Abstract
Objective
To examine the use of medical management, uterus-preserving surgery (UPS), and complementary treatments among women with uterine fibroids.
Study design
Prospective cohort study of 933 premenopausal women ages 31-54 years with symptomatic fibroids who participated in the Study of Pelvic Problems, Hysterectomy, and Intervention Alternatives (SOPHIA) for an average of 4.3 years (SD 2.5 years). Incident use of fibroid treatments was determined through annual interviews. Linear regression models were used to compare changes in fibroid-related symptoms among women who underwent UPS versus those who did not undergo surgery.
Results
Participants were racially and ethnically diverse, with a mean age of 43 years. During study follow-up, 531 participants (57%) did not undergo UPS or hysterectomy, 250 (27%) had at least one UPS, and 152 (16%) underwent hysterectomy. Complementary and alternative treatments were commonly used, including exercise (45%), diet (34%), herbs (37%), and acupuncture (16%): participants reported significant symptom improvement and few side effects with these interventions. In multivariable linear regression models, women who did not undergo surgery during the study reported improvement in dyspareunia (p<.001), pelvic pain (p<.001), and menstrual cramps (p<.001). However, women who underwent UPS reported greater overall resolution of “pelvic problems” compared with women who did not have surgical treatment (difference in change score 1.18 on a 4-point Likert scale, p<.001).
Conclusion
UPS are effective treatments for women with fibroids, but many women use hormonal or complementary treatments and report significant symptom improvement without surgical intervention.
Keywords: Uterine fibroids, Myomectomy, Uterine artery embolization, Complementary treatments
Introduction
Uterine fibroids occur in approximately 25% of reproductive-age women. Hysterectomy is the only definitive treatment, but many women seek uterus-preserving surgeries (UPS) such as myomectomy, uterine artery embolization and endometrial ablation, or a trial of medical management or complementary and alternative medicine (CAM) [1-5]. Despite the prevalence of fibroids and the myriad of available treatments, the current literature is limited in scope and quality to fully inform treatment decisions [6]. Several studies have reported the rate of an additional UPS or hysterectomy following a first UPS [7-11]. However, the likelihood of undergoing a first UPS or a hysterectomy among women with symptomatic fibroids is less well described and little is known about the effect of medications or CAM on fibroid-related symptoms [12,13].
We present an analysis of the use and effectiveness of UPS and nonsurgical treatments among 933 premenopausal women with symptomatic fibroids who were enrolled in the Study of Pelvic Problems, Hysterectomy, and Intervention Alternatives (SOPHIA). Our results will help guide and inform counseling for women who present with symptomatic fibroids, especially those who hope to avoid hysterectomy.
Materials and methods
Details of the SOPHIA methods and measures have been described [14,15]. In brief, SOPHIA was a prospective cohort study of premenopausal women, aged 31-54 years, presenting to gynecologic clinics in the San Francisco Bay area. For this analysis, all SOPHIA participants who self-reported a diagnosis of symptomatic fibroids with abnormal uterine bleeding or pelvic pressure at the time of their baseline interview and completed at least one follow-up interview were included. Women with a history gynecologic cancer were excluded. Participants were enrolled from 1998 to 2004. Institutional review boards at all hospital sites approved the study and all women gave informed consent for participation.
SOPHIA participants underwent yearly face-to-face interviews for up to 8 years. The questionnaires assessed a broad array of pelvic symptoms associated with fibroids. Participants were asked how bothered they were in the last 4 weeks by dyspareunia, pelvic pain, pelvic pressure, bladder pain, frequent urination, low back pain, and menstrual cramps. Answers to these symptom questions were reported on a 5-point severity scale which ranged from “not at all bothered” to “extremely bothered.” We also utilized a global question: “To what extent would you say your pelvic problems have been resolved?” Answers were reported on a 4-point scale which ranged from “not at all” to “completely.”
