Abstract
We have previously described a fluorometric method to measure ADP–ATP exchange rates in mitochondria of permeabilized cells, in which several enzymes that consume substantial amounts of ATP and other competing reactions interconverting adenine nucleotides are present. This method relies on recording changes in free extramitochondrial Mg2+ with the Mg2+-sensitive fluorescent indicator Magnesium Green (MgGr)™, exploiting the differential affinity of ADP and ATP for Mg2+. In particular, cells are permeabilized with digitonin in the presence of BeF3− and Na3VO4, inhibiting all ATP- and ADP-utilizing reactions but mitochondrial exchange of ATP with ADP catalyzed by the adenine nucleotide translocase. The rate of ATP appearing in the medium upon the addition of ADP to energized mitochondria is then calculated from the rate of change in free extramitochondrial Mg2+ using standard binding equations. Here, we describe a variant of this method involving an improved calibration step. This step minimizes errors that may be introduced during the conversion of the MgGr™ signal into free extramitochondrial [Mg2+] and ATP. Furthermore, we describe an approach for combining this methodology with the measurement of mitochondrial membrane potential and oxygen consumption in the same sample. The method described herein is useful for the study of malignant cells, which are known to thrive in hypoxic environments and to harbor mitochondria with profound functional alterations.
1. INTRODUCTION
Adenine nucleotide translocase (ANT) exchanges ATP for ADP across the inner mitochondrial membrane (Klingenberg, 1980; Pebay-Peyroula & Brandolin, 2004). Because ANT transports adenine nucleotides only in the Mg2+-free state (Klingenberg, 1980; Kramer, 1980), and Mg2+ has differential affinity for ADP and ATP, we are able to measure ANT activity using the membrane-impermeable Mg2+-sensitive fluorescent indicator “Magnesium Green (MgGr).” In this assay, the rate of change in free extramitochondrial [Mg2+] in the experimental medium is measured following the addition of ADP to mitochondria. The changes in free extramitochondrial [Mg2+] exhibit complete sensitivity to submicromolar amounts of the ANT inhibitor, carboxyatractyloside (cATR; Chinopoulos et al., 2009; Metelkin, Demin, Kovacs, & Chinopoulos, 2009). The rate of change in free [Mg2+] is converted to rate of ATP exported from mitochondria using standard binding equations (Chinopoulos et al., 2009). The ATP–ADP exchange rate mediated by the ANT from isolated mitochondria has been validated in Chinopoulos et al. (2009), especially in view of the contribution of the ATP-Mg2+/Pi carrier (Aprille, 1993) and a homolog of the Mrs2 protein originally described in yeast that mediates an electrophoretic uptake of Mg2+ in mitochondria (Kolisek et al., 2003).
In isolated mitochondria, besides ANT, adenylate kinase and creatine kinase in the intermembrane space interconvert adenine nucleotides, but the former is effectively inhibited by P1, P5-di(adenosine-5′) pentaphosphate (AP5A; Lienhard & Secemski, 1973), and the latter by excluding creatine or its phosphate derivatives in the medium (Chinopoulos et al., 2009). In permeabilized cells, however, numerous additional enzymes that interconvert adenine nucleotides exist. These include phosphorylases, phosphatases and kinases, as well as the Na+/K+ ATPase, the plasmalemmal, and endoplasmic Ca2+ ATPase, and in contractile cells the myosin ATPase. These reactions hamper the use of the binding equations that convert free [Mg2+] to ANT-dependent ADP–ATP exchange rate (Chinopoulos et al., 2009). Thus, in permeabilized cells, all competing adenine nucleotide inter-converting reactions need to be inhibited. This is accomplished by the use of BeF3− and vanadium compounds, which effectively inhibit ADP-and/or ATP-utilizing reactions (Baukrowitz, Hwang, Nairn, & Gadsby, 1994; Cantley et al., 1977; Davies & Hol, 2004; Gordon, 1991; Mukherjee et al., 2004; Robinson, Davis, & Steinberg, 1986; Werber, Peyser, & Muhlrad, 1992). In Kawamata, Starkov, Manfredi, and Chinopoulos (2010), we used this approach and demonstrated ANT-dependent ADP–ATP exchange rate in permeabilized cells, using BeF3− and Na3VO4. This method was also applied in determining ANT dysfunction in relation to mitochondrial membrane potential in myotubes expressing ANT1-harboring mutations linked to autosomal-dominant progressive external opthalmoplegia (Kawamata, Tiranti, Magrane, Chinopoulos, & Manfredi, 2011). In these studies, the conversion of dye emission signal to [Mg2+], and subsequently to ATP, was calibrated by obtaining the maximal fluorescence signal with excess [Mg2+] and the minimal fluorescence by the addition of the cation chelator, ethylenediaminetetraacetic acid (EDTA). However, because MgGr is a fluorimetric dye with single excitation and emission, it is subjected to the potential pitfalls of nonratiometric dyes related to variations in dye concentration and/or bleaching. In practice, this method introduced variability in the measurement of ADP–ATP exchange rate and a better calibration was required. Below we describe a step-by-step measurement of ANT-dependent adenine nucleotide exchange with a modified calibration method, allowing for an error-free conversion of MgGr signal to free extramitochondrial [Mg2+]. Furthermore, we present the methods to correlate ADP–ATP exchange rate to mitochondrial membrane potential and oxygen consumption. These could become particularly informative when studying bioenergetic parameters of cancer cell mitochondria that exhibit decreased or complete loss of electron flux associated with impaired respiration and ATP synthesis (Kwong, Henning, Starkov, & Manfredi, 2007).
