NNRTIs; 3) Regimen containing ritonavir and saquinavir in combination with one NRTI and no NNRTI; 4) An abacavir or tenofovir containing regimen of 3 or more NRTIs in the absence of both PI and NNRTIs, except for the three-NRTI regimens consisting of: abacavir + tenofovir + lamivudine or didanosine + tenofovir + lamivudine. Combination of zodovudine (AZT) and stavudine (d4T) with either a PIT or NNRTI were not considered HAART. Monotherapy is considered as taking one NRTI, or only PI, or only NNRTI. This definition of HAART use followed the guidelines of the US Department of Health and Human Services,35 the International AIDS-Society Panel Antiretroviral Guidelines36 and is consistent with previous analyses from WIHS.37,38
Statistical analysis
The distributions of socio-demographic characteristics were examined. We calculated mean and standard deviation of continuous variables and counts and their respective frequencies of categorical variables. Initially, CD4+ cell count and HIV viral load were provided as continuous variables. We used 10 copies/mL for those whose HIV viral load were suppressed to undetectable levels as it was identified and validated by Norteman et al.39 CD4+ cell count was categorized into three groups (<200, 200–500, and >500 cells/mm3) and HIV viral load was categorized into four groups (<4,000, 4,000–20,000, 20,001–100,000, and >100,000 copies/mL) as in previous WIHS studies.37,40–43
A linear mixed model was employed to determine the relationship between size of the largest anal wart and current smoking status at each visit. We chose this model over other statistical methods because of the following advantages. First, it is able to deal with missing values which are common in longitudinal studies. Second, it adjusts for the highly correlated nature of repeated measurements within and between individuals in longitudinal studies. Third, it is able to deal with the problem of unbalanced measurements (i.e., number of visit in our study) of subjects and the time interval between measurements.44 In WIHS study, the time interval between measurements was approximately equal (i.e., 6 months between visits).
An unadjusted model was first developed to determine the total variation of growth velocity.44 Next, an adjusted model was built including the following covariates: number of sex partners in the past six months, education level, marital status, enrollment phase, HAART use, and annual household income. Current smoking status (i.e., independent variable), was treated as a time-dependent variable in both the unadjusted and adjusted models. In the adjusted models, number of sex partners in the past six months, education level, marital status, annual household income, and HAART use were also treated as time-dependent variables. In other words, they were entered into the adjusted model both as a main effect and as a product with time (represented by visit number). The time-independent variables included race/ethnicity and enrollment phase and were entered in to the adjusted model as a main effect only. We use those covariates because they have been identified as potential confounders and been used consistently throughout other analyses in WIHS study.37,43,45–48 CD4+ cell count and HIV viral load were not included in the final model because we previously reported that there was no association between them and the size of anal warts.49 The PROC MIXED command of SAS 9.2 statistical package (Cary, NC) was used in the modeling process.50 All tests were two-sided and P = 0.05 was used as the cut-off for significance.
