Abstract
Individuals with 22q11.2 Deletion Syndrome (22qDS) have increased risk for psychiatric disorders. However, while medical geneticists self-report discussing psychiatric features of 22qDS with families (though often only when the child is older), most parents of children with 22qDS report receiving information about the psychiatric manifestations of 22qDS from non-medical sources. In an attempt to reconcile these previous findings, we sought to objectively determine the frequency with which medical geneticists discuss the potential psychiatric manifestations of 22qDS: a) in letters to referring physicians, and b) with families, and to explore plans for follow up. We abstracted data from charts of patients with 22qDS who were referred to a single medical genetics centre between January 1, 2000 and December 31, 2012. Psychiatric disorders were discussed in consult letters to referring physicians for n=57 (46%) of the 125 patients who met inclusion criteria – making them less frequently discussed than all other features of 22qDS. Despite exhaustive review of charts, the content of discussions with families was typically unclear. Follow-up in medical genetics was suggested for 50 people but only 18 (36%) of these patients returned. Disclosure of psychiatric features of 22qDS to families is necessary so that psychiatric disorders can be identified in time for early intervention to be implemented to achieve better prognosis for those affected. These empiric data offer some explanation as to why psychiatric services are underused by individuals with 22qDS.
Keywords: clinical genetics, Di-George, velo-cardiofacial syndrome, psychiatric disorders, psychiatric illness
INTRODUCTION
22q11.2 deletion syndrome (22qDS) is a common microdeletion syndrome affecting 1 of 4,000 births [Devriendt, Fryns, Mortier, van Thienen, & Keymolen, 1998]. 22qDS is a highly variable, multi-system condition with a range of clinical manifestations including congenital defects of heart and palate, calcium and immune deficiencies, speech, learning and cognitive disabilities as well as psychiatric illness [Shprintzen, 2008]. Individuals with this condition have a 25–30% chance of developing schizophrenia or other psychotic disorder, compared to a 3% general population risk for the same [Bassett et al., 2003]. In addition, individuals with 22qDS seem to have elevated rates of anxiety and mood disorders, attention-deficit disorder, oppositional defiant disorder, and autism spectrum disorders [Williams & Owen, 2004].
In 2008, Hercher and Bruenner found that psychiatric manifestations of the syndrome are of considerable concern to parents of children with 22qDS (causing more anxiety than any of the other features) but that parents reported finding information about this from non-medical sources instead of a healthcare provider [Hercher & Bruenner, 2008]. In a separate study, the most frequently reported source of information about psychiatric manifestations was the Internet [van den Bree et al., 2013]. In a 2013 study, medical geneticists (the group of healthcare professionals who are most often involved in diagnosing 22qDS [Hercher & Bruenner, 2008]) self-reported discussing potential psychiatric manifestations of the syndrome with families, but often only at follow-up appointments, when the child is older and of an age where these issues are perceived to be more relevant [Morris, Inglis, Friedman, & Austin, 2013]. These conflicting sets of data suggest different potential explanations. Perhaps, parents are usually told about the potential psychiatric manifestations of 22qDS by a medical geneticist or other physician, but do not remember. Alternatively, parents are not made aware of the potential psychiatric manifestations of the syndrome prior to their onset, perhaps because a) follow-up appointments in genetics after a 22qDS diagnosis (at which the potential for psychiatric disorders are more likely to be discussed [Hercher & Bruenner, 2008]) are not happening, or b) the medical geneticist expects the referring physician to follow up with the patient, but follow-up either does not happen at all; or follow up does happen, but the referring physician does not mention the potential psychiatric manifestations of the syndrome (perhaps due to lack of awareness) [Young, Shashi, Schoch, Kwapil, & Hooper, 2011].
Objectively exploring what transpires between medical geneticists, families, and referring physicians when a patient is diagnosed with 22qDS is of critical importance, because while early intervention for psychiatric conditions leads to the best long-term outcomes [Keshavan & Amirsadri, 2007], there is evidence that psychiatric services are underused by individuals with 22qDS [Young et al., 2011]. If in fact, families are not aware – in advance of psychiatric symptom onset – of this potential feature of the syndrome, they are perhaps less likely to seek and obtain timely and appropriate assistance for their child, which could lead to less optimal long term psychiatric outcomes. Thus, there is a need to definitively determine whether parents’ lack of information about the psychiatric manifestations of this condition is a result of gaps in clinical practice or informational overload. With this knowledge, strategies can be implemented to remedy the problem with the ultimate aim to improve utilization of psychiatric services within this population, which will improve outcomes for those affected.
