Abstract
Malignant tumors are often associated with an elevated fluid pressure due to the abnormal growth of vascular vessels, and thus an increased interstitial flow out of the tumor. Recent in vitro work revealed that interstitial flows critically regulated tumor cell migration within a three dimensional biomatrix, and breast cancer cell migration behavior depended sensitively on the cell seeding density, chemokine availability and flow rates. In this paper, we focus on roles of interstitial flows in modulating heterogeneity of cancer cell motility phenotype within a three dimensional biomatrix. Using a newly developed microfluidic model, we show that breast cancer cells (MDA-MB-231) embedded in a 3D type I collagen matrix exhibit both an amoeboid and a mesenchymal motility, and interstitial flows promote the cell population towards the amoeboid motility phenotype. Furthermore, the addition of exogenous adhesion molecules (fibronectin) within the extracellular matrix (type I collagen) partially rescues the mesenchymal phenotype in the presence of the flow. Quantitative analysis of cell tracks and cell shape shows distinct differential migration characteristics of amoeboid and mesenchymal cells. Notably, the fastest moving cells belong to the subpopulation of amoeboid cells. Together, these findings highlight the important roles of biophysical forces in modulating tumor cell migration heterogeneity and plasticity, as well as the suitability of microfluidic models in interrogating tumor cell dynamics at single-cell and subpopulation level.
Introduction
Interstitial flows are ubiquitous in human tissues. They are driven by the hydrostatic and osmotic pressure differences among the arterial, venous, and lymphatic vessels 1. In healthy tissue, interstitial flow rates are on the order of a few micrometers per second 2. Within malignant tumor, interstitial flow rates can reach as high as 10 μm/s in animal models 2-4, and up to 55 μm/s in human cancer patients 4, 5. A number of dynamically evolving tumor microenvironment factors have been identified to contribute to the elevated interstitial fluid flows, including the continual expansion of tumor mass which builds up the interstitial fluid pressure within the tumor 6, 7, the subsequent abnormal growth of vascular vessels via angiogenesis 8, 9 and/or lymphangiogenesis 10-12, as well as the denser extracellular matrix (ECM) deposited and remodeled by stromal cells with higher hydraulic conductivity 13, 14. Clinically, lymph nodes are known to be the first metastatic sites for many cancer types, including breast 15 and prostate cancers 16. Recognizing that interstitial flows drain towards lymph nodes, an emerging question is: whether and how interstitial flows guide and modulate tumor cell invasion into the lymph nodes 17. Indeed, pioneer work from the Swartz lab has demonstrated that interstitial flows (0.2 and 0.7 μm/s) can spatially redistribute chemokine secretions of breast and glioma tumor cells, and direct tumor cells invasion along the flow direction in a chemokine receptor CCR7/CXCR4 dependent manner using a modified Boyden Chamber model 17, 18.
Tumor cells are known to be heterogeneous (ensemble variability) and plastic (temporal variability) in response to the complex tumor microenvironment 19. In cancer metastasis, only a subpopulation of the tumor cells or rare cells break away from the primary tumor and migrate through the interstitial space, with only a fraction of those eventually establishing a secondary tumor at an ectopic site. Cancer cell heterogeneity and plasticity are also demonstrated through their diverse motility types. Single animal cell migration within a 3D architecture can be broadly categorized into amoeboid and mesenchymal motility phenotypes 20, 21. In amoeboid motility, cells appear rounded in shape, form actin protrusions and dynamically change their shapes to squeeze through pores within the collagen fiber network 22-24. Traction is distributed all around the cell surface through many short-lived adhesive contacts with the ECM 25, 26. In mesenchymal motility, cells appear elongated in shape, climb along the collagen fibers, and proceed by either remodeling or degrading the matrix in an integrin and/or proteolysis dependent manner 27, 28. Traction is exerted through long-lived, polarized and highly localized focal adhesion complexes 29-31. While leukocytes typically exhibit amoeboid motility, and fibroblasts assume mesenchymal motility, cancer cells are known to be able to switch between these two motility types depending on the microenvironment 32, 33. Wolf et al. discovered that fibrosarcoma cells switch from a mesenchymal to amoeboid motility when matrix metalloproteinase (MMPs) was inhibited in both 3D in vitro model and mouse model 32.
For understanding the heterogeneity and plasticity of tumor cell, there is a need for tools that can interrogate cancer cell invasion at single-cell or subpopulation level, and in real time. Although modified Boyden chamber models have played instrumental roles in revealing effects of interstitial flows on molecular mechanism governing tumor cell invasion 17, 18, 34, these results are limited in endpoints and population levels. Recently, microfluidic models have emerged for studying effects of interstitial flows on tumor cell invasion because of their compatibility with optical microscope, making it possible to follow single-cell dynamics in both time and space 35-37. In addition, microfluidic models have the advantage of providing well controlled microenvironments, such as fluid flows within a 3D ECM 38. Current microfluidic models have revealed that flow-guided cell migration depended on a number of critical parameters within the tumor microenvironment, including chemokine receptors, matrix stiffness, cell density, and flow rates 35, 36, 39. In a recent work, Polacheck et al. showed that breast tumor cells (MDA-MB-231) can migrate either along or against the interstitial flow direction depending on cell density and CCR-7 receptors 35. Haessler et al. demonstrated that interstitial flows modulate migration characteristics of a subpopulation of the MDA-MB-231 cells, highlighting the heterogenetic response of tumor cells under the influence of interstitial flows 36. In this article, we focus on the impact of interstitial flows on cell morphology and motility phenotypes.