RESULTS
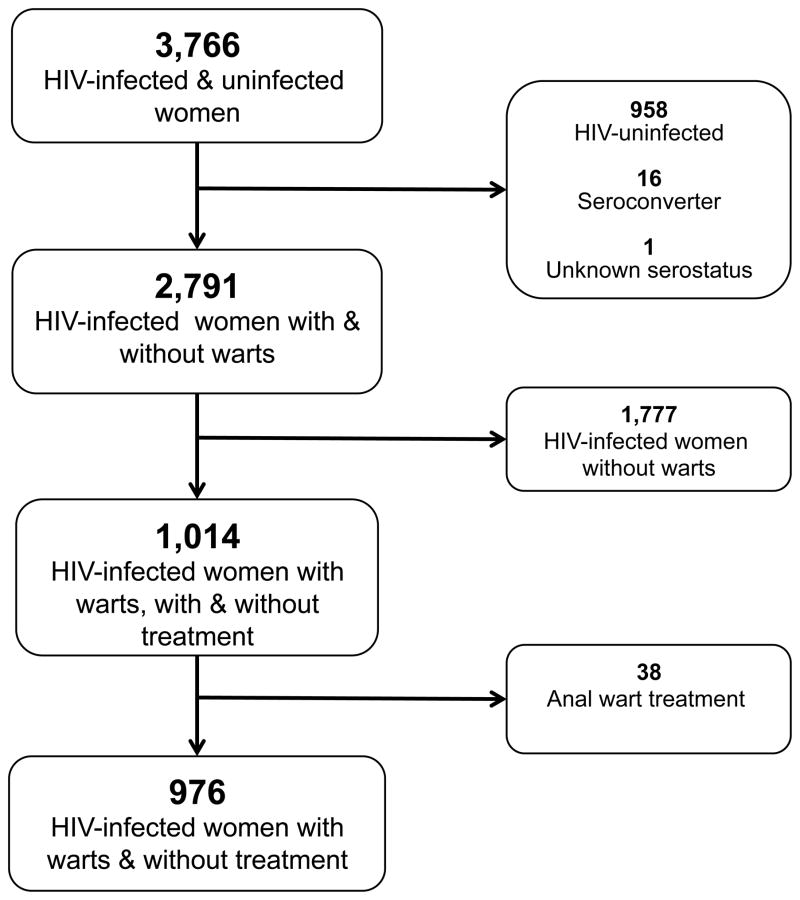
In the current study (between October 1994 and March 2006), the follow-up has 23 possible visits with 3,766 HIV-infected and -uninfected women. Exclusion criteria were HIV-uninfected women (n = 958), women who sero-converted during the study (n = 16) or those with unknown HIV sero-status (n = 1), women without anal warts during the entire follow-up period (n = 1,777), and women who underwent treatment for anal warts during the study (n = 38). Women who received treatment for anal warts were excluded because the various treatment modalities could greatly influence the size of anal warts in differing ways during follow-up, and there were not enough participants in this group to conduct a meaningful sub-group analysis. Finally, a sample of 976 women was available for this analysis (Figure 1).
Figure 1.
Flowchart of inclusion and exclusion of participants in current analysis.
Approximately 20% of participants had a CD4+ cell count less than 200 cells/mm3, and 50% had a HIV viral load more than 100,000 copies/mL. More than 65% of participants were current smokers at the baseline visit. Approximately 66% of study participants were African-American while the frequencies of Caucasian and the other race/ethnicity groups (i.e., Hispanic, Asian/Pacific Islanders, and Native America/Alaskan Native) were similar (19.42% and 19.94%, respectively) (Table 1).
Table 1.
Baseline Socio-demographic Characteristics of the WIHS HIV-infected Participants in the Current Study
| Characteristics | WIHS study (976) (n, %) |
|---|---|
| CD4+ cells count (cells/mm3) | |
| Mean CD4+ cell count±SD | 324.59±293.04 |
| <200 | 148 (19.79) |
| 200–500 | 328 (43.85) |
| >500 | 272 (36.36) |
| HIV RNA viral load (copies/mL) | |
| Mean viral load±SD | 181,175±1,039,797 |
| <4,000 | 331 (34.77) |
| 4,000–20,000 | 164 (17.23) |
| 20,001–100,000 | 215 (22.58) |
| >100,000 | 242 (25.42) |
| Cigarette smoking status | |
| Current smokers | 565 (65.39) |
| Not current smokers | 299 (34.61) |
| Number of cigarette smoked per day among current smokers | |
| <10 cigarettes/day | 288 (64.16) |
| 10–20 cigarettes/day | 44 (11.43) |
| ≥20 cigarettes/day | 94 (24.42) |
| Age (Median±SD) | 36.56±7.85 |
| ≤25 | 66 (6.77) |
| 26–35 | 383 (39.28) |
| 36–45 | 407 (41.74) |
| >45 | 119 (12.21) |
| Ethnicity | |
| Caucasian American | 189 (19.42) |
| African American | 590 (60.64) |
| Others | 194 (19.94) |
| Education | |
| <High school education | 317 (36.35) |
| High school education or GED | 295 (33.83) |
| Some college | 207 (23.74) |
| College graduate or graduate school | 53 (6.08) |
| Annual household income | |
| ≤$6,000 | 125 (25.61) |
| $6,001–$12,000 | 171 (35.04) |
| $12,001–$24,000 | 118 (24.18) |
| ≥24,001 | 74 (15.16) |
| Marital status | |
| Married or living with partner | 245 (35.00) |
| Widowed | 55 (7.86) |
| Separated or divorced | 146 (20.86) |
| Never married | 254 (36.29) |
| Number of male sex partners in the past 6 months | |
| 0 | 259 (27.52) |
| ≥1 | 682 (72.48) |
| HAART use at baseline | |
| No | 285 (97.60) |
| Yes | 7 (2.40) |
| Mean size of anal warts (mm2) ±SDa | 13.65±127.71 |
Abbreviations: GED, General education development; SD, Standard deviation.
