Abstract
Purpose
To examine community integration and contributing factors in people with aphasia (PWA) following stroke and to investigate the relationship between community integration and quality of life (QOL).
Materials and Methods
Thirty PWA and 42 age-and education-matched control subjects were involved. Main variables were as follows: socioeconomic status, mobility, and activity of daily living (ADL) (Modified Barthel Index), language function [Frenchay Aphasia Screening Test (FAST)], depression [Geriatric Depression Scale (GDS)], Community Integration Questionnaire (CIQ) and Stroke and Aphasia Quality of Life Scale-39 (SAQOL-39). Differences between aphasia and control groups and factors affecting community integration and QOL were analyzed.
Results
Home and social integration and productive activity were significantly decreased in the aphasia group compared to the control group; 8.5 and 18.3 points in total CIQ score, respectively. Amount of time spent outside the home and frequency of social contact were also significantly reduced in the aphasia group. Total mean score on the SAQOL-39 was 2.75±0.80 points and was significantly correlated with economic status, gait performance, ADL, depressive mood, and social domain score on the CIQ. Depression score measured by GDS was the single most important factor for the prediction of QOL, but the FAST score was significantly correlated only with the communication domain of the SAQOL-39.
Conclusion
Community activities of PWA were very limited, and depression was highly associated with decreased community integration and QOL. Enhancing social participation and reducing emotional distress should be emphasized for rehabilitation of PWA.
Keywords: Stroke, aphasia, quality of life, community integration
INTRODUCTION
Stroke brings crisis to patients and families due to abrupt changes in health status, functional capability, and degraded quality of life (QOL). Social consequences of stroke include negative impact on family relationships, deterioration in sexual life and leisure activities, and economic difficulties.1,2 Aphasia is one of the serious consequence following stroke, and significant aphasia persists in 10-38% of long-term survivors after stroke.3,4,5 Although aphasia is defined as a language disorder, emotional and psychosocial changes are commonly accompanied. 6,7 In addition, aphasia is reported to be a significant predictor of emotional distress, social isolation, and negative QOL after stroke.8,9,10
In 2001, the World Health Organization proposed the International Classification of Functioning, Disability and Health (ICF), composed with multiple interdependent domains (body function and structure, activities and participation, environment and personal factors) of which dynamically interact and overlap to create QOL.11 Several assessment tools have been used to measure QOL in aphasia, which include the Burden of Stroke Scale, Stroke and Aphasia Quality of Life Scale-39 (SAQOL-39) and the Assessment for Living with Aphasia.11,12 Communication with others is the first step in forming relationships within society. And most clinicians agree that QOL is significantly compromised in people with aphasia (PWA), and that efficient communication is an integral part of positive QOL.13,14 The Ultimate goal of aphasia rehabilitation is improving QOL; therefore, assessing outcome in aphasia should not be limited to measuring language impairment, but also include more effective outcome measures of life quality and related components.13
Despite the general agreement that decreased QOL is common in PWA, consensus on the extent of the impact of aphasia on community integration, and QOL in such population is lacking. Previous report indicates that time spent in the community outside the home is significantly decreased in chronic PWA, and severity of aphasia in particular has a negative impact on this factor.14,15 Dalemans, et al.16 reported that communication is an important predictor of social outcome in PWA, that functional performance, age and gender are the next important factors, and that promoting communication ability will increase community integration of the PWA. Other than communication difficulties, the community integration and quality aspects of life in PWA can be influenced by other various factors, such as personal, environmental or cultural factors. Studies on the relationship between the community integration and QOL in chronic aphasia are lacking.
The purpose of this study was twofold; 1) to examine the amount of community integration in PWA following stroke as compared to age-matched controls, 2) to investigate the relationship between various factors including personal, physical and activity level, and QOL in PWA.
MATERIALS AND METHODS
Participants
PWA who had visited the Department of Physical Medicine and Rehabilitation at Korea University Anam Hospital, Seoul, from May 2008 to November 2011 were enrolled in the study. All subjects were Korean and used Korean as a first language. Inclusion criteria were post-stroke aphasia confirmed by speech-language pathologists using a comprehensive aphasia test battery (Korean version of Western Aphasia Battery) and duration longer than 6 months from the onset of stroke. We have included only those who live in their home. Patients were excluded from the study if 1) they had premorbid psychiatric illness, 2) had cognitive disorders such as dementia (patients had been diagnosed with dementia, such as impaired cognitive function assessment, mini-mental state examination prior to the onset stroke), 3) had post stroke trouble serious enough to interfere with study participation in addition to motor weakness or aphasia (neglect, apraxia, executive dysfunction, etc.), 4) had other physical conditions like preexisting neurological disease, joint contracture, fracture, spasticity, and other musculoskeletal problem that could affect daily activities. In addition, patients with limited daily activities due to severe cardiovascular disease were also excluded. Among 47 PWA after stroke, 17 were excluded from the group, and finally, 30 PWA (mean age, 59.5 years) were selected for the study. The control group was selected from the people who visited public health care center, patients who visited our clinic with minor-musculo-skeletal problems (myofascial pain syndrome, arthritis, etc.) and hospital staffs. Thus, forty-two age- and education-matched control subjects (mean age, 61.5 years), who had no history of critical illness, and MMSE scores within 1 SD of the same age group, were recruited for the comparison.
