Abstract
Patients with chronic kidney disease (CKD) experience other diseases such as cardiovascular disease (CVD) and retinopathy. The purpose of this study was to assess whether retinopathy predicts future CVD events in a subgroup of the participants of the Chronic Renal Insufficiency Cohort (CRIC) study. In this ancillary investigation, 2605 CRIC participants were invited to participate and non-mydriatic fundus photographs were obtained in 1936 subjects. Using standard protocols, presence and severity of retinopathy (diabetic, hypertensive or other) and vessel diameter caliber were assessed at a central photograph reading center by trained graders masked to study participant’s information. Patients with a self-reported history of cardiovascular disease were excluded. Incident CVD events were adjudicated using medical records. Kidney function measurements, traditional and non-traditional risk factors for CVD were obtained. Presence and severity of retinopathy were associated with increased risk of development of any CVD in this population of CKD patients, and these associations persisted after adjustment for traditional risk factors for CVD. We also found a direct relationship between increased venular diameter and risk of development of CVD; however, the relationship was not statistically significant after adjustment for traditional risk factors. In conclusion, presence of retinopathy was associated with future CVD events, suggesting that retinovascular pathology may be indicative of macrovascular disease even after adjustment for renal dysfunction and traditional CVD risk factors. Assessment of retinal morphology may be valuable in assessing risk of CVD in patients with CKD, both clinically and in research settings.
Keywords: Retinopathy, chronic kidney disease, cardiovascular disease
Introduction
Patients with chronic kidney disease (CKD) experience high rates of co-morbid illnesses including cardiovascular disease (CVD) and retinopathy (1). We have previously reported the results of the Retinopathy in Chronic Renal Insufficiency (RCRIC) study on the cross-sectional association between retinopathy and cardiovascular disease (CVD) (1) in a group of patients with chronic kidney disease (CKD) enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study (2, 3). In this cohort, the prevalence of CVD was higher in patients with retinopathy and in patients with retinal venular dilation, and this association remained significant after controlling for traditional risk factors for CVD (1). This current paper evaluates the association between retinopathy and venular dilatation and the risk of subsequent CVD events.
Methods
The CRIC study design and methods have been reported previously (2, 3). Participants for the RCRIC study, an ancillary investigation of the CRIC study, were recruited at 6 of the 7 CRIC clinical centers. All 2605 CRIC participants from these 6 sites were invited to participate in the RCRIC study. From June 2006 to May 2008, 1936 participants enrolled in the RCRIC study and eye fundus photography were obtained at median time of 2 years after the CRIC baseline. The protocol was approved by an Institutional Review Boards at each of the participating institutions and all participants provided written informed consent.
Trained non-ophthalmic personnel obtained all photographs. To induce a physiologic, non-pharmacologic dilatation of the pupils, participants were seated in a darkened room for five minutes. Forty-five degree digital, color fundus photographs were obtained using a Canon CR-DGI, Non-Mydriatic Retinal Camera (Canon Inc, Tokyo Japan). Two photographs, one centered on the macula and the other on the optic disc, were obtained from each eye. A participant was included in the analysis if either the disc or macula photographs of one eye were of sufficient quality for classification by fundus reading center staff.
Trained graders and a retinal specialist, without knowledge of the participant’s clinical and demographic information, assessed all digital fundus photographs at the RCRIC Fundus Photograph Reading Center at the University of Pennsylvania. Fundus pathology including presence and severity of retinopathy of any cause (diabetic, hypertensive, or other) and measurement of the diameter of the major retinal vessels were assessed. Because the readers were unaware of the diabetic or hypertensive status of the participants, retinopathy was evaluated without assumption of cause.
The Atherosclerosis Risk in Communities (ARIC) fundus photographic (4) and the Early Treatment of Diabetic Retinopathy (ETDRS) grading protocols (5) were used to grade retinopathy due to diabetes mellitus, systemic hypertension, and other conditions. The Multi-Ethnic Study of Atherosclerosis (MESA) protocol was used for the evaluation of macular edema (6). These grading protocols have been previously validated in diabetic and non-diabetic populations. A single masked reader, using standard protocols with standardized photographic field definitions, evaluated digital fundus photographs displayed on color-calibrated monitors.
Retinal abnormalities were graded by comparing participant images to standard photographs. An EDTRS severity score was assigned for each eye (5). The ETDRS severity score is on an ordinal scale instead of a continuous scale. Scores were classified as normal (<14), very mild non-proliferative retinopathy (NPR) (14 to 20), non-proliferative retinopathy (35 to 53); and proliferative retinopathy (PR) (>60). The score of the eye with more advanced retinopathy or the score from a single eye, if only one eye was available, was used as the score of the participant. A total of 116 participants (6%) had photographs in which neither eye could be assessed. Among them, 38 participants had photographs in which retinopathy features could not be assessed due to poor image quality. The remaining 78 participants had photographs that were blurry or dark, and although some mild retinopathy features could be assessed, an accurate grading could not be assigned because more advanced and subtle retinopathy features were not discernible.