Among those with anal warts at baseline (n=417)
In both unadjusted and adjusted models (Table 2), there was no significant association between size of anal wart at the baseline visit and current smoking status over time. In the unadjusted model, at the baseline visit, women who were current smokers had anal warts that were 21.79 mm2 larger than women who were not current smokers. The growth rate of the largest anal wart after each visit in a woman who was also current smoker was 1.48 mm2 less than that of a woman who was not a current smoker. However, those results were not statistically significant (P = 0.41 and P = 0.56, respectively).
Table 2.
Linear Mixed Model of Size of Anal Warts and Current Smoking Status in the WIHS HIV-infected participants of the Current Study in Unadjusted and Adjusted Models
| Unadjusted model | Adjusted model† | |||
|---|---|---|---|---|
|
| ||||
| Coeff ±SE | p-value | Coeff ±SE | p-value | |
| Intercept | 5.20±21.25 | 0.81 | 59.26±33.38 | 0.07 |
| Visit | 4.07±2.02 | 0.04* | −6.76±6.79 | 0.32 |
| Not current smokers | Ref.a | . | Ref.c | . |
| Current smokers | 21.79±26.48 | 0.41 | −10.39±13.08 | 0.44 |
| Visit × (not current smokers) | Ref.b | . | Ref.d | . |
| Visit × (current smokers) | −1.48±2.51 | 0.55 | 0.87±2.71 | 0.75 |
Abbreviations: HAART, Highly active antiretroviral therapy; SE, Standard error.
Type 3 p=0.41;
Type 3 p=0.56;
Type 3 p=0.44;
Type 3 p=0.75;
Model adjusted for number of sex partner in the last 6 month, race/ethnicity, HAART use, enrollment, marital status, annual household income and education level.
Statistically significant at P value<0.05
DISCUSSION
In the current analysis, we did not find an association between smoking status and the size of the largest anal wart over time in HIV-infected women from an on-going prospective cohort study in the US. To our knowledge, this is the first study using a linear mixed model to investigate whether smoking status is a predictor for the size of anal warts over time among HIV-infected women. We, therefore, cannot compare our findings directly with any other study. There are, however, a few studies reporting the relationship between smoking status and presence of genital warts. In a previous analysis of a subset of WIHS participants, Feldman et al.31 reported that current smokers were 5.2 times (95% CI 1.02–26.0) more likely to develop genital warts than non-smokers. The major difference between our study and their study 31 is the outcome variable examined. In our study we examined anal warts only, while Feldman et al.30 also investigated genital warts. We looked into the changes of the size of anal wart over time (i.e., anal wart was already presented); whereas Feldman et al.31 examined the presence or absence of genital warts.
One interesting issue is that the largest wart in current smokers was larger than that in non-current smokers at baseline, even though it was insignificant in the adjusted model. It is plausible that when a current smoker has large wart at baseline, the growth rate (or speed of development) will be slower in subsequent visits when compared to non-smokers.
Even though we did not find an association between the size of the largest anal wart and current smoking status among HIV-infected women in the current analysis, this relationship should be further explored for several reasons. The effects of cigarette smoke by-products on HPV infection, in general, and the risk of cancer, in particular, have been examined previously with the majority of support coming from the cervical cancer literature.. Accordingly, McArdle et al.51 reported that in the occurrence of cervical neoplasia, there was a reduction of Langerhans cells in smokers that leads to mitigation of the effect of host immunity against HPV. Tobacco smoke likely exerts its actions via two classes of compounds: nitrosamines and polycyclic aromatic hydrocarbons (PAHs).52 In particular, nitrosamine 4-(methylnitrosamino)-1-(3-pryridyl)-1-butanone (NNK), which is the most active carcinogen in animal models,53 has been reported in high levels in the cervical mucus of women who smoke compared to non-smokers (mean±SD: 46.9±32.5ng/g vs. 13.0±9.3ng/g)54. Melikian et al.55 identified benzo[a]pyrene metabolites in cervical mucus and DNA adducts in cervical tissues and suggested that PAHs from tobacco smoke and other environmental sources can be transported to the cervix where they are then metabolized in the cervical epithelium. Recently, Alam et al.56 found that exposure of cervical cells to benzo[a]pyrene also induced high levels of HPV synthesis, thus facilitating the HPV-associated disease process. Since most of the literature on smoking and HPV disease has focused on cervical neoplasia, we have little to draw from for anal HPV disease. However, we feel confident that the interaction between HPV and smoking could be similar for the development of anal warts.