Procedures and measures
All the data were collected prospectively by interviewing the subjects and performing functional evaluation on them. All of the surveys and tests were initially performed by the subjects themselves. In a study performed using Greek SAQOL-39 generic version, reports by proxies rater who has regular contact with the patient is capable of providing information similar to that provided by the patient.17 Therefore, family members or caregivers were encouraged to participate and support in the interview for the subjects with severe aphasia who are incapable of participating in the interview.
To collect information about personal and environmental factors, aphasia and control groups were interviewed for basic demographic characteristics including age, economic status, marital status, and level of education. Based on monthly household income, economic status was divided into three levels: low (less than 1000000 Korean won), average (1000000-5000000 won) and high (above 5000000 won).
Onset and characteristics of stroke and type of aphasia were reviewed through medical records of PWA. Impairment of language function at the time of interview was evaluated using the Korean version of the Frenchay Aphasia Screening Test (FAST).18 The FAST is a valid and reliable aphasia screening test measuring four domains of language function; verbal expression, comprehension, reading, and writing. The score ranges from 0 to 30 and higher score implies better language function. 19 Physical impairments other than language were also assessed, including presence of hemiplegia and independence of gait. Gait was further evaluated with a Likert scale, scored from 1 (cannot walk at all) to 5 points (no problem while walking). Emotional factor is an important variable, and we assessed depression with the Geriatric Depression Scale (GDS). Despite the fact that GDS is an useful screening tool for depression in elderly people, a study showed that it is suitable for the people with impaired cognitive function or expression since it is in a simple form of yes/no questions.20 In addition, because GDS is focused more on affective aspect rather than somatic aspect, we considered it useful to apply it to the post stroke patients who have combined physical disability such as hemiplegia.21
Activity and participation levels of the PWA group were also evaluated. Activities of daily living (ADL) were evaluated using the Modified Barthel Index (MBI). The Community Integration Questionnaire (CIQ) was designed to quantify an individual's integration into home and family life, social activity, and productive activity.22,23 The CIQ is a 15-item questionnaire, which is a simple and reliable tool for assessing integration levels at home and in the community. It consists of three main domains, including home integration (i.e., market, meal preparation, household activities, finance), social integration (i.e., shopping, avocation, going out), and productive activity (i.e., work, school, volunteer activity). Most items are scored in a scale of 0 to 2 with 2 representing greater independence and integration. A total score can also be calculated, with a possible range of 0 to 29 points. A higher score indicates a higher level of community integration. Also, we collected information about the time spent in the community activities and number of persons and places visited during the activities, for items on the CIQ in both groups.
QOL was measured with the SAQOL-39, which is a reliable tool with acceptability and validity previously confirmed in PWA.12 The SAQOL-39 consists of 39 questions in four domains: physical (17 items), psychosocial (11 items), communication (7 items), and energy (4 items). The timeframe for all the questions was the past one week at the time of interview. Questions are in the form of 5-point scale, from 1 ('could not do it at all' or 'definitely yes') to 5 ('no trouble at all' or 'definitely no').
Informed consent for all procedures was obtained from each participant and caregiver, and the study was approved by Health Service Human Research Ethics Committee, and the Committee on Experimental Procedures Involving Human Subjects of Korea University Anam Hospital.
Statistical analysis
SPSS 12.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Student's t-test and chi-square analysis were used to compare demographic data, environmental differences, and CIQ scores between the aphasia and control groups. Pearson correlation analysis was used to assess the relationships among multiple personal and environmental factors, CIQ, and SAQOL-39 scores. In addition, multiple linear regression was performed between SAQOL-39 total score and multiple variables. A p-value less than 0.05 were considered as statistically significant.
