Abstract
Purpose
Colonoscopic polypectomy and surveillance are important to prevent colorectal cancer and identify additional relative risk factors for adequate surveillance. In this study, we evaluated risk factors related to recurrent high-risk polyps during the surveillance of patients with high-risk polyps.
Materials and Methods
We included 434 patients who had high-risk polyps (adenoma ≥10 mm, ≥3 adenomas, villous histology, or high-grade dysplasia) on the baseline colonoscopy and underwent at least one surveillance colonoscopy from 2005 to 2011 at Severance Hospital. Data regarding patient characteristics, bowel preparation and polyp size, location, number, and pathological diagnosis were retrospectively collected from medical records. Patients with recurrent high-risk polyps were compared with patients with low-risk or no polyps during surveillance.
Results
Patients were predominantly male (77.4%), with a mean age of 61.0±8.6 years and mean follow-up of 1.5±0.8 years. High-risk polyps recurred during surveillance colonoscopy in 51 (11.8%) patients. Results of multivariate analysis showed that male gender, poor bowel preparation, and a larger number of adenomas were independent risk factors for recurrent high-risk polyps (p=0.047, 0.01, and <0.001, respectively). Compared with high-risk polyps found during initial colonoscopy, high-risk polyps on surveillance colonoscopy had higher proportions of small adenomas, low-risk pathology, and fewer adenomas overall, but there was no difference in location.
Conclusion
Male patients and those with poor bowel preparation for colonoscopy or higher numbers of adenomas were more likely to experience recurrent high-risk polyps.
Keywords: Colon polyp, high-risk, polypectomy, surveillance
INTRODUCTION
Colonoscopic polypectomy and surveillance are important to prevent colorectal cancer.1,2,3 Guidelines recommend polypectomy surveillance intervals according to risk stratification, based on polyp characteristics.4 However, the present guidelines assume adequate bowel cleansing, proper withdrawal time, and complete cecal inspection at the qualifying colonoscopy, and did not consider other information about the patient or procedure.
Guidelines defined high-risk polyps as adenoma with villous histology, high-grade dysplasia (HGD), ≥10 mm, or 3 or more adenomas. The risk of high-risk polyps was 1.3-2.4% within 5 years of a negative colonoscopy, but this risk increases by 11.9% with three or more adenomas <10 mm.5,6,7,8,9,10 According to polyp size, the risk of high-risk polyp is 7.7% for polyps <5 mm, 15.9% for polyps 10-19 mm, and 19.3% for polyps >20 mm.11 Therefore, polypectomy surveillance guidelines recommend follow-up of less than 3 years for patients with high-risk polyps at baseline colonoscopy. Although not all patients with high-risk polyps progress clinically, many endoscopists tend to perform surveillance colonoscopy at intervals shorter than 3 years recommended by the guidelines. In fact, Martínez, et al.12 reported that the 1-year detection rate of high-risk polyps was 3.8% in lower-risk patients and 11.2% in high-risk patients. Therefore, a more detailed risk stratification of polypectomy surveillance guidelines appears to be needed.
Previous studies of recurrent high-risk polyps have defined the index colonoscopy as one or more adenomas or negative finding.5,6,7,8,9,10,11,12,13,14 However, we thought it necessary to evaluate the recurrence of high-risk polyps after an index colonoscopy finding of high-risk polyps. Therefore, we compared patients who had recurrent high-risk polyps with those with low-risk or no polyps during surveillance after an index colonoscopy with high-risk polyps. We then evaluated risk factors for recurrent high-risk polyps. Identification of more relative risk factors is important for adequate surveillance of high-risk polyps and will contribute to lower healthcare costs.