In our early work on breast cancer cell chemotaxis using a 3D microfluidic device with a gradient generator, we found that MDA-MB-231 cells switched between amoeboid and mesenchymal motility depending on whether or not the system was perfused 40. Inspired by this finding, we carried out a systematic study on the roles of interstitial flows on the morphology and motility of MDA-MB-231 cells using a 3D microfluidic model. In this article, we show that MDA-MB-231 cells embedded in a 3D collagen matrix are more rounded and execute mostly an amoeboid motility in the presence of interstitial flows; while they are more elongated and execute mostly mesenchymal motility in the absence of the flow. The addition of the exogenous adhesion molecule, fibronectin (FN), partially rescues a mesenchymal cell phenotype in the presence of the flow. Our work reveals that interstitial flows modulate cancer cell morphology and motility phenotypes, emphasizing roles of fluid flows in regulating cancer cell migration heterogeneity.
Materials & Methods
Microfluidic device preparation
The master mold for the microfluidic device was made using the standard photolithography technique at the Cornell Nanoscale Science and Technology Facility (CNF). The negative features on the silicon master were made using a two-step etching method. The layer with 5 μm height ridges was etched first, followed by a second layer etching for the 200 μm deep channels. The positive features shown in Figure 1 were made in the PDMS replica from the silicon mold. For the most recent generation of the device, we added two contact lines (10 μm in width and 5 μm in height) on the surface of the flow channel to avoid trapping air bubbles when initially introducing interstitial flows (See Fig. S1) into the flow channel 41. For details of the fabrication methods, please also see reference 37.
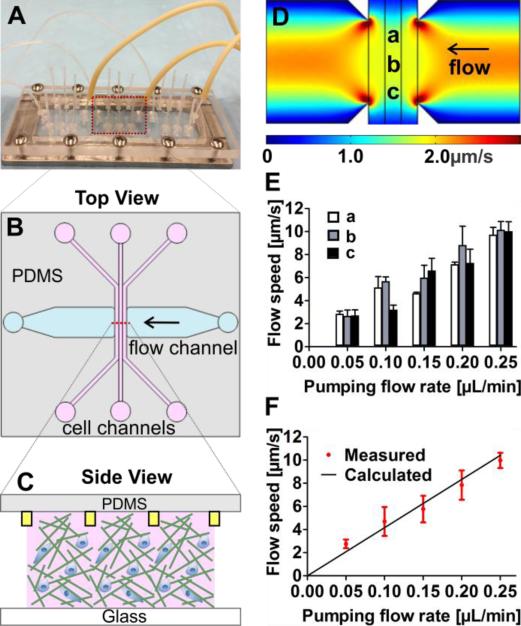
Figure 1. Microfluidic device setup and fluid flow characterization.
(A) Illustration of the microfluidic setup: three identical devices were patterned on a 1 mm thick PDMS membrane and sandwiched between a Plexiglas manifold and a 1” × 3” glass slide. (B) Within each device, cell embedded collagen is seeded in the three parallel cell channels, each with a cross section of 400 μm × 200 μm. Fluid flows are introduced in the horizontal flow channel with a cross section of 3 mm × 200 μm. (C) Collagen is confined within the three cell channels by the contact lines, marked by the yellow rectangles (not to scale), each with a cross section area of 10 μm × 5 μm. (D) Flow speed profile within the three cell channels along the flow path computed by COMSOL Multi-physics. The average speed through the cell channel is approximately 2.0 μm/s. (E) Measured flow speed at three spatial locations that are 1 mm apart (see position a, b, and c as marked in Figure 1D) using a fluorescence recovery after photobleaching (FRAP) method. (F) Measured flow speed in the cell channels is validated against the calculated speed using the known pumping flow rate and channel geometry. The red dots are experimental data, and the solid line (y = 41.7x) is the calculated flow speed.
A critical step to confine collagen successfully within the cell channels using the contact lines is to make sure that the hydrophilicity of the PDMS is optimal. The following steps have been optimized to achieve this goal. Before each experiment, PDMS devices were first made from the silicon mold and then autoclaved. Second, PDMS devices were treated with oxygen plasma (Harrick Plasma Cleaner PDC-001, Harrick Plasma, Ithaca, NY) for 30 seconds on high power mode and left in a laminar hood for 5 hours in room temperature. Finally, the PDMS device was sandwiched between a Plexiglas manifold and a standard 1” × 3” size glass slide, supported by a metal frame at the bottom. After assembling, 0.6% of agarose solution was prepared to fill the void space around the PDMS device to prevent medium from evaporating during cell experiment. In a typical experiment, two microfluidic chips were assembled in parallel and stored at 4°C for 30 minutes before cell seeding.
3D cell culture preparation
MDA-MB-231, a malignant breast tumor cell line, was provided by the Cornell Center of Microenvironment and Metastasis. DMEM medium (Invitrogen, Carlsbad, CA), supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA) and 1% antibiotics (Invitrogen, 100 units penicillin and 100 μg streptomycin) was used. Cells were cultured in a humidified incubator with 5% CO2 and 37°C environment and harvested at 50%-70% confluence for each experiment.
Type I collagen was extracted from rat tails (Pel-Freez, Rogers, AR) using a modified protocol 42 and stored at a concentration of 5 mg/mL in 0.1% acetic acid at 4°C . A volume of 60 μL collagen stock was titrated with 1.32μL 1N NaOH and 20 μL 10X M199 to yield a final pH of approximately 7.4 and then mixed with cell culture solution to a final volume of 200 μL. The final collagen concentration was 1.5 mg/mL. For some experiments, soluble form of fibronectin (human plasma, EMD Millipore, Calbiochem, Billerica, MA) was supplemented into the cell-collagen solution with a final concentration of 100 μg/mL. For all the experiments reported below, a cell concentration of 106 cells/mL was used.