RESULTS
Characteristics of the aphasia group
Clinical characteristics of the aphasia group are presented in Table 1. Mean duration from stroke onset to participation in this study was 29.1 months (range, 6 to 81 months). Twenty-eight subjects had brain lesions on the left hemisphere, one case had a crossed aphasia, and one had a bilateral hemispheric lesion. Among the 30 subjects of PWA, 27 were able to ambulate independently, and mean MBI score was 80.0 (range, 14-100). Thus, most participants were able to perform daily activities independently and were mobile. FAST score was 8.2 points in average, at the time of admission to the rehabilitation unit, which improved to 14.4 points at the time of enrollment in the study. Types of aphasia in order of frequency were anomic, global, and Broca's aphasia (Table 1).
Table 1. Clinical Characteristics of the Aphasia Group.
| n=30 | |
|---|---|
| Time post-stroke (months) | 29.1±20.6 |
| Lesion side | |
| Right | 1 |
| Left | 28 |
| Bilateral | 1 |
| Type | |
| Infarction | 17 |
| Hemorrhage | 13 |
| Hemiplegia | |
| Yes | 22 |
| No | 8 |
| Gait | |
| Independent | 27 |
| Dependent | 3 |
| Self-report of gait (5) | 3.7±0.9 |
| Modified Barthel Index (100) | 80.0±26.9 |
| Language evaluation | |
| K-FAST (30) (at admission) | 8.2±7.1 |
| K-FAST (30) (at recruitment) | 14.4±8.7 |
| Types of aphasia | |
| Global | 8 |
| Broca | 6 |
| Wernicke | 3 |
| Anomic | 11 |
| Conduction | 2 |
K-FAST, Korean-Frenchay Aphasia Screening Test.
Number in parentheses is maximum score.
Socioeconomic status and community integration in PWA
In the control group, 20 out of 42 subjects (47%) had paid work, but no one in the aphasia group did (p<0.001). Six subjects lost their jobs after onset of stroke. Number of subjects with paid work prior to stroke onset was 6 (20%), which showed statistically significant difference with the control group (p<0.01). Proportion of subjects with high monthly income was significantly high in the control group (p=0.007). In the CIQ, all three domains showed significantly low integration scores in the aphasia group as compared to the control group, especially in the domain of productive activity (p<0.001). Mean GDS score was 21.0 points in the aphasia group, which is significantly higher than the control group (Table 2).
Table 2. Comparison of Socioeconomic Factors and CIQ between Aphasia and Control Groups.
| Aphasia (n=30) | Controls (n=42) | |
|---|---|---|
| Age (yrs) | 59.2±7.2 | 61.5±10.7 |
| Gender | ||
| Male | 17 | 14 |
| Female | 13 | 28 |
| Duration of education (yrs) | 10.8±3.9 | 12.1±4.7 |
| Employment | ||
| Yes | 0* | 20 |
| No | 30 | 22 |
| Marital status | ||
| Married | 24 | 37 |
| Single | 2 | 1 |
| Widow | 3 | 3 |
| Divorced | 1 | 1 |
| Monthly income | ||
| Low | 13 | 15 |
| Average | 16 | 12 |
| High | 1* | 15 |
| CIQ | ||
| Home integration (10) | 2.6±3.0* | 5.6±3.0 |
| Social integration (14) | 5.7±3.0* | 9.9±2.2 |
| Productive activity (5) | 0.3±0.8* | 2.8±1.8 |
| Total (29) | 8.5±5.3* | 18.3±5.5 |
| GDS (30) | 21.0±6.3* | 8.3±5.9 |
CIQ, Community Integration Questionnaire; GDS, Geriatric Depression Scale.
Number in parentheses is maximum score.
*p<0.05, presented data are mean±SD.
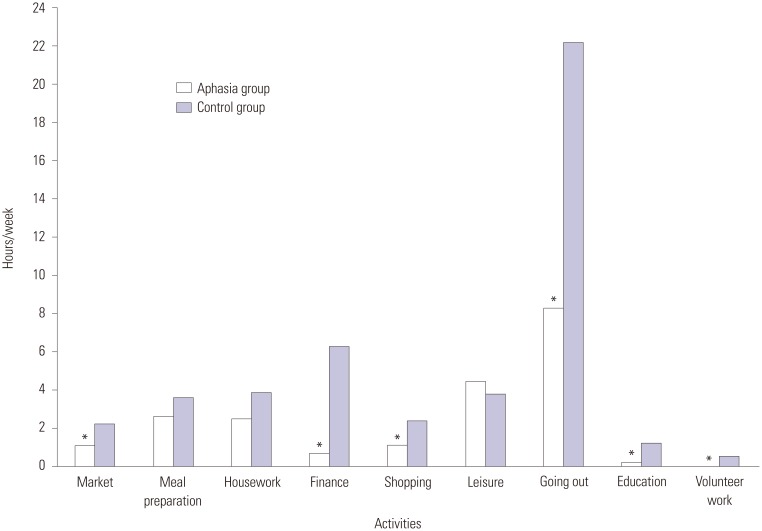
The quantity of time spent in each domain of home integration, social integration, and productive activity was also compared between the two groups (Fig. 1). The aphasia group spent less time on the activities of market (p=0.019), finance (p<0.001), shopping (p=0.003), going out (p<0.001), education (p=0.034), and volunteer activity (p=0.019) than the control group, but no significant difference was observed in meal preparation, household activities, or leisure activity (Fig. 1).