Another reason is that the relationship between smoking and HPV infection,15–18 cervical cancer,19–28 and anal cancer29,30 have been well studied in numerous epidemiologic studies. Accordingly, current smokers are 1.6–4.6 times more likely to have pre-cancerous and invasive cervical cancer than non-smokers and that the risk increases with the intensity or duration of smoking (Odds ratio [OR] 5.9, 95% CI 1.0–35.6) for those who smoke more than 10 cigarettes per day. Furthermore, cigarette smoking influences not only the incidence or prevalence but also the natural history and pathogenesis of HPV infection. Giuliano et al.57 found that “ever” smokers maintained an HPV infection significantly longer than women who never smoked (mean duration: 10.7 months vs. 8.5 months). Smokers were also found to have a lower probability of clearing oncogenic infections than women who never smoked (Hazard ratio [HR] 0.44, 95% CI 0.20–0.96, ≤8 cigarette/day). Recently, Matsumoto et al.58 reported that smokers has significantly lower regression probability of low-grade cervical abnormalities than non-smokers (55.0% vs. 68.8%, P = 0.004).
Smoking has been shown to be both an independent risk factor and a co-factor that interacts with other risk factors, such as CD4+ cell count or prior history of external genital warts to enhance the development of genital warts. In a study of 5,622 asymptotic men, Wiley et al.59 found that the risk of external genital wart development in a smoker who had a history of external genital warts and who had CD4+ cell count <200 cells/mm3 was 6.9 (95% CI 4.7–10.1), compared to those who did not have a history of external genital warts and who had CD4+ cell count less than 200 cells/mm3. They, however, did not report the difference in the incidence of genitals warts between smokers and non-smokers. Our study focuses on clinical outcomes, and the dataset obtained from the WIHS study did not allow us to investigate the molecular mechanisms of smoking on the size of anal warts. For this reason, further studies of this association at the molecular level are warranted.
Our study has two major strengths. First, using linear mixed modeling allows us to clearly address the association between the size of anal wart and current smoking status over time while other statistical methods cannot. The ability of the model to deal with the problems of high correlation of repeated measurements within and between individuals, missing values, nonlinear covariates, and unbalanced measurements greatly enhances our ability to utilize rich dataset to examine these important health outcomes among HIV-infected individuals. Furthermore, the use of linear mixed modeling also allows us to appropriately model the size of anal warts as a continuous outcome variable. Had we categorized the size of anal warts and used different approaches (i.e., logistic regression or Cox-proportional hazard regression), we would not have been able to detect subtle changes in the size of the wart during follow-up. One limitation to the current study is the use of the size of the largest anal wart at each visit as the outcome variable. This does not allow us to follow the same wart over time because the largest wart measured at one visit might not be the same in subsequent visits, especially when there are multiple warts. Even though studying the progression or regression of the same wart over time is not feasible with our data, we felt that studying overall disease burden (as measured by the largest wart) was the best proxy measure available. While we were unable to validate the largest anal wart size as a proxy measure for overall disease burden, no other studies to our knowledge have examined this relationship.
In summary, we did not find evidence for the association between the size of anal warts and current smoking status over time in HIV-infected women. However, our results suggest that, at baseline, women who smoke had much larger warts than those who did not smoke. Further exploration of the role of smoking, the interaction between smoking status with other risk factors (e.g., CD4+ cell count or HIV viral load), and the molecular study of the mechanism of smoking on anal warts over time are warranted.