We conducted a retrospective chart review of patients diagnosed with 22qDS between 2000 and 2012, in order to objectively determine how often medical geneticists discussed potential psychiatric manifestations with families and with referring physicians, and to gather information about follow-up appointments at medical genetics. We hypothesized that in families where the child with 22qDS was not already experiencing psychiatric problems, medical geneticists would more frequently discuss the potential for these problems to arise: a) within the timeframe 2010–2012 as compared to 2000–2002, and b) in adolescent/adult (≥12 years of age) as compared to pediatric (<12 years of age) patients. We expected psychiatric features of the syndrome to be more frequently discussed in 2010–2012 than 2000–2002 as a result of increasing awareness over time of psychiatric features being part of the 22qDS phenotype. We selected the earlier of the two time frames to capture clinical practice shortly after the association of psychiatric issues with the condition – initially reported in the early 1990s - started gaining traction [Murphy et al 1999]. We expected psychiatric features of the syndrome to be more frequently discussed with the families of older patients based on medical geneticists’ self-report in previous studies[Morris et al, 2014].
MATERIALS & METHODS
The charts of all patients referred to the BC Provincial Medical Genetics Program (BCPMGP) between January 1, 2000 and December 31, 2012 for an indication of 22qDS were identified from the program’s clinical database. From charts that met inclusion criteria (i.e. the patient received a cytogenetic diagnosis of 22qDS, and was seen by a medical geneticist who wrote a consult letter back to a referring physician), we abstracted the following data: patient demographic characteristics (sex, age, year of diagnosis), number of appointments, and which of the six common features of 22qDS (cardiac defects, craniofacial abnormalities, immune and calcium deficiencies, learning difficulties and psychiatric disorders) were discussed: a) in the consult letter back to the referring physician, and b) with the family. In order to determine if features were discussed with the family, the entire chart (including letters, progress notes, copies of emails, reading materials sent to patients, and any material from communication prior to 2000) was exhaustively reviewed. For all manifestations of 22qDS that were discussed – including psychiatric problems - we documented whether it was discussed because it was currently present in the patient (i.e. the chart notes, consult report, or referral indicated that the patient was symptomatic) or whether it was proactively presented as a possible future manifestation of the condition for an asymptomatic individual.
We used an inclusive approach to defining when “psychiatric disorders” had been discussed, and accepted a broad range of terms (e.g. “emotional disturbances”, “behavior problems”) in addition to more specific terms (e.g. psychosis, schizophrenia). In addition, we documented when referrals were suggested to mental health specialists, and the frequency with which probability estimates for the chance of psychiatric illness were provided to families and referring physicians. Follow-up visits at medical genetics (including return appointments, phone consultations or consult letters) were documented. Ethical approval for this study was obtained from the Institutional Review Board (IRB) of the University of British Columbia (H13 – 01166).
Data Analyses
Descriptive statistics were calculated for demographic data. We conducted four statistical tests, all of which involved considering only those patients who were not documented in any chart materials as already showing psychiatric features at the time of the medical genetics appointment. Specifically, first, we compared the frequency with which the psychiatric manifestations of 22qDS were discussed in letters to referring physicians in the timeframe 2010–2012 to the frequency with which they were discussed in 2000–2002. Second, we compared the frequency with which the psychiatric manifestations of 22qDS were discussed in the context of adolescent/adult patients (≥12 years of age) to the frequency with which they were discussed in the context of pediatric patients (<12 years of age). The remaining two statistical tests were repeats of those described above, but were performed using data regarding disclosure of psychiatric manifestations of 22qDS to families (rather than to referring physicians).
For all hypotheses, differences between categorical variables were analyzed using Fisher’s exact test using SPSS (Statistical Package for the Social Sciences). For all tests, a significance threshold (α) of p<0.01 was applied (to allow for the above four tests at a nominal overall significance level of 0.05).
RESULTS
A search of the BCPMGP database identified 436 patients who were referred for an indication of 22qDS, of whom 125 met study inclusion criteria (as described above), and for whom demographic information is shown in Table I. The remaining 311 patients were excluded from analysis for the following reasons: 18 charts could not be located, 254 patients tested negative for the microdeletion, 33 had not completed testing for the deletion, and 6 were not seen in medical genetics following 22qDS diagnosis.