2. METHODOLOGY
2.1. Reagents
Standard laboratory chemicals, AP5A, safranin O, and digitonin from Sigma (St. Louis, MO, USA). MgGr 5K+ salt from Life Technologies (Carlsbad, CA, USA). cATR from Calbiochem (San Diego, CA, USA). Tyrphostin 9, RG-50872, Malonaben, 3,5-di-tert-butyl-4-hydroxybenzylidenemalononitrile, 2,6-di-t-butyl-4-(2′, 2′-dicyanovinyl)phenol (SF 6847) from Biomol (catalog number EI-215; BIOMOL GmbH, Hamburg, Germany).
2.2. Stock reagents and buffers
25 mM Na3VO4, pH 8.7 (see below how to prepare)
0.2 M BeSO4 (see below how to prepare)
0.5 M NaF (see below how to prepare)
25 mM Ap5A, dissolved in distilled water
1 mM cATR, dissolved in distilled water
5 mM Oligomycin (if a mixture of oligomycins A, B, and Care used, that is, item # O4876 from Sigma-Aldrich, assume MW = 800), dissolved in ethanol
0.2 M EDTA
1 mM Safranin O, dissolved in distilled water
1.1 mM MgGr 5K+ salt, dissolved in distilled water
1 M Glutamate, dissolved in distilled water, pH 7 with KOH
0.5 M Malate, dissolved in distilled water, pH 7 with KOH
0.2 M ADP, K+ salt (see below how to prepare)
0.2 M ATP K+ salt (see below how to prepare)
2.5 mM Digitonin, dissolved in DMSO (see below)
1 M MgCl2
1 mM SF 6847, dissolved in ethanol. Do not substitute with FCCP, CCCP, or 2,4-dinitrophenol (see below).
Buffer A
8 mM KCl
110 mM K-gluconate
10 mM NaCl
10 mM Hepes
10 mM KH2PO4
0.005 mM EGTA
10 mM Mannitol
1 mM MgCl2
0.5 mg ml−1 Bovine serum albumin (fatty-acid-free)
pH 7.2
Buffer B
Buffer A without MgCl2, but containing 25 µM AP5A, 5 mM NaF, 0.2 mM BeSO4, 30 µM Na3VO4, 5 µM EDTA (to chelate nominal amount of Mg2+ in the buffer) and 50 µM digitonin.
Buffer C
Same as buffer A, but without MgCl2, and containing 4 µM cATR, 2 µM oligomycin, and 5 µM EDTA.
2.3. Preparation of reagents
ADP and ATP are purchased as a K+ salt of the highest purity available and titrated to pH 6.9 with KOH to make stocks of 0.2 M. Concentration of ADP and ATP stock solutions is corrected by measuring absorbance at 260 nm using an extinction coefficient εM = 15,400 M−1 cm−1.
All other reagents should be dissolved in distilled water, except SF 6847 and oligomycin which should be dissolved in ethanol, and digitonin which should be dissolved in DMSO.
2.4. Preparation of sodium orthovanadate (Na3VO4) and beryllium trifluoride (BeF3−)
A 25 mM Na3VO4 solution is prepared in distilled water (>17 mΩ resistance). The pH of the solution is adjusted to 8.7 with HCl, upon which it turns yellow. This solution is boiled until it turns colorless and cooled to room temperature. The pH is reassessed and readjusted to pH 8.7 with HCl, upon which the solution turns yellow again. This cycle of boiling until colorless and readjusting the pH is repeated until the solution remains colorless at pH 8.7. Finally, the solution is brought up to the initial volume with distilled water and stored in aliquots at −80 °C. This treatment removes all decavanadate ions present in the Na3VO4 solution, which induces mitochondrial membrane depolarization and inhibition of oxygen consumption (Aureliano & Crans, 2009). Orthovanadate inhibits the oxidation of only disrupted mammalian mitochondria (Byczkowski, Zychlinski, & Tluczkiewicz, 1979). Likewise, fluoroberyllium nucleoside diphosphate complexes inhibit only the exposed F1F0-ATPase (Issartel, Dupuis, Lunardi, & Vignais, 1991). BeSO4 and NaF are prepared as aqueous stock solutions of 0.2 M and 0.5 M, respectively, and kept at +4 °C for several years. BeF3− is formed immediately in solution upon mixing of BeSO4 and NaF, provided that NaF is in excess in the working solution.
Vanadate, beryllium, and fluoride salts are highly toxic to tissues and to the environment, and thus require proper handling and disposal. The combination of orthovanadate and BeF3− will inhibit kinases, mutases, phosphatases, and ATPases (Climent, Bartrons, Pons, & Carreras, 1981; Ray, Moore, & Rao, 1990). However, some kinases, such as pyruvate kinase, will remain uninhibited (Lord & Reed, 1990). In this respect, upon permeabilization of the cells one has to totally separate pyruvate kinase from its substrate, phosphoenol pyruvate, that is, there must be no glucose present in the medium prior to permeabilization, and a few minutes lag time must be allowed prior to ADP–ATP exchange rate measurements in order for the remaining reactions by kinases to “die-out.”
