Abstract
Objectives
Relapse to drug misuse may follow exposure to drug cues that elicit craving. The learned associations, or “emotional memories,” that underlie responses to cues may be attenuated or erased by the beta-adrenergic antagonist propranolol during a “reconsolidation window” shortly after the memories are reactivated by cues.
Methods
We evaluated the effects of propranolol on cue-induced drug cravings in healthy opioid-dependent individuals who used cocaine while receiving methadone maintenance (n = 33). Participants were asked to recall specific cocaine-use and neutral events in an interview; these events were used to develop personalized auditory script/cue sets. Approximately one week later, propranolol (40 mg) or placebo (random assignment, double-blind) was administered orally before presentation of the script/cue sets; the presentation of the script/cue sets were tested 1 and 5 weeks after the propranolol/placebo session. Ongoing drug use was monitored via urine screens and self-report in twice-weekly visits.
Results
Cue reactivity, as assessed by craving scales and physiological responses, was unexpectedly greater in the propranolol group than in the placebo group. This counter-hypothesized group difference was present acutely during propranolol administration and appeared to persist (without reaching statistical significance) during the subsequent test sessions.
Conclusions
Our results do not support the use of propranolol for cue-induced cocaine craving in opioid-maintained patients.
Keywords: cocaine, cue-induced craving, memory reconsolidation, propranolol, imagery scripts
Relapse to drug misuse may follow exposure to cues that elicit drug-related memories (Sinha, Fuse, et al., 2000). The term memories is used here not in its everyday sense (conscious, narrative recollections), but in a sense that refers to Pavlovian associations—for example, between the sight of a syringe and a set of responses such as tachycardia and craving. Such memories (sometimes called “emotional memories”) may be actively re-stored, or reconsolidated, when they are reactivated. Reconsolidation is thought to be a dynamic reprocessing of an established memory (Alberini and Ledoux 2013); a rough analogy would be the resaving of a currently open document on a computer: the text of the document can be updated, or even erased, before the document is resaved with the same filename. Reconsolidation may also occur with some types of declarative memory (Chan and LaPaglia 2013), but in the work reported here, we are dealing only with emotional memory. Disruption of an emotional memory during reconsolidation does not erase knowledge of an event; it only attenuates the link between that knowledge and an emotional response (Kindt, Soeter, et al., 2009).
Emotional-memory reconsolidation involves beta-adrenergic receptors (Przybyslawski and Sara 1997; Chamberlain, Muller, et al., 2006). In rats, blockade of beta-receptors with propranolol either five minutes or two hours after memory reactivation disrupted reconsolidation in both a food-reinforced task and a footshock-punished task (Przybyslawski, Roullet, et al., 1999). In humans (Schwabe, Nader, et al., 2012; Soeter and Kindt 2012), propranolol disrupted reconsolidation of recently learned emotional associations when administered before or after reactivation.
Reconsolidation also occurs with appetitive associations. For example, propranolol given immediately after re-exposure to a sucrose-associated cue reduced sucrose seeking in rats three weeks later (Diergaarde, Schoffelmeer, et al., 2006). Propranolol administered immediately after memory reactivation similarly disrupted conditioned place preference (CPP) for cocaine (Bernardi, Lattal, et al., 2006). Propranolol showed promise for blocking response to cocaine cues in a human laboratory study (Saladin, Gray, et al., 2013). These results have implications for the treatment of addiction: a propranolol/reactivation intervention may overcome the limitations of extinction-based relapse-prevention procedures, which do not weaken underlying associations and thus rarely generalize outside the context where they are administered (Bouton 2002).
We evaluated a propranolol/reactivation intervention for cue-induced drug cravings in polydrug users during methadone maintenance. This is an unlabeled use of propranolol. We hypothesized that propranolol administered in a memory-reactivation session would reduce cocaine-cue reactivity weeks later.
Materials and Methods
Participants and setting
We enrolled cocaine-abusing outpatients who were also being maintained on methadone for heroin dependence. We chose this population because cocaine abuse and dependence are common in methadone patients, and because concurrent cocaine and heroin use are the modal pattern of use of these drugs in our geographical region. We recruited volunteers who met these criteria: age 18-55, cocaine use for at least one year and at least once in the previous 30 days (confirmed by urine), and on a stable dose of methadone (>30 mg/day) for at least 30 days. Exclusions were hypersensitivity to propranolol, cognitive impairment, physical dependence on sedatives, pregnancy, and medical contraindications such as hepatic impairment, uncompensated congestive heart failure, pulmonary edema, asthma, COPD, history of severe allergic reactions (seasonal, environmental, food, medications, etc.), Raynaud's disease; second- or third-degree atrioventricular block, arrhythmias other than sinus arrhythmia, thyroid dysfunction, diabetes mellitus, or renal impairment. Screening included physical examination, standard laboratory tests, and the Addiction Severity Index (ASI) (McLellan, Luborsky, et al., 1985). The study was registered at www.clinicaltrials.gov (Clinical trial #NCT00688805) and was conducted at NIDA in Baltimore, MD from 2008 to 2012. Participants gave written informed consent as approved by the Addictions IRB of the NIDA Intramural Research Program.