Table 1.
Demographic characteristics of patients and the appointments
| Characteristic | n | % |
|---|---|---|
|
| ||
| Sex of patient
| ||
| Male | 76 | 61 |
| Female | 49 | 39 |
|
| ||
| Age of patient
| ||
| Prenatal | 17 | 14 |
| 0–2 | 47 | 38 |
| 3–5 | 14 | 11 |
| 6–8 | 13 | 10 |
| 9–11 | 6 | 5 |
| 12–14 | 12 | 10 |
| 15–17 | 3 | 2 |
| 18+ | 13 | 10 |
|
| ||
| Type of appointment
| ||
| Prenatal | 17 | 14 |
| Pediatric | 95 | 76 |
| Adult | 13 | 10 |
|
| ||
| Psychiatric symptoms
| ||
| Patients <12 years old | ||
| Current symptoms | 3 | 4 |
| No current symptoms | 77 | 96 |
|
| ||
| Patients ≥12 years old | ||
| Current symptoms | 11 | 39 |
| No current symptoms | 17 | 61 |
Discussion of psychiatric and other manifestations following diagnosis of 22qDS
Letters to referring physicians
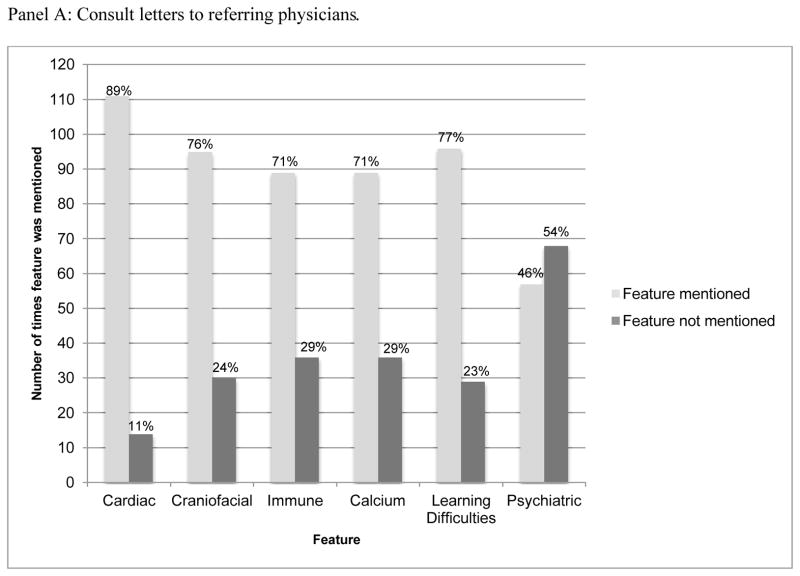
Psychiatric illness was the only feature of 22qDS that was discussed in <50% of consult letters (57/125) from medical geneticists to referring physicians (Figure 1, Panel A). In 14/57 of these cases, the patient was already manifesting psychiatric problems, in one case whether the patient was already symptomatic or not was ambiguous, and in the remaining 42 cases, the patient appeared to be asymptomatic for psychiatric symptoms. When considering only the 42 consult letters related to asymptomatic patients with whom psychiatric features were discussed, there was no statistically significant difference between the frequency of disclosure of psychiatric manifestations of 22qDS either over time (comparing the 2000–2002 timeframe to the 2010–2012 timeframe, (p= 0.41, Fisher’s exact test) or for adolescent/adult as compared to pediatric patients (p= 0.39, Fisher’s exact test).
Figure 1. Frequency with which the most common features of 22qDS were mentioned in Medical Genetics during consults with 125 patients.