As previously reported (7), grade re-grade reliability was assessed in 200 RCRIC participants. Weighted Kappa for participant’s ETDRS score was 0.77 (95% CI: 0.67–0.88), a value consistent with the reproducibility assessment reported by the ETDRS study (5).
Image measurements of vascular arteriolar and venular calibers were performed according to the ARIC protocol, using interactive vessel analysis (IVAN) software developed at the University of Wisconsin (4). Vessels were measured within an annulus spanning 0.5 to 1 disc diameter from the edge of the disc. Graders identified major arterioles and venules and chose segments most suitable for measurement. The diameter of up to 6 arterioles and 6 venules were averaged (4).
CRIC participants were queried about any cardiovascular events at the time of enrollment to the CRIC study. This information was not ascertained by investigating patient’s medical records. Subsequently, during semiannual CRIC study visits participants were queried again about possible cardiovascular events, onset of end-stage kidney disease (ESRD), all hospitalizations, and a selected set of diagnostic tests/procedures. ICD-9 discharge codes were recorded for all hospitalizations. When codes included any from a pre-selected list suggesting a cardiovascular event (congestive heart failure (CHF), myocardial infraction (MI), stroke, atrial fibrillation (AF), and peripheral arterial disease (PAD)), or participants died during a hospitalization, medical records were retrieved for detailed review using event-specific criteria. Two physicians performed these reviews and classified each hospitalization with respect to the probability of the events of CHF, MI, stroke, AF. Trained study staff reviewed medical records classified with ICD-9 codes that suggested PAD and abstracted data onto a study form.
The criteria used for adjudication of CHF were based on: clinical symptoms, radiographic evidence of pulmonary congestion, physical examination of the heart and lungs, central venous hemodynamic monitoring data, and echocardiographic imaging. The criteria for MI were based on symptoms of angina, cardiac biomarkers, and electrocardiographic data. The criteria for AF were based on electrocardiographic data, rhythm strips, and selected medical record notes. Two neurologists reviewed all hospitalizations suggestive of stroke. The criteria for stroke were based on neurological symptoms, tests/procedures including Magnetic Resonance Imaging of the brain, Computerized Axial Tomography of the brain, transthoracic echocardiographic and trans-esophageal echocardiographic imaging, and any history of prior stroke. The guidelines for MI were based on symptoms of angina, cardiac biomarkers, and electrocardiographic data. The guidelines for AF were based on electrocardiographic data, rhythm strips, and selected medical record notes. Hypertension was defined as either systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or use of antihypertensive medications. Diabetes was defined as either fasting glucose ≥126 mg/dl, random glucose ≥200 mg/dl, or use of insulin or anti-diabetic medication.3 ESRD was defined as the initiation of chronic dialysis or kidney transplantation, and ascertainment of ESRD was supplemented by linkage to the US Renal Data System. Estimated glomerular filtration rate (eGFR) was calculated using the CRIC eGFR equation (8).
Only the 1245 patients who did not report a history of CVD at baseline were considered at risk of developing a CVD event. Participant’s characteristics at the time of fundus photography were analyzed for all participants, and stratified by diabetes status. Data values from the annual visit closest in time to fundus photography were used as baseline characteristics for the RCRIC study. The associations between retinopathy and incidence of each cardiovascular condition (AF, CHF, stroke, MI, and PAD), as well as, a composite of all conditions with the exception of AF, termed “any CVD”, was assessed using Kaplan-Meier curves and Cox proportional hazards models to estimate hazard ratios (HR) and their 95% confidence intervals (95% CI). AF was excluded from the composite of any CVD because it is most probably caused by a different pathophysiological pathway. P-values were calculated for any difference between retinopathy severity categories, and also for linear trends across categories among subjects with gradable photographs. Multivariate models were adjusted by traditional risk factors for CVD including age, sex, low density lipoprotein, high density lipoprotein, previous CVD history, systolic blood pressure, smoking status (never/former/current), diabetes, hypertension, hemoglobin A1C, eGFR and log-transformed 24 hour urine protein. Similar analyses were performed for the association between any retinopathy or baseline retinal vascular calibers (categorized into quartiles) and any CVD (the composite cardiovascular outcome), for all participants together, and then, after a stratification by diabetes status. All data analyses were performed in SAS v9.4 (SAS Institute Inc., Cary, NC), and two-sided p-value <0.05 was considered to be statistically significant.