2.5. Digitonin stock
Our digitonin powder stocks were purchased as “approximately 50% estimated by thin layer chromatography”; therefore, we cannot be certain of the exact concentration of digitonin present in the measuring chamber. Optimum digitonin amount added to the chamber is determined by measuring oxygen consumption in nonpermeabilized cells using succinate as respiratory substrate, as detailed in Kawamata et al. (2010) and is the one conferring the highest respiratory control ratio. The entire study should be performed using the same digitonin stock solution.
2.6. Equipment
Oroboros Oxygraph-2k (Oroboros Instruments, Innsbruck, Austria) equipped with an LED exhibiting a wavelength maximum of 465 ± 25 nm (current for light intensity adjusted to 2 mA, i.e., level ‘4’) and either an <505-nm shortpass excitation filter (dye-based, filter set “safranin") or an <495-nm shortpass excitation filter (dye-based, filter set “MgG/CaG”). Emitted light is detected by a photodiode (range of sensitivity: 350–700 nm), through either an >560-nm longpass emission filter (dye-based) for safranin O or an >525-nm longpass emission filter for MgGr. A cuvette-based fluorimeter equipped with the capability of stirring and thermoregulation would also be suitable (e.g., Hitachi F-7000 spectrofluorimeter; Hitachi High Technologies, Maidenhead, UK).
2.7. Culturing and harvesting of the cells
The amount of cells required varies from one type to another. In cell types that contain extremely high amounts of mitochondria, such as in differentiated myotubes from mouse C2C12 myoblasts, plating 140,000 myocytes for differentiation (by exchanging horse for fetal bovine serum at the time when myocytes are over 90% confluent) is sufficient for the assay. For other cells, such as human fibroblasts, 1–2 million cells are required. Below, we present results obtained from HEK293 cells (~400,000 cells per assay). Regardless of the cell type, cells are washed once in phosphate-buffered saline and harvested with 0.1 ml of 0.25% trypsin–EDTA, inactivated by 0.9 ml calf serum, followed by centrifugation at 1100 g for 2 min. Next, cells are washed once in buffer A without disturbing the pellet. After the wash, cells are resuspended in 0.2 ml of buffer B. This buffer contains the same base composition as buffer A described above, but includes substrates (glutamate and malate) as well as inhibitors. See steps below for specific concentrations of substrates and inhibitors to include. The rationale for using this particular buffer composition is elaborated in Chinopoulos et al. (2009). Glutamate and malate as mitochondrial substrates were chosen on the basis that they support mitochondrial substrate-level phosphorylation, and as such, they contribute to greater ATP efflux rates (Chinopoulos et al., 2010; Kiss et al., 2013).
2.8. Kd determination of ATP and ADP for Mg2+
First, one should measure the apparent Kd values of ADP for Mg2+ and ATP for Mg2+ for the pertaining conditions (media, temperature, ionic strength, type and amount of cells, etc.), steps 1–7.
Cells resuspended in 2 ml of buffer C are added to a chamber of an Oroboros Oxygraph-2k. The presence of cATR and oligomycin in the buffer is only required for the Kd determination. MgGr fluorescence is recorded by the O2k-Fluorescence LED2-Module at a 1-Hz acquisition rate. Experiments are performed at 37 °C. Digitonin (see above regarding remarks about its concentration) and MgGr 5K+ salt (MgGr; 1 µM) are subsequently added to the chamber.