MATERIALS AND METHODS
Among 5472 patients who underwent more than two colonoscopies from 2005 to 2011 at Severance Hospital, 2672 patients had polyps found during their first colonoscopy. Of these, we enrolled 434 consecutive patients with high-risk polyps (adenoma ≥10 mm, ≥3 adenomas, ≥20% villous histology, or HGD) during first colonoscopy. The following clinical data were collected retrospectively from medical records: 1) patient characteristics including gender, age, body mass index, family history of colorectal cancer (CRC) or polyps, and personal history of aspirin and/or nonsteroidal anti-inflammatory drug (NSAID) use; 2) procedure information including bowel cleanliness, endoscopist experience, colonoscopy withdrawal time, presence of diverticular disease, and success/failure of colonoscopy; and 3) polyp information including size, location, number, pathological diagnosis, and interval between initial and surveillance colonoscopies. Bowel cleansing was accomplished with 4 L of a polyethylene glycol-electrolyte solution (Colonlyte; Taejun, Seoul, Korea). Adequacy of bowel cleansing was classified using a three-point scale (good: dry colon or small volume of clear liquid; fair: large volume of clear liquid or minimal semi-solid residue with clear liquid; poor: significant amount of solid residue).15 Endoscopists were classified as experienced (>1000 colonoscopic cases) or inexperienced (≤100 colonoscopic cases). Withdrawal time was defined as time required for withdrawal of the colonoscope from cecum to anus, including polypectomy. We evaluated all removed polyps without disuse. Polyp size was determined by comparison with an open biopsy forceps. In cases with multiple polyps, polyp size was defined as size of the largest polyp. Location was categorized relative to the splenic flexure: 1) right colon (cecum, ascending and transverse colons) or 2) left colon (rectum, sigmoid and descending colons). In cases with multiple polyps, polyp location was defined as that of the index adenoma. Index polyp was based on the largest polyp, or polyps with HGD or villous histology if all polyps were <1 cm.16 Baseline colonoscopy was defined as the first colonoscopy showing a high-risk polyp. For patients examined in less than 6 months of the baseline colonoscopy, the additional polyps were included as part of the baseline colonoscopy. Surveillance colonoscopy was defined as a colonoscopy repeated within more than 1 year of the index colonoscopy. To evaluate factors affecting recurrence of highrisk polyps after a previous colonoscopy finding of high-risk polyp, patients with recurrent high-risk polyps were compared with those who had low-risk or no polyps during surveillance after an index colonoscopy with high-risk polyps.
Patient characteristics are presented as mean±standard deviation or n (%), as appropriate. The independent t-test or Mann-Whitney U test was used to compare continuous variables, and χ2 test or Fisher's exact test was used to compare categorical variables. Multivariate logistic regression was performed to identify risk factors for recurrence of high-risk polyps. Variables that were predictive at the 0.05 level by using a univariate analysis were entered into the final multivariate analysis. A p-value of <0.05 was considered significant, and all statistical analyses were performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics
A total of 434 patients (336 men, 98 women) underwent surveillance colonoscopy after a previous colonoscopy finding of high-risk polyp. Baseline characteristics of the patients are summarized in Table 1. Mean age was 61.0±8.6 years, and mean follow-up was 17.6±9.6 months. Recurrent high-risk polyps during surveillance colonoscopy were observed in 51 (11.8%) patients. Initial colonoscopy results of 51 patients with recurrent high-risk polyps were compared with those of 383 patients with low-risk or no polyps during surveillance. Gender, bowel preparation, withdrawal time, and polyp number were different between two groups.
Table 1. Baseline Characteristics of the Study Patients.