Participants were asked about their use of surgical and nonsurgical treatments during the annual interviews. Myomectomy, endometrial ablation, or uterine artery embolization were classified as a UPS. Nonsurgical treatments were categorized as western medicine (hormonal contraception with estrogen and/or progestin, nonsteroidal analgesics, narcotic pain medication) or CAM (exercise, herbs, diet, acupuncture, physical therapy). For all western medicines, participants were asked whether they used a treatment specifically for bleeding and/or pelvic pain, and not for contraception or other symptoms. For CAM, participants were asked whether or not they were using the treatment for “pelvic problems.” When a participant reported use of a treatment, two follow-up questions further explored her experience with the treatment: (1) “What effect did (the treatment) have on your symptoms?” and (2) How bothered were you by side effects of this treatment? For the effectiveness question, 5 response options ranging from “made them a lot better” to “made them a lot worse” were offered, and for the side effects question, 4 response options ranging from from “a lot” to “not at all” were used.
Associations between baseline sociodemographic characteristics and symptoms with the most invasive surgical interventions that participants underwent during the study period were tested via chi-squares and ANOVAs. Nonsurgical treatment effect is reported as the percentage of women using the treatment who stated that treatment made their symptoms “a lot better.” Side effects are reported as the percentage of women using the treatment who answered that side effects bothered them “a lot” or “some.” We calculated the change in symptoms from baseline to the last interview among women who did not undergo surgery during the study period and among women who underwent any UPS. We compared the difference in change scores between these two groups of participants using linear regression in a model that controlled for age, race/ethnicity, educational attainment, year of recruitment, length of follow-up period, and entry into menopause. For questions that addressed changes in bleeding symptoms, we excluded women who reported the onset of menopause during the study period. All analyses were conducted using SAS for Windows, version 9.
Results
Among 1,503 SOPHIA participants, this analysis includes the 933 women (62%) who had presented for care with symptomatic fibroids in the year prior to study enrollment and completed at least one follow-up interview. Participants were followed for an average of 4.2 years (SD 2.5 years): 9% were lost to follow-up after the baseline interview. Over the course of the study period, 531 participants (57%) did not undergo UPS or hysterectomy, 250 (27%) had at least one UPS, and 152 (16%) underwent hysterectomy with or without an antecedent UPS (Table 1). The study cohort was racially and ethnically diverse, with a mean age of 43 years. At baseline, study participants reported high rates of fibroid-related symptoms including frequent bleeding (66%) and/or pelvic pressure (49%), and 57% reported pelvic pain as a result of fibroids (Table 1). There were no statistically significant differences in baseline symptoms among women who did not undergo surgery during study follow-up compared with women who underwent UPS or hysterectomy.
Table 1.
Baseline characteristics of SOPHIA participants with symptomatic fibroids by what procedure they underwent during the study period.