Results
Characteristics of the participants recruited have been described before (9). All 1245 participants who had baseline retinal photographs and did not self-report any CVD at baseline were included in the study. Out of these participants 1081 had retinal vascular caliber measurements. Median age at the time of photography was 60 years (range: 22 to 77 years), 53.1% were white, 40.4% were black, 52.3% were male, and 495 (39.8%) had diabetes. At baseline, 896 (72.0%) participants had no retinopathy, 77 (6.2%) had mild non-proliferative retinopathy, 135 (10.8%) had non-proliferative retinopathy, 87 (7.0%) had proliferative retinopathy, and the remaining 50 (4.0%) were not assessed due to poor image quality. Table 1 shows participant’s characteristics overall and by presence or absence of diabetes mellitus.
Table 1.
Patient characteristics at the time of fundus photography by diabetes status
| Characteristic | Non-diabetics (n=750) | Diabetics (n=495) | Total (n=1245) | |
|---|---|---|---|---|
| Age (years) | Mean (SD) | 56.5 (11.8) | 59.5 (10.4) | 57.7 (11.4) |
| Sex | Male | 378 (50.4%) | 273 (55.2%) | 651 (52.3%) |
| Female | 372 (49.6%) | 222 (44.8%) | 594 (47.7%) | |
| Smoking status | Non-smoker | 410 (54.7%) | 230 (46.5%) | 640 (51.4%) |
| Former smoker | 271 (36.1%) | 209 (42.2%) | 480 (38.6%) | |
| Current smoker | 69 (9.2%) | 56 (11.3%) | 125 (10.0%) | |
| Hypertension | Yes | 589 (78.5%) | 464 (93.7%) | 1053 (84.6%) |
| Systolic Blood Pressure (mmHg) | Mean (SD) | 121.8 (19.0) | 130.0 (21.4) | 125.0 (20.4) |
| High-density Lipoprotein (mg/dL) | Mean (SD) | 52.2 (17.5) | 46.8 (14.1) | 50.1 (16.4) |
| Low-density Lipoprotein (mg/dL) | Mean (SD) | 110.3 (31.3) | 93.1 (31.7) | 103.5 (32.5) |
| Triglycerides (mg/dL) | Mean (SD) | 143.2 (107.2) | 151.9 (105.3) | 146.7 (106.5) |
| Hemoglobin A1C (%)a | Mean (SD) | 5.6 (0.49) | 7.3 (1.50) | 6.3 (1.33) |
| eGFR (ml/min per 1.73 m2) | Mean (SD) | 50.4 (19.7) | 43.1 (17.9) | 47.6 (19.3) |
| 24-h Urine Protein (g/24H) | Mean (SD) | 0.55 (1.25) | 0.93 (1.71) | 0.70 (1.46) |
| Retinal Level | No NPR | 658 (87.7%) | 238 (48.1%) | 896 (72.0%) |
| Mild NPR | 49 (6.5%) | 28 (5.7%) | 77 (6.2%) | |
| NPR | 20 (2.7%) | 115 (23.2%) | 135 (10.8%) | |
| PR | 7 (0.9%) | 80 (16.2%) | 87 (7.0%) | |
| Ungradable | 16 (2.1%) | 34 (6.9%) | 50 (4.0%) | |
| Arteriole diameter (μm) | Mean (SD) | 148.6 (14.0) | 151.0 (14.0) | 149.4 (14.1) |
| Venule diameter (μm) | Mean (SD) | 218.0 (21.8) | 221.6 (23.9) | 219.3 (22.6) |
| Arteriole/Venule ratio | Mean (SD) | 0.68 (0.063) | 0.69 (0.073) | 0.69 (0.067) |
Hemoglobin A1C was not available at baseline fundus photography, and therefore, the CRIC baseline was used.
NPR=Non-Proliferative Retinopathy, PR=Proliferative Retinopathy, SD=Standard Deviation, eGFR=Estimated Glomerular Filtration Rate
Among participants with gradable fundus photographs, retinopathy was present in 223 (48%) of 461 participants with diabetes compared to 76 (10%) of 734 participants without diabetes (p<0.001), and 283 (28%) of 1008 participants with hypertension compared to 16 (10%) of 187 without hypertension (p<0.001). Among 161 participants with neither diabetes mellitus nor hypertension, 3 (2%) had mild retinopathy, and another 4 had ungradable photographs.
During a median follow up of 5.01 years, 103 (8.3%) of the 1245 participants developed one or more cardiovascular events. They included 67 AF events, 52 CHF events, 22 stroke events, 32 MI events, and 17 peripheral arterial disease. New any CVD events were more common in participants with diabetes; 62 (12.5%) of 495 persons with diabetes and 41 (5.5%) of 750 persons without diabetes had a new any CVD event.