General Procedures
We used a between-subjects design; participants were randomized to placebo or 40 mg propranolol; medications were administered double-blind. A dose of 40 mg propranolol produced relevant mnemonic effects in other studies (Kroes, Strange, et al., 2010; Schwabe, Nader, et al., 2012; Sevenster, Beckers, et al., 2012; Soeter and Kindt 2012; Saladin, Gray, et al., 2013). Randomization was done by random-numbers table and stratified by sex by an investigator (KLP) who had no contact with participants. This was an outpatient study with two overnight stays. There were 4 sessions: one for admission and auditory-script development; one for a reactivation intervention with propranolol or placebo (overnight stay before and after), and two test sessions 1 and 5 weeks after intervention.
Outcome measures
Craving
We used two cocaine-craving measures, designated as co-primary outcomes: the 14-item Cocaine Craving Questionnaire (CCQ) and a Visual Analog Scale (VAS). The VAS was worded “Please rate the intensity of your desire to use cocaine AT THIS MOMENT,” rated on a line anchored with “none at all” and “extremely.” The single-item VAS generates especially robust and reliable results, perhaps on account of its minimal response burden. The 14-item CCQ assesses craving as a multidimensional construct (Tiffany, Carter, et al., 2000).
Mood
To assess a broader spectrum of changes in mood during sessions, we had participants fill out the Positive Affect and Negative Affect Schedule (PANAS) (Watson, Clark, et al., 1988).
Heart rate and blood pressure
Sitting (feet flat) blood pressure and heart rate were assessed with a LabLinc V (Coulbourn Instruments, Whitehall, PA) and blood pressure cuff placed on the upper nondominant arm.
Drug use
For two weeks before and four weeks after the intervention session, participants gave urine specimens twice a week to be tested for cocaine metabolites.
Baseline session
In the first session, participants completed the CCQ, VAS, and PANAS, and, to check ability to follow guided-imagery instructions, the Questionnaire on Mental Imagery (QMI) (Sheehan 1967). Participants were then interviewed to generate two imagery scripts—one for cocaine craving, and one neutral, using the method of Sinha and Tuit (2012). Each cocaine script was based on a specific occasion of cocaine use, usually the most recent. Participants also viewed pictures of neighborhoods, people engaging in drug-related behaviors, and cocaine paraphernalia to identify those that best represented their own experiences.
Approximately 1 week later, participants were admitted to a residential research unit for two nights for the intervention/reactivation session. Before the session, they ate a standardized breakfast. Methadone was administered before the session for participants who usually took their methadone before 11am, or after the session otherwise.
After baseline measures (prereactivation), participants swallowed a capsule containing propranolol or placebo, with 8 ounces of water. Propranolol 40 mg immediate-release tablets were administered inside capsules packed with dextrose. Placebo capsules contained only dextrose. The cue-exposure session (described below) was timed so administration occurred 120 min before the cocaine script, so the script would approximately coincide with peak propranolol concentration and propranolol effects would last at least 120 min after the script (Shand, Nuckolls, et al., 1970; Altamura, Moliterno, et al., 2013). After the session, participants stayed in the residential unit overnight.
Two subsequent test sessions occurred approximately 1 week and 5 weeks later, as described below.
Intervention and Two Subsequent Test Sessions
Before each session, participants reported the number of days since last use of heroin or cocaine, were evaluated for intoxication, underwent breath-alcohol testing, and gave urine that was tested for pregnancy (women only), cocaine, opiates, benzodiazepines, and amphetamines. No participant tested positive. Participants who were smokers had a smoking break 35 min after capsule administration (and assessment of vital signs) and smoked their last pre-session cigarette 30 minutes before pre-neutral-script measurements; this was intended to minimize and standardize nicotine withdrawal. During the two overnight stays, participants had access to cigarettes and a smoking area.
The intervention session consisted of pre-drug baseline and safety assessment, propranolol/placebo dosing, neutral script, cocaine script, and guided relaxation and safety assessment. The test sessions consisted of neutral script, cocaine script, and guided relaxation and safety assessment.
Script/Cue Presentation and Data Collection
The two individualized, pre-recorded imagery scripts, neutral and cocaine-cue, were approximately 210 s long and presented through headphones with a 45-minute interval in between. The neutral script was presented first to prevent carryover effects from the cocaine script.
Before each script, participants were shown approximately 10 images that they had identified as best evoking their own cocaine or relaxation experiences. In addition, before the cocaine script, participants were given tactile cues (e.g., a crack pipe, a baggie containing imitation crack, a lighter). There was no tactile component to the neutral cues. Before each script, participants heard recorded instructions telling them to close their eyes during the script, imagine themselves in the scene, and then continue imagining until told to stop (90 s after the script ended).