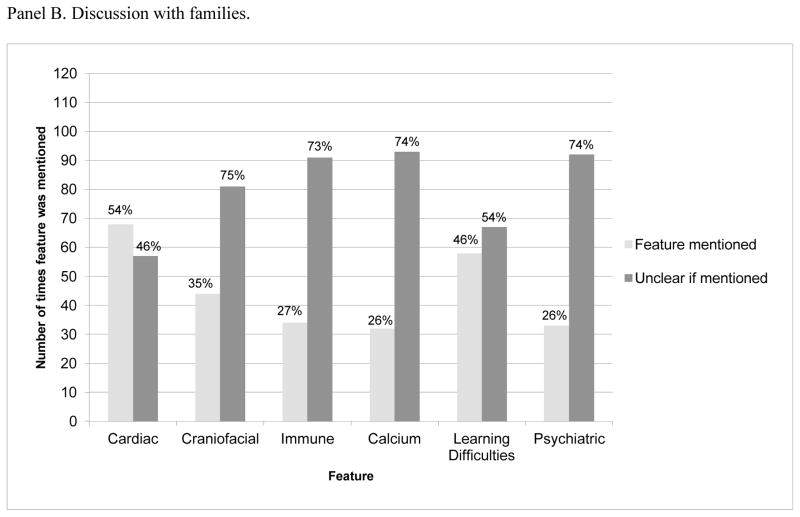
In both panels, the percentages at the top of each bar represent the proportion of the time that the given feature was discussed, or not discussed. Data shown in Panel A were drawn from consult letter to the referring physician. Data shown in Panel B were drawn from exhaustive review of entire chart. When psychiatric features were discussed with families, this was typically at initial diagnosis (27/33), rather than at a follow up appointment.
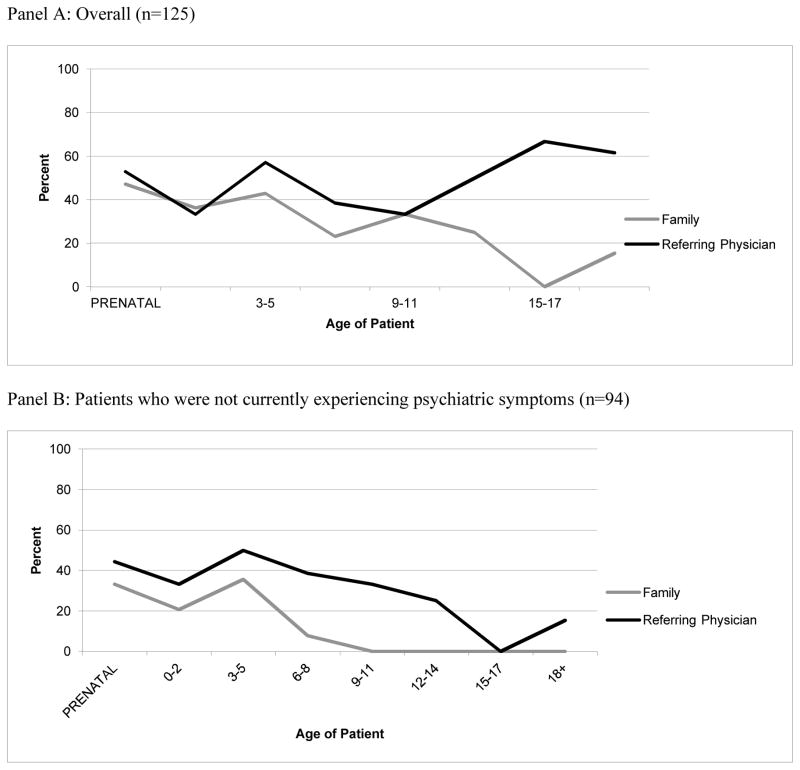
The frequency with which psychiatric manifestations of 22qDS were discussed in letters to referring physicians, for patients of different ages is shown in Figure 2.
Figure 2. Frequency with which psychiatric manifestations of 22qDS were discussed in letters to the referring physician, and with the family according to the age of the patient.
Both panels show the frequency of disclosure of psychiatric features of 22qDS according to age of the patient. Panel A shows the frequency of disclosure overall (i.e. including patients who were already manifesting symptoms), while Panel B shows the frequency of disclosure only for patients who were not showing symptoms of psychiatric illness. Figures are provided for descriptive purposes only, and do not relate to the hypotheses tested. No statistical analyses were performed.
When psychiatric illness was discussed proactively in letters to referring physicians (in the context of asymptomatic patients), risk estimates were disclosed in 11/42 cases.
Discussion with families
Based on data abstracted from the entire chart, psychiatric features were discussed with 26% (33/125) of families at some point during their care in medical genetics (see Figure 1, Panel B). In 11/33 of these cases, the patient was already manifesting psychiatric problems, in one case, whether the patient was already symptomatic or not was ambiguous, and in 21 cases the patient appeared to be asymptomatic for psychiatric symptoms.
When considering only the 21 charts of asymptomatic patients where it was clear that psychiatric features had been discussed with families (pediatric cases, n=15; prenatal cases, n=6), there was no statistically significant difference between the frequency of disclosure of psychiatric manifestations of 22qDS either over time (comparing 2000 to 2012, p= 0.58, Fisher’s exact test) or for adolescent/adult as compared to pediatric patients (p= 0.02, Fisher’s exact test).