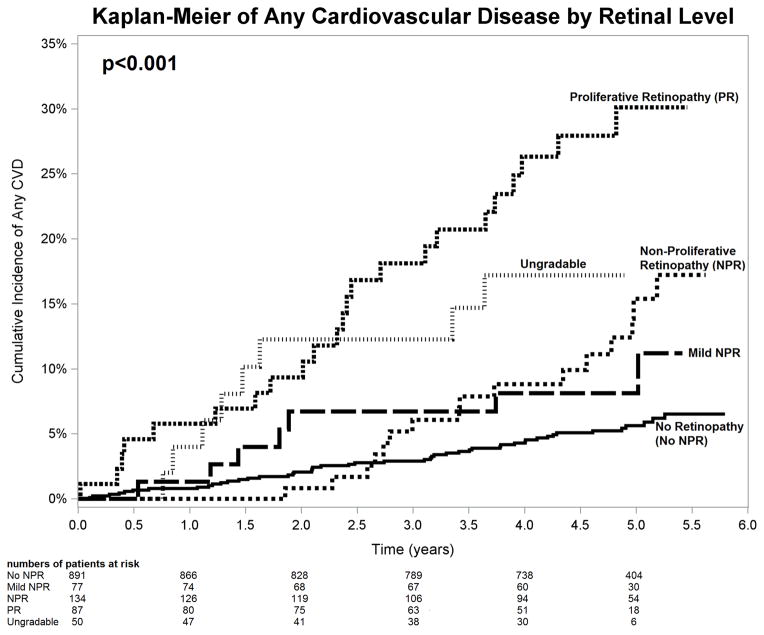
Retinopathy was associated with increased risk of development of new any CVD event in the univariate analysis (p<0.001; Table 2, Figure 1). Although less strong, this association remained statistically significant after adjustment for other CVD risk factors such as age, LDL, HDL, systolic pressure, urinary protein, eGFR, sex, diabetes, hypertension, smoking (multivariate analysis, p=0.002; Table 2). Presence of any retinopathy (mild NPR, NPR or PR) was significantly associated with increased risk of any CVD (HR 1.76 (1.03, 2.99), p=0.02, Table 2).
Table 2.
Association of retinopathy and retinal vascular calibers with any cardiovascular disease
| Characteristicd | # of events/N (%) | Univariate | Multivariatee | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |||
| Retinal Level | No NPR | 48/896 (5%) | 1 | <0.001a <0.001b |
1 | 0.002a 0.008b |
| Mild NPR | 7/77 (9%) | 1.76 (0.80, 3.89) | 1.20 (0.47, 3.08) | |||
| NPR | 16/135 (12%) | 2.40 (1.36, 4.22) | 1.33 (0.64, 2.75) | |||
| PR | 23/87 (26%) | 6.05 (3.68, 9.96) | 3.40 (1.71, 6.78) | |||
| Ungradable | 9/50 (18%) | 4.22 (2.07, 8.61) | 3.14 (1.40, 7.06) | |||
| Any Retinopathy | No Retinopathy | 48/896 (5%) | 1 | <0.001a <0.001c |
1 | 0.02a 0.04c |
| Any Retinopathy | 46/299 (15%) | 3.18 (2.12, 4.77) | 1.76 (1.03, 2.99) | |||
| Ungradable | 9/50 (18%) | 4.20 (2.06, 8.57) | 2.92 (1.31, 6.53) | |||
| Arteriole diameter quartile (μm) | 1 (≤140) | 20/272 (7%) | 1 | 0.86a 0.91b |
1 | 0.61a 0.69b |
| 2 (>140, ≤149) | 15/269 (6%) | 0.76 (0.39, 1.49) | 0.59 (0.28, 1.26) | |||
| 3 (>149, ≤158) | 19/270 (7%) | 0.94 (0.50, 1.76) | 0.78 (0.38, 1.60) | |||
| 4 (>158) | 20/270 (7%) | 0.98 (0.52, 1.81) | 0.78 (0.37, 1.62) | |||
| Venule diameter quartile (μm) | 1 (≤204) | 14/271 (5%) | 1 | 0.04a 0.009b |
1 | 0.49a 0.12b |
| 2 (<204, ≤218) | 13/270 (5%) | 0.93 (0.44, 1.98) | 1.23 (0.53, 2.83) | |||
| 3 (>218, ≤234) | 19/270 (7%) | 1.36 (0.68, 2.72) | 1.45 (0.64, 3.28) | |||
| 4 (>234) | 28/270 (10%) | 2.08 (1.10, 3.96) | 1.79 (0.83, 3.88) | |||
| Arteriole-Venule ratio quartile | 1 (≤0.64) | 28/271 (10%) | 1 | 0.02a 0.02b |
1 | 0.23a 0.37b |
| 2 (>0.64, ≤0.68) | 19/270 (7%) | 0.67 (0.37, 1.20) | 0.83 (0.43, 1.62) | |||
| 3 (>0.68, ≤0.73) | 10/270 (4%) | 0.33 (0.16, 0.67) | 0.42 (0.18, 0.97) | |||
| 4 (>0.73) | 17/270 (6%) | 0.58 (0.32, 1.07) | 0.89 (0.45, 1.76) | |||
P value for evaluation of differences between categories.
