Abstract
Background
Policymakers have proposed risk-adjusted bundled payment as the single-most promising method of linking reimbursement to value rather than to quantity of service. Our objective was to assess the relationship between risk and cost to develop a model for forecasting cardiac surgery costs under a bundled payment scheme.
Methods
All patients undergoing adult cardiac surgery operations for which there was a Society of Thoracic Surgeons (STS) risk score over a 5-year period (2008–2013) at a tertiary care, university hospital were reviewed. Patients were stratified into 5 groups based on preoperative risk as a basis for negotiating risk-adjusted bundles. A multivariable regression model was developed to analyze the relationship between risk and log-transformed costs. Monte Carlo simulation was performed to validate the model by comparing predicted to actual FY2013 costs.
Results
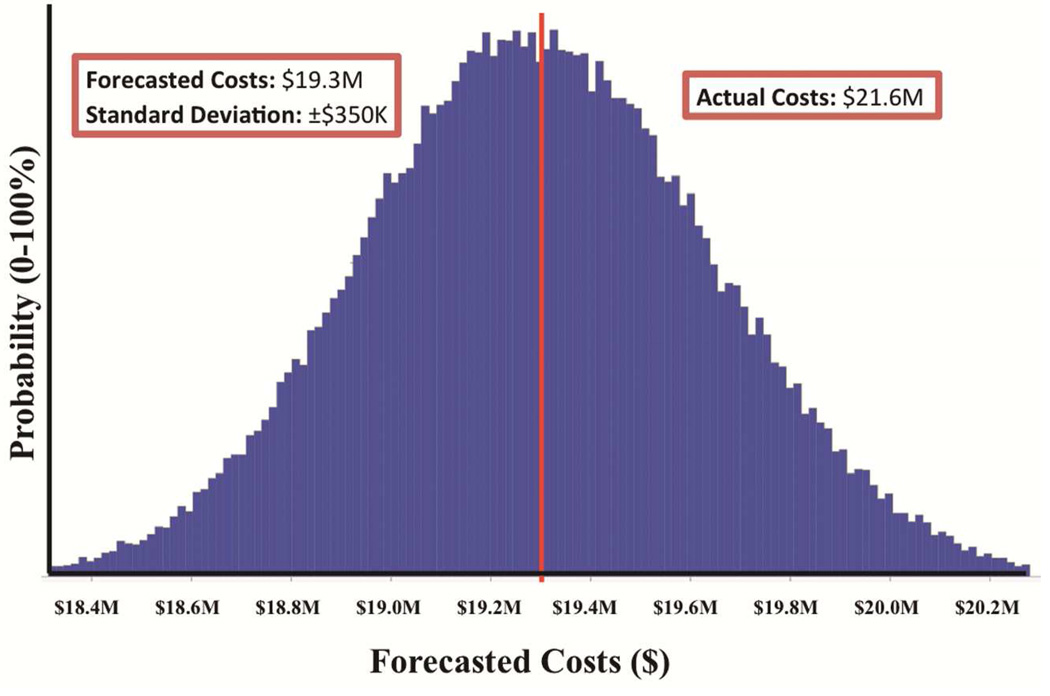
Among the 2514 patients analyzed, preoperative risk was strongly correlated with costs (p<0.001) but was only able to explain 28% (R2=0.28) of the variation in costs between individual patients. Using bundling to diffuse and adjust for risk improved prediction to only 33% (R2=0.33). Actual costs in 2013 were $21.6M compared to predicted costs of $19.3M (±$350K), which is well outside the forecast’s 95% confidence interval.
Conclusion
Even among the most routine cardiac surgery operations using the most widely validated surgical risk score available, much of the variation in costs cannot be explained by preoperative risk or surgeon. Consequently, policymakers should re-examine whether individual practices or insurers are best suited to manage the residual financial risk.
Keywords: Health economics (cost analysis, insurance, relative value); Health provider (arrangements, delivery/reimbursements); Health policy (includes government regulation, Obama Care); Health professional affairs, advocacy, regulation
INTRODUCTION
There is strong consensus that the U.S. health care system fails to create the value that it should, and the current fee-for-service reimbursement model is widely believed to create incentives that reward providers simply for delivering more care—not necessarily better care [1,2,3,4,5]. To preserve financial solvency, new solutions to reform the payment model to reward value are urgently needed [6].