The frequency with which psychiatric manifestations of 22qDS were discussed with families, for patients of different ages is shown in Figure 2.
Despite exhaustive review, in the majority of charts (92/125), we were unable to determine whether psychiatric features of 22qDS had been discussed.
When psychiatric illness was discussed proactively with asymptomatic pediatric patients, risk estimates were disclosed to families in only 2/21 cases.
Follow-up after a 22qDS diagnosis
In 20% (25/125) of consult letters, there was no stated plan for follow-up; in 39% (n=49) of cases, responsibility for follow-up was given back to the referring or primary care physician, with no expectation to see the patient back in medical genetics; and in the remaining 41% (n=51) of cases, the medical genetics team expected to be involved in follow-up. When the medical geneticist intended follow-up, only 36% (n=18) of patients returned. When follow-up did occur, there were typically a total of two visits to medical genetics per patient. For most patients (93%, n=114), referrals to mental health specialists and resources were not made. When they were made, it was usually in the context of a patient who was already symptomatic (83%, n=5).
DISCUSSION
This study was the first to attempt to objectively explore clinical practice around disclosure of psychiatric manifestations of 22qDS in medical genetics. We found no support for the hypotheses that the psychiatric manifestations would be discussed more over time (comparing 2000–2002, to 2010–2012) and or with increasing age of the patient. Even employing a very broad definition of what constitutes disclosure of psychiatric manifestations (e.g. “emotional disturbances”), psychiatric features of 22qDS were the only manifestations of the condition that were more likely to be omitted from rather than mentioned in consult reports to referring physicians. Further, even with exhaustive review of complete charts, we could only definitively identify that psychiatric disorders had been discussed with a quarter of families. These findings are broadly consistent with recent studies showing that both genetic counselors and medical geneticists self-report disclosing psychiatric manifestations of 22qDS less often than other manifestations of the condition [Martin et al., 2012; Morris et al., 2013].
Though medical geneticists have previously self-reported that they more frequently discuss psychiatric disorders at appointments when the affected individual is an adolescent or an adult as compared to when the patient is a child [Morris et al., 2013], our objective data did not support this. When visualizing data relating to communications between medical geneticists and referring physicians, a subtle trend towards increased frequency of disclosure of psychiatric features of 22qDS with age of patient seemed possible (Figure 2, Panel A). However, when we excluded discussions related to patients who were already experiencing psychiatric symptoms (39% of patients in this study who were ≥12 years old were already manifesting psychiatric symptoms at the time of the appointment), this trend disappeared. In fact, we could not find evidence that psychiatric disorders were ever discussed with families when the patient was ≥12 years old and was not already manifesting symptoms. Though it is of course possible that the practice of the medical geneticists at the clinic we studied here is divergent from the practice of medical geneticists who responded to the self-report survey, or that the self-report study was affected by social desirability bias, the only other way of reconciling our data with those of Morris et. al. (2013) is to assume that when medical geneticists self-report discussing this feature more often with adolescents or adults, they are implicitly referring to discussion of psychiatric illness in an already-symptomatic patient. It is critically important to consider the conceptual and practical differences between discussion of psychiatric features of 22qDS with families where the patient has not yet developed symptoms, and discussing this issue in the context of already-present symptoms. Fundamentally, discussing psychiatric manifestations of the condition prior to symptom-onset is crucial in alerting families to the potential need for psychiatric evaluation and treatment, so that they can seek out timely intervention. Timely intervention in the context of psychiatric disorders improves long-term prognosis [Emsley, Chiliza, & Schoeman, 2008].
Medical geneticists have also previously self-reported feeling that follow-up appointments were a more appropriate time to discuss psychiatric features of 22qDS than at initial diagnosis, when families may be overwhelmed with information [Morris et al., 2013]. However, our results show that medical genetics follow-up visits after a diagnosis of 22qDS were suggested for less than half of patients, and that only a third of those patients for whom follow-up is suggested actually attend subsequent visits, sometimes despite multiple attempts to follow up. While it may not be necessary for all patients with 22qDS to return to medical genetics, it becomes very important that medical geneticists communicate with physicians and families about the potential for psychiatric problems associated with 22qDS. If this communication does not happen, as our results suggest, the medical professionals providing long-term, ongoing care for individuals with 22qDS are likely to be unaware of the association, which precludes their ability to raise the issue with families. If families are not informed about the psychiatric manifestations of 22qDS from either a geneticist or family physician, it creates a barrier to accessing early intervention for psychiatric symptoms. Taken together, our data support parents’ reports that they are not told about the psychiatric manifestations of 22qDS by healthcare providers [Hercher & Bruenner, 2008] and offer insight into the reasons for which psychiatric services are underutilized by individuals with this condition, as has been demonstrated by other work [Young et al., 2011].