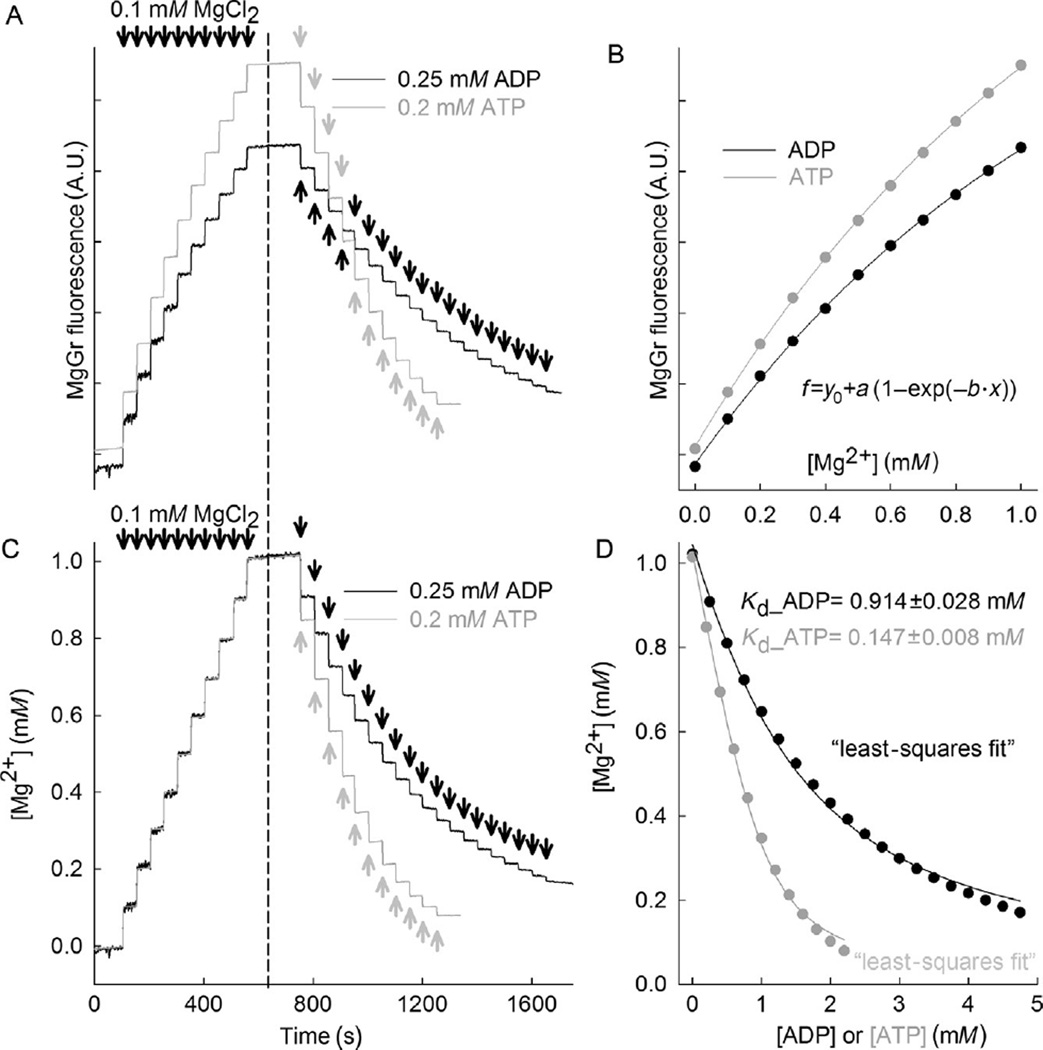
MgGr fluorescence signal is recorded upon stepwise additions of 0.1 mM MgCl2 for a total of 10 additions (about 50 s of recording time per addition). This is shown in Fig. 17.1A, on the left side of the dashed line.
Continuing in same cells in the chamber, add 0.25 mM ADP in subsequent steps for a total of 19 steps (about 50 s of recording time per addition). This is shown in Fig. 17.1A, on the right side of the dashed line.
Likewise, in a new cell preparation, repeat steps 1–3, but with 11 additions of 0.2 mM ATP instead of ADP, also shown in Fig. 17.1A.
Convert the MgGr signal of both left and right part of Fig. 17.1A to free [Mg2+]. To do this, plot the steady states of MgGr after each addition of MgCl2 (as seen in the left part of Fig. 17.1A) on the y-axis as a function of [Mg2+] (x-axis) and apply the following fit equation (you can use either trace): f = y0 + a(1 − exp(−b·x)). This is an exponential rise to maximum equation with a three-parameter function, where y0, a, and b are coefficients. The results are shown in Fig. 17.1B.
Calibrate the right part of Fig. 17.1A, using the coefficients y0, a, and b determined from step 5, shown in Fig. 17.1B, by solving the following exponential function for x: x = (−1/b)ln(1− ((f−y0)/a)). After calibration of MgGr signals obtained in Fig. 17.1A, it should look like Fig. 17.1C. If calibration was performed correctly, the left parts of both traces should be almost identical.
- Next, from the calibrated right part of Fig. 17.1C, the Kd of ADP for Mg2+ and the Kd of ATP for Mg2+ is estimated by fitting the following equation with the least squares method to the data points:
where [Mg2+]t is the total [Mg2+] (1 mM), [L]t is the total concentration of added ADP (or ATP). The least square fitting method can be performed by a customized Excel tool or other freely available software. The fitted curves are shown in Fig. 17.1D for both ADP and ATP. The Kd determined are Kd_ADP = 0.914 ± 0.028 mM, and Kd_ATP = 0.147 ± 0.008 mM. Since Kd values are now known for the pertaining experimental conditions, ADP–ATP exchange rates can be calculated from the MgGr recordings.
Figure 17.1. Magnesium Green fluorescence calibration and estimation of Kd of ATP and ADP for Mg2+.
(A) Reconstructed time recordings of MgGr raw fluorescence traces in permeabilized HEK293 cells as a function of extramitochondrial [Mg2+] (left part of the traces), and as a function of extramitochondrial ADP or ATP (right parts of the traces). (B) MgGr fluorescence changes are dependent on extramitochondrial [Mg2+]. (C) Calibrated time recordings of extramitochondrial [Mg2+] (left part of the traces), and as a function of extramitochondrial ADP and ATP (right parts of the traces) are shown. (D) Calibrated extramitochondrial Mg2+ plots as a function of ADP or ATP are shown, from which we estimated Kd of ADP and ATP for Mg2+ using the least squares method to fit the data.
2.9. [Mg2+]free determination from MgGr fluorescence in permeabilized cells and conversion to ADP–ATP exchange rate
Add 1.1 µM of MgGr in 1.8 ml of buffer B. Record MgGr fluorescence for a few minutes and allow the signal to stabilize. In the meantime, harvest cells by trypsinization as elaborated above. Cells are washed once with buffer A and then resuspended in 0.2 ml of buffer B.