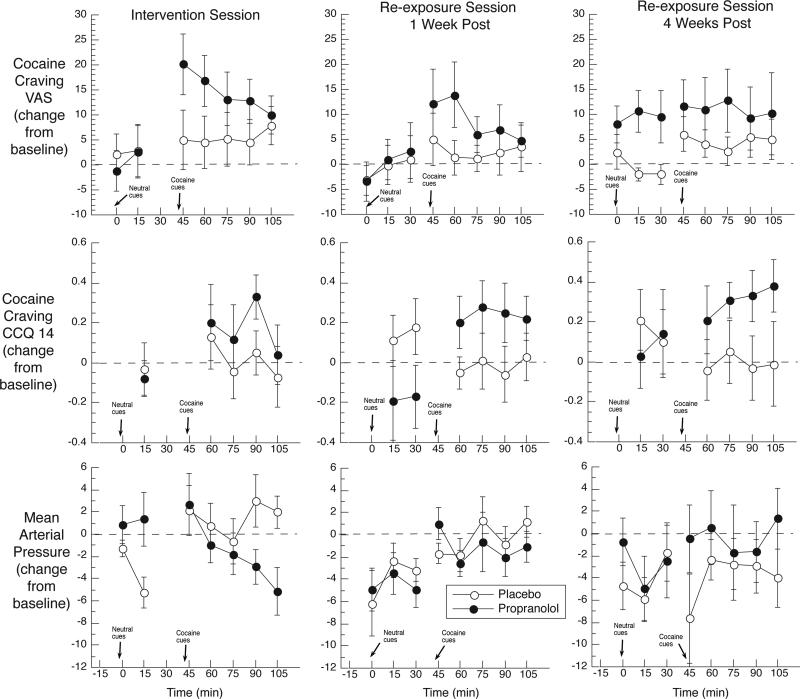
The VAS, CCQ, PANAS, heart rate, and blood pressure were collected 5 minutes before the neutral script, and again at time points shown in figure 2 (including collection within 2 min after each script for VAS, blood pressure, and heart rate).
Figure 2.
Mean change scores (each time point minus baseline from beginning of session) for the cocaine-craving Visual Analog Scale (VAS), the 14-item Cocaine Craving Questionnaire (CCQ-14), and mean arterial blood pressure (MABP). Solid symbols: propranolol group (n = 18). Open symbols: placebo group (n = 15). Propranolol/placebo was administered in the intervention session only (left-hand panels), 75 min before the neutral script. Error bars show standard error of the mean. Results show unexpectedly strong cocaine craving in the propranolol group in the intervention session (for which we had hypothesized no acute effect of propranolol on craving), which seemed to persist, though not to a statistically significant degree, in the re-exposure/test sessions (for which we had hypothesized a beneficial effect of prior propranolol exposure).
Data Analysis
We initially aimed to collect data from 30 participants per group, providing 80% power to detect a Drug x Script interaction of f = 0.18 or greater (equivalent to a Pearson r of 0.18) on any outcome measure at a two-tailed alpha of .05. Due to a change in availability of residential beds, we stopped the study early, with data from 15 and 18 participants randomized to the placebo and propranolol groups, respectively, providing 80% power to detect a Drug x Script interaction of f = 0.25 or greater (equivalent to a Pearson r of 0.24).
Demographic/baseline measures were compared between groups using t-tests, chi-squares, or exact tests. Three drug-use-history measures that differed between groups at p < .10 were included as covariates, separately and together, in the main outcome analyses. This did not substantially change the results, so we report the results without them.
Our hypothesis was that propranolol in the intervention/reactivation session would reduce cocaine-cue reactivity in the two subsequent drug-free re-exposure sessions, so, in our outcome analyses, we used data from those two sessions. For each measure, change scores were calculated separately for the neutral and cocaine scripts; the timepoint just before each script served as its baseline. The change scores were the dependent variables in repeated-measures regression analyses (SAS Proc Mixed). A separate model was run for each dependent variable in each of the two re-exposure sessions. In each model, the between-subjects predictor was Drug (propranolol or placebo, reflecting which one the participant had received in the intervention session), and the time-varying within-subjects predictor was Script (cocaine or neutral, with the time points collapsed to generate a single least-squares mean indicating reactivity to that type of script). A first-order autoregressive error structure was used. A significant Drug x Script interaction, with the appropriate 4 × 4 pattern of means, would reflect the hypothesized attenuation of cue reactivity by prior exposure to propranolol.
The urine data were collapsed into “weeks”; week -1 and -2 were the two weeks before the intervention, week 0 was the day of intervention, and weeks 0.5, 1, 2, 3, and 4 were the weeks after intervention. To analyze the results, we ran three different repeated-measures logistic regressions (SAS Proc Glimmix): (1) To check for baseline differences, we analyzed the pre-intervention time points, with Group as the between-subjects predictor and Week at the within-subjects predictor. (2) To test the hypothesis that propranolol would reduce subsequent use of cocaine, we analyzed the post-intervention time points, controlling for each participant's baseline percentage of cocaine-negative urines. (3) To check for nonspecific changes over time, we analyzed all eight time points.