P value for evaluation of linear trend across categories, excluding ungradable.
P value for evaluation of a difference between any retinopathy and no retinopathy.
Retinal level based on worst eye, retinal vascular calibers based on mean of both eyes.
Adjusted by age, gender, low-density lipoprotein, high-density lipoprotein, systolic blood pressure, smoking status, diabetes, hypertension, triglycerides, hemoglobin A1C, eGFR, and log of 24-hour urine protein.
CI confidence interval; HR hazard ratio.
Figure 1.
Kaplan Meier estimates of cumulative incidence of any CVD (cardiovascular disease) by retinal level. NPR=Non proliferative retinopathy, PR=Proliferative retinopathy.
We detected no significant association between retinopathy and risk of development of AF (Table 3). For CHF and peripheral arterial disease, although strong associations were detected in the univariate analysis (p<0.001, Table 3) these associations were not statistically significant after adjustment for other CVD risk factors (p= 0.34 for CHF, and p=0.07 for peripheral arterial disease). Among the outcomes that we studied, the risk of stroke showed the strongest relationship with retinopathy. Participants with proliferative retinopathy had a 9.09 (95% CI 2.18, 37.8) times higher risk of future stroke than participants without retinopathy (Table 3). For MI and peripheral arterial disease, participants with proliferative retinopathy had a 5.43 (95% CI 1.37, 21.5) and 5.73 (95% CI 1.14, 28.9) times higher risk than participants without retinopathy.
Table 3.
Association of retinal level with cardiovascular diseases
| Univariate | Multivariated | |||||
|---|---|---|---|---|---|---|
| Condition | Retinal Levelc | # of events/N (%) | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Atrial fibrillation (n=67) | No NPR | 52/896 (6%) | 1 | 0.76a 0.42b |
1 | 0.49a 0.26b |
| Mild NPR | 5/77 (6%) | 1.14 (0.46, 2.86) | 1.26 (0.49, 3.22) | |||
| NPR | 6/135 (4%) | 0.81 (0.35, 1.88) | 0.59 (0.22, 1.59) | |||
| PR | 3/87 (3%) | 0.63 (0.20, 2.03) | 0.51 (0.14, 1.87) | |||
| Ungradable | 1/50 (2%) | 0.37 (0.05, 2.68) | 0.29 (0.04, 2.13) | |||
| Congestive heart failure (n=52) | No NPR | 26/896 (3%) | 1 | 0.001a <0.001b |
1 | 0.34a 0.63b |
| Mild NPR | 4/77 (5%) | 1.85 (0.65, 5.31) | 1.71 (0.56, 5.26) | |||
| NPR | 8/135 (6%) | 2.18 (0.99, 4.82) | 0.48 (0.15, 1.49) | |||
| PR | 9/87 (10%) | 4.02 (1.88, 8.59) | 1.11 (0.39, 3.18) | |||
| Ungradable | 5/50 (10%) | 3.96 (1.52, 10.3) | 1.68 (0.53, 5.26) | |||
| Stroke (n=22) | No NPR | 7/896 (1%) | 1 | <0.001a <0.001b |
1 | 0.04a 0.004b |
| Mild NPR | 2/77 (3%) | 3.39 (0.70, 16.3) | n/ae | |||
| NPR | 4/135 (3%) | 4.04 (1.18, 13.8) | 3.17 (0.70, 14.4) | |||
| PR | 7/87 (8%) | 11.7 (4.10, 33.4) | 9.09 (2.18, 37.8) | |||
| Ungradable | 2/50 (4%) | 5.77 (1.20, 27.9) | 6.49 (1.14, 37.0) | |||
| Myocardial infarction (n=32) | No NPR | 12/896 (1%) | 1 | <0.001a <0.001b |
1 | 0.06a 0.01b |
| Mild NPR | 2/77 (3%) | 2.03 (0.45, 9.05) | 2.26 (0.48, 10.6) | |||
| NPR | 8/135 (6%) | 4.89 (2.00, 12.0) | 5.49 (1.54, 19.5) | |||
| PR | 7/87 (8%) | 6.66 (2.62, 16.9) | 5.43 (1.37, 21.5) | |||
| Ungradable | 3/50 (6%) | 5.23 (1.47, 18.6) | 5.36 (1.05, 27.4) | |||
| Peripheral arterial disease (n=17) | No NPR | 5/896 (1%) | 1 | <0.001a <0.001b |
1 | 0.07a 0.06b |
| Mild NPR | 0/77 (0%) | n/ae | n/ae | |||
| NPR | 2/135 (1%) | 2.83 (0.55, 14.6) | 1.37 (0.20, 9.51) | |||
| PR | 7/87 (8%) | 16.5 (5.23, 52.2) | 5.73 (1.14, 28.9) | |||
| Ungradable | 3/50 (6%) | 12.9 (3.06, 54.2) | 7.30 (1.47, 36.3) | |||
P value for evaluation of differences between retinal level categories, including ungradable.