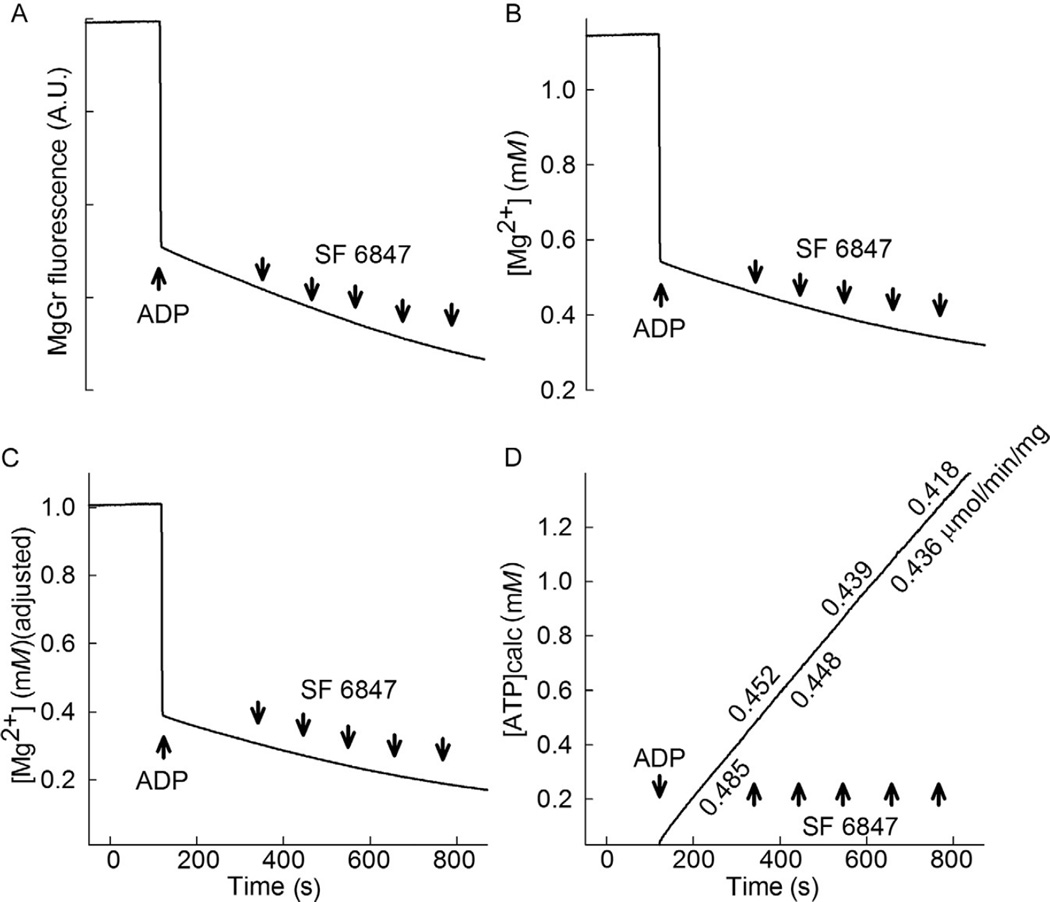
Upon addition of cells to the measuring chamber, wait for 3 min and resume recording. After 2 min, add a known amount of ADP; we suggest 2 mM ADP. When ADP is added, the fluorescence signal will drop, as shown in Fig. 17.2A. Subsequently, the uncoupler SF 6847 is added to the chamber in 10 nM increments (~100 s between each interval); this will be used later on to correlate the ATP efflux rates with membrane potential changes, see below. It is important to use SF 6847 and not the more widely used FCCP, CCCP, or 2,4-dinitrophenol, because SF 6847 is the only uncoupler that does not quench MgGr fluorescence up to a concentration of 1 µM (SF 6847 is also appropriate to use with Calcium Green, a Ca2+-sensitive dye; this is not unexpected as both Calcium Green and MgGr are fluorescein derivatives).