LIMITATIONS
This was a study of a single medical genetics clinic, and therefore we cannot generalize these results to infer practice at other centers. All data, including information regarding whether the patient had experienced psychiatric symptoms already, were drawn from chart review. Despite exhaustive review, it was difficult to definitively ascertain the specific nature of the psychiatric issues an individual with 22qDS had experienced when these issues were documented as being present, and it is possible that some patients who had experienced psychiatric problems already were not documented as such in the charts. Thus, it is possible that - while already low - the frequencies with which psychiatric disorders were proactively discussed with families that we report here, are overestimated. While we can be confident about the features of the syndrome that were disclosed to the referring physician, it was also difficult (again, despite exhaustive chart review), to discern the content of discussions with families about the manifestations of 22qDS, and thus it is possible that in some cases, psychiatric disorders were discussed with families but that this was not documented anywhere in the chart. However, our data are consistent with previous studies indicating that parents report not being told about the psychiatric manifestations of the condition [Hercher and Bruenner, 2008, van den Bree et al, 2013]. Regardless, the difficulty we encountered in discerning the content of discussions with families from exhaustive chart review raises interesting and potentially important questions about the purpose of medical genetics charts, and in particular, whether it should be possible to definitively identify from a chart which aspects of a syndrome were discussed with families, so as to allow for comprehensive follow up and co-ordination of care. Though reports about the association between 22qDS and psychiatric conditions started emerging in the 1990s, it could be argued that perhaps physicians would not have yet known about this by 2000–2002 (the first timeframe we chose for our comparison). This serves to make the observation that we found no statistically significant difference in the frequency with which the psychiatric manifestations of the condition were discussed between the two timeframes more striking.
It is possible that concerns about the potential implications for insurance or privacy inhibit clinical staff in medical genetics from disclosing or documenting psychiatric risks in the medical record. But this concern should be carefully weighed against the potential duty to warn families, given that clinical guidelines clearly recommend disclosure of psychiatric risks, and that early intervention can improve prognosis. Without evidence that psychiatric disorders were discussed, medical genetics practices may be vulnerable.
CONCLUSION
Parents of children with 22qDS and individuals with the condition themselves cite consultation with specialists as one of the benefits of a genetic diagnosis [Costain, Chow, Ray, & Bassett, 2011]. It has also been suggested that the greatest potential benefit of early diagnosis of 22qDS is the ability to recognize the early stages of schizophrenia or other psychiatric illness, allowing prompt consultation or diagnosis from experts, followed by effective treatment [Bassett & Costain, 2012]. Clinical practice guidelines for the management of patients with 22qDS recommend repeated psychiatric assessments beginning as early as age 1, suggesting medical providers pay vigilant attention to changes in behavior or emotional state [Bassett et al., 2011, Habel et al. 2011]. Cumulatively, these studies establish the desire for, and importance of, awareness of psychiatric risk associated with 22qDS, referral to specialists, and ongoing assessment for psychiatric illness in order to ensure prompt treatment. Our objective data show that medical geneticists do not typically discuss psychiatric manifestations of 22qDS at the time of diagnosis or in follow up appointments, and that follow up appointments are not always suggested. Further, information about the psychiatric manifestations of the syndrome are typically not communicated back to referring or primary care physicians. While medical geneticists have self-reported that they were more likely to discuss psychiatric risk when patients are older [Morris et al., 2013], our data show that this is not happening – at least in this clinical setting. Considering that the duration of untreated psychiatric illness is associated with poorer prognosis [Emsley et al., 2008], there is potentially a duty to warn patients or their parents of psychiatric risk associated with their genetic diagnosis. Without this knowledge, early symptoms will likely be missed and therapeutic interventions will be delayed [Shah et al., 2013].