Acknowledgments
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington, DC, Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1- HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. HN Luu is received funding from the Vietnam Education Foundation (VEF) under VEF Fellowship, the NIH/Fogarty Training Fellowship – grant # D43-TW007669 though the Center for International Training and Research (CITAR), School of Public Health, the University of Texas Health Science Center at Houston, and UTHealth Innovation for Cancer Prevention Research Pre- and Post-doctoral Fellowship, The University of Texas School of Public Health-Cancer Prevention and Research Institute of Texas grant #RP101503. The content is solely responsibility of the authors and does not necessarily represent official views of the Cancer Prevention and Research Institute of Texas.
ABBREVIATIONS
- AZT
Zodovudine
- CI
Confidence interval
- d4T
Stavudine
- GED
General education development
- HAART
Highly active antiretroviral therapy
- HPV
Human papillomavirus
- PI
Protease inhibitor
- NNRTIs
Non-nucleoside reverse transcriptase inhibitors
- NRTIs
Nucleoside reverse transcriptase inhibitors
- OR
Odds ratio
- SD
Standard deviation
- WIHS
Women’s Interagency HIV Study
References
- 1.Koutsky LA, Galloway DA, Holmes KK. Epidemiology of genital Human Papillomavirus infection. Epidemiol Rev. 1988;10:122–63. doi: 10.1093/oxfordjournals.epirev.a036020. [DOI] [PubMed] [Google Scholar]
- 2.Minkoff HL, Eisenberger-Matityahu D, Feldman J, et al. Prevalence and incidence of gynecologic disorders among women infected with Human Immunodeficiency Virus. Am J Obstet Gynecol. 1999;180:824–36. doi: 10.1016/s0002-9378(99)70653-8. [DOI] [PubMed] [Google Scholar]
- 3.Beutner KR, Reitano MV, Richwald GA, et al. External genital warts: Report of the American Medical Association Consensus Conference. AMA Expert Panel on External Genital Warts. Clin Infect Dis. 1998;27:796–806. doi: 10.1086/514964. [DOI] [PubMed] [Google Scholar]
- 4.Palefsky J, del Rio C. Is high-grade dysplasia on anal pap a high-grade problem? AIDS Clin Care. 2002;14:44–5. [PubMed] [Google Scholar]
- 5.Lacey CJ. Therapy for genital Human Papillomavirus-related disease. J Clin Virol. 2005;32:S82–90. doi: 10.1016/j.jcv.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Vandepapeliere P, Barrasso R, Meijer CJ, et al. Randomized controlled trial of an adjuvanted Human Papillomavirus (HPV) type 6 L2E7 vaccine: Infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis. 2005;192:2099–107. doi: 10.1086/498164. [DOI] [PubMed] [Google Scholar]
- 7.Carter PS, de Ruiter A, Whatrup C, et al. Human immunodeficiency virus infection and genital warts as risk factors for anal epithelial neoplasia in homosexual men. Br J Surg. 1995;4:473–4. doi: 10.1002/bjs.1800820414. [DOI] [PubMed] [Google Scholar]
- 8.Blomberg M, Friis S, Munk C, et al. Genital warts and risk of cancer: a Danish study of nearly 50,000 patients with genital warts. J Infect Dis. 2012;205:1544–53. doi: 10.1093/infdis/jis228. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2009 Sexually transmitted disease surveillance. US Centers for Disease Control and Prevention, US Department of Health Services, Public Health Services; Atlanta: 2009. [last accessed 20–10–2011]. http://www.cdc.gov/std/stats09/default.htm. [Google Scholar]
- 10.Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis. 2003;36:1397–403. doi: 10.1086/375074. [DOI] [PubMed] [Google Scholar]
- 11.Chandler MG. Genital warts: A study of patient anxiety and information needs. Br J Nurs. 1996;5:174–9. doi: 10.12968/bjon.1996.5.3.174. [DOI] [PubMed] [Google Scholar]