- Figure 17.2B shows the free [Mg2+] calibrated from MgGr raw fluorescence. One may notice that initial total [Mg2+] was measured to be 1.1486 mM, that is, not exactly 1 mM, which was the actual amount added into the chamber. After the addition of 2 mM ADP, free [Mg2+] was measured to attain the value of 0.5553 mM, that is, not the exact value of 0.3958 mM, which is what would be expected. The value of 0.3958 mM was derived from the equation below:
where [Mg2+]t is the total [Mg2+] (1 mM), [L]t is the total concentration of added ADP (or ATP), and Kd is the fitted value of KADP or KATP, respectively. These errors stem from the fact that MgGr is not a ratiometric dye, and thus it is subject to the pitfalls elaborated above. It is therefore necessary to adjust the whole trace shown in Fig. 17.2B so that the value just prior to the addition of 2 mM ADP is 1 mM [Mg2+] (the total), and the first value of [Mg2+] after the addition of 2 mM ADP is 0.3958 mM. From Fig. 17.1B, it is apparent that the relation between free [Mg2+] and MgGr fluorescence signal in the 0–0.5 mM range is fairly linear, see also Chinopoulos et al. (2009). Thus, one may apply a series of simple arithmetic operations to correct the errors: the difference between the measured total [Mg2+] (1.1486 mM) and the measured first value of [Mg2+] after the addition of 2 mM ADP (0.5553 mM) is 0.5933 mM, while the difference between the actual total [Mg2+] (1 mM) and the expected first value of [Mg2+] after the addition of 2 mM ADP (0.3958 mM) is 0.6042 mM; therefore, the measured changes in free [Mg2+] are underestimated by a factor of 0.6042/0.5553 = 1.01837. Thus, the whole trace of Fig. 17.2B is multiplied by 1.01837. This would generate a trace in which the value of [Mg2+] just prior to the addition of 2 mM ADP is 1.169 mM (the estimated total), and the first value of [Mg2+] after the addition of 2 mM ADP is 0.5655 mM (not shown). By subtracting the difference of 1.169–1 = 0.169 mM from this whole trace, the final trace should look like the one depicted in Fig. 17.2C. Note that in the trace shown in Fig. 17.2C, the value just prior to the addition of 2 mM ADP is 1 mM [Mg2+] (the total), and the first value of [Mg2+] after the addition of 2 mM ADP is 0.3958 mM. The trace depicting the estimated [Mg2+] has therefore been corrected, and the conversion to ATP appearing in the medium can now be applied, as shown in the next step. - We convert the corrected [Mg2+] values to ATP appearing in the medium, using the equation below:
Figure 17.2. Determination of extramitochondrial [Mg2+] and conversion to ATP.
(A) Reconstructed time recording of MgGr raw fluorescence in permeabilized HEK293 cells upon addition of 2 mM ADP (where indicated), followed by incremental 10 nM additions of the uncoupler SF 6847. (B) Calibrated time recording of extramitochondrial [Mg2+] obtained from panel (A). (C) Corrected calibrated trace of panel (B), as described in the text. (D) Calculated amount of ATP appearing in the medium converted from panel (C). The rate of ATP appearing in the medium is indicated in µmol min−1 mg protein−1.
The above equation is available for download as an executable file at http://www.tinyurl.com/ant-calculator. The rate of ATP appearing in the medium can be calculated by making a linear regression for the ATP values as a function of time, as shown in Fig. 17.2D.
2.10. Mitochondrial membrane potential (ΔΨm) determination in in situ mitochondria of permeabilized cells
ΔΨm is estimated using fluorescence quenching of the cationic dye safranin O due to its accumulation inside energized mitochondria (Akerman & Wikstrom, 1976), also taking into account the considerations discussed in Perevoshchikova, Sorochkina, Zorov, and Antonenko (2009) and Figueira, Melo, Vercesi, and Castilho (2012).
Cells are treated exactly as described for free [Mg2+] determination, except that MgGr is replaced by 5 µM safranin O.
Fluorescence is recorded in an Oroboros Oxygraph-2k at a 1-Hz acquisition rate, using the 495-nm excitation and 585-nm emission wavelengths. Experiments are performed at 37 °C. After the baseline signal has stabilized, 2 mM ADP is added to the chamber and fluorescence is allowed to stabilize. The raw signal of safranin O fluorescence is shown in Fig. 17.3A.
After the addition of ADP, ΔΨm is further depolarized stepwise by the addition of 10 nM SF 6847 until the signal after depolarization becomes stable (~100 s). Safranin O fluorescence is converted to mV (Fig. 17.3B) using the Nernst equation and assuming a matrix [K+] = 120 mM, which was determined by a voltage-fluorescence calibration curve of safranin O fluorescence in the presence of 2 nM valinomycin and increasing [K+] (0.2–120 mM; Akerman & Wikstrom, 1976). An important note is that cancer cells express very variable levels of IF1 (Sanchez-Arago et al., 2013), which inhibits the ATP hydrolytic function of the ATPase, thus hindering reversal rates. Therefore, it is not recommended to study ATP influx rates in mitochondria from cancer cells, that is, at very depolarized values. The method is suitable for studying cancer mitochondria during ATP synthesis.
Figure 17.3. Mitochondrial membrane potential and oxygen consumption determination in permeabilized cells.
(A) Reconstructed time recording of safranin O raw fluorescence in permeabilized HEK293 cells upon addition of 2 mM ADP (where indicated), followed by incremental 10 nM additions of the uncoupler SF 6847. (B) Calibrated time recording of ΔΨm obtained from panel (A). (C) Reconstructed time recording of oxygen concentration in the medium (black trace) and oxygen flux (gray trace) recorded simultaneously with either MgGr signal (panel A) or safranin O signal (panel A).
2.11. Mitochondrial respiration
Oxygen consumption is simultaneously measured with safranin O reflecting ΔΨm or MgGr fluorescence reflecting ADP–ATP exchange rates in the same samples polarographically using the Oxygraph-2k. Oxygen concentration (black trace of Fig. 17.3C) and oxygen flux (gray trace of Fig. 17.3C expressed as pmol s−1 mg−1; negative time derivative of oxygen concentration, divided by tissue mass per volume and corrected for instrumental background oxygen flux arising from oxygen consumption of the oxygen sensor and back-diffusion into the chamber) were recorded using DatLab software (Oroboros Instruments).