| All N=933 N (%) | No surgery N=531 N (%) | Uterus-preserving surgery N=250 N (%) | Hysterectomy N=152 N (%) | p-value | |
|---|---|---|---|---|---|
| Characteristic | |||||
| Age (mean (SD)) | 43 (4) | 43 (4) | 43 (4) | 43 (3) | 0.34 |
| Married/Living wit partner | 490 (54) | 278 (55) | 130 (53) | 82 (54) | 0.88 |
| Parity >= 1 | 242 (26) | 140 (26) | 55 (22) | 47 (31) | 0.13 |
| Race/Ethnicity | 0.29 | ||||
| Asian | 107 (11) | 67 (13) | 27 (11) | 13 (9) | |
| Black/African-American | 280 (31) | 155 (30) | 79 (32) | 46 (31) | . |
| Latina, Latin American | 112 (12) | 73 (14) | 28 (11) | 11 (7) | . |
| White | 370 (40) | 198 (38) | 100 (40) | 72 (48) | . |
| Other | 48 (5) | 26 (5) | 14 (6) | 8 (5) | |
| Education | 0.002 | ||||
| High school or less | 148 (16) | 94 (18) | 30 (12) | 24 (16) | |
| Some college | 295 (32) | 186 (36) | 69 (28) | 40 (27) | . |
| College degree or greater | 479 (52) | 242 (46) | 150 (60) | 87 (58) | |
| Household income | 0.52 | ||||
| < $25,000 | 108 (27) | 64 (30) | 25 (23) | 19 (23) | |
| $25,001-$50,000 | 121 (30) | 63 (29) | 32 (30) | 26 (31) | . |
| $50,001-$100,000 | 121 (30) | 63 (29) | 36 (34) | 22 (27) | . |
| > $100,000 | 55 (14) | 25 (12) | 14 (13) | 16 (19) | |
| Symptoms | |||||
| Major depression (PHQ)‡ | 55 (11) | 37 (13) | 11 (8) | 7 (11) | 0.43 |
| Heavy or frequent bleeding | 619 (66) | 340 (64) | 168 (67) | 111 (73) | 0.11 |
| Pelvic pain | 536 (57) | 310 (58) | 141 (56) | 85 (56) | 0.80 |
| Fibroids cause pelvic pressure | 450 (49) | 253 (48) | 122 (49) | 75 (49) | 0.92 |
| Dyspareunia on most or almost all days | 47 (10) | 29 (10) | 10 (8) | 8 (13) | 0.51 |
The majority of participants had not undergone surgery for fibroids prior to enrollment in the study (Table 2, n=715, 77% of cohort). Among these women, 74% did not undergo any surgery over an average of 3.7 years of follow-up, while 7% had a myomectomy and 15% underwent hysterectomy (4.8 years and 5 years of follow-up respectively). Women with a history of myomectomy had a reoperation rate of 11% for myomectomy and 19% for hysterectomy (6 years and 5.8 years of follow-up respectively).
Table 2. Uterus-preserving surgeries and hysterectomy during study follow-up, stratified by surgical procedures received prior to enrollment.