Health policy experts shaping current reform efforts have identified bundled payments as the single-most promising mechanism for achieving cost savings, potentially reducing health care expenditure by 5.4% over the next decade [7,8]. An underlying philosophy behind creating Accountable Care Organizations (ACOs) in the Affordable Care Act (ACA) is that aggregating payments into broader bundles of care will address problems inherent in fee-for-service reimbursement [9].
In the case of acute diagnoses, bundling occurs based on discrete episodes of care [6]. In essence, the system replaces payments for each procedure (e.g., cardiac catheterization, echocardiogram, surgical operation) with a single bundled payment for an entire hospitalization, including acute and post-acute services [10]. For example, for a patient undergoing coronary artery bypass grafting (CABG), an insurer would pay an ACO a single price for all services required to diagnose acute coronary syndrome, perform surgery, recover the patient in the hospital, rehabilitate the patient at a facility or at home, and establish follow-up care [11]. Geisinger’s ProvenCare CABG program stands out as the most visible model of bundled payments in cardiac surgery [12,13,14].
By accepting an episode-based bundled payment in lieu of a series of fee-for-service payments, provider organizations are incentivized to improve care coordination and efficiency by focusing on highest impact interventions [8]. For insurers, outlier payments are eliminated, risk is reduced, and the payment infrastructure is simpler and more transparent [15]. Overall, the system is a variation on capitation; insurers and providers negotiate the price of the bundle, but financial risk is transferred from insurers to providers [16].
For bundled care to succeed, revenues paid to providers must to some degree correlate with the costs providers encounter in caring for patients [8]. The rough mechanism CMS has used in its pilot programs has involved pricing based on historical claims data [17]. Recognizing the potential financial incentives for providers to skimp on care or avoid high-risk patients, policymakers have proposed riskadjustment in pricing bundles [6,8,14]. The purpose of our study was to assess the relationship between risk and cost to develop a model for forecasting our institution’s cardiac surgery costs under a risk-adjusted bundled payment scheme.
PATIENTS AND METHODS
Patients
The study was approved by the University of Virginia Institutional Review Board, including a waiver for the need to obtain patient consent. All adult patients undergoing cardiac operations were retrospectively reviewed from fiscal years (FY) 2009–2013 (5-year period) using our Society of Thoracic Surgeons (STS) Adult Cardiac Surgery database. Only patients for whom there was a calculated STS Predicted Risk of Mortality (PROM) were included, thus limiting the study to the most common types of cardiac operations: isolated CABG, isolated aortic valve replacement (AVR), isolated mitral valve repair or replacement (MVR), combined CABG+AVR, and combined CABG+MVR.
Patients were divided into model development (FY2009–2012) or model validation (FY2013) cohorts given that most bundled payment pilot projects have used recent historical cost data as the basis for generating bundled pricing. The two cohorts were analyzed for differences in preoperative, operative, and postoperative characteristics.
Cost Data
Cost data were abstracted from our Clinical Data Repository (CDR), which captures all inpatient and outpatient encounters within the University of Virginia Health System; its accuracy and validity have been published elsewhere and shown to be comparable nationally [18]. Briefly, the CDR uses microcosting algorithms to capture cost data within an actual utilization framework. Thus, our cost figures represent the cost of care as estimated by the institution rather than charges relayed to patients and insurers or actual reimbursement.
Model Development
To understand the predictive relationship between patient characteristics and cost, multivariable linear regression models were built with total hospitalization costs (log-transformed, given that they follow a log-normal distribution) as the dependent variable. Performance of the models was assessed by R2. Two separate models were developed: a preoperative and a preoperative-postoperative model.