| All patients | High-risk → no or low-risk | High-risk → high-risk | p value | |
|---|---|---|---|---|
| Number (%) | 434 | 383 (88.2) | 51 (11.8) | |
| Patient factors | ||||
| Male (%) | 336 (77.4) | 290 (75.7) | 46 (90.2) | 0.02 |
| Age (yrs) | 61.0±8.6 | 60.7±8.7 | 63.2±7.9 | 0.058 |
| Body mass index (kg/m2) | 24.0±2.9 | 24.1±2.8 | 23.5±3.0 | 0.183 |
| Family history of CRC (%) | 37 (8.5) | 36 (9.4) | 1 (2.0) | 0.074 |
| Family history of polyps (%) | 23 (5.3) | 20 (5.2) | 3 (5.9) | 0.843 |
| Aspirin and/or NSAID use (%) | 70 (16.1) | 60 (15.7) | 10 (19.6) | 0.477 |
| Procedure factors | ||||
| Bowel preparation (%) | 0.005 | |||
| Good or fair | 351 (81.0) | 317 (83.0) | 34 (66.7) | |
| Poor | 82 (19.0) | 65 (17.0) | 17 (33.3) | |
| Endoscopist (%) | 0.133 | |||
| Experienced colonoscopist | 230 (53.0) | 208 (54.3) | 22 (43.1) | |
| Fellow | 204 (47.0) | 175 (45.7) | 29 (56.9) | |
| Withdrawal time (min) | 24.2±17.8 | 23.6±17.7 | 28.9±17.6 | 0.043 |
| Diverticular disease (%) | 57 (13.1) | 50 (13.1) | 7 (13.7) | 0.894 |
| Failed colonoscopy (%) | 7 (1.6) | 5 (1.3) | 2 (3.9) | 0.164 |
| Polyp factors | ||||
| Interval period (yrs) | 1.5±0.8 | 1.5±0.8 | 1.6±0.9 | 0.27 |
| Size (max) | 14.5±9.1 | 14.8±9.3 | 12.2±7.7 | 0.054 |
| Site (%) | 0.859 | |||
| Left (DC-R) | 255 (59.0) | 226 (59.3) | 29 (58.0) | |
| Right (C-SF) | 176 (41.0) | 155 (40.7) | 21 (42.0) | |
| Polyp number | ||||
| Total number (%) | 3.7±3.2 | 3.5±3.1 | 5.7±3.6 | <0.001 |
| 1-2 | 186 (42.9) | 180 (47.0) | 6 (11.8) | <0.001 |
| 3-9 | 225 (51.8) | 189 (49.3) | 36 (70.6) | |
| ≥10 | 23 (5.3) | 14 (3.7) | 9 (17.6) | |
| Number of polyps ≥1 cm | 1.0±1.1 | 1.0±1.0 | 0.9±1.5 | 0.593 |
| Pathology (%) | 0.166 | |||
| Adenoma | 187 (43.1) | 158 (41.3) | 29 (56.9) | |
| Villous | 83 (19.1) | 76 (19.8) | 7 (13.7) | |
| High grade | 164 (37.8) | 149 (38.9) | 15 (29.4) |
CRC, colorectal cancer; NSAID, nonsteroidal anti-inflammatory drug; DC, descending colon; R, rectum; C, cecum; SF, splenic flexure.
Factors associated with recurrent high-risk polyps
We analyzed risk factors of recurrent high-risk polyps using logistic regression (Table 2). Results of univariate and multivariate analysis showed that male gender, poor bowel preparation, and a higher number of adenoma were independent risk factors for recurrence (p=0.047, 0.01, and <0.001, respectively). Endoscopist experience and polyp size, location, and pathology were not risk factor of recurrent high-risk polyps.