P value for evaluation of linear trend across retinal level categories, excluding ungradable.
Based on worst eye.
Adjusted by age, gender, low-density lipoprotein, high-density lipoprotein, systolic blood pressure, smoking status, diabetes, hypertension, triglycerides, hemoglobin A1C, eGFR, and log of 24-hour urine protein.
Not estimable due to no events in this category. For multivariate analysis of stroke, 2 events in mild NPR were not included in the model because these 2 patients had a missing value in one or more covariates.
CI confidence interval; HR hazard ratio; NPR Non-proliferative retinopathy; PR Proliferative retinopathy.
No significant association was observed between arteriolar diameter and increased risk of any CVD in either univariate or adjusted analyses. Larger venular diameter, on the other hand, was associated with increased risk of any CVD event in the univariate analysis (p=0.009 for linear trend, Table 2). Similar results were observed for the arteriole-venule ratio, although in this case the risk decreased with larger arteriole-venule ratio because a larger venule value in the denominator resulted in a smaller ratio (p=0.02, Table 2). However, these associations were not statistically significant in the multivariate analysis of venous diameter (p=0.12) and arteriole-venule ratio (p=0.37, Table 2)
Table 4 shows the data stratified for the presence or absence of diabetes mellitus. No interaction was observed between diabetes status and the relationship between retinopathy and any CVD (p=0.65, not shown). In other words, the association between retinopathy and risk for any CVD was not significantly different between persons with and without diabetes. However, there was a significant association between larger venous diameter and increased risk of any CVD in persons without diabetes (p=0.02; Table 4) but not in persons with diabetes (p=0.66). The interaction between diabetes and the relationship between venule diameter and any CVD was also not significant (p=0.18, not shown).
Table 4.
Association of retinopathy and retinal vascular calibers with any cardiovascular disease, by diabetes status
| Characteristic | Diabetics | Non-Diabetics | |||||
|---|---|---|---|---|---|---|---|
| # of events/N (%) | HRe (95% CI) | p-value | # of events/N (%) | HRe (95% CI) | p-value | ||
| Any Retinopathy | No Retinopathy | 19/238 (8%) | 1 | 0.38a 0.21b |
29/658 (4%) | 1 | 0.01a 0.75b |
| Any Retinopathy | 38/223 (17%) | 1.54 (0.79, 3.03) | 8/76 (11%) | 1.18 (0.43, 3.20) | |||
| Ungradable | 5/34 (15%) | 1.81 (0.58, 5.65) | 4/16 (25%) | 5.70 (1.77, 18.4) | |||
| Arteriole diameter quartile (μm) | 1 (≤140) | 7/74 (9%) | 1 | 0.22a 0.69c |
13/198 (7%) | 1 | 0.43a 0.95c |
| 2 (>140, ≤149) | 6/99 (6%) | 0.29 (0.08, 1.10) | 9/170 (5%) | 1.02 (0.40, 2.61) | |||
| 3 (>149, ≤158) | 13/101 (13%) | 0.86 (0.32, 2.32) | 6/169 (4%) | 0.44 (0.12, 1.62) | |||
| 4 (>158) | 11/102 (11%) | 0.49 (0.16, 1.49) | 9/168 (5%) | 1.43 (0.51, 3.99) | |||
| Venule diameter quartile (μm) | 1 (≤204) | 6/84 (7%) | 1 | 0.89a 0.66c |
8/187 (4%) | 1 | 0.14a 0.02c |
| 2 (<204, ≤218) | 6/79 (8%) | 1.33 (0.41, 4.38) | 7/191 (4%) | 1.17 (0.34, 3.97) | |||
| 3 (>218, ≤234) | 11/101 (11%) | 0.94 (0.30, 2.98) | 8/169 (5%) | 2.02 (0.62, 6.60) | |||
| 4 (>234) | 14/112 (13%) | 0.86 (0.29, 2.57) | 14/158 (9%) | 3.11 (1.01, 9.59) | |||
| Arteriole-Venule ratio quartile | 1 (≤0.64) | 12/107 (11%) | 1 | 0.17a 0.35c |
16/164 (10%) | 1 | 0.46a 0.19c |
| 2 (>0.64, ≤0.68) | 9/83 (11%) | 0.73 (0.27, 1.98) | 10/187 (5%) | 1.16 (0.45, 3.01) | |||
| 3 (>0.68, ≤0.73) | 4/79 (5%) | 0.47 (0.12, 1.83) | 6/191 (3%) | 0.54 (0.18, 1.61) | |||
| 4 (>0.73) | 12/107 (11%) | 1.74 (0.66, 4.56) | 5/163 (3%) | 0.57 (0.18, 1.85) | |||
P value for evaluation of differences between categories.
P value for evaluation of a difference between any retinopathy and no retinopathy.