Experimental procedures
First, cell-embedded collagen solution was introduced to fill two or three cell channels in each device (See Figure 1B,C) on an ice pack. The assembled microfluidic chips were then placed in an incubator (100% humidity, 37 °C, 5% CO2) to allow collagen polymerization for 45 minutes. To prevent cells from gravitationally settling down to the bottom of the device, the microfluidic chips were positioned up-side-down for the first 11 minutes and then flipped over for 34 more minutes in the incubator. Second, 37 °C medium was introduced into the empty flow channels until the outlet reservoirs were full, both the inlets and outlets of the flow channel were then plugged with PDMS filled gel loading tips. Third, the microfluidic chip was transferred to the microscope stage enclosed by an environmental control chamber, which is kept at 37 °C with 100% humidity and 5% CO2. The chip was typically left on the stage for an hour for reaching equilibrium before imaging. Finally, interstitial flows were pumped through the flow channel and the cell channels were imaged. We defined t = 0 as the time when the flows and imaging were started, which typically was three hours after the cell seeding. In each experiment, one device was used as control (no flow) and the other two were connected to a syringe pump to introduce interstitial flows of 0.05μL/min (2.0 μm/s). This speed was chosen to represent the high end of interstitial flows in healthy tissue.
Staining for acting filaments
Actin filaments were stained using Actin Cytoskeleton and Focal Adhesion Staining Kit (EMD Millipore). MDA-MB-231 cells were first seeded in 1.5 mg/mL collagen matrix placed in 96-well plate and incubated in a humidified condition with 5% CO2 at 37°C for at least 5 hours to allow cells to spread and attach to the collagen matrix. Cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature and washed twice with 1X wash buffer (1X PBS with 0.05% Tween-20). Cells were then permeated with 0.1% Triton X-100 for 5 minutes and washed twice, followed by applying the blocking solution (1X PBS with 1% BSA) for 40 minutes. TRITC-conjugated phalloidin with 1:100 dilution was applied to the cell culture for 60 minutes at room temperature. The samples were then imaged using a Zeiss LSM 710 confocal microscope after being washed three times.
Fluid flow measurements
Fluorescence recovery after photobleaching (FRAP) method was used to measure the flow speeds through the collagen matrix (see Figure 1D-F and Ref. 37). For each measurement, 1.5 mg/mL collagen solution was first introduced into the three cell channels and polymerized in the same way as discussed. Second, a 10−4M fluorescein sodium (Fisher Scientific, Pittsburgh, PA) solution was pumped into the flow channel, and through collagen matrix using a syringe pump (KDS-230, KD Scientific, Holliston, MA). Third, a focused light spot (~400μm in diameter) was brought to the location of interest (See positions a, b, c in Fig. 1D) for 6-8 seconds using a high magnification lens (40X, NA=0.6, Olympus) together with a xenon lamp (LB-LS-30 Sutter Instrument, Novato, CA, USA). A series of 11 images (on GFP mode) were immediately captured at an interval of 1.5 second using a low magnification lens (4X, NA=0.13, Olympus). Finally, the images were post processed using ImageJ to obtain the flow speeds. This measurement was repeated at five different pumping flow rates (0.05 – 0.25 μL/min).
Imaging and data analysis
An inverted microscope (IX81, Olympus America, Center Valley, PA, USA) with a CCD camera (Orca-ER, Hamamatsu Photonics, Japan) was used for all the experiments. In cell experiments, images at three or more positions of the cell channel were taken using 10X objective (Olympus, NA=0.3) in bright field mode. All the images were taken in the mid-z plane of the channel. Here z refers to the vertical direction. A sequence of 192 images was captured every 5 minutes for a total of 16 hours. t = 0 is defined as the time when the first image was taken, at which the flow was applied.
To quantify cell morphology, the cell shape was fitted to an ellipse outline using a ROI manager tool from ImageJ. The aspect ratio of each cell is defined as the ratio of the major to the minor axis of the ellipse. Cells with aspect ratio smaller than 2.0 were considered as amoeboid cells and otherwise were considered as mesenchymal cells to be consistent with previous work 43.
To quantify cell motility, cell migration trajectories were first tracked using manual tracking in ImageJ. The cell speed (total distance traveled divided by 16 hours of imaging time), x-velocity (displacement along the flow direction divided by 16 hours), persistence (displacement divided by the entire length of the track), x-persistence (displacement in the flow direction divided by the entire length of the track), and mean square displacements (MSDs) were computed using an in house MATLAB program. The x -velocity and x -persistence were converted in all experiments, with positive values indicating motion against the flow and negative values indicating motion along with the flow. To minimize the variations from experiment to experiment, speed was normalized by the average speed of the control group (no flow) and the normalized x-velocity was the average velocity along flow direction subtracted by that of the control group, and divided by the average speed of the control group. We note that although cells embedded within a 3D collagen matrix, and images were taken in the mid-plane along the vertical direction of the cell channel, almost all the tracked cells remained in the field of view during the 16 hour imaging time (See Smovie 1&2). Thus, only 2D trajectories were obtained and presented. Cells migrate faster than a threshold speed of 0.2 μm/min were considered to be motile cells. 60 motile cells were randomly selected from each condition for computing all the motility parameters. Student t-test was performed for two-group analysis using Prism GraphPad. All experiments were repeated three to four times independently.