- 12.Persson G, Dahlof LG, Krantz I. Physical and psychological effects of anogenital warts on female patients. Sex Transm Dis. 1993;20:10–3. doi: 10.1097/00007435-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Naguib SM, Lundin FE, Jr, Davis HJ. Relation of various epidemiologic factors to cervical cancer as determined by a screening program. Obstet Gynecol. 1966;28:451–9. [PubMed] [Google Scholar]
- 14.Sasson IM, Haley NJ, Hoffmann D, et al. Cigarette smoking and neoplasia of the uterine cervix: Smoke constituents in cervical mucus. N Engl J Med. 1985;312:315–6. doi: 10.1056/nejm198501313120516. [DOI] [PubMed] [Google Scholar]
- 15.IARC Working Group on the Evaluation of Carcinogenic Risks to Human. Human papillomaviruses. Lyon, France: International Agency for Research on Cancer; 2005. [Google Scholar]
- 16.Deacon JM, Evans CD, Yule R, et al. Sexual behaviour and smoking as determinants of cervical HPV infection and of CIN3 among those infected: A case-control study nested within the Manchester cohort. Br J Cancer. 2000;83:1565–72. doi: 10.1054/bjoc.2000.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiley DJ, Wiesmeier E, Masongsong E, et al. Smokers at higher risk for undetected antibody for oncogenic Human Papillomavirus type 16 infection. Cancer Epidemiol Biomarkers Prev. 2006;15:915–20. doi: 10.1158/1055-9965.EPI-05-0963. [DOI] [PubMed] [Google Scholar]
- 18.Syrjanen K, Shabalova I, Petrovichev N, et al. Smoking is an independent risk factor for oncogenic Human Papillomavirus (HPV) infections but not for high-grade CIN. Eur J Epidemiol. 2007;22:723–35. doi: 10.1007/s10654-007-9180-8. [DOI] [PubMed] [Google Scholar]
- 19.Clarke EA, Morgan RW, Newman AM. Smoking as a risk factor in cancer of the cervix: Additional evidence from a case-control study. Am J Epidemiol. 1982;115:59–66. doi: 10.1093/oxfordjournals.aje.a113279. [DOI] [PubMed] [Google Scholar]
- 20.Northfelt DW. Anal neoplasia in persons with HIV infection. AIDS Clin Care. 1996;8:63–6. [PubMed] [Google Scholar]
- 21.Engeland A, Bjorge T, Haldorsen T, et al. Use of multiple primary cancers to indicate associations between smoking and cancer incidence: An analysis of 500,000 cancer cases diagnosed in Norway during 1953–93. Int J Cancer. 1997;70:401–7. doi: 10.1002/(sici)1097-0215(19970207)70:4<401::aid-ijc5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Kanetsky PA, Gammon MD, Mandelblatt J, et al. Cigarette smoking and cervical dysplasia among non-Hispanic black women. Cancer Detect Prev. 1998;22:109–19. doi: 10.1046/j.1525-1500.1998.cdoa17.x. [DOI] [PubMed] [Google Scholar]
- 23.Roteli-Martins CM, Panetta K, Alves VA, et al. Cigarette smoking and high-risk HPV DNA as predisposing factors for high-grade cervical intraepithelial neoplasia (CIN) in young Brazilian women. Acta Obstet Gynecol Scand. 1998;77:678–82. doi: 10.1034/j.1600-0412.1998.770617.x. [DOI] [PubMed] [Google Scholar]
- 24.Winkelstein W., Jr Smoking and cervical cancer--current status: A review. Am J Epidemiol. 1990;131:945–57. doi: 10.1093/oxfordjournals.aje.a115614. [DOI] [PubMed] [Google Scholar]
- 25.Lacey JV, Jr, Frisch M, Brinton LA, et al. Associations between smoking and adenocarcinomas and squamous cell carcinomas of the uterine cervix (United States) Cancer Causes Control. 2001;12:153–61. doi: 10.1023/a:1008918310055. [DOI] [PubMed] [Google Scholar]
- 26.Hakama M, Luostarinen T, Hallmans G, et al. Joint effect of HPV16 with chlamydia trachomatis and smoking on risk of cervical cancer: Antagonism or misclassification (Nordic countries) Cancer Causes Control. 2000;11:783–90. doi: 10.1023/a:1008976703797. [DOI] [PubMed] [Google Scholar]
- 27.Castle PE, Wacholder S, Lorincz AT, et al. A prospective study of high-grade cervical neoplasia risk among Human Papillomavirus-infected women. J Natl Cancer Inst. 2002;94:1406–14. doi: 10.1093/jnci/94.18.1406. [DOI] [PubMed] [Google Scholar]