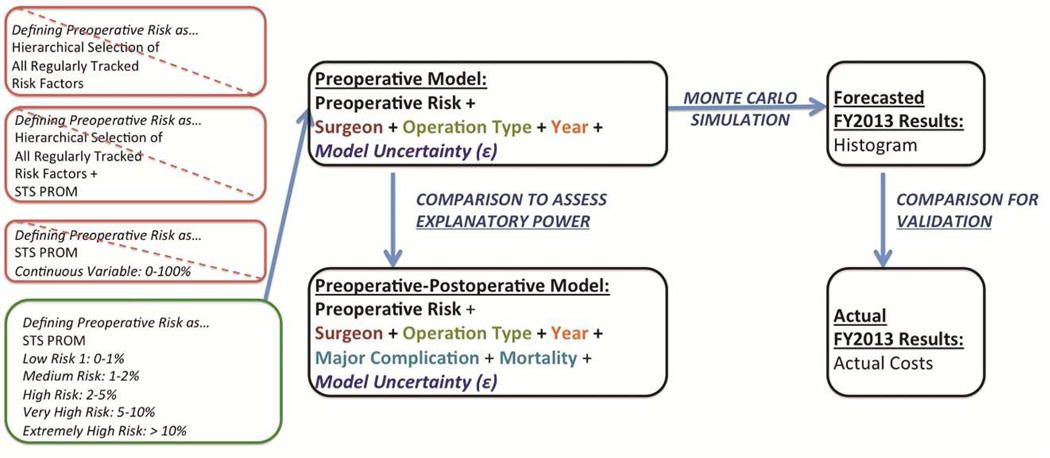
The preoperative model included only variables (e.g., patient risk factors) that were known before the operation (in addition to primary surgeon and operation type). Notably, several regression models incorporating preoperative risk factors were attempted. However, given the minor difference in model performance between the hierarchal model built based using all regularly tracked risk factors compared to the model built aggregating the majority of these risk factors into STS PROM, we elected to use the latter given its simplicity. Furthermore, STS PROM was attempted as a continuous variable versus bundling patients into risk quintiles, deciles, or logical breakpoints observed on a STS-PROM versus cost plot (which yielded the best explanatory model). This overall process is outlined in Figure 1.
FIGURE 1.
Overview of methodology.
The preoperative-postoperative model included the same variables in addition to postoperative events, represented by two dummy variables: morbidity and mortality. Morbidity was defined by a composite outcome of major adverse events using standard STS definitions (e.g., prolonged mechanical ventilation, renal failure, re-exploration, etc.).
Model Validation
To validate our model, we used Monte Carlo simulation, a technique widely used in financial analysis, to forecast FY2013 costs. Briefly, Monte Carlo simulation takes advantage of the fact that all regression models assume a degree of uncertainty (e.g., in their coefficients, intercepts, and residual error) that can be quantified or approximated (based on historical data, subjective judgment, etc.) by defining assumptions as probability distributions [19]. Using several thousand iterations (i.e., repeated sampling from the assumed probability distribution), Monte Carlo simulation in essence allows for the multiplication and addition of probability distributions. Consequently, instead of generating a single forecast value, it allows for more realistic financial prediction by producing a range of possible values and their probabilities to better illustrate risk and uncertainty. In our Monte Carlo simulation, we inputted the characteristics of the actual patients who presented to our institution in FY2013 into the regression model, proxied model uncertainty using the standard error of the residual of the multivariable regression, and ran the simulation through 100,000 iterations to forecast predicted FY2013 costs. We then compared forecasted to actual FY2013 costs for model validation.
Statistical Analysis
All categorical variables are expressed as a percentage of the group of origin; continuous variables are expressed as the mean ± standard deviation (SD). Univariate comparisons were performed using the Pearson χ2 test for dichotomous variables and Student’s t-test for continuous variables. All reported p values are 2-tailed, and statistical significance was defined as p ≤ 0.05. Univariate and multivariable analyses were performed using Microsoft Excel (Redmond, WA) and open-source R statistical software (http://www.R-project.org). Monte Carlo simulation was performed using Oracle Crystal Ball (Redwood City, CA).
RESULTS
Patient Characteristics, Clinical Outcomes, and Costs
The total cohort consisted of 2514 patients. Patient characteristics, risk factors, and adverse events for the development and validation cohorts are presented in Tables 1–2. While minor variations existed, there were no clinically significant differences in patient characteristics, comorbidities, case-mix, or outcomes between the model and validation cohorts. Actual costs in FY2013 were $21.6M.
TABLE 1.