Results and Discussion
Generation of spatially uniform interstitial flows within a 3D ECM using a microfluidic device
Interstitial flows were generated within a 3D collagen matrix using a microfluidic device developed previously in our lab (see Fig. 1 and also Ref. 37). Briefly, three identical devices were patterned in a 1mm thick PDMS membrane, which was subsequently sandwiched between a glass slide and a Plexiglas manifold (Fig. 1A). In a typical experiment, collagen was first introduced into three parallel cell channels. After the collagen was polymerized, medium was introduced through the flow channel that is perpendicular to the cell channels via a syringe pump (Fig. 1B). The key feature of the device was that it used a contact line pinning technique to confine collagen matrix within the wall-less cell channels (Fig. 1C), such that interstitial flows could run through the collagen matrix without obstructions and were spatially uniform.
The flow within the device was characterized and validated using both computational and experimental methods (Fig. 1D-F). The computed flow speed using a multiphysics program (COMSOL) within the device showed that the flow speed was uniform within the central portion of the three cell channels. The speed variation was less than 8.0% in the central 80% of the cell channels (Fig. 1D). This spatial uniformity of the flow was verified in experimental measurements as shown in Fig. 1E. Here, the interstitial flow speeds at three different spatial locations a, b, and c (as marked in Fig. 1D) within the collagen matrix were obtained using a FRAP technique 37, 44. The measured flow speeds at three spatial locations were essentially the same within experimental uncertainties, confirming the spatial uniformity of the flow speed within the device. Furthermore, the experimentally measured flow rates were validated against the calculated values as shown in Fig. 1F. We note here that the advantage of using the contact line pinning based method is the spatial uniformity of the flow speed within the collagen matrix.
Interstitial flows promote an amoeboid cell morphology and motility
A distinct effect of interstitial flows on MDA-MB-231 cells is that it promotes an amoeboid over mesenchymal cell motility phenotype (Fig. 2 and Smovies 1 & 2). Initially, almost all the cells (or ~100%) were round or in an amoeboid shape at the moment they were mixed with the un-polymerized collagen. In the absence of flow, most cells appeared to be round at t = 0. As time evolved, cells stretched, adhered to the collagen fibers, became elongated, and executed a mesenchymal motility phenotype (Fig. 2A, Smovie 1, and Fig. S2A). We observed that about 30% of the MDA-MB-231 cells dynamically evolved from amoeboid to mesenchymal motility within a time duration of 16 hours. In the presence of flow, most cells remained round and executed an amoeboid motility phenotype (Fig. 2A, Smovie 2 and Fig. S2B). The distribution of the cell aspect ratio was found to have a distinct shift towards smaller aspect ratio values for the case with the flow in comparison to the case with no flow at t = 16 hr (Fig. 2B). At all time points between t = 0 and 16 hr, we found that cells in the absence of flow had a larger percentage of mesenchymal cells than those with flow (Fig. 2C). For example, the percentage of mesenchymal cells at t = 16 hr was 58 ± 4% with no flow, in contrast to 27 ± 4% with a flow of 2 μm/s. It should be noted that the initial fraction of about 25% of the mesenchymal cells were a result of the 3 hours time difference between the time of cell seeding and the time of imaging.
Figure 2. Interstitial flows promote an amoeboid cell morphology and motility.
(A) Micrographs of MDA-MB-231 cells embedded in a collagen matrix at two time points (t = 0 and t = 16 hr) in the absence (top row) and presence (bottom row) of the flow. The flow speed is 2 μm/s (or a flow rate of 0.05 μL/min). (B) The distribution of the aspect ratio of MDA-MB-231 cells in the absence (black) and presence (blue) of the flow at t = 16 hr. Cells with aspect ratio (a/b) less than 2.0 are defined as amoeboid cells, and cells with aspect ratio greater than 2.0 are defined as mesenchymal cells. (C) Percentage of mesenchymal cells versus time in the absence and presence of flow. 600 cells from three independent experiments are used towards the computation of each data point. (D) Micrographs of amoeboid and mesenchymal cells taken at one z-plane. The red color shows actin staining. All scale bars are 50μm.
Cell aspect ratio (long axis divided by short axis of the cell shape, see insert of Fig. 2B) was used for quantifying cell morphology. Cells with aspect ratio less than 2.0 were defined as amoeboid cells, and larger than 2.0 as mesenchymal cells. This definition was derived from our own observations and is consistent with previous literature 32, 43, 45. In our own experiments, round or amoeboid cells extended their protrusions in all directions, formed short lived adhesion to the fibers, and squeezed through the collagen matrix when finding a suitable path (Smovie 3). The elongated or mesenchymal cells, on the other hand, were highly polarized. They formed long-lived adhesions and climbed along the fibers (Smovie 4). Additionally, we visualized spatial distribution of actin filaments within cells of these two types using actin staining. Clearly, the round cells displayed an amoeboid phenotype where the actin filaments were mostly distributed around the cell peripherals and the elongated cells demonstrated a mesenchymal phenotype where the actin filaments formed a highly polarized bundle (Fig. 2D). In the previous literature, aspect ratio of 2.0 was used as a threshold for the definition of amoeboid (round) versus mesenchymal (elongated) cells migrating within a 3D matrix for a variety of cell types, including, breast tumor cells (MDA-MB-231) 32, melanoma cells (A375M2) 45, and fibrosarcoma cells (HT-1080) 43. This definition was based on the observations of cell motility types, proteolytic activities and the co-cluster of integrins 32, GTPase Rac or Rho-kinase signaling activities 45, and localization of actin or focal adhesion molecule paxillin 43. For the remaining of the paper, we will refer to round cells (or cells with aspect ratio less than 2.0) as amoeboid cells, and elongated cells (or cells with aspect ratio larger than 2.0) as mesenchymal cells.