Fig. 1. Comparison of amount of time spent in performing the Community Integration Questionnaire subunits between aphasia and control groups. Most activities outside home were significantly decreased in the aphasia group (*p<0.05).
The average number of places visited during community activities was significantly lower in the aphasia group as compared with controls; 1.8±0.8 in the aphasia group and 2.8±1.3 in controls (p<0.001). Places visited by the aphasia group were limited to medical facilities and markets. In addition, the frequencies of social contact with friends and attendance in meetings were also decreased (Fig. 2).
Fig. 2. Mean number of activities performed during a week in aphasia and control groups. Number of social contacts was significantly decreased in the aphasia group (*p=0.00).
Factors affecting CIQ
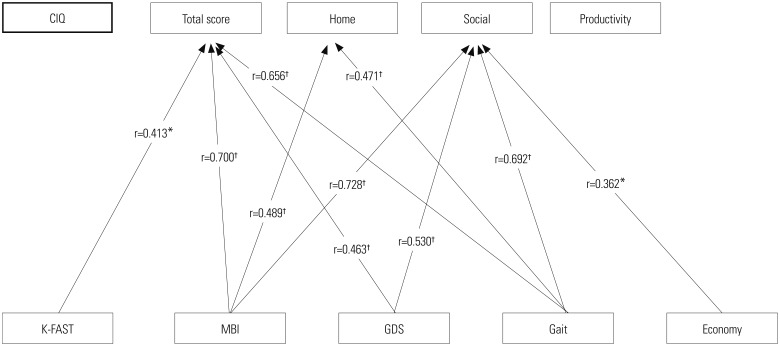
Total score of CIQ showed a positive correlation with FAST, MBI, GDS, and gait score. Home integration of CIQ was significantly correlated with ADL and mobility (MBI and gait score). FAST score was significantly correlated with total scores of the CIQ, but did not show significant correlation with any of the subdomains of the CIQ. The productive activity domain had no correlation with any of the listed factors (Fig. 3).
Fig. 3. Correlation between CIQ and multiple factors in the aphasia group. The factors which showed significant correlation are shown in the diagram. FAST score level of PWA, activity of daily living and mobility, depressive mood, and economic status had different influences on CIQ. *p<0.05, †p<0.01. CIQ, Community Integration Questionnaire; K-FAST, Korean-Frenchay Aphasia Screening Test; MBI, Modified Barthel Index; GDS, Geriatric Depression Scale.
QOL and contributing factors
SAQOL-39 scores of the aphasia group are listed in Table 3. Economic status, MBI, gait, GDS, and CIQ total and subunit scores were significantly correlated with total scores of the SAQOL-39. Depressive mood as measured by GDS revealed a significant negative correlation with all the domains of the SAQOL-39 (p<0.001). FAST score of PWA was not significantly correlated with the total SAQOL-39 score, but was correlated positively with the subdomain of communication (r=0.422, p<0.01). In the CIQ, social integration score was significantly correlated with all the domains of the SAQOL-39. The multiple linear regression model using the above variables predicted that total SAQOL-39 score (R2=0.786, p<0.001) and GDS score was the only significant explanatory factor for the total score of SAQOL-39 (p<0.001) (Table 4).
Table 3. SAQOL-39 Scores in the Aphasia Group.
| Domain | Mean±SD |
|---|---|
| Physical (5) | 3.13±1.11 |
| Communication (5) | 2.44±0.78 |
| Psychosocial (5) | 2.47±0.73 |
| Energy (5) | 2.43±0.70 |
| Total (5) | 2.75±0.80 |
SAQOL-39, Stroke and Aphasia Quality of Life Scale-39.