For all analyses, alpha was p ≤ .05, two-tailed.
Results
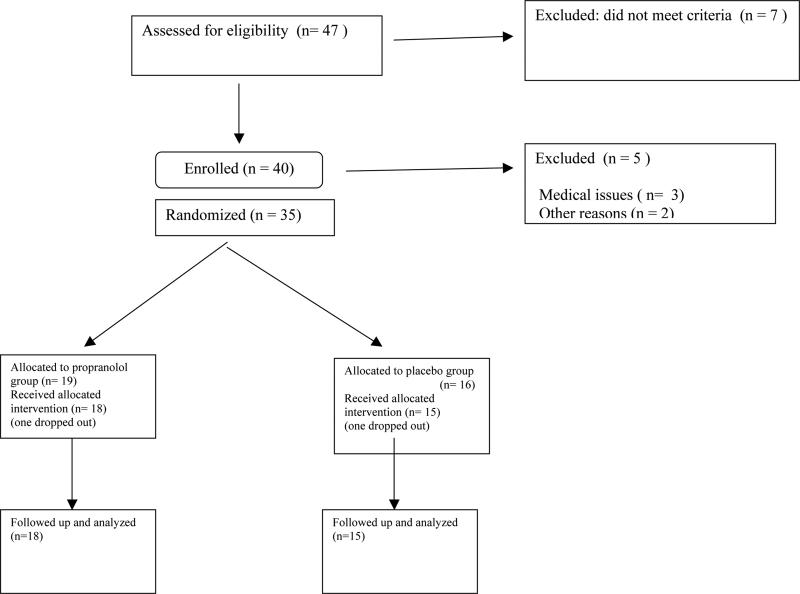
Forty participants enrolled; 35 completed (Figure 1). Data from 33 (15 placebo, 18 propranolol) were included in the analyses; data from two were excluded because one was discovered to have left methadone maintenance during the study and the other was noncompliant.
Figure 1.
Participants’ flow through the study.
The only statistically significant baseline difference between groups (Table 1) was days of polydrug use; two other items differed at trend level. As noted above, including these as covariates did not affect the results.
Table 1.
Participants' demographic characteristics, drug-use history, and scores on a test of the capacity for mental imagery.
| Measure | Placebo Group | Propranolol Group | p-value |
|---|---|---|---|
| N | 15 | 18 | |
| Age (yrs) mean; range | 42.1; 24-54 | 41.6; 26-55 | .96 |
| Sex n (%) | .23 | ||
| Male | 9 (60%) | 7 (38.9%) | |
| Female | 6 (40%) | 11 (61.1%) | |
| Race n (%) | .46 | ||
| African American | 12 (80%) | 12 (66.7%) | |
| European American | 2 (13.3%) | 5 (27.8%) | |
| Other | 1 (6.7%) | 1 (5.6%) | |
| Education (yrs) | 11.9 | 11.9 | .60 |
| Employment n (%) | .40 | ||
| Full time | 5 (33.3%) | 5 (27.8%) | |
| Part time regular hours | 0 | 3 (16.7%) | |
| Part time irregular hours | 4 (26.7%) | 2 (11.1%) | |
| Unemployed | 6 (40%) | 7 (38.9%) | |
| In Controlled Environment | 0 | 1 (5.6%) | |
| Cigarette smoking | 14 (93%) | 16 (89%) | .99 |
| Cigarettes per day (average ± SD) | 14.93 ± 10.87 | 12.50 ±9.28 | .49 |
| Cocaine | |||
| Route of Administration n (%) | .09* | ||
| Intranasal | 1 (7.1%) | 0 (0%) | |
| Smoked | 9 (64.3%) | 17 (94.4%) | |
| Intravenous | 4 (28.6%) | 1 (5.6%) | |
| Days used in last 30 (mean; range) | 10.8; 0-28 | 16.9; 1-30 | .06* |
| Years of use (mean; range) | 9.9; 0-30 | 10.0; 0-20 | .96 |
| Heroin | |||
| Route of Administration | .99 | ||
| Intranasal | 8 (53.3%) | 10 (55.6%) | |
| Intravenous | 7 (46.7%) | 8 (44.4%) | |
| Days used in last 30 (mean; range) | 1.0; 0-7 | 1.6; 0.6 | .43 |
| Years of use (mean; range) | 17.5; 1-30 | 13.3; 2-28 | .10. |
| More than one substance | |||
| Days used in last 30 (mean; range) | 9.2; 0-28 | 17.2; 0-30 | .02* |
| Years of use (mean; range) | 4.7; 0-23 | 3.7; 0-15 | .65 |
| Questionnaire on Mental Imagery (mean; range) | 75.9; 41-116 | 77.7; 40-130 | .85 |
Results for the two re-exposure sessions are summarized in Table 2 and described below.