Table 2. Univariate and Multivariate Logistic Regression for the Factors Associated with Recurrent High-Risk Polyps.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| p value | OR | 95% CI | p value | |
| Patient factors | ||||
| Gender | 0.026 | |||
| Female | 1 | |||
| Male | 2.757 | 1.015-7.489 | 0.047 | |
| Age | 0.059 | |||
| Body mass index | 0.183 | |||
| Family history of CRC | 0.108 | |||
| Family history of polyps | 0.843 | |||
| Aspirin and/or NSAID use | 0.478 | |||
| Procedure factors | ||||
| Bowel preparation | 0.006 | |||
| Good or fair | 1 | |||
| Poor | 2.408 | 1.238-4.662 | 0.010 | |
| Endoscopist (experienced colonoscopist, fellow) | 0.135 | |||
| Withdrawal time | 0.052 | |||
| Diverticular disease | 0.894 | |||
| Failed colonoscopy | 0.185 | |||
| Polyp factors | ||||
| Interval period | 0.270 | |||
| Size (max) | 0.055 | |||
| Site [left (DC-R), right (C-SF)] | 0.831 | |||
| Polyp number | ||||
| Total number | <0.001 | 1.150 | 1.069-1.237 | <0.001 |
| 1-2 | <0.001 | |||
| 3-9 | ||||
| ≥10 | ||||
| Number of polyps ≥1 cm | 0.592 | |||
| Pathology (adenoma, villous, high grade) | 0.079 | |||
OR, odds ratio; CI, confidence interval; CRC, colorectal cancer; NSAID, nonsteroidal anti-inflammatory drug; DC, descending colon; R, rectum; C, cecum; SF, splenic flexure.
Detection rate of recurrent high-risk polyp in cases without three risk factors
We evaluated the recurrence rate of high-risk polyp in cases without the three risk factors described above (Table 3). Recurrence rates of high-risk polyps for the non-risk factors such as female gender, proper bowel preparation, and 1-2 polyps were 5.0%, 9.6%, and 3.2%, respectively. Evaluation of combinations of non-risk factors showed that the recurrence rate of high-risk polyps decreased by 3.7% in females with proper bowel preparation, 3.0% in females with 1-2 polyps, and 1.9% for males with proper bowel preparation and 1-2 polyps. Recurrent high-risk polyps were not observed in patients with all three non-risk factors.
Table 3. Actual Risk for Recurrence of High-Risk Polyp in Cases without Three Risk Factors.
| Factor number | Non-risk factors | High risk polyp/non-risk factor, n (%) |
|---|---|---|
| 1 | Female | 5/99 (5.0) |
| Proper bowel preparation | 34/352 (9.6) | |
| 1-2 polyps | 6/187 (3.2) | |
| 2 | Female+proper bowel preparation | 3/80 (3.7) |
| Female+1-2 polyps | 2/66 (3.0) | |
| Proper bowel preparation+1-2 polyps | 3/157 (1.9) | |
| 3 | Female+proper bowel preparation+1-2 polyps | 0/54 (0) |
Comparison of polyp characteristics between high-risk polyps in initial colonoscopy and those in surveillance colonoscopy
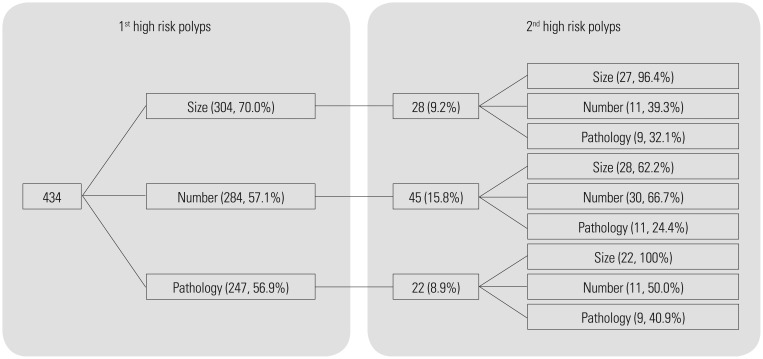
High-risk polyps that recurred during surveillance had increased numbers of small adenomas, low-risk pathology, and fewer total adenomas, but did not differ in location compared with polyps found during initial colonoscopy (Table 4). Of the 434 patients with high-risk polyps, 304 patients had adenomas ≥10 mm (70.0%), 284 patients had >3 adenomas (57.1%), and 247 patients had high-risk pathology such as villous histology or HGD (56.9%) (Fig. 1). Rates of recurrence for high-risk polyps was 28/304 (9.2%) patients with adenoma ≥10 mm, 45/284 (15.8%) patients with >3 adenomas, and 22/247 (8.9%) patients with high-risk histology. In addition, 96.4% of patients with high-risk polyps with adenoma ≥10 mm at initial colonoscopy had recurrent high-risk polyps with adenomas ≥10 mm, 66.7% of patients with high-risk polyps with >3 adenomas had recurrent high-risk polyps with >3 adenomas, and 100% of patients with high-risk polyps with high-risk pathology had recurrent high-risk polyps with adenoma ≥10 mm. In cases of high-risk pathology, recurrence rates of high-risk polyps were 7/83 (8.4%) patients with villous histology and 15/165 (9.1%) patients with HGD (data not shown).