From the above measured parameters, one is able to estimate the rate of ATP appearing in the medium as a function of ΔΨm or oxygen consumption rate.
3. CONCLUSIONS AND COMMENTS
Using this technique, we have previously addressed ANT-dependent adenine nucleotide exchange in disease conditions (Kawamata et al., 2011). By generating ADP–ATP exchange rate/ΔΨm profile for myotubes expressing ANT1 with pathogenic mutations, we found that the mutations caused a reduced ADP-induced depolarization of membrane potential. This suggested that mutant ANT1-expressing cells require more ΔΨm to generate the same amount of ATP. In the same cells, ATP synthesis measured by a luciferase-based method did not show significant differences between mutant and wild-type ANT1-expressing cells. Thus, this is a sensitive method to determine ATP production from mitochondria. Furthermore, with the presently described methodology, one is provided the capacity to measure ADP–ATP exchange rate in a kinetic mode together with oxygen consumption, in addition to recording changes in ΔΨm. This may be particularly informative when studying bioenergetic parameters of cancer cell mitochondria, as these are known to exhibit decreased or complete loss of electron flux, which leads to impaired respiration and ATP synthesis (Kwong et al., 2007).
ACKNOWLEDGMENTS
This work was supported by the National Institute of Health/National Institute of Neurological Disease and Stroke Grant F31 NS054554 to H. K., PO AG014930 grant by NIH/NIA to A. A. S, the Országos Tudományos Kutatási Alapprogram Grants NNF 78905, NNF2 85658, K 100918, and the MTA-SE Lendület Neurobiochemistry Research Division Grant 95003 to C. C.
ABBREVIATIONS
- ANT
adenine nucleotide translocase
- AP5A
P1, P5-di(adenosine-5′) pentaphosphate
- cATR
carboxyatractyloside
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol-bis(2-aminoethylether)-N,N,N′, N′-tetraacetic acid
- MgGr
Magnesium Green
- SF 6847
tyrphostin 9, RG-50872, Malonaben, 3,5-di-tert-butyl-4-hydroxybenzylidenemalononitrile, 2,6-di-t-butyl-4-(2′,2′-dicyanovinyl)phenol
REFERENCES
- Akerman KE, Wikstrom MK. Safranine as a probe of the mitochondrial membrane potential. FEBS Letters. 1976;68:191–197. doi: 10.1016/0014-5793(76)80434-6. [DOI] [PubMed] [Google Scholar]
- Aprille JR. Mechanism and regulation of the mitochondrial ATP-Mg/P(i) carrier. Journal of Bioenergetics and Biomembranes. 1993;25:473–481. doi: 10.1007/BF01108404. [DOI] [PubMed] [Google Scholar]
- Aureliano M, Crans DC. Decavanadate (V10 O28 6-) and oxovanadates: Oxometalates with many biological activities. Journal of Inorganic Biochemistry. 2009;103:536–546. doi: 10.1016/j.jinorgbio.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Hwang TC, Nairn AC, Gadsby DC. Coupling of CFTR Cl- channel gating to an ATP hydrolysis cycle. Neuron. 1994;12:473–482. doi: 10.1016/0896-6273(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Byczkowski J, Zychlinski L, Tluczkiewicz J. Interaction of vanadate with respiratory chain of rat liver and wheat seedling mitochondria. The International Journal of Biochemistry. 1979;10:1007–1011. doi: 10.1016/0020-711x(79)90081-8. [DOI] [PubMed] [Google Scholar]
- Cantley LC, Jr, Josephson L, Warner R, Yanagisawa M, Lechene C, Guidotti G. Vanadate is a potent (Na, K)-ATPase inhibitor found in ATP derived from muscle. The Journal of Biological Chemistry. 1977;252:7421–7423. [PubMed] [Google Scholar]
- Chinopoulos C, Gerencser AA, Mandi M, Mathe K, Torocsik B, Doczi J, et al. Forward operation of adenine nucleotide translocase during F0F1-ATPase reversal: critical role of matrix substrate-level phosphorylation. FASEB Journal. 2010;24:2405–2416. doi: 10.1096/fj.09-149898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinopoulos C, Vajda S, Csanady L, Mandi M, Mathe K, Adam-Vizi V. A novel kinetic assay of mitochondrial ATP-ADP exchange rate mediated by the ANT. Biophysical Journal. 2009;96:2490–2504. doi: 10.1016/j.bpj.2008.12.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Climent F, Bartrons R, Pons G, Carreras J. Effect of vanadate on phosphoryl transfer enzymes involved in glucose metabolism. Biochemical and Biophysical Research Communications. 1981;101:570–576. doi: 10.1016/0006-291x(81)91297-3. [DOI] [PubMed] [Google Scholar]
- Davies DR, Hol WG. The power of vanadate in crystallographic investigations of phosphoryl transfer enzymes. FEBS Letters. 2004;577:315–321. doi: 10.1016/j.febslet.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Figueira TR, Melo DR, Vercesi AE, Castilho RF. Safranine as a fluorescent probe for the evaluation of mitochondrial membrane potential in isolated organelles and permeabilized cells. Methods in Molecular Biology. 2012;810:103–117. doi: 10.1007/978-1-61779-382-0_7. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods in Enzymology. 1991;201:477–482. doi: 10.1016/0076-6879(91)01043-2. [DOI] [PubMed] [Google Scholar]