Table 2.
Additional outcome measures from the 1-week and 5-week re-exposure/test sessions in participants who had previously been exposed to a propranolol intervention session (n = 18) or a placebo control session (n = 15). Results generally indicated, at best, no benefit.
| Measures | Script | Group | Interaction | Comments on change scores |
|---|---|---|---|---|
| 1 week post-intervention | ||||
| PANAS - Positive | F(1,31)=0.97, p=.33 | F(1,31)=2.99, p=.09* | F(1,31)=3.56, p=.07* | Propranolol group: decreases in positive affect (Group effect); decreases in positive affect after neutral script (Interaction) |
| PANAS - Negative | F(1,31)=1.83, p=.19 | F(1,31)=2.04, p=.16 | p=.81 | |
| Mean arterial pressure | F(1,23)=12.52, p=.002* | F(1,24)=0.35, p=.56 | F(1,23)=0.03, p=.87 | Greater decreases after neutral script than after cocaine |
| Diastolic pressure | F(1,23)=11.15, p=.003* | F(1,24)=0.27, p=.61 | F(1,23)=0.28, p=.60 | Greater decreases after neutral script than after cocaine script |
| Heart rate | F(1,23)=3.04, p=.09* | F(1,24)=0.01, p=.92 | F(1,23)=1.90, p=.18 | Greater increases after cocaine script |
| 5 weeks post-intervention | ||||
| PANAS - Positive | F(1,29)=0.76, p=.39 | F(1,29)=0.46, p=.50 | F(1,29)=0.01, p=.91 | |
| PANAS - Negative | F(1,29)=5.91, p=.02* | F(1,29)=1.89, p=.18 | F(1,29)=2.61, p=.12 | Cocaine script increased negative affect |
| Mean arterial pressure | F(1,20)=0.15, p=.70 | F(1,20)=2.03, p=.17 | F(1,20)=0.91, p=.35 | |
| Diastolic pressure | F(1,20)=0.40, p=.54 | F(1,20)=0.30, p=.59 | F(1,20)=0.04), p=.85 | |
| Heart rate | F(1,20)=0.07, p=80 | F(1,20)=0.04, p=.85 | F(1,20)=4.10, p=.06* | Greater increases in propranolol group after cocaine script |
Cocaine-Craving VAS
VAS scores for the intervention session are shown in Figure 2 (top left panel). The figure suggests that propranolol acutely increased reactivity to the cocaine script; this was reflected in a Group x Script interaction, F(1,31) = 8.25, p < .001.
One week later, the cocaine script increased VAS ratings of craving in both groups, F(1,31) = 6.10, p = .02, with mean change scores of +5.41 (SEM 2.12) for the cocaine script and –1.18 (SEM 2.43) for the neutral script. The means (Figure 2, top middle panel) suggest that, contrary to our hypothesis, reactivity to the cocaine script was higher in the propranolol group, though this was not reflected in a Group x Script interaction.
Five weeks later, the cocaine script still tended to increase VAS ratings of craving, F(1,29) = 3.35, p = .08, though the specificity of this effect was dampened, with mean change scores of +8.39 (SEM 2.54) for the cocaine script and +3.89 (SEM 2.72) for the neutral script. The means (Figure 2, top right panel) suggest that the propranolol group was especially reactive to both scripts, though this was not reflected in a Group x Script interaction.
Cocaine Craving CCQ
One week post-intervention, the cocaine script increased CCQ scores (and the neutral script decreased them) in the propranolol group only, as reflected in a Group x Script interaction, F(1,31) = 10.58, p = .003 (Figure 2, center row, center column). Mean change scores were: Placebo group, neutral script: +0.16 (SEM 0.12); Placebo group, cocaine script: -0.02 (SEM 0.10); Propranolol group, neutral script: -0.17 (SEM 0.11); Propranolol group, cocaine script: +0.23 (SEM 0.09). Five weeks post-intervention, the propranolol group seemed to remain especially reactive to the cocaine-craving script (Figure 2, center row, right column). However, the interaction was no longer statistically significant (Table 2). There were no main effects of Script or Group in either post-intervention session.
PANAS
The only effect on the PANAS was an increase in negative affect following the cocaine script 5 weeks post-intervention (Table 2).
Vital Signs
Figure 2 (bottom) shows one of the vital-sign measures, systolic blood pressure. The only effect on it during the two re-exposure sessions was a main effect of Group 5 weeks post-intervention, F(1,20)=5.74, p = .03. There were significant main effects of Script on MAP and diastolic blood pressure 1 week post-intervention (Table 2).