| Procedure during study follow-up | N (%) | Mean years of follow-up |
|---|---|---|
| No prior UPS at baseline, N=719 | ||
| No surgery | 530 (74) | 3.7 |
| Myomectomy | 49 (7) | 4.8 |
| Uterine artery embolization | 22 (3) | 5.1 |
| Endometrial ablation | 8 (1) | 4.2 |
| Hysterectomy 110 (15) | 5.0 | |
| Prior myomectomy, N=159 | ||
| No surgery | 104 (65) | 3.8 |
| Myomectomy | 17 (10) | 6.0 |
| Uterine artery embolization | 5 (3) | 3.9 |
| Endometrial ablation | 2 (1) | 8.0 |
| Hysterectomy | 31 (19) | 5.8 |
| Prior uterine artery embolization, N=29 | ||
| No surgery | 23 (79) | 3.3 |
| Myomectomy | 0 | |
| Endometrial ablation | 0 | |
| Hysterectomy | 6 (20) | 3.7 |
| Prior endometrial ablation N=59 | ||
| No surgery | 47 (80) | 3.0 |
| Myomectomy | 2 (3) | 8.0 |
| UAE | 1 (2) | 3.2 |
| Hysterectomy | 9 (15) | 6.2 |
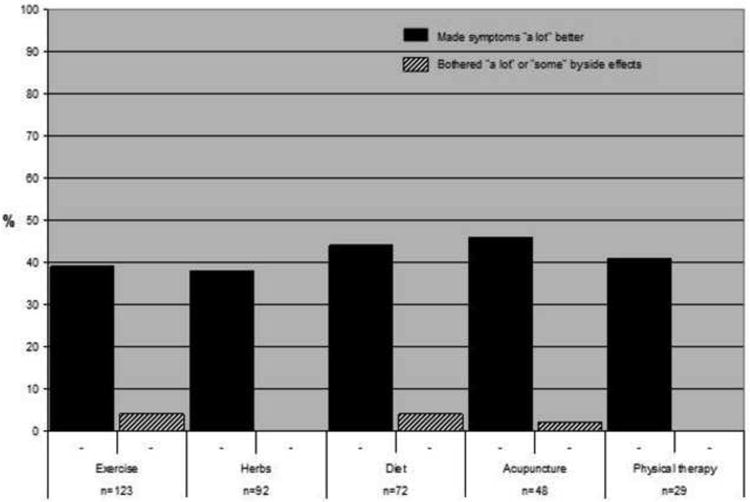
Western medication was commonly used among women who did not undergo surgery during the study period and had no prior history of UPS (Fig. 1). Anti-inflammatory analgesics were the most frequently used medication (70%), followed by narcotic pain medication (29%). CAM was used by many women to relieve fibroid-related symptoms: exercise (40%), herbs (37%), and diet (34%) were the most common. Among participants who used hormonal contraception, 55% reported that combined hormonal contraception made them a lot better, but 22% were bothered by side effects of these medicines (Fig. 2). The progestin intrauterine device (IUD) had the highest proportion of women who reported feeling a lot better as a result of its use (71%) and a low rate of bothersome side effects (24%). For CAM (Fig. 3), improvement in symptoms was somewhat lower than for hormonal medication (exercise 39%, herbs 38%, physical therapy 41%), but bothersome side effects were rare (<5% for all therapies).
Fig. 1. Use of medical treatments for symptomatic fibroids among women who did not undergo surgery.
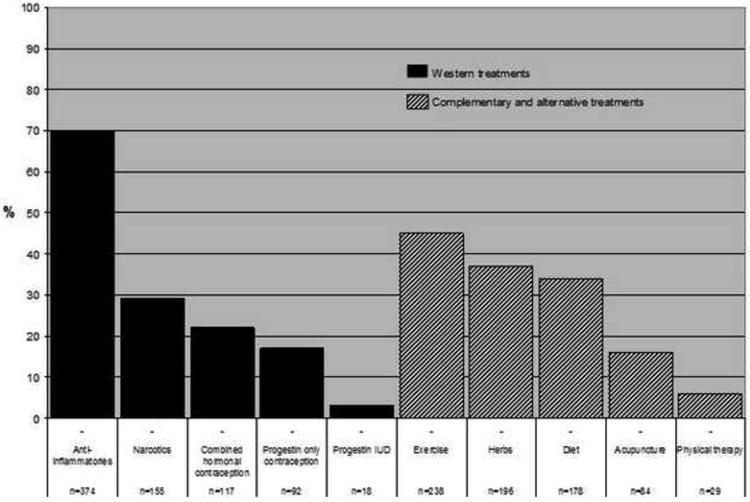
Fig. 2. Effectiveness of western treatments and bothersome side effects among women who did not undergo surgery.
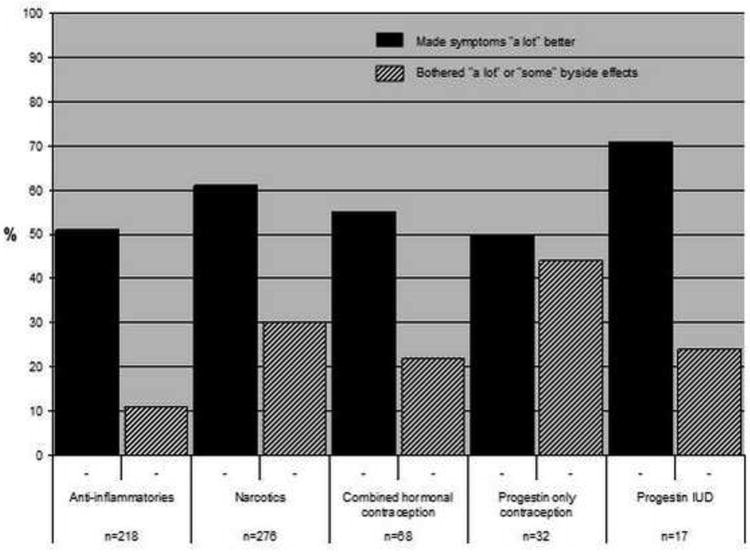
Fig. 3. Effectiveness of complementary and alternative treatments and bothersome side effects among women who did not undergo surgery.