Table 4. Comparison of Polyp Characteristics between High-Risk Polyps Detected by Initial Colonoscopy and Those Detected by Surveillance Colonoscopy.
| First high-risk polyp | Second high-risk polyp | p value | |
|---|---|---|---|
| n | 434 | 51 | |
| Size (max), mm | 14.5±9.1 | 11.8±7.2 | 0.015 |
| Site | |||
| Left (DC-R) | 255 (58.8%) | 23 (45.1%) | 0.055 |
| Right (C-SF) | 176 (40.6%) | 28 (54.9%) | |
| Polyp number | |||
| Total number | 3.7±3.2 | 3.1±1.9 | 0.05 |
| 1-2 | 186 (42.9%) | 20 (39.2%) | 0.451 |
| 3-9 | 225 (51.8%) | 30 (58.8%) | |
| ≥10 | 23 (5.3%) | 1 (2.0%) | |
| Number of polyps >1 cm | 1.0±1.1 | 0.7±0.5 | 0.031 |
| Pathology | |||
| Adenoma | 187 (43.1%) | 38 (74.5%) | <0.001 |
| Villous | 83 (19.1%) | 2 (3.9%) | |
| High-grade | 164 (37.8%) | 11 (21.6%) |
DC, descending colon; R, rectum; C, cecum; SF, splenic flexure.
Fig. 1. Distribution of repeated high-risk polyps according to size, number, and pathology. The recurrence rate of high-risk polyps was higher in patients with higher numbers of adenomas (15.8%) than those with larger adenomas (9.2%) or high-risk pathology (8.9%).
DISCUSSION
The present study is novel in that the baseline colonoscopy finding was high-risk polyps, whereas most previous studies defined index colonoscopy as one or more adenomas or negative finding.5,6,7,8,9,10,11,12,13,14 We compared patients with recurrent high-risk polyps with those who had low-risk or no polyps during surveillance. In addition, we identified relative risk factors for recurrent high-risk polyps.
The 2013 post-polypectomy surveillance guidelines developed by the European Society of Gastrointestinal Endoscopy recommended a 3-year repetition of surveillance colonoscopy in patients with high-risk adenomas at first or subsequent surveillance examinations. However, colonoscopy is not always performed optimally. Therefore, polypectomy surveillance guidelines should consider not only polyp characteristics but also factors related to the procedure and patient. In this study we identified for the first time the following risk factors related to recurrence of high-risk polyps during surveillance: male gender, poor bowel preparation, and higher number of adenoma.
The 2012 post-polypectomy surveillance guidelines established by the United States Multi-Society Task Force (USMSTF) recommended repeat colonoscopy within 1 year if the bowel preparation was poor.4 Poor bowel preparation occurs in >20% of all colonoscopic examinations.17,18,19 Previous studies reported that the rate of missing advanced neoplasia was 18-27% after colonoscopy with poor bowel preparation.20,21 In the present study, 82 of all patients (18.9%) had poor bowel preparation and the mean interval of repeated colonoscopy was 1.5 years. Nineteen of 82 patients (23.2%) with poor bowel preparation underwent repeated colonoscopy within 1 year. The rate of poor bowel preparation for patients with recurrent high-risk polyps was higher than that of patients with low-risk or no polyps during surveillance (33.3% vs. 17.0%, respectively). Therefore, patients with poor bowel preparation and high-risk polyps need a shorter surveillance interval.