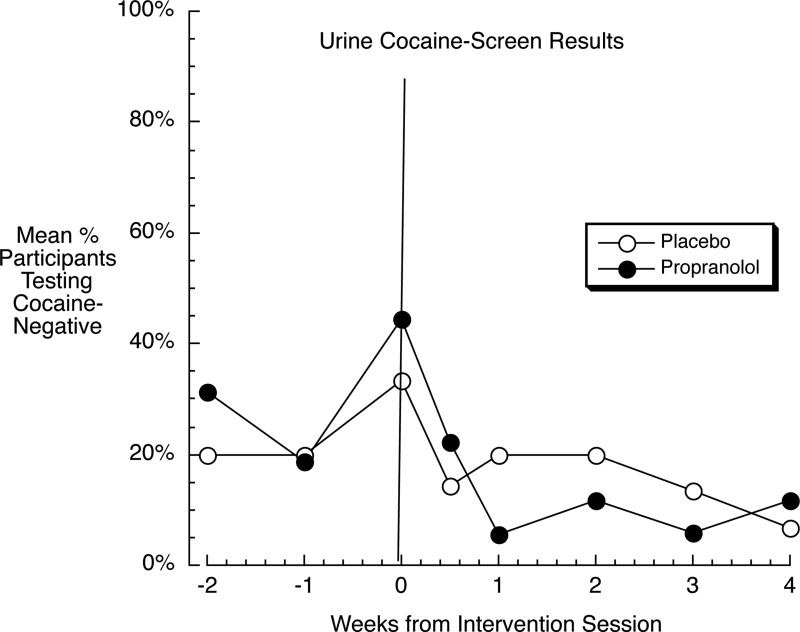
Cocaine use
Pre-intervention, the groups did not differ, F(1,31) = 0.15, p = .70 (Figure 3). Post-intervention, controlling for baseline, the groups still did not differ, F(1,30) = 2.71, p = .11, nor was there a Group x Week interaction, F(4,120) = 1.61, p = .17. In a third analysis with all 8 time points collapsed into two phases (pre versus post), all participants deteriorated across phase, F(1,31) = 7.21, p = .01, but this deterioration did not differ by group: Group x Phase F(1,31) = 0.10, p = .75.
Figure 3.
Percentage of participants whose urine specimens consistently tested negative for cocaine in (usually) twice-weekly testing, in the groups that received propranolol (solid symbols) or placebo (open symbols) in the intervention session (horizontal line). Results indicate, at best, no beneficial effect of the propranolol intervention on cocaine use.
Discussion
Cue reactivity was unexpectedly greater in the propranolol group than in the placebo group. This occurred both acutely during propranolol and one and five weeks later, when the prior propranolol/reactivation session should have had a protective effect. There was also no indication that the intervention reduced cocaine use.
These results differ from those of an earlier study by Saladin and colleagues (2013), in which a single 40 mg dose of propranolol administered two hours after exposure to cocaine cues reduced subsequent cue reactivity in opioid-free cocaine users in a test session conducted after an overnight stay on an inpatient unit. The benefit from propranolol vanished after the participants had returned to their home environments for a week. We first tested our participants for a persistent benefit after they had spent a week in their home environments. Thus, at the time point where our results can be compared to Saladin's, the two sets of findings are both negative.
In the Saladin study, propranolol did not acutely increase cue reactivity. It is possible that the effects of propranolol on cocaine-cue responses are different during opioid maintenance. For example, rats’ CPP for morphine was more difficult to disrupt with a propranolol/reactivation procedure if the rats had undergone more initial morphine/place pairings (Robinson and Franklin 2010) and was not disruptable if morphine exposure had occurred daily for two weeks, even if morphine was no longer present (Robinson, Armson, et al., 2011). In the latter study, rats that continued to receive chronic morphine during testing showed an exacerbation of CPP after propranolol/reactivation (Robinson, Armson, et al., 2011). We do not know whether this paradox is specific to opioids; if it is, it would help explain our findings.
We have some other tentative explanations for the absence of a lasting benefit from propranolol. When we started our study, expecting a lasting benefit from the reactivation session, we did so partly on the basis of findings with propranolol's disruption of reconsolidation in cocaine-trained rats (Bernardi, Lattal, et al., 2006). One barrier to translating the findings of such studies is that of dose scaling; for example, the study just cited (Bernardi, Lattal, et al., 2006) used a propranolol dose of 10 mg/kg intraperitoneally, whereas we used a fixed total dose of 40 mg given orally, which is the dose typically used in reconsolidation studies (Kindt, Soeter, et al., 2009; Saladin, Gray, et al., 2013; Spring, Wood, et al., 2015).
There is now evidence that propranolol can disrupt human reconsolidation of longstanding traumatic memories (Brunet, Poundja, et al., 2012), but that evidence was obtained under open-label conditions, using six propranolol/reactivation sessions (versus one in our study), and using doses of propranolol as high as 100 mg (versus 40 mg in our study). When similarly high doses of propranolol were tested in post-combat PTSD under double-blind conditions—and using only one reactivation session—they were no more effective than propranolol given in a no-reactivation control condition (Pitman 2011).