Our results point to opportunity for improved care between diagnosis of 22qDS and intervention and treatment for psychiatric illness. While there is a strong justification for guidelines around disclosing the risk of psychiatric illness associated with 22qDS [Cancrini et al., 2014], best practices for communicating the information have not been established. Genetic counselors are ideally placed to address the psychiatric manifestations of 22qDS [Austin & Honer, 2008; Costain, Esplen, Toner, Hodgkinson, & Bassett, 2012a; Costain et al., 2012b; Inglis, Koehn, McGillivray, Stewart, & Austin, 2014; Peay & Austin, 2011]. Genetic counselors can also help patients with 22qDS and their families to understand how the deletion confers risk for psychiatric illness and providing strategies that can be employed to mitigate that risk. Indeed, genetic counseling - repeated at various life stages - is recommended as part of clinical management of individuals diagnosed with 22qDS, to provide updated information and address evolving concerns [Bassett et al., 2011]. However, it has been suggested that disclosing psychiatric risk as part of the 22qDS phenotype at the time of diagnosis is too much information during an already stressful time that can involve heart and/or craniofacial repair and careful management of immune systems and calcium levels, and indeed in some circumstances (e.g. in the context of a complex and significant congenital heart defect where a child’s survival is uncertain), discussing psychiatric disorders may not be appropriate). Future conversation between medical professionals, families, and patients themselves could improve and streamline clinical practice around disclosure of psychiatric risk for those affected with 22qDS with the ultimate aim of improving utilization of mental health services within this population, which will improve outcomes for these patients.
Acknowledgments
This study was made possible by Microgrant funding from the Rare Disease Foundation and the UBC Genetic Counseling program. JA was supported by the Canada Research Chairs Program, the Michael Smith Foundation for Health Research, the Canadian Institutes of Health Research, and BC Mental Health and Substance Use Services. None of the direct or indirect sponsors of this study had any role in the study design, data collection/analysis/interpretation, writing the report, or the decision to submit this manuscript for publication. All authors declare that they have no other conflicts of interests. Serena Talcott Baughman wrote the first draft of this manuscript, and did not receive any honorarium, grant, or other form of payment to do so. The authors thank Patricia Birch for her input including help with the departmental database, and all members of the Translational Psychiatric Genetics Group for their manifold support, insight, guidance, and commitment.
References
- Austin JC, Honer WG. Psychiatric genetic counselling for parents of individuals affected with psychotic disorders: a pilot study. Early Intervention in Psychiatry. 2008;2(2):80–89. doi: 10.1111/j.1751-7893.2008.00062.x. [DOI] [PubMed] [Google Scholar]
- Bassett A, Costain G. Clinical applications of schizophrenia genetics: genetic diagnosis, risk, and counseling in the molecular era. The Application of Clinical Genetics. 2012:1–18. doi: 10.2147/TACG.S21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EW, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The schizophrenia phenotype in 22q11 deletion syndrome. American Journal of Psychiatry. 2003;160(9):1580–1586. doi: 10.1176/appi.ajp.160.9.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, Habel A, Marino B, Oskarsdottir S, Philip N, Sullivan K, Swillen A, Vorstman J International 22q11.2 Deletion Syndrome Consortium. Practical Guidelines for Managing Patients with 22q11.2 Deletion Syndrome. The Journal of Pediatrics. 2011;159(2):332–339. e1. doi: 10.1016/j.jpeds.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancrini C, Puliafito P, Digilio M, Soresina A, Martino S, Rondelli R, Consolini R, Ruga E, Cardinale F, Finocchi A, Romiti ML, Martire B, Bacchetta R, Albano V, Carotti A, Specchia F, Montin D, Cirillo E, Cocchi G, Trizzino A, Bossi G, Milanesi O, Azzari C, Corsello G, Pignata C, Aiuti A, Pietrogrande MC, Marino B, Ugazio AG, Plebani A, Rossi P Italian Network for Primary Immunodeficiencies. Clinical Features and Follow-Up in Patients with 22q11.2 Deletion Syndrome. The Journal of Pediatrics. 2014;164(6):1475–1480.e2. doi: 10.1016/j.jpeds.2014.01.056. [DOI] [PubMed] [Google Scholar]