In the multivariable model, we observed improvements over time in pelvic symptoms among women who did not undergo surgery during the study follow-up, as well as among those who underwent UPS (Table 3). In a global assessment of whether or not pelvic problems had resolved, women who underwent UPS reported greater improvement compared with women who did not undergo surgery (difference in change score 1.18, p<.001). At baseline, scores for this question were not significantly different between groups (mean 1.65 for no surgery, 1.33 for UPS). Women who did not undergo a surgical intervention reported significant improvements in dyspareunia (change score -1.31, p<.001), bladder pain (-0.56, p<.001), and menstrual cramps (-0.39, p<.001). There were no statistically significant differences between groups for improvement in the duration or quantity of bleeding, pelvic or low back pain, pelvic pressure, or menstrual cramps. Women who underwent UPS reported greater improvements in bladder pain (difference in change scores equaled -0.67, p=.003), and frequent urination (difference in change scores equaled -0.83, p<.001) compared with women who did not have surgery.
Table 3. Changes in symptoms among women with no prior fibroid surgery: no surgery versus uterus-preserving surgery during study follow-up*.
| No surgery during study follow-up Mean change from baseline to end of study (SE) |
Uterus-preserving surgery during study follow-up Mean change from baseline to end of study (SE) |
Difference in change score (SE) | P value for difference in change score* | |
|---|---|---|---|---|
| Pelvic problems resolved | 0.43 (.06)† | 1.62 (.17) † | 1.18 (.18) | <.001 |
| Menstruation‡ | ||||
| Duration of menses (days) | 0.18 (.31) | -0.64 (.83) | -.82 (.89) | .35 |
| Duration of heavy flow/passing clots (days) | 0.0 (.21) | -.88 (.56) | -.88 (.46) | .14 |
| Spotting or bleeding between periods (days) | -.37 (.33) | -.77 (.88) | -.39 (.77) | .68 |
| In the past 4 weeks, how bothered were you by…. | ||||
| Dyspareunia (painful sex) | -1.31 (.09) † | -1.7 (.25) † | -.39 (0.27) | .14 |
| Pelvic pain at other times | .19 (.08) † | .04 (.22) | -.15 (.23) | .52 |
| Tightness or pelvic pressure | -0.09 (.08) | -.0.04 (.22) | 0.04 (.23) | .85 |
| Bladder pain | -.56 (.08) † | -1.24 (.21) † | -.67 (.22) | .003 |
| Frequent urination | .56 (.08) † | -.27 (.20) | -.83 (.24) | <.001 |
| Low back pain | .08 (.09) | -.40 (.26) | -.48 (.27) | .07 |
| Menstrual cramps | -39 (.09) † | -.39 (.12) | .01 (.27) | .98 |
Multivariable model controlled for age, ethnicity, education, cohort 1/cohort 2, length of follow-up, entry into menopause
p<.001 for the change from baseline to the end of the study within each group
These questions exclude women who entered menopause during the study period (N=268, 232 in no surgery group and 36 in UPS group).
Comment
In this diverse cohort of 933 premenopausal women with symptomatic fibroids, we report several novel findings for nonsurgical management. At baseline, study participants were a symptomatic group with high rates of abnormal bleeding, pelvic pain, or pelvic pressure attributed to fibroids. Despite these symptoms, 57% of participants did not undergo any surgical intervention throughout the study period. Many of these women utilized hormonal contraception and/or analgesics to relieve symptoms and reported that these medications significantly improved their symptoms. CAM was also commonly used to relieve fibroid-related symptoms, and 38-46% reported that these treatments made their symptoms “a lot better”. As expected, women who underwent a UPS during the study reported a decrease in many bothersome symptoms and an overall improvement in their “pelvic problems”. However, we also observed significant improvements over time in “pelvic problems” as well as dyspareunia, pelvic pressure, bladder pain, and menstrual cramps among women who did not undergo surgery. Surgery provided greater relief compared with no surgery in only a few symptom areas in a multivariable model, although both groups had similar levels of symptom severity at baseline.