- 28.Hildesheim A, Herrero R, Castle PE, et al. HPV co-factors related to the development of cervical cancer: Results from a population-based study in Costa Rica. Br J Cancer. 2001;84:1219–26. doi: 10.1054/bjoc.2001.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daling JR, Weiss NS, Hislop TG, et al. Sexual practices, sexually transmitted diseases, and the incidence of anal cancer. N Engl J Med. 1987;317:973–7. doi: 10.1056/NEJM198710153171601. [DOI] [PubMed] [Google Scholar]
- 30.Holly EA, Whittemore AS, Aston DA, et al. Anal cancer incidence: Genital warts, anal fissure or fistula, hemorrhoids, and smoking. J Natl Cancer Inst. 1989;81:1726–31. doi: 10.1093/jnci/81.22.1726. [DOI] [PubMed] [Google Scholar]
- 31.Feldman JG, Chirgwin K, Dehovitz JA, et al. The association of smoking and risk of condyloma acuminatum in women. Obstet Gynecol. 1997;89:346–50. doi: 10.1016/S0029-7844(97)00011-2. [DOI] [PubMed] [Google Scholar]
- 32.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS collaborative study group. Epidemiology. 1998;9:117–25. [PubMed] [Google Scholar]
- 33.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–9. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WIHS Data Management and Analysis Center (WDMAC) Women’s Interagency HIV Study (WIHS) dossier. Baltimore, MD: WIHS Data Management and Analysis Center (WDMAC); May, 2010. [last accessed 20–10–2011]. https://statepiaps.jhsph.edu/wihs/Invest-info/dossier.pdf. [Google Scholar]
- 35.Panels on Clinical Practices for the Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV infected adults and adolescents. US Department of Health and Human Services and Henry J Kaiser Family Foundation; May 17, 2002. [last accessed 20–10–2011]. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5107a1.htm. [Google Scholar]
- 36.Yeni PG, Hammer SM, Carpenter CC, et al. Antiretroviral treatment for adult HIV infection in 2002: Updated recommendations of the International AIDS Society-USA Panel. JAMA. 2002;288:222–35. doi: 10.1001/jama.288.2.222. [DOI] [PubMed] [Google Scholar]
- 37.Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on Human Papillomavirus infection and squamous intraepithelial lesions in Human Immunodeficiency Virus-positive women. J Infect Dis. 2010;201:681–90. doi: 10.1086/650467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahdieh-Grant L, Li R, Levine AM, et al. Highly active antiretroviral therapy and cervical squamous intraepithelial lesions in Human Immunodeficiency Virus-positive women. J Natl Cancer Inst. 2004;96:1070–6. doi: 10.1093/jnci/djh192. [DOI] [PubMed] [Google Scholar]
- 39.Notermans DW, de Wolf F, Oudshoorn P, et al. Evaluation of a second-generation nucleic acid sequence-based amplification assay for quantification of HIV type 1 RNA and the use of ultrasensitive protocol adaptations. AIDS Res Hum Retroviruses. 2000;16:1507–17. doi: 10.1089/088922200750006038. [DOI] [PubMed] [Google Scholar]
- 40.Palefsky JM. Anal squamous intraepithelial lesions: Relation to HIV and Human Papillomavirus infection. J Acquir Immune Defic Syndr. 1999;21:S42–8. [PubMed] [Google Scholar]
- 41.Palefsky JM, Holly EA, Ralston ML, et al. Prevalence and risk factors for anal Human Papillomavirus infection in Human Immunodeficiency Virus (HIV)-positive and high-risk HIV-negative women. J Infect Dis. 2001;183:383–91. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 42.Holly EA, Ralston ML, Darragh TM, et al. Prevalence and risk factors for anal squamous intraepithelial lesions in women. J Natl Cancer Inst. 2001;93:843–9. doi: 10.1093/jnci/93.11.843. [DOI] [PubMed] [Google Scholar]
- 43.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of Human Papillomavirus in Human Immunodeficiency Virus-positive women. J Natl Cancer Inst. 2005;97:577–86. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 44.Fitzmaurice GM, Laird NM, Ware JH. In: Applied longitudinal analysis. Fitzmaurice GM, Laird NM, Ware JH, editors. Hoboken, New Jersey: Wiley-Interscience, John Wiley & Sons, Inc; 2004. [Google Scholar]