Preoperative Characteristics.
| Preoperative Variables n |
FY2009–2013 2008 |
FY2013 506 |
P |
|---|---|---|---|
| Age (years) | 66.9 | 67.8 | 0.175 |
| BMI (kg/m2) | 29.7 | 30.5 | 0.350 |
| Creatinine (mg/mL) | 1.3 | 1.2 | 0.009 |
| Ejection Fraction (%) | 51.4 | 53.5 | 0.041 |
| Female (%) | 31.7% | 30.4% | 0.574 |
| Smoker (%) | 20.1% | 16.0% | 0.035 |
| Diabetes (%) | 38.7% | 41.3% | 0.281 |
| Hypertension (%) | 82.9% | 85.4% | 0.180 |
| Peripheral Arterial Disease (%) | 16.4% | 16.2% | 0.920 |
| Dialysis-Dependent (%) | 3.5% | 1.8% | 0.045 |
| Chronic Lung Disease (%) | 21.5% | 24.3% | 0.177 |
| Prior PCI (%) | 21.6% | 20.4% | 0.531 |
| Prior Sternotomy (%) | 32.6% | 31.4% | 0.608 |
| Congestive Heart Failure (%) | 35.0% | 41.5% | 0.006 |
| NYHA II | 5.5% | 11.5% | 0.000 |
| NYHA III | 18.5% | 19.0% | 0.802 |
| NYHA IV | 10.9% | 11.1% | 0.928 |
| Status | |||
| Elective (%) | 58.3% | 55.9% | 0.339 |
| Urgent (%) | 41.7% | 44.1% | 0.339 |
| STS PROMM (%) | 19.9% | 19.8% | 0.915 |
| STS PROM (%) | 3.4% | 3.1% | 0.414 |
Comparison of preoperative characteristics between the development and validation cohorts.
TABLE 2.
Clinical Outcomes.
| Clinical Outcomes n |
FY2009–2012 2008 |
FY2013 506 |
P |
|---|---|---|---|
| Operative Duration (min) | 224.6 | 231.6 | 0.310 |
| Cardiopulmonary Bypass Time (min) | 99.1 | 103.4 | 0.140 |
| Cross-clamp time (min) | 71.0 | 74.1 | 0.118 |
| Intraoperative Blood Transfusion (%) | 32.6% | 38.7% | 0.009 |
| Postoperative Length of Stay (days) | 7.2 | 7.3 | 0.530 |
| Intensive Care Length of Stay (hours) | 73 | 79 | 0.915 |
| Intensive Care Readmission (%) | 4.1% | 5.1% | 0.296 |
| Atrial Fibrillation (%) | 21.2% | 23.7% | 0.218 |
| Major Complication (%) | 13.0% | 15.0% | 0.239 |
| Deep Sternal Wound Infection (%) | 0.3% | 0.4% | 0.722 |
| Prolonged Ventilation (%) | 7.6% | 8.3% | 0.607 |
| Pneumonia (%) | 2.6% | 3.4% | 0.346 |
| Sepsis (%) | 0.5% | 0.9% | 0.290 |
| Renal Failure (%) | 5.1% | 5.1% | 0.955 |
| Dialysis (%) | 2.3% | 3.4% | 0.182 |
| Reoperation (%) | 2.0% | 2.8% | 0.279 |
| Stroke (%) | 2.3% | 1.0% | 0.068 |
| Myocardial Infarction (%) | 0.1% | 0.0% | 0.455 |
| Cardiac Arrest (%) | 2.0% | 1.6% | 0.503 |
| Mortality (%) | 2.5% | 2.4% | 0.830 |
| Observed:Expected Ratio | |||
| Morbidity | 0.65 | 0.76 | - |
| Mortality | 0.74 | 0.76 | - |
Comparison of clinical outcomes between the development and validation cohorts.
Cost Model Explanatory Power
A hierarchical multivariable regression using all available individual preoperative risk factors resulted in an R2 of 0.36. Body mass index (BMI) was the only significant variable (p<0.001) when STS PROM was also included in the model. Models using individual STS PROM scores alone were able to explain only 28% (R2=0.28) of the variation in costs among individual patients, but using STS PROM to bundle patients into risk cohorts to diffuse and adjust for risk improved explanatory power to 35% (R2 = 0.35). Given this model’s simplicity, it was selected as the best preoperative model for multivariable regression (Figure 1), the results of which are presented in Table 3. Preoperative risk is strongly correlated with perioperative hospital costs (p<0.001) after controlling for surgeon, year, and operation type.