The change in fibroid symptoms over time in premenopausal women without surgical intervention is not well understood. DeWaay et al. reported spontaneous regression and even complete resolution of some fibroids among premenopausal women examined with serial sonography over 2.5 years [16]. Another longitudinal study found that 7% of fibroids decreased in size among 72 premenopausal women evaluated with repeated magnetic resonance imaging (MRI) [17]. This natural decrease in fibroid size over time among some women may help explain the symptom improvements we observed for women who did not undergo surgery. Hormonal therapy, used by many participants who did not undergo surgery, may have an effect on fibroid growth. Oral contraceptives and depoprovera have been shown to decrease the risk of developing fibroids in some studies [18-20]. The clinical improvements among women who did not undergo surgery may therefore reflect the effectiveness of hormonal medication, or analgesics, or CAM to decrease fibroid-related symptoms by controlling heavy bleeding and pelvic discomfort.
There are few studies that report the proportion of symptomatic women who undergo a first surgery to treat fibroids over several years of follow-up. In our cohort, among women with no prior surgery at study enrollment, 27% reported a first UPS and 16% had a hysterectomy. This is a similar rate of hysterectomy to a cohort of women in North Carolina with fibroids ≥5 centimeters followed for 1 year (15%), but those women had a higher rate of myomectomy (17% vs. 7%) [12]. In a retrospective chart review of 421 women with fibroids in Cleveland who had a median follow-up of 29 months, the hysterectomy rate was 29% [13]. Although the rate of surgery is likely influenced by many factors including fibroid size, number and location, symptom severity, and local practice patterns, these prior studies combined with our data indicate that the majority of women with symptomatic fibroids are successfully managed without surgical intervention.
The likelihood of reoperation following a UPS informs counseling for women considering surgery for fibroids. Among women with a prior myomectomy, 11% underwent a repeat myomectomy over 6 years of follow-up and 19% underwent hysterectomy over 5.8 years of follow-up. These rates are consistent with prior studies that have found 10-25% of women require an additional major surgery to treat fibroids after myomectomy [8,9,11]. Women with prior uterine artery embolization had a 20% chance of undergoing hysterectomy in our cohort over 3.7 years of follow-up, similar to the hysterectomy rate in a large retrospective cohort study in the same local geographic area as our study [10]. The overall low rates of reoperation for all UPS highlight the effectiveness of UPS to avoid hysterectomy in most patients with symptomatic fibroids.
Medical management may also be an effective option for women with symptomatic fibroids, although there is limited evidence to support this approach. Studies of hormonal contraceptives to treat abnormal bleeding or pelvic pain have generally excluded women with fibroids. In our study, approximately half of the women who were using either combined contraceptives with estrogen and progestin or a progestin-only method reported that their symptoms improved “a lot” with these therapies. The progestin IUD was even more effective, with 71% of users reporting that their symptoms became “a lot better”. This strong effect is consistent with prior studies that have reported a decrease in menstrual bleeding and pain in women with fibroids who utilize the progestin IUD [22].