Table 4. Correlation between SAQOL-39 Sub-Domain Scores and Significant Variables in the Aphasia Group.
| SAQOL-39 | |||||
|---|---|---|---|---|---|
| Physical | Communication | Psychosocial | Energy | Total | |
| Economic status | NS | NS | 0.436* | 0.387* | 0.417* |
| K-FAST | NS | 0.422* | NS | NS | NS |
| MBI | 0.744† | 0.547† | 0.380* | NS | 0.675† |
| Gait | 0.671† | 0.544* | 0.366* | 0.389* | 0.633† |
| GDS | -0.703† | -0.647† | -0.814† | -0.595† | -0.803† |
| CIQ | |||||
| Home | NS | NS | NS | NS | NS |
| Social | 0.679† | 0.613† | 0.407* | 0.366* | 0.657† |
| Productive activity | NS | 0.414* | NS | NS | NS |
| Total | 0.557† | 0.540† | NS | 0.364* | 0.557† |
CIQ, Community Integration Questionnaire; GDS, Geriatric Depression Scale; K-FAST, Korean-Frenchay Aphasia Screening Test; MBI, Modified Barthel Index; SAQOL-39, Stroke and Aphasia Quality of Life Scale-39; NS, data was statistically not significant.
*p<0.05, †p<0.01.
DISCUSSION
Purpose of this study was to investigate the amount of activities and relationship between QOL and various factors in PWA after stroke. In this study, PWA performed daily home activities relatively well, but instrumental ADL and social participation were significantly restricted as compared with age-matched controls. Reduced community integration in the aphasia group was closely related with mobility, language and ADL performance, emotional distress, and socioeconomic factors. Also, QOL measured by SAQOL-39 was decreased in PWA, and economic status, MBI, gait, GDS, and CIQ total and subunit scores were significantly correlated with total scores of the SAQOL-39. Among the multiple factors, the presence of depression was the only factor that significantly correlated with QOL in PWA after stroke.
Depression, social isolation, decrease in productive activity and change in family role are frequently reported in chronic aphasia patients after stroke.24 Social isolation is a serious issue for stroke patients. Dalemans, et al.25,26 defined social participation as 'the performance of people in actual activities in social life domains through interaction with others in the context in which they live'. Forming social network with others is reduced in stroke patients, and a previous study showed that socialization outside the home was decreased to 50.4% after stroke, and that 38% of the patients showed a decrease in hobby activities and other interests.27 Especially, the meaningful performance is limited after stroke due to communicative difficulty and brings social discouragement and isolation in PWA, which leads life to less satisfactory and PWA experience more hurtful negative responses from others and found it more difficult to retain their friends.10,26 Code15 reported that chronic PWA spend a significant amount of time only with familiar people such as families, and most of the time spent outside the home is used for visiting medical facilities. In our results, most participants were able to perform daily activities independently and were mobile. Time spent in indoor activities such as preparing meals and house work was not significantly reduced in the aphasia group as compared to the control group. However, outdoor activities and social participation such as going out, going to the market, shopping, school, and volunteer activities were markedly decreased. In addition, most subjects with aphasia spent the majority of their time with their families and went out to familiar places such as market and hospitals. The frequency of these activities, however, was markedly reduced. In a previous study, PWA after stroke showed tendency for active participation in outdoor activities when contacting with more familiar people in a quiet (suburban) environment and when living in a smaller town.26 However, majority of our subjects lived in urban areas, which lead to stress of making social interaction with unfamiliar people. It is because most of our study subjects are psychologically withdrawn from the society and find it difficult to interact with the community due to their aphasia, despite their ability to walk or perform daily living activities. Dalemans, et al.16 claimed that the factors associated with social participation in PWA are age, gender, functional performance and severity of aphasia. The severity of aphasia was reported as one of the main factors affecting social and community activity, and our results also suggested that language performance was significantly associated with community integration.13,14 Cruice, et al.14 reported that social activity, social networks, and social support were significantly correlated with communication ability in PWA, and better communication ability corresponded with better social participation and higher QOL.
Return to work may be the important goal in PWA. The rate of returning to work following stroke has been reported to range between 19-73%.28 The factors positively related to return to work in stroke patients were stroke severity measured by ADL, age less than 65 years, high education level, and whitecollar employment.28,29 And most patients returned to work during the early period of 3-6 months after stroke onset and significant additional returns were not observed at one year follow-up. Low rate of paid work in patients with aphasia after stroke was also reported, and the return rate was reported to be around 28.4% (range, 6.7-53.3%) in younger stroke patients with aphasia.30 In the present study, comparison of employment rate between the aphasia patients before stroke and the control group showed statistically significant difference. This is due to selection bias occurred from collecting the control group in a typical social group rather than general population. However, despite the fact that most of PWA after stroke was able to walk independently and perform daily activities and that no significant difference in education level was observed from the control group, none of PWA could return to their work. It implies that aphasia is a critical factor in jobs that require higher level of communication and intellect. Also, previous studies suggest that successful returning to work was mostly achieved within 1 or 2 years after onset of stroke.28,31 Therefore, we believe that long duration of stroke in our study subjects is another factor that influenced such low rate of returning to work. Pedersen, et al.4 reported that aphasia severity and predicting prognosis in stroke is closely related to general stroke severity. Long duration of rehabilitation could mean that the patient has severe aphasia, along with other post stroke troubles which resulted in isolation from the community.