Mesenchymal to amoeboid transitions have been reported in cancer cell invasion in previous literature in response to the changes in their 3D microenvironments 32, 46, 47. Wolf et al. discovered that blocking pericellular proteolysis of HT-1080 fibrosarcoma cells and MDA-MB-231 carcinoma cells embedded in collagen encouraged mesenchymal to amoeboid transition 32. Yamazaki et al. showed that Rac signaling was correlated with elongated cell morphology, and inhibiting Rac1 led to a mesenchymal to amoeboid transition of HT1080 cells in 3D collagen matrix 47. Sahai et al. demonstrated that Rho was correlated with round cell morphology, and inhibiting Rho signaling of the A375m2 cells in a 3D environment, in contrast, promoted elongated mesenchymal phenotype 48. Kumar's group reported a mesenchymal to amoeboid transition by modulating the matrix stiffness of the ECM. More specifically, they found that U373-MG human glioma cells changed from a mesenchymal to an amoeboid morphology when the collagen matrix was stiffened by the addition of the agarose gel 46. Here, our experimental results revealed that interstitial flows promoted an amoeboid phenotype of MDA-MB-231 cells. It remains to be explored whether other cell lines also undergo motility phenotype transitions under the influences of interstitial flows.
In contrast to the amoeboid cell migration, mesenchymal cells require integrin-based adhesion to migrate. It is known that mesenchymal cells form long-lived adhesions with the ECM fiber bundles, which trigger the downstream signaling that activates actin remodeling and thus cell migration 49. We hypothesized that interstitial flows modulate cell motility through adhesion. Fibronectin (FN) is an important adhesion molecule in mediating mammalian cell migration 50. In the absence of flow, cell secreted FNs assemble into fibrillar form, and bound with collagen, which promote a mesenchymal cell phenotype. In the case of flow, the interstitial-flow-induced amoeboid cell motility was likely caused by the lack of assembled endogenous adhesion molecules such as FN. More specifically, the flows carried away the cell-secreted adhesion molecules before they were assembled into fibrils and anchored to the collagen fibers. To test this hypothesis, we carried out experiments to investigate the roles of exogenous FN on cell morphology and motility in the absence/presence of the flows.
Exogenous FN promotes a mesenchymal cell phenotype
To study the cooperative roles of interstitial flows and adhesion molecules on breast tumor cell motility phenotype, we investigated cell aspect ratio under four different flow and FN conditions. Overall, cells were more elongated in the presence of FN than those without (Fig. 3A). Cell aspect ratio distribution shifted distinctively towards larger aspect ratio values in the presence of FN (Fig. 3B) in the case of no flow. Furthermore, the percentage of mesenchymal cells was significantly higher with FN than no FN at all time points (Fig. 3C). For example, the percentage of mesenchymal cells at t = 16 hr was 76 ± 4% with FN, in contrast to 58 ± 4% with no FN. In the presence of the flow, this shift towards mesenchymal phenotype due to the presence of FN was mild, and was less evident than the case with no flow (Fig. 3C). The percentage of mesenchymal cells at t = 16 hr was 32 ± 4% with FN, similar to 27 ± 4% with no FN.
Figure 3. Exogenous FN promote a mesenchymal cell phenotype.
(A) Micrographs of MDA-MB-231 cells embedded in a collagen matrix at two time points (t = 0 hr and t = 16 hr) in the absence (top row) and presence (bottom row) of FN. The FN concentration is 100 μg/mL. There is no flow in all four cases. Scale bar is 50μm. (B) Histograms of the aspect ratio in the absence (black) and presence (red) of FN at t = 16 hr with no flow. (C) Percentage of mesenchymal cells versus time for four flow and FN conditions. Number of cells tracked in each group is 600 cells from three independent experiments.
The presence of exogenous FN provides cell adhesion sites to cells, encourages actin filament polarization and thus promotes a mesenchymal cell phenotype. The observed shift towards mesenchymal cell phenotype due to FN was more evident in the absence of the flow than with the flow possibly due to the fact that flows carried away the exogenous FN with time. The results here indicate that the MDA-MB-231 cell motility type switch depends on the availability of the adhesion molecules, which can be modulated through the interstitial flows. We note that FN is added to the cell-embedded collagen prior to the introduction of the flow. An alternative explanation of this phenomena is that the shear flow activates membrane surface receptor β1-integrin 39, which renders the receptors less sensitive to FN in the presence of low. Future experiments using in-soluble form of fibronectin will help elucidating the exact roles that FN play in cell motility phenotypes switch in the presence of flow.
Interstitial flows mildly enhance cell speed but no significant directional cell migration is observed
Cell motility was examined via the tracking of each individual cell, which was subsequently used to compute cell migration parameters, including cell speed and velocity along the flow, as shown in Figure 4. Figure 4A shows the cell migration trajectories under four combinations of flow and FN experimental conditions (For detailed trajectories of three repeating independent experiments, see Fig. S3).
Figure 4. Interstitial flows slightly enhance cell speed but no significant directional migration is observed.
(A) Cell trajectories under various flow and FN conditions. Each plot has 60 cell trajectories, and each colored line represents one cell trajectory of 16 hours long. Flow is from left to right in this data set. Scale bar is 100μm. Normalized speed (B) and normalized velocity along flow direction Vx (C) along the direction of the flow for four conditions. 180 cells combined from three experiments in each condition are used here. * *: P<0.01, and * * *: P < 0.0001.
Using normalized cell speed, we observed that interstitial flows enhanced cell migration speed mildly, with a percentage increase of 10% and 20% for the case of no FN and FN respectively (Fig. 4B) and a mild shift towards the larger speed reveled in a speed histogram (Fig. S4A). The normalized cell velocity did not show a significant deviation from zero in any of the four flow and FN experimental conditions (Fig. 4C, Fig. S4C), indicating no significant directed cell migration in parallel to the flow was observed at the population level. This was further supported by more detailed calculations as shown in supplementary materials (Fig. S5-7 and Fig. S4D). A detailed comparison of our results on MDA-MB-231 cell motility along with the experimental conditions from three different labs was included in the supplementary materials section (Table S1) 35, 36.