The use of CAM to treat symptoms associated with fibroids is a unique finding in our study. Nearly 50% of women in the United States use CAM, with almost $19 billion in sales in 2002 [24,25]. Prior studies have focused on the use of CAM to treat dysmenorrhea, infertility, and pregnancy-related nausea [26,27]. To our knowledge, however, CAM use to specifically address “pelvic problems” among women with symptomatic fibroids has not been previously reported. In our study, 40% of women were using exercise to treat their pelvic problems, 30% used specific foods or herbs to manage symptoms, and 15% used acupuncture. The effectiveness of CAM to improve pelvic symptoms was only moderate (36-43%), but bothersome side effects were extremely rare. The mechanism of how CAM may improve fibroid-related symptoms has not been elucidated, but some foods, vitamins and minerals have been found to decrease endometrial production of prostaglandins that are associated with vasoconstriction and myometrial contractions [27].
There are several limitations to our study. The diagnosis of fibroids is by self-report without specification of size, location, or the number of tumors. A prospective cohort study reported 93% accuracy in the diagnosis of fibroids by self-report [28]. However, based on self-report, we were unable to confirm that a patient's fibroids were the primary cause of her pelvic symptoms. In addition, we could not determine specific fibroid characteristics that may have influenced satisfaction with medical management or symptom improvement among women who did not undergo surgery: selection bias may impact these results if, for instance, women with smaller fibroids were managed preferentially with these modalities. The reporting of surgery may have some inaccuracies without verification of the medical record. Finally, our results may not be generalizable to other patient populations, given the lack of geographic diversity in our sample and the effect of local practice patterns on the rates of hysterectomy and UPS to treat fibroids.
In this longitudinal study of women with symptomatic fibroids, we have described the natural history of fibroid-related symptoms among women who do and do not undergo fibroid surgery and the rate of an initial surgical intervention. Our findings indicate that nonsurgical management may be a viable option for many women and that medical treatment, including CAM, may significantly improve symptoms. Further research on the effectiveness of western medication and CAM, as well as expectant management, is needed to better inform counseling for women who wish to avoid surgery.
Acknowledgments
Financial support: This study was funded by grants from the Agency for Healthcare Research and Quality (U01 HS09478, R01 HS011657, U01 HS07373) and the National Institute on Aging and Office of Research in Women's Health, National Institutes of Health (U01 HS09478). Dr. Jacoby was supported by the Women's Reproductive Health Research Career Development Program (Grant K12 HD001262) while she was preparing this manuscript. The funding sources had no role or involvement in the design and conduct of the study; the collection, management, analysis or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
COI: The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005;48:312–24. doi: 10.1097/01.grf.0000159538.27221.8c. [DOI] [PubMed] [Google Scholar]
- 2.Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104:393–406. doi: 10.1097/01.AOG.0000136079.62513.39. [DOI] [PubMed] [Google Scholar]
- 3.Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol. 2008;198:357–66. doi: 10.1016/j.ajog.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 4.Qidwai GI, Caughey AB, Jacoby AF. Obstetric outcomes in women with sonographically identified uterine leiomyomata. Obstet Gynecol. 2006;107:376–82. doi: 10.1097/01.AOG.0000196806.25897.7c. [DOI] [PubMed] [Google Scholar]
- 5.Stovall DW. Clinical symptomatology of uterine leiomyomas. Clin Obstet Gynecol. 2001;44:364–71. doi: 10.1097/00003081-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan M, Hartmann K, McKoy N, et al. Management of uterine fibroids: an update of the evidence. Evid Rep Technol Assess (Full Rep) 2007:1–122. [PMC free article] [PubMed] [Google Scholar]