In a recent meta-analysis, emotional distress, aphasia severity, communication and activity limitations, other medical problems, and social factors were the predictive factors of poor health-related QOL in aphasia.32 In the present study, FAST score was significantly correlated with total CIQ score and communication domains of the SAQOL-39. However, it was not significantly correlated with the subdomain of the CIQ or physical and psychosocial domain scores. Therefore, language impairment may not seem to predict poor QOL. Based on our results, only depression was correlated significantly with negative QOL. Previous studies reported that longterm mood disorders, including depression, exist in up to one-third of stroke patients, and that such disorders affect QOL of the patients.10,27,31,33 If stroke patients lack ability to perform daily activities independently for a long period during their rehabilitation, they fall into depression and it may affect their recovery as much as their physical disability.34 Therefore, early diagnosis and treatment of depression in stroke patients is an important aspect of rehabilitation.
Due to limited means of communication, diagnosis of depression with aphasia patients is even harder and caution is needed to observe progress in treatment. Cruice, et al.35 reported that participants with aphasia after stroke showed significantly higher GDS score, compared to unaffected participants. They claimed that such mood disorders affect general well-being of the subjects, suggesting intervention for psychological well-being and general mood. According to Code and Herrmann,36 depression can be developed in PWA related to brain lesion during the acute phase, and secondary depression may be caused by a reaction to psychosocial, neuropsychological and functional impairment and disability in the subacute phase. In addition, depression may be provoked by psychosocial alterations and insufficient coping strategies. However, these emotional problems can often be underestimated due to communication impairment and difficulties to assess. Our results also suggested that PWA after stroke are at high risk for depression. The negative correlation between CIQ (total and social integration) and GDS scores suggests that depression exaggerates social isolation of PWA, in addition to communication difficulty. However, these emotional problems can often be underestimated in PWA due to communication impairment and difficulties in assessment.
Discriminative feature of our study is the recruitment of age- and education-matched control subjects. In addition, we investigated predicting factors and the relationship between both community integration and QOL in post-stroke aphasia patients. A limitation of this study is the small sample size and the fact that subjects were recruited from a single university hospital. Participants included in this study had been followed-up via outpatient clinic, therefore, subjects with more severe aphasia might have been included. In addition, the fact that we did not consider possibility of underestimating CIQ and GDS due to only basic physical test performed, and K-FAST, GDS and questionnaire were used without re-evaluating other post stroke troubles related to cognitive function at the time of interview, is also a limitation of this study. Although most studies have similar problems in interviews with aphasia patients, interviews in the present study were conducted by caregivers for 13 subjects in the aphasia group due to communication problems, which is about 40% of the total subjects; despite communication difficulty in severe aphasia subjects, interviews were performed in the presence of main caregivers, encouraging and answering for the patients to minimize limitations of this study. Further studies on QOL and community integration of a larger number of stroke patients with and without aphasia are needed to clarify the pure impact of communication disability on community integration and QOL in aphasia.
However, we found that aphasia after stroke influences physical functioning, emotion, and social participation and also decreases QOL. Poor social participation and decreased QOL are very serious issues for stroke patients, and many factors lead to poor community integration and QOL in these people. Particularly in aphasia patients, such issues tend to be underestimated due to limited communication ability, which easily leads to vicious cycle of social isolation and decreased QOL. In this study, regardless of relatively high level of independence in ambulation and ADL, community integration beyond home was found to be very limited. In addition, lowered community integration had negative effect on all the domains of measured QOL. Among the various contributing factors, depressive mood in particular was correlated highly with community integration and QOL. Thus, comprehensive evaluation of daily functioning and social participation are essential for PWA after stroke. Together with treatment focused on communication improvement for PWA, rehabilitation programs to enhance social participation and management of emotional distress are important for successful outcomes in PWA.
We believe that in the future, comparative study with home bound post-stroke patients with and without aphasia under the control of other post stroke troubles will provide more information about social participation and QOL of aphasia patients.