- Issartel JP, Dupuis A, Lunardi J, Vignais PV. Fluoroaluminum and fluoroberyllium nucleoside diphosphate complexes as probes of the enzymatic mechanism of the mitochondrial F1-ATPase. Biochemistry. 1991;30:4726–4733. doi: 10.1021/bi00233a013. [DOI] [PubMed] [Google Scholar]
- Kawamata H, Starkov AA, Manfredi G, Chinopoulos C. A kinetic assay of mitochondrial ADP-ATP exchange rate in permeabilized cells. Analytical Biochemistry. 2010;407:52–57. doi: 10.1016/j.ab.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata H, Tiranti V, Magrane J, Chinopoulos C, Manfredi G. adPEO mutations in ANT1 impair ADP-ATP translocation in muscle mitochondria. Human Molecular Genetics. 2011;20:2964–2974. doi: 10.1093/hmg/ddr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss G, Konrad C, Doczi J, Starkov AA, Kawamata H, Manfredi G, et al. The negative impact of alpha-ketoglutarate dehydrogenase complex deficiency on matrix substrate-level phosphorylation. The FASEB Journal. 2013;27:2392–2406. doi: 10.1096/fj.12-220202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. The ADP-ATP translocation in mitochondria, a membrane potential controlled transport. Journal of Membrane Biology. 1980;56:97–105. doi: 10.1007/BF01875961. [DOI] [PubMed] [Google Scholar]
- Kolisek M, Zsurka G, Samaj J, Weghuber J, Schweyen RJ, Schweigel M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. The EMBO Journal. 2003;22:1235–1244. doi: 10.1093/emboj/cdg122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. Influence of divalent cations on the reconstituted ADP, ATP exchange. Biochimica et Biophysica Acta. 1980;592:615–620. doi: 10.1016/0005-2728(80)90104-8. [DOI] [PubMed] [Google Scholar]
- Kwong JQ, Henning MS, Starkov AA, Manfredi G. The mitochondrial respiratory chain is a modulator of apoptosis. The Journal of Cell Biology. 2007;179:1163–1177. doi: 10.1083/jcb.200704059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienhard GE, Secemski II. P 1, P 5 -Di(adenosine-5’)pentaphosphate, a potent multisubstrate inhibitor of adenylate kinase. The Journal of Biological Chemistry. 1973;248:1121–1123. [PubMed] [Google Scholar]
- Lord KA, Reed GH. Vanadyl(IV) complexes with pyruvate kinase: Activation of the enzyme and electron paramagnetic resonance properties of ternary complexes with the protein. Archives of Biochemistry and Biophysics. 1990;281:124–131. doi: 10.1016/0003-9861(90)90421-t. [DOI] [PubMed] [Google Scholar]
- Metelkin E, Demin O, Kovacs Z, Chinopoulos C. Modeling of ATP-ADP steady-state exchange rate mediated by the adenine nucleotide translocase in isolated mitochondria. The FEBS Journal. 2009;276:6942–6955. doi: 10.1111/j.1742-4658.2009.07394.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee B, Patra B, Mahapatra S, Banerjee P, Tiwari A, Chatterjee M. Vanadium—An element of atypical biological significance. Toxicology Letters. 2004;150:135–143. doi: 10.1016/j.toxlet.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Pebay-Peyroula E, Brandolin G. Nucleotide exchange in mitochondria: Insight at a molecular level. Current Opinion in Structural Biology. 2004;14:420–425. doi: 10.1016/j.sbi.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Perevoshchikova IV, Sorochkina AI, Zorov DB, Antonenko YN. Safranine O as a fluorescent probe for mitochondrial membrane potential studied on the single particle level and in suspension. Biochemistry (Mosc) 2009;74:663–671. doi: 10.1134/s000629790906011x. [DOI] [PubMed] [Google Scholar]
- Ray BD, Moore JM, Rao BD. 31P NMR studies of enzyme-bound substrate complexes of yeast 3-phosphoglycerate kinase: III. Two ADP binding sites and their Mg(II) affinity; effects of vanadate and arsenate on enzymic complexes with ADP and 3-P-glycerate. Journal of Inorganic Biochemistry. 1990;40:47–57. doi: 10.1016/0162-0134(90)80039-z. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Davis RL, Steinberg M. Fluoride and beryllium interact with the (Na + K)-dependent ATPase as analogs of phosphate. Journal of Bioenergetics and Biomembranes. 1986;18:521–531. doi: 10.1007/BF00743148. [DOI] [PubMed] [Google Scholar]
- Sanchez-Arago M, Formentini L, Martinez-Reyes I, Garcia-Bermudez J, Santacatterina F, Sanchez-Cenizo L, et al. Expression, regulation and clinical relevance of the ATPase inhibitory factor 1 in human cancers. Oncogenesis. 2013;2:e46. doi: 10.1038/oncsis.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werber MM, Peyser YM, Muhlrad A. Characterization of stable beryllium fluoride, aluminum fluoride, and vanadate containing myosin subfragment 1-nucleotide complexes. Biochemistry. 1992;31:7190–7197. doi: 10.1021/bi00146a023. [DOI] [PubMed] [Google Scholar]