Martínez, et al.13 and Bonithon-Kopp, et al.14 earlier reported that the risk of high-risk polyps during surveillance was higher among male. In addition, USMSTF guidelines added male gender as a risk factor for high-risk polyps.4 The present study also found that male patients were more likely to have recurrent high-risk polyps than low-risk or no polyps during surveillance. Thus, male gender is an independent risk factor for recurrent high-risk polyps, as well as occurrence of high-risk polyps.
The pooling of eight prospective studies with the finding of adenomas at baseline colonoscopy revealed that with each additional adenoma, there is a linear increase in risk for advanced metachronous neoplasia: 8.6% for 1 adenoma, 12.7% for 2 adenomas, 15.3% for 3 adenomas, 19.6% for 4 adenomas, and 24.1% for >5 adenomas.13 A previous study of 895 patients with a baseline neoplasia of <10 mm reported that the risk of advanced neoplasia was 4.6% for 1-2 adenomas and 11.9% for >3 adenomas.5 In the present study, the risk of recurrent high-risk polyps was 3.2% for 1-2 adenomas, 16% for 3-9 adenomas, and 39.1% for >10 adenomas. As shown in Fig. 1, the recurrence rate of high-risk polyps was higher in patients with higher numbers of adenomas (15.8%) than those with larger adenomas (9.2%) or high-risk pathology (8.9%).
Larger polyps were a risk factor for recurrent high-risk polyps in numerous previous studies, defining the index colonoscopy as one or more adenomas or a negative finding.3,5,11,22,23 Although most studies reported size as a significant factor, some did not. Neither van Stolk, et al.24 nor Bonithon-Kopp, et al.14 found size to be a significant predictor of metachronous advanced adenomas. Likewise, polyp size was not a risk factor for recurrent high-risk polyps in the present study. These results suggest that a multiplicity of polyps might be considered a more important factor than size or pathology for surveillance intervals for high-risk polyps.
Interestingly, the combination of three non-risk factors decreased recurrence of high-risk polyps, and there were no recurrent high-risk polyps in patients with all three non-risk factors (Table 3). The results confirm these three risk factors and suggest that risk stratification is needed to determine surveillance intervals for patients with high-risk polyps. At a minimum, the surveillance interval for patients without any of the three risk factors should differ from that of patients with one or more risk factors.
This study has several limitations. First, we could not precisely analyze withdrawal time as a possible risk factor for recurrent high-risk polyps, because withdrawal time included polypectomy time. Second, we had no information of alcohol taking and smoking in our data. Third, this study was retrospective in design with a relatively small sample size. Therefore, a large, multicenter, prospective study will be necessary in the future to confirm these risk factors for recurrence of high-risk polyps.