ACKNOWLEDGEMENTS
This study was supported by a Korea University Grant.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Niemi ML, Laaksonen R, Kotila M, Waltimo O. Quality of life 4 years after stroke. Stroke. 1988;19:1101–1107. doi: 10.1161/01.str.19.9.1101. [DOI] [PubMed] [Google Scholar]
- 2.Daniel K, Wolfe CD, Busch MA, McKevitt C. What are the social consequences of stroke for working-aged adults? A systematic review. Stroke. 2009;40:e431–e440. doi: 10.1161/STROKEAHA.108.534487. [DOI] [PubMed] [Google Scholar]
- 3.Yoon TH, Han SJ, Yoon TS, Kim JS, Yi TI. Therapeutic effect of repetitive magnetic stimulation combined with speech and language therapy in post-stroke non-fluent aphasia. NeuroRehabilitation. 2015;36:107–114. doi: 10.3233/NRE-141198. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen PM, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Aphasia in acute stroke: incidence, determinants, and recovery. Ann Neurol. 1995;38:659–666. doi: 10.1002/ana.410380416. [DOI] [PubMed] [Google Scholar]
- 5.Wade DT, Hewer RL, David RM, Enderby PM. Aphasia after stroke: natural history and associated deficits. J Neurol Neurosurg Psychiatry. 1986;49:11–16. doi: 10.1136/jnnp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemsley G, Code C. Interactions between recovery in aphasia, emotional and psychosocial factors in subjects with aphasia, their significant others and speech pathologists. Disabil Rehabil. 1996;18:567–584. doi: 10.3109/09638289609166318. [DOI] [PubMed] [Google Scholar]
- 7.Cahana-Amitay D, Albert ML, Pyun SB, Westwood A, Jenkins T, Wolford S, et al. Language as a Stressor in Aphasia. Aphasiology. 2011;25:593–614. doi: 10.1080/02687038.2010.541469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyun SB, Kim SH, Hahn MS, Kwon HK, Lee HJ. Quality of life after stroke. J Korean Acad Rehabil Med. 1999;23:233–239. [Google Scholar]
- 9.Thomas SA, Lincoln NB. Predictors of emotional distress after stroke. Stroke. 2008;39:1240–1245. doi: 10.1161/STROKEAHA.107.498279. [DOI] [PubMed] [Google Scholar]
- 10.Northcott S, Hilari K. Why do people lose their friends after a stroke? Int J Lang Commun Disord. 2011;46:524–534. doi: 10.1111/j.1460-6984.2011.00079.x. [DOI] [PubMed] [Google Scholar]
- 11.Simmons-Mackie N, Kagan A. Application of the ICF in aphasia. Semin Speech Lang. 2007;28:244–253. doi: 10.1055/s-2007-986521. [DOI] [PubMed] [Google Scholar]
- 12.Hilari K, Byng S, Lamping DL, Smith SC. Stroke and Aphasia Quality of Life Scale-39 (SAQOL-39): evaluation of acceptability, reliability, and validity. Stroke. 2003;34:1944–1950. doi: 10.1161/01.STR.0000081987.46660.ED. [DOI] [PubMed] [Google Scholar]
- 13.Worrall LE, Holland AL. Editorial: Quality of life in aphasia. Aphasiology. 2003;17:329–332. [Google Scholar]
- 14.Cruice M, Worrall L, Hickson L, Murison R. Finding a focus for quality of life with aphasia: social and emotional health, and psychological well-being. Aphasiology. 2003;17:333–353. [Google Scholar]
- 15.Code C. The quantity of life for people with chronic aphasia. Neuropsychol Rehabil. 2003;13:379–390. doi: 10.1080/09602010244000255. [DOI] [PubMed] [Google Scholar]
- 16.Dalemans RJ, De Witte LP, Beurskens AJ, Van Den Heuvel WJ, Wade DT. An investigation into the social participation of stroke survivors with aphasia. Disabil Rehabil. 2010;32:1678–1685. doi: 10.3109/09638281003649938. [DOI] [PubMed] [Google Scholar]
- 17.Ignatiou M, Christaki V, Chelas EN, Efstratiadou EA, Hilari K. Agreement between people with aphasia and their proxies on health-related quality of life after stroke, using the Greek SAQOL-39g. Psychology. 2012;3:686–690. [Google Scholar]
- 18.Pyun SB, Hwang YM, Ha JW, Yi H, Park KW, Nam K. Standardization of Korean Version of Frenchay Aphasia Screening Test in normal adults. J Korean Acad Rehabil Med. 2009;33:436–440. [Google Scholar]
- 19.Salter K, Jutai J, Foley N, Hellings C, Teasell R. Identification of aphasia post stroke: a review of screening assessment tools. Brain Inj. 2006;20:559–568. doi: 10.1080/02699050600744087. [DOI] [PubMed] [Google Scholar]