The fastest migrating cells are within the subpopulation of amoeboid cells
We observed that MDA-MB-231 cells dynamically changed their cell shape during the observation period. To investigate the relationship between cell aspect ratio and cell speed, we computed the hourly average speed of each individual cell together with its cell aspect ratio. Fig. 5A-D shows the scatter plots of hourly cell speed versus the corresponding aspect ratio under four different experimental conditions (For detailed scatter plots of three repeating independent experiments, see Fig. S8). Interestingly, we saw a common feature from all four plots in that the maximum speed of a sub-population (within a narrow range of aspect ratio) is inversely proportional to its aspect ratio as indicated by the red dashes lines in Fig. 5 and Fig.S9. By closely examining the spread of the scatter plots along x-axis in Fig. 5A-D, we also found that the cell aspect ratio distribution spanned in a wider range (or more heterogeneous) in the absence of flow than with the flow. In the presence of the flow, more cells had smaller aspect ratios (or assume an amoeboid motility type), which was consistent with the computed average values shown in Figure 3C. To understand whether interstitial flows have differential effects on amoeboid versus mesenchymal cells, we compared the normalized hourly speed for amoeboid versus mesenchymal cells. The result indicated interstitial flows enhanced the speed of both amoeboid and mesenchymal cells (Fig. S10). It is interesting to note that our discovery, that the fastest migrating cells are within the amoeboid cell phenotype, is consistent with intravital imaging where the fast migrating cells in vivo are also seen to be amoeboid cells 21, 22.
Figure 5. Maximum cell speed is inversely correlated to the aspect ratio of cells.
(A-D) MDA-MD-231 cell speed versus aspect ratio under four flow and FN conditions. Each dot represents an hourly average speed at a defined aspect ratio. Number of dots for each experiment is 960. Dashed lines are linear fits to the measured maximum speed at a narrow range of aspect ratio (A.R. range = 1) versus cell aspect ratio.
Interstitial flows impair the mean squared displacements (MSDs) and the persistence of the cell tracks
An important characteristic for cancer cell invasion within a 3D ECM is its ability to spread to a distant location. Towards this end, we computed the mean squared distances (MSDs) using the cell trajectories as shown in Figure 4A for all four experimental conditions. We found that the MSDs were lower in the presence of flow compared to the case with no flow over the observation period (Fig. 6A), indicating interstitial flows impaired the average displacement of the whole cell population, and thus the ability to spread. This was also observed in the two cases with FN. In addition, all the MSD profiles display an exponent (measured from a fit of MSDs ~tα) greater than 1 (Fig. 6B), indicating that cell displacements in 3D did not follow a Gaussian distribution, and can be described by a super diffusive model. Our observation on MDA-MB-231 cell motility was consistent with previous work on the non-Gaussian random motility of HT1080 cells in a 3D type I collagen matrix 51. The lower exponent for the case with flow, α = 1.27 ± 0.013, in contrast to α = 1.46 ± 0.013 for the case of no flow, also shows that cell migration is closer to a random walk ( e. g. α = 1) in the presence of flow. Again, flow impairs the cells ability to spread. We note that this observation was derived from combining all three sets of experimental results.
Figure 6. Interstitial flows impair MDA-MB-231 mean squared displacements and persistence.
(A) Linear scale plot and (B) logarithmic scale plot of the mean square displacements (MSDs) for four flow and FN conditions over 16 hours. The exponent, α, is measured from a fit of MSDs ~tα and are equal to 1.46 ± 0.013, 1.27 ± 0.013, 1.45 ± 0.025, and 1.48 ± 0.007 for four conditions. 180 cells combined from three experiments in each condition are used. (C) Scatter plots of cell migration persistence for four flow and FN conditions in a typical experiment. 60 cells are used for each condition. (D) Scatter plots of cell migration persistence for amoeboid versus mesenchymal cells in one experiment. Approximately 35 amoeboid or mesenchymal cells are used for analysis. *: P < 0.05, * *: P < 0.01, and * * *: P < 0.0001.
To understand the impaired MSDs in the presence of the flow, we computed persistence of the cells in the presence and absence of the flow. Fig. 6C shows that interstitial flows impaired the average cell migration persistence, from 0.26 ± 0.02 with no flow to 0.16 ± 0.01 with flow, for the case of no FN. The two groups with FN also exhibited the same trend, from 0.20 ± 0.02 with no flow to 0.14 ± 0.01 with flow (Fig. 6C). In addition, the persistence histogram also indicates a slight shift towards smaller persistence when flow was present (Fig.S4B).
This decrease in persistence in the presence of flow was consistent with our observation that interstitial flows promote amoeboid motility. Amoeboid cell motility are less persistent than that of mesenchymal cells because amoeboid cells migrate via squeezing through the pore structure of the collagen fiber in a path-finding fashion while mesenchymal cells migrate via climbing along collagen fibers in a path-generating fashion 52. Mesenchymal cells exhibited larger average persistence than amoeboid cells, with 0.21 ± 0.02 compared to 0.13 ± 0.02 (Fig. 6D).
In order to metastasize, cancer cells need to break away from the primary tumor and migrate through interstitial space before entering vascular vessels. It is important to understand how environmental cues influence cancer cells’ ability to disseminate. Here, we propose to use MSD, the average distance squared of all the cells within a population to characterize cell dissemination. This is important, because cell speed or cell persistence alone is not sufficient to describe cell spreading 53. Through MSD measurements, we observed that although interstitial flows enhanced average cell speed, they nonetheless reduced the cancer cells’ probability of migrating persistently. The combined outcome, characterized by MSD, is the impaired ability to spread to a distant site in the presence of flow.