What is clearer now than when we started our study is that, for well-established memories especially, reconsolidation processes are not necessarily engaged upon every episode of reactivation. In studies of newly acquired fear associations in humans, reconsolidation seemed to be engaged only when the outcome of the reactivation trial was not predictable—when participants thought they might learn something new (Sevenster, Beckers, et al., 2012). Similar findings are seen with other types of memory (Hupbach, Hardt, et al., 2008; Winters, Tucci, et al., 2009; Hupbach, Gomez, et al., 2011). We gave participants no reason to expect that they might receive cocaine. Our participants may have inferred, correctly, that the conditioned stimuli (the drug cues) were not going to be associated with the unconditioned stimulus (cocaine intoxication), and therefore, they may not have engaged memory-updating mechanisms.
None of this explains the other unexpected aspect of our findings: the seeming exacerbation of cocaine-cue reactivity by propranolol, acutely (to a statistically significant degree) and perhaps during propranolol-free follow-up tests (though there was too much variability for the exacerbation to be statistically significant). In terms of acute effects, propranolol is not reliably anxiolytic in the way that benzodiazepines are, at least not in the presence of a stressor (Papadopoulos, Rich, et al., 2010). But in healthy, unstressed volunteers, 80 mg of propranolol increased subjective ratings of gregariousness and optimism (and reduced ratings of anxiety) relative to placebo (Landauer, Pocock, et al., 1979), and 40 mg increased subjective ratings of “detachment” on a VAS (Salem and McDevitt 1984). Perhaps these mood changes reflect a subtle emotional disinhibition inducible by propranolol. In our participants, that disinhibition might have manifested as a greater craving response to appetitive cues, or a greater willingness to report it.
One limitation of our study is that we did not include a “propranolol without reactivation” control condition. With a few exceptions (Kroes, Strange, et al., 2010; Saladin, Gray, et al., 2013), human studies of propranolol and memory have begun to include such a condition (Pitman 2011; Schwabe, Nader, et al., 2012) to strengthen a reconsolidation-based interpretation of the findings. Also, we administered propranolol before reactivation (so peak plasma levels would coincide with cue exposure), rather than immediately afterward. With some exceptions (Pitman 2011; Brunet, Poundja, et al., 2012; Schwabe, Nader, et al., 2012; Sevenster, Beckers, et al., 2012), the trend in human reconsolidation studies has been to administer propranolol after memory reactivation (Pitman 2011; Soeter and Kindt 2012; Saladin, Gray, et al., 2013) to help rule out drug effects on retrieval. These limitations would have impeded interpretation of a beneficial effect of propranolol if we had detected one. They do not explain the counter-hypothesized effects we found.
Another limitation is that we stopped the study early (and with a smaller number of participants than intended); our results might have been different if we had continued. However, our extant data do not show even a trend toward any benefit from propranolol. Our groups had differences in drug-use history and some baseline differences in craving during the intervention session—but our results were unchanged when we used the drug-history measures as covariates. Our negative findings complement just-published negative findings in smokers (Pachas, Gilman, et al., 2015).
Acknowledgments
This research was supported by the Intramural Research Program (IRP) of the National Institute on Drug Abuse (NIDA), National Institutes of Health and a NIDA R01 [grant number DA-020647] (Aharonovich). The authors wish to thank Dr. Robert Brooner, the treatment and research staff of Archway and the study participants.
References
- Alberini CM, Ledoux JE. Memory reconsolidation. Curr Biol. 2013;23(17):R746–750. doi: 10.1016/j.cub.2013.06.046. [DOI] [PubMed] [Google Scholar]
- Altamura AC, Moliterno D, Paletta S, Maffini M, Mauri MC, Bareggi S. Understanding the pharmacokinetics of anxiolytic drugs. Expert Opin Drug Metab Toxicol. 2013;9(4):423–440. doi: 10.1517/17425255.2013.759209. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17(13):1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52(10):976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Brunet A, Poundja J, Tremblay J, et al. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. J Clin Psychopharmacol. 2012;31(4):547–550. doi: 10.1097/JCP.0b013e318222f360. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Blackwell AD, Robbins TW, Sahakian BJ. Noradrenergic modulation of working memory and emotional memory in humans. Psychopharmacology. 2006;188(4):397–407. doi: 10.1007/s00213-006-0391-6. [DOI] [PubMed] [Google Scholar]
- Chan JC, LaPaglia JA. Impairing existing declarative memory in humans by disrupting reconsolidation. Proc Natl Acad Sci USA. 2013;110(23):9309–9313. doi: 10.1073/pnas.1218472110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Schoffelmeer AN, De Vries TJ. Beta-adrenoceptor mediated inhibition of long-term reward-related memory reconsolidation. Behav Brain Res. 2006;170(2):333–336. doi: 10.1016/j.bbr.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Nadel L. Episodic memory updating: the role of context familiarity. Psychon Bull Rev. 2011;18(4):787–797. doi: 10.3758/s13423-011-0117-6. [DOI] [PubMed] [Google Scholar]