TABLE 3.
Multivariable regression model using only preoperative characteristics.
| Variable | Coefficient | P-value | Lower 95% | Upper 95% |
|---|---|---|---|---|
| Intercept | 4.405 | <0.001 | 4.383 | 4.426 |
| Medium Risk (STS PROM 1–2%) | 0.059 | <0.001 | 0.038 | 0.079 |
| High Risk (STS PROM 2–5%) | 0.122 | <0.001 | 0.101 | 0.143 |
| Very High Risk (STS PROM >5%) | 0.234 | <0.001 | 0.207 | 0.260 |
| Extremely High Risk (STS PROM >10%) | 0.302 | <0.001 | 0.270 | 0.334 |
| Surgeon 2 | 0.032 | <0.001 | 0.014 | 0.050 |
| Surgeon 3 | 0.053 | <0.001 | 0.035 | 0.071 |
| Isolated aortic valve | −0.001 | <0.001 | −0.022 | 0.019 |
| Isolated mitral valve | 0.055 | <0.001 | 0.029 | 0.081 |
| CABG+aortic valve | 0.054 | <0.001 | 0.028 | 0.080 |
| CABG+mitral valve | 0.030 | 0.123 | −0.008 | 0.068 |
| FY2010 | −0.042 | <0.001 | −0.064 | −0.021 |
| FY2011 | −0.015 | 0.059 | −0.036 | 0.006 |
| FY2012 | 0.022 | 0.043 | 0.001 | 0.04 |
The intercept was defined as a low risk (STS PROM < 1%) isolated CABG in FY2009 performed by Surgeon 1. The dependent variable was log-transformed total hospital costs. Coefficients reflect the mean impact of an increase in or the presence of each variable on log-transformed costs. The R2 of the regression was 0.33.
While preoperative risk clearly contributes to increased costs, the R2 value of the model that considered only preoperative variables was considerably lower than the R2 for the model that considered both preoperative and postoperative variables (0.73). Although the increased power is not unexpected, such a sharp increase for the inclusion of only two dummy variables is dramatic, suggesting that although patient characteristics and comorbidities are important, they may not be the main drivers of increased costs.
Cost Model Validation
While the preoperative-postoperative model is instructive for demonstrating that preoperative risk is insufficient to predict costs, it is not feasible to negotiate contracts using postoperative complications, as the primary goal of bundled payment is to incentivize care in which complications are minimized rather than rewarded. Consequently, the results of the Monte Carlo simulation using the preoperative model alone were analyzed for validation (Figure 1). Actual costs in 2013 were $21.6M compared to predicted costs of $19.3M ± $350K, which is well outside the 95% confidence interval of the forecast and implies a poorly calibrated model. Consequently, a bundling scheme using preoperative patient characteristics and comorbidities to risk-adjust payments for the most common cardiac operations would have produced an annual loss of approximately $2.3M ± $350K at our institution in 2013.
COMMENT
Clinical outcomes, including 30-day mortality and morbidity, were worse among patients in higher risk categories. Actual FY2013 costs were well outside the 95% confidence interval of the forecast, but it is worth noting that a potentially achievable reduction of costs by 10% would bring actual costs to within the 95% confidence interval of our forecast. Consequently, it is difficult to determine the degree of forecasting accuracy required to propose a validated method for risk-adjusting payments.
However, it is clear that preoperative risk alone poorly explained differences in costs between patients. Even among the most common cardiac operations, much of the variation in costs cannot be explained by preoperative risk or surgeon, and bundling patients into risk-adjusted groups only moderately improved explanatory power. Consequently, it is not surprising that risk-adjustment inadequately compensates providers for the substantial financial risk they assume in a bundled payment scheme, as evidenced by the results of our Monte Carlo simulation.
Comparison to Prior Literature
The literature regarding cost prediction in cardiac surgery is still evolving. A multi-institutional study found that higher risk patients (based on EuroScore) were associated with increased costs but that preoperative variables alone could not explain a reasonable degree of variation in costs among patients [20]. After including postoperative complications, they were able to develop a better, but far from perfect, model. Similar results at a single institution were found using STS PROM [21].