As different microfluidic and modified Boyden chamber models emerge for studying roles of interstitial flows on cancer cell invasion, we notice that cell migration characteristics sensitively depend on experimental conditions. In our work, we did not observe the flow-guided cell migration that had been observed in a microfluidic model by Polacheckl et al.35 and in a modified Boyden chamber 17. One critical factor could be the different stiffness of the ECM or substrata cells were embedded in. In Polacheck et al., cells were embedded in type I collagen matrix with a pH of 8.9, and higher pH condition have been shown to increase the mechanical properties such as the stiffness of the ECM 54. In Shields et al., cell-embedded matrix was placed on a cell culture insert within the Boyden chamber. The cell culture insert was made of a porous polycarbonate with a stiffness on the order of GPa. In these two cases, interstitial flows coupled with the stiffer matrix or substrata may promote rheotaxis along the flow direction. In our experiment, cells were embedded in a 1.5mg/mL collagen matrix, with a Young's modulus close to 50Pa 55, 56. We find this apparent impact of matrix stiffness on motility particularly interesting because this is consistent with the recent reports on the effects of the altered tumor mechanical microenvironment. Specifically, it has been observed that stiffer stroma contributed from the cell-aligned collagen fibers57 and high density of crosslinking fibers 58 promote tumor cell invasion 59.
Conclusion and future perspectives
Tumor microenvironment is complex and evolves constantly in space and time. It is thus critical to develop in vitro models that can recapitulate this spatially and temporally changing landscape. Using a microfluidic model, we reveal that interstitial flows promote an amoeboid cell motility phenotype, and the fast moving cells belong to the subpopulation of amoeboid cells. Using cell shape analysis, we find that maximum cell speed within a specific cell aspect ratio range is inversely related to cell aspect ratio. Using cell migration trajectory analysis, we find that interstitial flows impair the cell ability to migrate persistently and subsequently their ability to spread. These results raise a number of important questions at molecular level with respect to how interstitial flows influence cell migration. For example, how interstitial flows impact the GTPase RhoA and Rac1 signaling activities, which are known to directly correlate with amoeboid and mesenchymal cell motility.
Cell adhesions to the ECM via integrins are central to cell migration within a 3D architecture. There is still much to explore before we fully understand how cell adhesion is coupled with fluid flow to alter cell migration. The work presented here demonstrates that exogenous FN partially rescues a mesenchymal phenotype in the presence of the flow. This result indicates that fluid flow may influence cell migration through carrying away adhesion molecules or through direct shear activated integrins 39. The next level of inquiry requires the development on utilization of biosensors 60, 61 that are compatible with dynamic imaging in microfluidic device to allow for direct monitoring of molecular activities and at the same time cell migration behavior.
Supplementary Material
Acknowledgments
Wu would like to thank Melody A. Swartz for insightful discussions throughout this project. This work was primarily supported by the National Cancer Institute (Award No R21CA138366), partially supported by the Cornell Center on the Microenvironment & Metastasis (Award No U54CA143876 from the National Cancer Institute), the Cornell NanoScale Science and Technology Facility, Cornell University Biotechnology Resource Center and the Cornell Nanobiotechnology Center.
References
- 1.Jain RK. Cancer Research. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 2.Chary SR, Jain RK. Proc Natl Acad Sci U S A. 1989;86:5385–5389. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler TP, Grantham FH, Gullino PM. Cancer Res. 1975;35:3084–3088. [PubMed] [Google Scholar]
- 4.Hompland T, Ellingsen C, Ovrebo KM, Rofstad EK. Cancer Res. 2012;72:4899–4908. doi: 10.1158/0008-5472.CAN-12-0903. [DOI] [PubMed] [Google Scholar]
- 5.Munson JM, Shieh AC. Cancer Manag Res. 2014;6:317–328. doi: 10.2147/CMAR.S65444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heldin CH, Rubin K, Pietras K, Ostman A. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 7.Less JR, Posner MC, Boucher Y, Borochovitz D, Wolmark N, Jain RK. Cancer Res. 1992;52:6371–6374. [PubMed] [Google Scholar]
- 8.Boucher Y, Leunig M, Jain RK. Cancer Res. 1996;56:4264–4266. [PubMed] [Google Scholar]
- 9.Weidner N, Semple JP, Welch WR, Folkman J. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 10.Swartz MA, Skobe M. Microsc Res Tech. 2001;55:92–99. doi: 10.1002/jemt.1160. [DOI] [PubMed] [Google Scholar]
- 11.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 12.Harrell MI, Iritani BM, Ruddell A. The American Journal of Pathology. 2007;170:774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butcher DT, Alliston T, Weaver VM. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reed RK, Rubin K. Cardiovasc Res. 2010;87:211–217. doi: 10.1093/cvr/cvq143. [DOI] [PubMed] [Google Scholar]