P value for evaluation of linear trend across categories, excluding ungradable.
Retinal level based on worst eye, retinal vascular calibers based on mean of both eyes.
Adjusted by age, gender, low-density lipoprotein, high-density lipoprotein, systolic blood pressure, smoking status, diabetes, hypertension, triglycerides, hemoglobin A1C, eGFR, and log of 24-hour urine protein.
CI confidence interval; HR hazard ratio.
Discussion
The burden of retinopathy associated with CKD, especially among persons with diabetes, has been well established. To our knowledge this is the first study to look at the association between retinopathy and future development of a number of cardiovascular diseases in a large cohort of CKD patients with different degrees of well-defined renal function impairment. Our current results show an association between retinopathy and future development of any cardiovascular disease. This association remains robust even after adjustment for well-known risk factors for cardiovascular disease suggesting that presence of retinopathy offers risk information beyond that provided by those established risk factors These results are similar to those of Liew et al (10) who found that retinopathy was associated with increased risk of coronary heart disease mortality in a population-based cohort of persons with and without diabetes.
The association of retinopathy with development of stroke was particularly noteworthy. Patients with proliferative retinopathy had a stroke hazard ratio of 9.09 (95% CI 2.18–37.8) in comparison to patients without retinopathy. Associations between retinal vascular changes and stroke have been previously described (11) and this link is probably due to the fact that retinal vessels share many characteristics with cerebral vessels. Furthermore, our results also show that patients with proliferative retinopathy had a hazard ratio for myocardial infarction of 5.4 in comparison to those without retinopathy suggesting that vascular changes in the retina may have commonalities with vascular pathology in the heart.
We did not find any association between retinal arteriolar diameters and future development of cardiovascular disease. On the other hand, we detected an association between larger venous diameter and development of any CVD in the univariate analysis. Participants within the quartile with the largest venous diameter had a hazard ratio of 2.08 for any CVD in comparison with those within the quartile with the smallest venous diameter even after adjustment for other risk factors. In the multivariate analysis, the odds ratio increased with higher quartile of diameter but the trend was not statistically significant (p=0.12) Retinal venular dilatation has been previously associated with poor glycemic control (12), obesity, inflammation, endothelial dysfunction (13), progression of diabetic retinopathy (14) and genetic markers (15), all conditions that may play a role in the development of CVD.
Although we did not find any significant interaction between diabetes and the relationship between venular diameter and risk of any CVD, our results show that this relationship is only statistically significant in persons without diabetes (Table 4). Diabetes mellitus is known to be associated with venous dilatation and this dilatation predicts progression of retinopathy (16). Possibly, the lack of statistically significant relationship found in our study in participants with diabetes may be due to an effect of diabetes on venous caliber that may modulate the relationship between vasodilatation and any CVD. This absence of a relationship may also be due to our relatively small sample size.
Our results in this cohort of patients with kidney disease are consistent with other population-based studies showing associations between larger retinal venous diameter and coronary heart disease and stroke (17–19). However, a recent study did not detect any significant association between retinal vascular diameter and the development of CKD (20).
Strengths of our study include a relatively large sample size of patients with well characterized CKD, a prospective study design with a median follow up of about 5 years, a standardized ascertainment of outcomes, a high proportion of African Americans, and a standardized protocol for photographic capture and grading system performed by readers masked to participants clinical information. Our findings should be considered in light of the fact that some participants had photographs of poor quality that did not allow for definitive evaluation. A limitation of our study is that baseline CVD was self-reported by the participants.
Acknowledgments
Funding: This study was supported by NIH grant RO1 DK 74151. CRIC was funded by NIDDK cooperative agreements (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902), University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131, Vivian S. Lasko Research Fund, Nina C. Mackall Trust, and Research to Prevent Blindness.