A more contemporary statewide analysis of outcomes after AVR in Virginia demonstrated that higher risk cohorts were significantly associated with higher costs, but there was no regression analysis to show the association’s predictive value [22]. Costs were also determined by cost-to-charge ratios based on billing, which may not be reflective of a provider’s true cost. Additionally, costs were assumed to follow a normal distribution in contrast to a lognormal or gamma distribution. Also, in contrast to our analysis, they used only 3 risk cohorts, with a small minority of patients comprising the high-risk cohort—which may not be viable for risk-adjustment if applied to centers specializing in high-risk patients.
A subsequent statewide analysis of patients in Virginia undergoing CABG revealed that predictive costing performed poorly when only preoperative variables were known (R2 = 0.14), and postoperative complications dramatically increased the explanatory power of their model (R2 = 0.47) [23]. Costs were determined on the basis of cost-to-charge ratios and assumed to follow a gamma distribution. Their model performed poorly in predicting costs for individual patients, and observed costs were higher than predicted, similar to our results. The authors, however, justified their model given the degree of calibration in their validation cohort—i.e., that while individual patient prediction was poor, prediction for the entire cohort appeared reasonable. While such a justification is tempting from the perspective of a policymaker able to average out uncertainty over 40,000+ patients for a single operation type over a 10-year period in a large state, it may be unrealistic for individual practices or hospitals under pressure to meet a bottom line each year. We believe our use of Monte Carlo simulation better accounts for how uncertainty in model performance could impact a hospital financially if accrued over several thousand patients during a fiscal year.
In the context of this literature, our study contributes to the very recent and important efforts to create a financial model to predict costs among patients undergoing the most common cardiac surgical operations. However, in contrast to the prior literature, we are unique in using such a model as the basis for developing a risk-adjusted bundled payment scheme and then examining its financial impact on individual hospital systems. We also propose a methodology to quantify the transfer of financial risk from health insurers to providers in bundled care, which previously has remained in the realm of theoretical speculation.
Our results also add to the literature that preoperative risk factors alone do not predict cost or resource utilization. We agree with the existing literature that the ability to identify costly postoperative complications remains important for helping surgeons and administrators highlight areas for potential improvement and develop quality benchmarks. However, such complications are only known “after-thefact,” and thus not suitable for inclusion in a predictive model to serve as the basis for a potential payment scheme.
Implications for Policy
Indeed, the architects of Medicare’s bundled payment program acknowledge the significant financial risks faced by participating hospitals, and our results mirror their concerns. In their pilot, they observed substantial year-to-year variation in the severity of illness in, and costs for, patients requiring treatment at even large hospitals [14]. They speculated that annual winners and losers in the payment scheme could easily have been the result of random or systematic variation in illness severity rather than marks of true success or failure. Consequently, they recently proposed risk-adjustment to mitigate the problem. Given that the STS has garnered international acclaim for developing one of the most widely validated measures of risk available to any specialty [24], our results imply that risk-adjustment mechanisms alone inadequately compensate providers for the degree of financial risk they accept in entering into bundled payment agreements. Moreover, validated risk-adjustment only exists for the most common types of cardiac operations, yet our results show that even here the financial risk appears unacceptable.
Given the inadequacy of risk-adjustment alone, providers will need to pursue other strategies to better bear the residual financial risk (previously borne by Medicare and insurers who can pool risks across a much larger group of patients with more diverse health problems). Stop-loss provisions (for costly outlier patients) or risk-corridors (offsetting high losses via profit sharing by one party) represent potential opportunities to improve bundled reimbursement, but complete indemnification of providers may defeat the primary problem bundled reimbursement attempts to solve. Providers may also choose to merge into larger, presumably better-capitalized organizations or acquire insurers themselves [25], which could also buttress some of the proposed benefits of bundled care by capturing economies of scale and creating centers of excellence. However, there is also legitimate debate regarding whether such consolidation benefits patients [26]. For example, a recent analysis in Virginia markets experiencing rapid health care consolidation revealed that pricing increased from 20% below the state average to 25% above average [27]. Moreover, the most recent economic recession illustrated that unprecedented consolidation likely cannot overcome an underappreciation of financial risk. Finally, providers must examine whether the success of bundling thus far (e.g., Geisinger) has equally been the result of local traditions, superior management, and physician leadership—capabilities that are difficult to prescribe through legislation.