- Hupbach A, Hardt O, Gomez R, Nadel L. The dynamics of memory: context-dependent updating. Learn Mem. 2008;15(8):574–579. doi: 10.1101/lm.1022308. [DOI] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12(3):256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Kroes MC, Strange BA, Dolan RJ. Beta-adrenergic blockade during memory retrieval in humans evokes a sustained reduction of declarative emotional memory enhancement. J Neurosci. 2010;30(11):3959–3963. doi: 10.1523/JNEUROSCI.5469-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landauer AA, Pocock DA, Prott FW. Effects of atenolol and propranolol on human performance and subjective feelings. Psychopharmacology. 1979;60(2):211–215. doi: 10.1007/BF00432296. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola JS, et al. New data from the Addiction Severity Index: reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Pachas GN, Gilman J, Orr SP, et al. Single dose propranolol does not affect physiologic or emotional reactivity to smoking cues. Psychopharmacology. 2015;232(9):1619–1628. doi: 10.1007/s00213-014-3797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos A, Rich A, Nutt DJ, Bailey JE. The effects of single dose anxiolytic medication on the CO2 models of anxiety: differentiation of subjective and objective measures. J Psychopharmacol. 2010;24(5):649–656. doi: 10.1177/0269881108097716. [DOI] [PubMed] [Google Scholar]
- Pitman RK. [March 5, 2013];Developing memory reconsolidation blockers as novel PTSD treatments. 2011 Available at: http://www.dtic.mil/dtic/tr/fulltext/u2/a540931.pdf.
- Pitman RK. [March 5, 2013];A psychophysiologic study of weakening traumatic combat memories with post-reactivation propranolol. 2011 Available at: http://www.dtic.mil/dtic/tr/fulltext/u2/a553813.pdf.
- Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 1999;19(15):6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyslawski J, Sara SJ. Reconsolidation of memory after its reactivation. Behav Brain Res. 1997;84(1-2):241–246. doi: 10.1016/s0166-4328(96)00153-2. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Armson M, Franklin KB. The effect of propranolol and midazolam on the reconsolidation of a morphine place preference in chronically treated rats. Front Behav Neurosci. 2011;5:42. doi: 10.3389/fnbeh.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Franklin KB. Reconsolidation of a morphine place preference: impact of the strength and age of memory on disruption by propranolol and midazolam. Behav Brain Res. 2010;213(2):201–207. doi: 10.1016/j.bbr.2010.04.056. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Gray KM, McRae-Clark AL, et al. A double blind, placebo-controlled study of the effects of post-retrieval propranolol on reconsolidation of memory for craving and cue reactivity in cocaine dependent humans. Psychopharmacology. 2013;226(4):721–737. doi: 10.1007/s00213-013-3039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem SA, McDevitt DG. Central effects of single oral doses of propranolol in man. Br J Clin Pharmacol. 1984;17(1):31–36. doi: 10.1111/j.1365-2125.1984.tb04995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Wolf OT, Beaudry T, Pruessner JC. Neural signature of reconsolidation impairments by propranolol in humans. Biol Psychiatry. 2012;71(4):380–386. doi: 10.1016/j.biopsych.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Neurobiol Learn Mem. 2012;97(3):338–345. doi: 10.1016/j.nlm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Shand DG, Nuckolls EM, Oates JA. Plasma propranolol levels in adults with observations in four children. Clin Pharmacol Ther. 1970;11(1):112–120. doi: 10.1002/cpt1970111112. [DOI] [PubMed] [Google Scholar]
- Sheehan PW. A shortened form of Betts’ questionnaire upon mental imagery. J Clin Psychol. 1967;23(3):386–389. doi: 10.1002/1097-4679(196707)23:3<386::aid-jclp2270230328>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152(2):140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Tuit K. Imagery script development procedures manual. CreateSpace; Charleston, SC: 2012. [Google Scholar]
- Soeter M, Kindt M. Stimulation of the noradrenergic system during memory formation impairs extinction learning but not the disruption of reconsolidation. Neuropsychopharmacology. 2012;37(5):1204–1215. doi: 10.1038/npp.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring JD, Wood NE, Mueller-Pfeiffer C, Milad MR, Pitman RK, Orr SP. Prereactivation propranolol fails to reduce skin conductance reactivity to prepared fear-conditioned stimuli. Psychophysiology. 2015;52(3):407–415. doi: 10.1111/psyp.12326. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL, Singleton EG. Challenges in the manipulation, assessment and interpretation of craving relevant variables. Addiction. 2000;95(Suppl 2):S177–187. doi: 10.1080/09652140050111753. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34(1):19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Winters BD, Tucci MC, DaCosta-Furtado M. Older and stronger object memories are selectively destabilized by reactivation in the presence of new information. Learn Mem. 2009;16(9):545–553. doi: 10.1101/lm.1509909. [DOI] [PubMed] [Google Scholar]