Footnotes
Financial Disclosures:
Dr. Raymond Townsend is a consultant for Medtronic and Janssen. Dr. Lo has received prior research funding from Amgen and ongoing research funding from Sanofi. All other coauthors have no financial conflict of interest regarding the contents of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grunwald JE, Ying GS, Maguire MG, Pistilli M, Daniel E, Alexander J, Whittock-Martin R, Parker CR, Mohler E, Lo JC, Townsend R, Gadegbeku CA, Lash JP, Fink JC, Rahman M, Feldman H, Kusek J, Xie D, Coleman M, Keane MJ The CRIC Study Group. Association Between Retinopathy and Cardiovascular Disease in Patients with Chronic Kidney Disease (From the Chronic Renal Insufficiency Cohort [CRIC] Study) Am J Cardiology. 2012;110:246–253. doi: 10.1016/j.amjcard.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Rahman M, Townsend RR, Wright JT Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. The Chronic Renal Insufficiency Cohort (CRIC) study: Design and methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 3.Lash JP, Go AS, Appel LG, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Andrew Narva A, Robinson N, Teal V, Feldman HI Chronic Renal Insufficiency Cohort (CRIC) Study Group. Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, Sharrett AR, Davis MD, Cai J Atherosclerosis Risk in Communities Study Group. Atherosclerosis Risk in Communities Study Group: Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 5.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs - an extension of the Modified Airlie House classification. ETDRS Report Number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 6.Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, Sharrett AR, Shea S. Diabetic Retinopathy in a Multi-ethnic Cohort in the United States. Am J Ophthalmol. 2006;141:446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunwald JE, Alexander J, Ying GS, Maguire M, Daniel E, Whittock-Martin R, Parker C, McWilliams K, Lo JC, Go A, Townsend R, Gadegbeku CA, Lash JP, Fink JC, Rahman M, Feldman H, Kusek J, Xie D, Jaar BG The CRIC Study Group. Retinopathy and Chronic Kidney Disease in the Chronic Renal Insufficiency Cohort Study (CRIC) Arch Ophthalmol. 2012;130:1136–1144. doi: 10.1001/archophthalmol.2012.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI CRIC Study Investigators. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grunwald JE, Alexander J, Maguire M, Whittock R, Parker C, McWilliams K, Lo JC, Townsend R, Gadegbeku CA, Lash JP, Fink JC, Rahman M, Feldman H, Kusek J, Ojo A The CRIC Study Group. Prevalence of Ocular Fundus Pathology in Subjects with Chronic Kidney Disease: The Chronic Renal Insufficiency Cohort (CRIC) Study. Clin J Am Soc Nephrol. 2010;5:867–873. doi: 10.2215/CJN.08271109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liew G, Wong TY, Mitchell P, Cheung N, Wang JJ. Retinopathy predicts coronary heart disease mortality. Heart. 2009;95:391–394. doi: 10.1136/hrt.2008.146670. [DOI] [PubMed] [Google Scholar]
- 11.Baker ML, Hand PJ, Wang jj, Wong TY. Retinal Signs and Stroke: Revisiting the Link Between the Eye and Brain. Stroke. 2008;39:1371–1379. doi: 10.1161/STROKEAHA.107.496091. [DOI] [PubMed] [Google Scholar]
- 12.Ikram MK, Janssen JA, Roos AM, Rietveld I, Witteman JC, Breteler MM, Hofman A, van Duijn CM, de Jong PT. Retinal vessel diameters and risk of impaired fasting glucose or diabetes: the Rotterdam study. Diabetes. 2006;44:506–510. doi: 10.2337/diabetes.55.02.06.db05-0546. [DOI] [PubMed] [Google Scholar]
- 13.Klein BE, Knudtson MD, Tsai MY, Klein R. The relation of markers of inflammation and endothelial dysfunction to the prevalence and progression of diabetic retinopathy. Wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol. 2009;127:1175–1182. doi: 10.1001/archophthalmol.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy MS, Klein R, Janal MN. Retinal venular diameter as an early indicator of progression to proliferative diabetic retinopathy with and without high-risk characteristics in African Americans with Type 1 diabetes mellitus. Arch Ophthalmol. 2011;129:8–15. doi: 10.1001/archophthalmol.2010.340. [DOI] [PubMed] [Google Scholar]
- 15.Fahi SJ, Sun C, Zhu G, Healey PR, Spector TD, Martin NG, Mitchell P, Wong TY, Mackey DA, Hammond CJ, Andrew T. The relationship between retinal arteriolar and venular calibers is genetically mediated, and each is associated with risk of cardiovascular disease. Invest Ophthalmol and Vis Sci. 2011;52:975–981. doi: 10.1167/iovs.10-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein R, Myers CE, Lee KE, Gangnon R, Klein BE. Changes in retinal vessel diameter and incidence and progression of diabetic retinopathy. Arch Ophthalmol. 2012;130:749–755. doi: 10.1001/archophthalmol.2011.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, Cushman M, Duncan BB. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch Intern Med. 2006;166:2388–2394. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- 18.Ikram MK, de Jong FJ, Bos MJ, Vingerling JR, Hofman A, Koudstaal PJ, de Jong PT, Breteler MM. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006;66:1339–1343. doi: 10.1212/01.wnl.0000210533.24338.ea. [DOI] [PubMed] [Google Scholar]
- 19.McClintic BR, McClintic JI, Bisogniano JD. The relationship between retinal microvascular abnormalities and coronary heart disease: A review. Am J Med. 2010;123:374e1–7. doi: 10.1016/j.amjmed.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabanayagam C, Shankar A, Klein BE, Lee KE, Muntner P, Nieto FJ, Tsai MY, Cruickshanks KJ, Schubert CR, Brazy PC, Coresh J, Klein R. Bidirectional association between retinal vessel diameters and estimated GFR decline: The beaver Dam CKD Study. Am J Kidney Dis. 2011;57:682–691. doi: 10.1053/j.ajkd.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]