- 7.Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6:595–602. doi: 10.1093/humupd/6.6.595. [DOI] [PubMed] [Google Scholar]
- 8.Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105:877–81. doi: 10.1097/01.AOG.0000156298.74317.62. [DOI] [PubMed] [Google Scholar]
- 9.Acien P, Quereda F. Abdominal myomectomy: results of a simple operative technique. Fertil Steril. 1996;65:41–51. doi: 10.1016/s0015-0282(16)58025-x. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson GF, Shaber RE, Armstrong MA, Hung YY. Changes in rates of hysterectomy and uterine conserving procedures for treatment of uterine leiomyoma. Am J Obstet Gynecol. 2007;196:601 e1–5. doi: 10.1016/j.ajog.2007.03.009. discussion e5-6. [DOI] [PubMed] [Google Scholar]
- 11.Malone LJ. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34:200–3. [PubMed] [Google Scholar]
- 12.Davis BJ, Haneke KE, Miner K, et al. The fibroid growth study: determinants of therapeutic intervention. J Womens Health (Larchmt) 2009;18:725–32. doi: 10.1089/jwh.2008.0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber AM, Mitchinson AR, Gidwani GP, Mascha E, Walters MD. Uterine myomas and factors associated with hysterectomy in premenopausal women. Am J Obstet Gynecol. 1997;176:1213–7. doi: 10.1016/s0002-9378(97)70337-5. discussion 7-9. [DOI] [PubMed] [Google Scholar]
- 14.Kuppermann M, Learman LA, Schembri M, et al. Effect of noncancerous pelvic problems on health-related quality of life and sexual functioning. Obstet Gynecol. 2007;110:633–42. doi: 10.1097/01.AOG.0000279153.56275.b5. [DOI] [PubMed] [Google Scholar]
- 15.Kuppermann M, Learman LA, Schembri M, et al. Predictors of hysterectomy use and satisfaction. Obstet Gynecol. 2010;115:543–51. doi: 10.1097/AOG.0b013e3181cf46a0. [DOI] [PubMed] [Google Scholar]
- 16.DeWaay DJ, Syrop CH, Nygaard IE, Davis WA, Van Voorhis BJ. Natural history of uterine polyps and leiomyomata. Obstet Gynecol. 2002;100:3–7. doi: 10.1016/s0029-7844(02)02007-0. [DOI] [PubMed] [Google Scholar]
- 17.Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci U S A. 2008;105:19887–92. doi: 10.1073/pnas.0808188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiaffarino F, Parazzini F, La Vecchia C, Marsico S, Surace M, Ricci E. Use of oral contraceptives and uterine fibroids: results from a case-control study. Br J Obstet Gynaecol. 1999;106:857–60. doi: 10.1111/j.1471-0528.1999.tb08409.x. [DOI] [PubMed] [Google Scholar]
- 19.Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed) 1986;293:359–62. doi: 10.1136/bmj.293.6543.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wise LA, Palmer JR, Harlow BL, et al. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol. 2004;159:113–23. doi: 10.1093/aje/kwh016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derman SG, Rehnstrom J, Neuwirth RS. The long-term effectiveness of hysteroscopic treatment of menorrhagia and leiomyomas. Obstet Gynecol. 1991;77:591–4. [PubMed] [Google Scholar]
- 22.Kaunitz AM. Progestin-releasing intrauterine systems and leiomyoma. Contraception. 2007;75:S130–3. doi: 10.1016/j.contraception.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Roy SN, Bhattacharya S. Benefits and risks of pharmacological agents used for the treatment of menorrhagia. Drug Saf. 2004;27:75–90. doi: 10.2165/00002018-200427020-00001. [DOI] [PubMed] [Google Scholar]
- 24.Dennehy CE. The use of herbs and dietary supplements in gynecology: an evidence-based review. J Midwifery Womens Health. 2006;51:402–9. doi: 10.1016/j.jmwh.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280:1569–75. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 26.Fugh-Berman A, Kronenberg F. Complementary and alternative medicine (CAM) in reproductive-age women: a review of randomized controlled trials. Reprod Toxicol. 2003;17:137–52. doi: 10.1016/s0890-6238(02)00128-4. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd KB, Hornsby LB. Complementary and alternative medications for women's health issues. Nutr Clin Pract. 2009;24:589–608. doi: 10.1177/0884533609343001. [DOI] [PubMed] [Google Scholar]
- 28.Marshall LM, Spiegelman D, Goldman MB, et al. A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril. 1998;70:432–9. doi: 10.1016/s0015-0282(98)00208-8. [DOI] [PubMed] [Google Scholar]