Study Limitations
Our understanding of preoperative risk is still evolving, and some risk factors (e.g., frailty, pulmonary hypertension) are not captured in current risk models but appear to be important. Additionally, STS PROM was intended to estimate risk of 30-day mortality in large groups of patients rather than the financial risk posed by individual patients. Nevertheless, it is precisely such logic that mandates caution in designing risk-adjustment mechanisms for bundled care. Furthermore, hierarchical models did not yield an especially more explanatory model than STS PROM in predicting financial risk.
Second, cost data is highly dependent on accounting practices employed by individual hospitals [281]. Nevertheless, the same could be said for almost any study utilizing cost data, and our methodology represents an attempt to capture dollars reflective of our institution’s resource utilization rather than arbitrary patient charges. As time-driven, activity-based costing begins to gain acceptance, we can improve these estimates [29]. Ideally, professional associations should begin to standardize a methodology for accounting to make estimates comparable between different provider organizations.
Finally, the analysis is limited to a single-institution and to retrospective data. Consequently, we cannot assess how a bundled payment incentive structure may reduce duplicative costs or wasteful resource consumption, which is undoubtedly embedded in—and thus biases upward—our calculation of actual FY2013 costs. Ultimately, however, it is important to analyze how bundled payments in their current proposed form could impact the practice of cardiac surgery at a relatively large statewide center.
Conclusion
Even among the most common cardiac operations, much of the variation in costs cannot be explained by preoperative risk or surgeon. Given that STS PROM is the most widely validated measure of risk among surgical specialties, a more extensive understanding of the relationship between preoperative risk and financial risk must be developed. For bundled payments to succeed, strategies beyond riskadjustment are required to compensate providers, and there should be robust debate regarding whether providers or insurance companies are better suited to manage the residual financial risk.
FIGURE 2.
Forecast of fiscal year 2013 costs.
Predicted costs are displayed as a forecasted histogram (± standard deviation). The x-axis displays a given cost level, and the y-axis displays the probability of the forecast at that cost level.
ACKNOWLEDGEMENTS
This publication was supported by the NIH (5T32HL007849-13). The authors would also like to thank Judy Smith and Curtis Klann for their diligent data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Society of Thoracic Surgeons 51th Annual Meeting, San Diego, CA, January 26, 2015
REFERENCES
- 1.Mitchell JB, Wedig G, Cromwell J. The Medicare physician fee freeze: what really happened? Health Aff. 1989;8(1):21–33. doi: 10.1377/hlthaff.8.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Inglehart J. Health policy report: the recommendations of the physician payment review commission. N Engl J Med. 1989;320:1156–1160. doi: 10.1056/NEJM198904273201732. [DOI] [PubMed] [Google Scholar]
- 3.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 4.Gawande A. The cost conundrum: what a Texas town can teach us about health care. New Yorker. 2009 Jun 1; [Google Scholar]
- 5.Feder J. Bundle with care – rethinking Medicare incentives for post-acute services. N Engl J Med. 2013;369:400–401. doi: 10.1056/NEJMp1302730. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder SA, Frist W. Phasing out fee-for-service payment. N Engl J Med. 2013;368:2029–2032. doi: 10.1056/NEJMsb1302322. [DOI] [PubMed] [Google Scholar]
- 7.Hussey PS, Eibner C, Ridgely MS, McGlynn EA. Controlling U.S. health care spending—separating promising from unpromising approaches. N Engl J Med. 2009;361:2109–2111. doi: 10.1056/NEJMp0910315. [DOI] [PubMed] [Google Scholar]