In conclusion, patients who are male, and have poor bowel preparation and multiplicity of polyps are more likely experience recurrent high-risk polyps compared with patients without these factors, suggesting that risk stratification for adequate surveillance is needed, based on these three risk factors.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 2.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 3.Bertario L, Russo A, Sala P, Pizzetti P, Ballardini G, Andreola S, et al. Predictors of metachronous colorectal neoplasms in sporadic adenoma patients. Int J Cancer. 2003;105:82–87. doi: 10.1002/ijc.11036. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–1085. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Imperiale TF, Glowinski EA, Lin-Cooper C, Larkin GN, Rogge JD, Ransohoff DF. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med. 2008;359:1218–1224. doi: 10.1056/NEJMoa0803597. [DOI] [PubMed] [Google Scholar]
- 7.Leung WK, Lau JY, Suen BY, Wong GL, Chow DK, Lai LH, et al. Repeat-screening colonoscopy 5 years after normal baseline-screening colonoscopy in average-risk Chinese: a prospective study. Am J Gastroenterol. 2009;104:2028–2034. doi: 10.1038/ajg.2009.202. [DOI] [PubMed] [Google Scholar]
- 8.Brenner H, Haug U, Arndt V, Stegmaier C, Altenhofen L, Hoffmeister M. Low risk of colorectal cancer and advanced adenomas more than 10 years after negative colonoscopy. Gastroenterology. 2010;138:870–876. doi: 10.1053/j.gastro.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 9.Miller HL, Mukherjee R, Tian J, Nagar AB. Colonoscopy surveillance after polypectomy may be extended beyond five years. J Clin Gastroenterol. 2010;44:e162–e166. doi: 10.1097/MCG.0b013e3181e5cd22. [DOI] [PubMed] [Google Scholar]
- 10.Chung SJ, Kim YS, Yang SY, Song JH, Kim D, Park MJ, et al. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut. 2011;60:1537–1543. doi: 10.1136/gut.2010.232876. [DOI] [PubMed] [Google Scholar]
- 11.Martínez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–841. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez ME, Thompson P, Messer K, Ashbeck EL, Lieberman DA, Baron JA, et al. One-year risk for advanced colorectal neoplasia: US versus UK risk-stratification guidelines. Ann Intern Med. 2012;157:856–864. doi: 10.7326/0003-4819-157-12-201212180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez ME, Sampliner R, Marshall JR, Bhattacharyya AK, Reid ME, Alberts DS. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology. 2001;120:1077–1083. doi: 10.1053/gast.2001.23247. [DOI] [PubMed] [Google Scholar]
- 14.Bonithon-Kopp C, Piard F, Fenger C, Cabeza E, O'Morain C, Kronborg O, et al. Colorectal adenoma characteristics as predictors of recurrence. Dis Colon Rectum. 2004;47:323–333. doi: 10.1007/s10350-003-0054-1. [DOI] [PubMed] [Google Scholar]
- 15.Kim WH, Cho YJ, Park JY, Min PK, Kang JK, Park IS. Factors affecting insertion time and patient discomfort during colonoscopy. Gastrointest Endosc. 2000;52:600–605. doi: 10.1067/mge.2000.109802. [DOI] [PubMed] [Google Scholar]
- 16.Hong SN, Yang DH, Kim YH, Hong SP, Shin SJ, Kim SE, et al. [Korean guidelines for post-polypectomy colonoscopic surveillance] Korean J Gastroenterol. 2012;59:99–117. doi: 10.4166/kjg.2012.59.2.99. [DOI] [PubMed] [Google Scholar]
- 17.Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76–79. doi: 10.1067/mge.2003.294. [DOI] [PubMed] [Google Scholar]
- 18.Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55:2014–2020. doi: 10.1007/s10620-009-1079-7. [DOI] [PubMed] [Google Scholar]
- 19.Kazarian ES, Carreira FS, Toribara NW, Denberg TD. Colonoscopy completion in a large safety net health care system. Clin Gastroenterol Hepatol. 2008;6:438–442. doi: 10.1016/j.cgh.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73:1207–1214. doi: 10.1016/j.gie.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75:1197–1203. doi: 10.1016/j.gie.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc. 2006;64:614–626. doi: 10.1016/j.gie.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 23.Noshirwani KC, van Stolk RU, Rybicki LA, Beck GJ. Adenoma size and number are predictive of adenoma recurrence: implications for surveillance colonoscopy. Gastrointest Endosc. 2000;51(4 Pt 1):433–437. doi: 10.1016/s0016-5107(00)70444-5. [DOI] [PubMed] [Google Scholar]
- 24.van Stolk RU, Beck GJ, Baron JA, Haile R, Summers R. Adenoma characteristics at first colonoscopy as predictors of adenoma recurrence and characteristics at follow-up. The Polyp Prevention Study Group. Gastroenterology. 1998;115:13–18. doi: 10.1016/s0016-5085(98)70359-2. [DOI] [PubMed] [Google Scholar]