- Costain G, Chow EWC, Ray PN, Bassett AS. Caregiver and adult patient perspectives on the importance of a diagnosis of 22q11.2 deletion syndrome. Journal of Intellectual Disability Research. 2011;56(6):641–651. doi: 10.1111/j.1365-2788.2011.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costain G, Esplen MJ, Toner B, Hodgkinson KA, Bassett AS. Evaluating Genetic Counseling for Family Members of Individuals With Schizophrenia in the Molecular Age. Schizophrenia Bulletin. 2012a doi: 10.1093/schbul/sbs124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costain G, Esplen MJ, Toner B, Scherer SW, Meschino WS, Hodgkinson KA, Bassett AS. Evaluating Genetic Counseling for Individuals With Schizophrenia in the Molecular Age. Schizophrenia Bulletin. 2012b doi: 10.1093/schbul/sbs138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devriendt K, Fryns JP, Mortier G, van Thienen MN, Keymolen K. The annual incidence of DiGeorge/velocardiofacial syndrome. Journal of Medical Genetics. 1998;35(9):789–790. doi: 10.1136/jmg.35.9.789-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley R, Chiliza B, Schoeman R. Predictors of long-term outcome in schizophrenia. Current Opinion in Psychiatry. 2008;21(2):173–177. doi: 10.1097/YCO.0b013e3282f33f76. [DOI] [PubMed] [Google Scholar]
- Habel A, Herriot R, Kumararatne D, Allgrove J, Baker K, Baxendale H, Bu’Lock F, Firth H, Gennery A, Holland A, Illingworth C, Mercer N, Pannebakker M, Parry A, Roberts A, Tsai-Goodman B. Towards a safety net for management of 22q11.2 deletion syndrome: guidelines for our times. European Journal of Pediatrics. 2011;173:757–65. doi: 10.1007/s00431-013-2240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercher L, Bruenner G. Living with a child at risk for psychotic illness: The experience of parents coping with 22q11 deletion syndrome: An exploratory study. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;146A(18):2355–2360. doi: 10.1002/ajmg.a.32466. [DOI] [PubMed] [Google Scholar]
- Inglis A, Koehn D, McGillivray B, Stewart SE, Austin J. Evaluating a unique, specialist psychiatric genetic counseling clinic: uptake and impact. Clinical Genetics. 2014 doi: 10.1111/cge.12415. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Amirsadri A. Early intervention in schizophrenia: current and future perspectives. Current Psychiatry Reports. 2007;9(4):325–328. doi: 10.1007/s11920-007-0040-8. [DOI] [PubMed] [Google Scholar]
- Martin N, Mikhaelian M, Cytrynbaum C, Shuman C, Chitayat DA, Weksberg R, Bassett AS. 22q11.2 Deletion Syndrome: Attitudes towards Disclosing the Risk of Psychiatric Illness. Journal of Genetic Counseling. 2012 doi: 10.1007/s10897-012-9517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris E, Inglis A, Friedman J, Austin J. Discussing the psychiatric manifestations of 22q11.2 deletion syndrome: an exploration of clinical practice among medical geneticists. Genetics in Medicine. 2013;15(9):713–720. doi: 10.1038/gim.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velocardio facial syndrome. Archives of General Psychiatry. 1999;56(10):940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Peay HL, Austin JC. How to Talk with Families About Genetics and Psychiatric Illness. W. W. Norton & Company; 2011. [Google Scholar]
- Shah SK, Hull SC, Spinner MA, Berkman BE, Sanchez LA, Abdul-Karim R, Hsu AP, Claypool R, Holland SM. What Does the Duty to Warn Require? The American Journal of Bioethics. 2013;13(10):62–63. doi: 10.1080/15265161.2013.828528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome: 30 Years of study. Developmental Disabilities Research Reviews. 2008;14(1):3–10. doi: 10.1002/ddrr.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bree MBM, Miller G, Mansell E, Thapar A, Flinter F, Owen MJ. The internet is parents’ main source of information about psychiatric manifestations of 22q11.2 deletion syndrome (22q11.2DS) European Journal of Medical Genetics. European Journal of Medical Genetics. 2013;56(8):439–441. doi: 10.1016/j.ejmg.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NM, Owen MJ. Genetic abnormalities of chromosome 22 and the development of psychosis. Current Psychiatry Reports. 2004;6(3):176–182. doi: 10.1007/s11920-004-0062-4. [DOI] [PubMed] [Google Scholar]
- Young AS, Shashi V, Schoch K, Kwapil T, Hooper SR. Discordance in diagnoses and treatment of psychiatric disorders in children and adolescents with 22q11.2 deletion syndrome. Asian Journal of Psychiatry. 2011;4(2):119–124. doi: 10.1016/j.ajp.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]