- 15.Carter CL, Allen C, Henson DE. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Cai T, Nesi G, Tinacci G, Giubilei G, Gavazzi A, Mondaini N, Zini E, Bartoletti R. J Surg Res. 2011;167:267–272. doi: 10.1016/j.jss.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Munson JM, Bellamkonda RV, Swartz MA. Cancer Res. 2013;73:1536–1546. doi: 10.1158/0008-5472.CAN-12-2838. [DOI] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg Robert A. Cell. 144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Friedl P, Wolf K. The Journal of cell biology. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Condeelis J, Segall JE. Nat Rev Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 22.Pinner S, Sahai E. J Microsc. 2008;231:441–445. doi: 10.1111/j.1365-2818.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 23.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 24.Guck J, Lautenschlager F, Paschke S, Beil M. Integrative Biology. 2010;2:575–583. doi: 10.1039/c0ib00050g. [DOI] [PubMed] [Google Scholar]
- 25.Ricart BG, Yang MT, Hunter CA, Chen CS, Hammer DA. Biophys J. 2011;101:2620–2628. doi: 10.1016/j.bpj.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renkawitz J, Sixt M. EMBO Rep. 2010;11:744–750. doi: 10.1038/embor.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nabeshima K, Inoue T, Shimao Y, Sameshima T. Pathol Int. 2002;52:255–264. doi: 10.1046/j.1440-1827.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 28.Zaman MH, Trapani LM, Sieminski AL, MacKellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Proceedings of the National Academy of Sciences. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedl P, Wolf K. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 30.Friedl P, Zanker KS, Brocker EB. Microsc Res Tech. 1998;43:369–378. doi: 10.1002/(SICI)1097-0029(19981201)43:5<369::AID-JEMT3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Cukierman E, Pankov R, Stevens DR, Yamada KM. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 32.Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Brocker EB, Friedl P. The Journal of cell biology. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells A, Grahovac J, Wheeler S, Ma B, Lauffenburger D. Trends Pharmacol Sci. 2013;34:283–289. doi: 10.1016/j.tips.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shieh AC, Rozansky HA, Hinz B, Swartz MA. Cancer Res. 2011;71:790–800. doi: 10.1158/0008-5472.CAN-10-1513. [DOI] [PubMed] [Google Scholar]
- 35.Polacheck WJ, Charest JL, Kamm RD. Proceedings of the National Academy of Sciences. 2011;108:11115–11120. doi: 10.1073/pnas.1103581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haessler U, Teo JC, Foretay D, Renaud P, Swartz MA. Integr Biol (Camb) 2012;4:401–409. doi: 10.1039/c1ib00128k. [DOI] [PubMed] [Google Scholar]
- 37.Tung C.-k., Krupa O, Apaydin E, Liou J-J, Diaz-Santana A, Kim BJ, Wu M. Lab on a chip. 2013;13:3876–3885. doi: 10.1039/c3lc50489a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu M, Swartz MA. Journal of Biomechanical Engineering. 2014;136:021011–021011. doi: 10.1115/1.4026447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polacheck WJ, German AE, Mammoto A, Ingber DE, Kamm RD. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2447–2452. doi: 10.1073/pnas.1316848111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim BJ, Hannanta-anan P, Chau M, Kim YS, Swartz MA, Wu M. PloS one. 2013;8:e68422. doi: 10.1371/journal.pone.0068422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vulto P, Podszun S, Meyer P, Hermann C, Manz A, Urban GA. Lab on a chip. 2011;11:1596–1602. doi: 10.1039/c0lc00643b. [DOI] [PubMed] [Google Scholar]
- 42.Cross VL, Zheng Y, Won Choi N, Verbridge SS, Sutermaster BA, Bonassar LJ, Fischbach C, Stroock AD. Biomaterials. 2010;31:8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrie RJ, Gavara N, Chadwick RS, Yamada KM. The Journal of cell biology. 2012;197:439–455. doi: 10.1083/jcb.201201124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chauhan VP, Lanning RM, Diop-Frimpong B, Mok W, Brown EB, Padera TP, Boucher Y, Jain RK. Biophys J. 2009;97:330–336. doi: 10.1016/j.bpj.2009.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 46.Ulrich TA, Jain A, Tanner K, MacKay JL, Kumar S. Biomaterials. 2010;31:1875–1884. doi: 10.1016/j.biomaterials.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 47.Yamazaki D, Kurisu S, Takenawa T. Oncogene. 2009;28:1570–1583. doi: 10.1038/onc.2009.2. [DOI] [PubMed] [Google Scholar]
- 48.Sahai E, Marshall CJ. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 49.Parsons JT, Horwitz AR, Schwartz MA. Nature reviews Molecular cell biology. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pankov R, Yamada KM. Journal of Cell Science. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 51.Wu P-H, Giri A, Sun SX, Wirtz D. Proceedings of the National Academy of Sciences. 2014;111:3949–3954. doi: 10.1073/pnas.1318967111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pathak A, Kumar S. Integr Biol (Camb) 2011;3:267–278. doi: 10.1039/c0ib00095g. [DOI] [PubMed] [Google Scholar]
- 53.Berg HC. Random walks in biology. Princeton University Press; Princeton, N.J.: 1983. [Google Scholar]
- 54.Raub CB, Unruh J, Suresh V, Krasieva T, Lindmo T, Gratton E, Tromberg BJ, George SC. Biophysical Journal. 2008;94:2361–2373. doi: 10.1529/biophysj.107.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL. Journal of Biomechanical Engineering. 2002;124:214–222. doi: 10.1115/1.1449904. [DOI] [PubMed] [Google Scholar]
- 56.Hall MS, Long R, Feng X, Huang Y, Hui C-Y, Wu M. Experimental cell research. 2013;319:2396–2408. doi: 10.1016/j.yexcr.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, Tsao MS, Khokha R, Martin L, Boyd N. Cancer Epidemiol Biomarkers Prev. 2005;14:343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 59.Ulrich TA, de Juan Pardo EM, Kumar S. Cancer Res. 2009;69:4167–4174. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spiering D, Hodgson L. In: Rho GTPases. Rivero F, editor. Vol. 827. Springer; New York: 2012. pp. 215–234. ch. 15. [Google Scholar]
- 61.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.