- 20.Gillen R, Tennen H, McKee TE, Gernert-Dott P, Affleck G. Depressive symptoms and history of depression predict rehabilitation efficiency in stroke patients. Arch Phys Med Rehabil. 2001;82:1645–1649. doi: 10.1053/apmr.2001.26249. [DOI] [PubMed] [Google Scholar]
- 21.Carod-Artal FJ, Ferreira Coral L, Trizotto DS, Menezes Moreira C. Poststroke depression: prevalence and determinants in Brazilian stroke patients. Cerebrovasc Dis. 2009;28:157–165. doi: 10.1159/000226114. [DOI] [PubMed] [Google Scholar]
- 22.Hosomi A, Nagakane Y, Yamada K, Kuriyama N, Mizuno T, Nishimura T, et al. Assessment of arcuate fasciculus with diffusion-tensor tractography may predict the prognosis of aphasia in patients with left middle cerebral artery infarcts. Neuroradiology. 2009;51:549–555. doi: 10.1007/s00234-009-0534-7. [DOI] [PubMed] [Google Scholar]
- 23.Dalemans RJ, de Witte LP, Beurskens AJ, van den Heuvel WJ, Wade DT. Psychometric properties of the community integration questionnaire adjusted for people with aphasia. Arch Phys Med Rehabil. 2010;91:395–399. doi: 10.1016/j.apmr.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Hinckley JJ, Packard ME. Family education seminars and social functioning of adults with chronic aphasia. J Commun Disord. 2001;34:241–254. doi: 10.1016/s0021-9924(01)00049-1. [DOI] [PubMed] [Google Scholar]
- 25.Dalemans R, de Witte LP, Lemmens J, van den Heuvel WJ, Wade DT. Measures for rating social participation in people with aphasia: a systematic review. Clin Rehabil. 2008;22:542–555. doi: 10.1177/0269215507087462. [DOI] [PubMed] [Google Scholar]
- 26.Dalemans RJ, de Witte L, Wade D, van den Heuvel W. Social participation through the eyes of people with aphasia. Int J Lang Commun Disord. 2010;45:537–550. doi: 10.3109/13682820903223633. [DOI] [PubMed] [Google Scholar]
- 27.Labi ML, Phillips TF, Greshman GE. Psychosocial disability in physically restored long-term stroke survivors. Arch Phys Med Rehabil. 1980;61:561–565. [PubMed] [Google Scholar]
- 28.Treger I, Shames J, Giaquinto S, Ring H. Return to work in stroke patients. Disabil Rehabil. 2007;29:1397–1403. doi: 10.1080/09638280701314923. [DOI] [PubMed] [Google Scholar]
- 29.Wozniak MA, Kittner SJ. Return to work after ischemic stroke: a methodological review. Neuroepidemiology. 2002;21:159–166. doi: 10.1159/000059516. [DOI] [PubMed] [Google Scholar]
- 30.Hinckley JJ. Investigating the predictors of lifestyle satisfaction among younger adults with chronic aphasia. Aphasiology. 1998;12:509–518. [Google Scholar]
- 31.van Velzen JM, van Bennekom CA, Edelaar MJ, Sluiter JK, Frings-Dresen MH. How many people return to work after acquired brain injury?: a systematic review. Brain Inj. 2009;23:473–488. doi: 10.1080/02699050902970737. [DOI] [PubMed] [Google Scholar]
- 32.Hilari K, Needle JJ, Harrison KL. What are the important factors in health-related quality of life for people with aphasia? A systematic review. Arch Phys Med Rehabil. 2012;93(1 Suppl):S86–S95. doi: 10.1016/j.apmr.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 33.Code C, Hemsley G, Herrmann M. The emotional impact of aphasia. Semin Speech Lang. 1999;20:19–31. doi: 10.1055/s-2008-1064006. [DOI] [PubMed] [Google Scholar]
- 34.Tateno A, Kimura M, Robinson RG. Phenomenological characteristics of poststroke depression: early-versus late-onset. Am J Geriatr Psychiatry. 2002;10:575–582. [PubMed] [Google Scholar]
- 35.Cruice M, Worrall L, Hickson L. Reporting on psychological well-being of older adults with chronic aphasia in the context of unaffected peers. Disabil Rehabil. 2011;33:219–228. doi: 10.3109/09638288.2010.503835. [DOI] [PubMed] [Google Scholar]
- 36.Code C, Herrmann M. The relevance of emotional and psychosocial factors in aphasia to rehabilitation. Neuropsychol Rehabil. 2003;13:109–132. doi: 10.1080/09602010244000291. [DOI] [PubMed] [Google Scholar]