- 45.Cook JA, Cohen MH, Grey D, et al. Use of highly active antiretroviral therapy in a cohort of HIV-seropositive women. Am J Public Health. 2002;92:82–7. doi: 10.2105/ajph.92.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palefsky JM, Minkoff H, Kalish LA, et al. Cervicovaginal Human Papillomavirus infection in Human Immunodeficiency Virus-1 (HIV)-positive and high-risk HIV-negative women. J Natl Cancer Inst. 1999;91:226–36. doi: 10.1093/jnci/91.3.226. [DOI] [PubMed] [Google Scholar]
- 47.Nowicki MJ, Karim R, Mack WJ, et al. Correlates of CD4+ and CD8+ lymphocyte counts in high-risk immunodeficiency virus (HIV)-seronegative women enrolled in the Women’s Interagency HIV Study (WIHS) Hum Immunol. 2007;68:342–9. doi: 10.1016/j.humimm.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Hessol NA, Holly EA, Efird JT, et al. Anal intraepithelial neoplasia in a multisite study of HIV-infected and high-risk HIV-uninfected women. AIDS. 2009;23:59–70. doi: 10.1097/QAD.0b013e32831cc101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luu HN, Amirian ES, Chan W, Beasley RP, Piller LB, Scheurer ME. CD4+ cell count and HIV load as predictors of size of anal warts over time in HIV-infected women. J Infect Dis. 2012;205:578–85. doi: 10.1093/infdis/jir813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.SAS. Version 9.2. SAS Institute; Cary, North Carolina: 2000–2008. [Google Scholar]
- 51.McArdle JP, Muller HK. Quantitative assessment of Langerhans’ cells in human cervical intraepithelial neoplasia and wart virus infection. Am J Obstet Gynecol. 1986;154:509–15. doi: 10.1016/0002-9378(86)90592-2. [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann DHI. Tobacco consumption and lung cancer. In: Hansen HH, editor. Lung cancer. Advances in basic and clinical research. Boston: Kluwer Academic Publ; 1995. pp. 1–42. [DOI] [PubMed] [Google Scholar]
- 53.Hoffmann D, Brunnemann KD, Prokopczyk B, et al. Tobacco-specific N-nitrosamines and areca-derived N-nitrosamines: Chemistry, biochemistry, carcinogenicity, and relevance to humans. J Toxicol Environ Health. 1994;41:1–52. doi: 10.1080/15287399409531825. [DOI] [PubMed] [Google Scholar]
- 54.Prokopczyk B, Cox JE, Hoffmann D, et al. Identification of tobacco-specific carcinogen in the cervical mucus of smokers and nonsmokers. J Natl Cancer Inst. 1997;89:868–73. doi: 10.1093/jnci/89.12.868. [DOI] [PubMed] [Google Scholar]
- 55.Melikian AA, Sun P, Prokopczyk B, et al. Identification of benzo[a]pyrene metabolites in cervical mucus and DNA adducts in cervical tissues in humans by gas chromatography-mass spectrometry. Cancer Lett. 1999;146:127–34. doi: 10.1016/s0304-3835(99)00203-7. [DOI] [PubMed] [Google Scholar]
- 56.Alam S, Conway MJ, Chen HS, et al. The cigarette smoke carcinogen benzo[a]pyrene enhances Human Papillomavirus synthesis. J Virol. 2008;82:1053–8. doi: 10.1128/JVI.01813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giuliano AR, Sedjo RL, Roe DJ, et al. Clearance of oncogenic Human Papillomavirus (HPV) infection: Effect of smoking (United States) Cancer Causes & Control. 2002;13:839–46. doi: 10.1023/a:1020668232219. [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto K, Oki A, Furuta R, et al. Tobacco smoking and regression of low-grade cervical abnormalities. Cancer Sci. 2010;10:2065–73. doi: 10.1111/j.1349-7006.2010.01642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiley DJ, Elashoff D, Masongsong EV, et al. Smoking enhances risk for new external genital warts in men. Int J Environ Res Public Health. 2009;6:1215–34. doi: 10.3390/ijerph6031215. [DOI] [PMC free article] [PubMed] [Google Scholar]