- 8.Mechanic RE, Altman SH. Payment reform options: episode payment is a good place to start. Health Aff. 2009;28(2):w262–w271. doi: 10.1377/hlthaff.28.2.w262. [DOI] [PubMed] [Google Scholar]
- 9.Cutler DM, Ghosh K. The Potential for Cost Savings through Bundled Episode Payments. N Engl J Med. 2012;366(12):1075–1077. doi: 10.1056/NEJMp1113361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hackbarth G, Reischauer R, Mutt A. Collective accountability for medical care – toward bundled Medicare payments. N Engl J Med. 2008;359:3–5. doi: 10.1056/NEJMp0803749. [DOI] [PubMed] [Google Scholar]
- 11.Paulus RA, Davis K, Steele GD. Continuous innovation in health care: implications of the Geisinger experience. Health Aff. 2008;27(5):1235–1245. doi: 10.1377/hlthaff.27.5.1235. [DOI] [PubMed] [Google Scholar]
- 12.Weeks WB, Rauh SS, Wadsworth EB, et al. The unintended consequences of bundled payments. Ann Intern Med. 2013;158(1):62–64. doi: 10.7326/0003-4819-158-1-201301010-00012. [DOI] [PubMed] [Google Scholar]
- 13.Abelson R. In a Bid for Better Care, Surgery with a Warranty. NY Times. 2007 May 17; [Google Scholar]
- 14.Lee TH. Pay for performance, version 2.0? N Engl J Med. 2007;357:531–533. doi: 10.1056/NEJMp078124. [DOI] [PubMed] [Google Scholar]
- 15.Casale AS, Paulus RA, Selna MJ, et al. “ProvenCareSM”: a provider-driven pay-for-performance program for acute episodic cardiac surgical care. Ann Surg. 2007;246:613–623. doi: 10.1097/SLA.0b013e318155a996. [DOI] [PubMed] [Google Scholar]
- 16.Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011;365:777–779. doi: 10.1056/NEJMp1105963. [DOI] [PubMed] [Google Scholar]
- 17.Mechanic RE. Lessons learned preparing for Medicare bundled payments. N Engl J Med. 2012;367:1873–1875. doi: 10.1056/NEJMp1210823. [DOI] [PubMed] [Google Scholar]
- 18.Yount KW, Turrentine FE, Lau CL, Jones RS. Putting the value framework to work in surgery. J Am Coll Surg. 2015 Apr;220(4):596–604. doi: 10.1016/j.jamcollsurg.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 19.Carraway RL, Jenkins R. Quick-start guide for Crystal Ball. Darden Case Collection: UVA-QA-0658 [Google Scholar]
- 20.Sokolovic E, Schmidlin D, Schmid ER, et al. Determinants of costs and resource utilization associated with open heart surgery. Eur Heart J. 2002;23:574–578. doi: 10.1053/euhj.2001.3031. [DOI] [PubMed] [Google Scholar]
- 21.Riordan CJ, Engoren M, Zacharias A, et al. Resource utilization in coronary artery bypass operation: does surgical risk predict cost? Ann Thorac Surg. 2000;69:1092–1097. doi: 10.1016/s0003-4975(99)01562-3. [DOI] [PubMed] [Google Scholar]
- 22.Osnabrugge RLJ, Speir A, Head S, et al. Costs for Surgical Aortic Valve Replacement According to Preoperative Risk Categories. Annals Thorac Surg. 2013;96(2):500–506. doi: 10.1016/j.athoracsur.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 23.Osnabrugge RL, Speir AM, Head SJ, et al. Prediction of costs and length of stay in coronary artery bypass grafting. Ann Thorac Surg. 2014;98(4):1286–1293. doi: 10.1016/j.athoracsur.2014.05.073. [DOI] [PubMed] [Google Scholar]
- 24.Teisberg EO, Wallace S. Creating a high-value delivery system for health care. Semin Thorac Cardiovascular Surg. 2009;21(1):35–42. doi: 10.1053/j.semtcvs.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Dafny L. Hospital industry consolidation—still more to come? N Engl J Med. 2014;370(3):198–199. doi: 10.1056/NEJMp1313948. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez E. Antitrust enforcement in health care—controlling costs, improving quality. N Engl J Med. 2014;371(24):2245–2247. doi: 10.1056/NEJMp1408009. [DOI] [PubMed] [Google Scholar]
- 27.Carreyrou J. Nonprofit Hospitals Flex Pricing Power. Wall St J. 2008 Aug 28;:1A. [Google Scholar]
- 28.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2005. [Google Scholar]
- 29.Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011 Sep;89(9):46–61. [PubMed] [Google Scholar]