Abstract
Background
External counterpulsation (ECP) increases perfusion to a variety of organs, and may be helpful for acute stroke.
Methods
This was a single-blinded, prospective, randomized controlled feasibility and safety trial of ECP for acute middle cerebral artery (MCA) ischemic stroke, in which 23 patients presenting within 48 hours of symptom onset were randomized into one hour of either 1) ECP at a pressure up to 300mmHg (“full-pressure”), while determining the highest tolerable pressure that would augment MCA mean flow velocity (MFV) by 15%, or 2) ECP at 75mmHg (“sham-pressure”). Transcranial Doppler MCA flow velocities and NIH Stroke Scale (NIHSS) scores were checked before, during, and after ECP, and outcomes were assessed at 30 days after randomization.
Results
While procedures were feasible to implement, there was a frequent inability to augment MFV by 15% despite maximal pressures in full-pressure patients, whereas in sham-pressure patients, MFV frequently increased due to increases in PSV and EDV. In both groups, starting ECP was often associated with contemporaneous improvements in NIHSS stroke scores. There were no between group differences in NIHSS, modified Rankin Scores and Barthel Indices, and no device or treatment-related serious adverse events, deaths, intracerebral hemorrhages, or episodes of acute neuro-worsening.
Conclusions
ECP was safe and feasible to apply in acute ischemic stroke. It was associated with unanticipated effects on flow velocity, and contemporaneous improvements in NIHSS score regardless of pressure used, with a possibility that even very low ECP pressures were physiologically active. Further study is warranted.
Keywords: external counterpulsation, ischemic stroke, transcranial Doppler, cerebral blood flow velocity
Introduction
External counterpulsation (ECP) increases blood flow in organs such as the brain, eye, kidney, skin and liver1, 2, 3, 4, and improves outcome in heart disease5,6,7, and has potential as a perfusion-optimization treatment for ischemic stroke. It has been approved for use in ischemic heart disease and congestive heart failure, and utilizes electrocardiogram-triggered inflations of cuffs to apply pressures ranging from 75 – 300 mmHg sequentially to the lower extremities and buttocks, inducing retrograde aortic blood flow and increased diastolic blood flow2,3, 8, 9, 10. ECP augmented mean flow velocity (MFV) on transcranial Doppler (TCD) in a study in five healthy human volunteers11, and - used as a 14-week long regimen of daily one-hour treatments - was feasible, safe, and improved functional outcome in subacute ischemic stroke patients12. The utility of ECP as an acute treatment for ischemic stroke remains unestablished, however. The Counterpulsation to Upgrade Forward Flow in Stroke (CUFFS) trial was designed to evaluate the safety and feasibility of instituting a one-hour treatment of ECP in adult stroke patients presenting within 48 hours of onset of a middle cerebral artery (MCA) stroke, while exploring its impacts on MCA flow velocity and acute neurological deficit.
Materials and Methods
Study Design
CUFFS was a prospective, randomized, controlled, single (patient) blinded study in which adults with acute strokes were randomly assigned to either full-pressure or sham-pressure ECP. TCD was performed prior to, during and immediately after ECP to assess changes in MCA flow velocity, with NIHSS evaluated at the same time-points. Subsequent NIHSS scores were obtained during each patient’s hospital stay and again at 30 days post-randomization, with adverse events (AEs) and serious adverse events (SAEs) monitored out to 2 days and 30 days post-randomization, respectively. The study was performed at three urban comprehensive stroke centers, and all study procedures were supported by a U.S. Food and Drug Administration investigational device exemption and approved by the Institutional Review Boards (IRBs) at each of the participating sites.
Procedures and assessments
Screening, enrollment and randomization
We enrolled awake patients 18 years of age and older with acute MCA distribution strokes13 so as to optimize the uniformity and interpretation of results (since having a relatively narrower distribution of potential deficits would allow more robust comparison between treatment groups). Inclusion criteria were an ability to initiate ECP within 48 hours of stroke onset, with no acute reperfusion therapy or other experimental therapy planned. Exclusion criteria included rapidly resolving symptoms; an NIHSS > 22; current or prior intracranial hemorrhage; brain tumor or abscess; a presentation consistent with subarachnoid hemorrhage; vascular anomalies such as known or suspected aortic dissection, aneurysm, or other anomaly of the heart or great vessels; cardiac issues such as non-trivial aortic regurgitation, symptomatic valvular heart disease, acute symptomatic congestive heart failure, or a known left ventricular ejection fraction < 30%; issues that would interfere with ECP triggering such as a pacemaker, rapid atrial fibrillation, or frequent PVCs; conditions that might be affected by or limit repeated cuff inflations, such as known collagen vascular disease, significant obesity, a history of significant chronic low back pain, ongoing lumbar radiculopathy, symptomatic lower extremity peripheral vascular occlusive disease, phlebitis, stasis ulcer, severe varicosities, or a diagnosis of deep vein thrombosis (DVT) within the past month; known coagulopathy such as thrombocytopenia with platelet count < 100K, or an INR > 2.0; blood pressure > 180/100 despite treatment; and an inadequate temporal window for TCD imaging.
Written consent was prior to initiating study procedures. After enrollment but before randomization, all patients underwent duplex ultrasound scanning of the lower extremities to rule out DVT, along with an assessment of temporal window adequacy for TCD; patients meeting these criteria were randomly assigned to one of two treatment arms: 1) full-pressure ECP or 2) sham-pressure ECP, in a 1:1 allocation ratio using random permuted block treatment assignment, stratified by stroke severity and site. The randomization plan was created and developed by the trial biostatistician and was implemented centrally in real time using an online database. Since this was a safety trial, group assignment – though concealed from study subjects - was not concealed from study personnel.
Intervention and assessments immediately prior to, during, and immediately after ECP
Depending on his or her randomization assignment, each patient underwent one of two treatments regimens using the ViaCare™ ECP system made by Scottcare Inc (Cleveland, OH): (1) one hour of ECP at full pressure, which was applied in a tiered, dose-escalating manner, starting at 200mmHg and increasing up to 300mmHg as measured by the device, or (2) one hour of ECP at a sham pressure of 75mmHg, replicating the sham pressure used in a seminal trial of ECP for ischemic heart disease5. Paired assessments of TCD velocities and an NIHSS score were made within the hour before starting ECP, twice again during ECP (at 10 and 40 minutes), and immediately after an hour of ECP. A 2MHz probe was used at a constant angle and depth of insonation to determine peak systolic velocity (PSV), end-diastolic velocity (EDV), as well as an additional velocity peak during ECP, the peak diastolic augmented velocity (PDAV); MFVs were calculated based of these values using our previously published methods11. Since subjects’ legs were wrapped in cyclically inflating cuffs while on ECP, we based comparisons between off-ECP and on-ECP neurological deficit on a “conditional” NIHSS score, which excluded patient’s leg-exam findings. All other NIHSS comparisons were performed on scores calculated in the standard fashion. Tolerability was assessed continuously, and pre-treatment blood pressure, pulse, respiratory rate, and SpO2 were recorded immediately before and after ECP.
In full-pressure patients, if the TCD assessment 10 minutes into treatment did not reveal at least a 15% augmentation in MFV from pre-ECP, the pressure was increased from 200 mmHg to 300mg Hg, and subsequent assessments made as per protocol. Otherwise, the pressure was routinely increased to 300mg Hg halfway through the hour. If at any time a patient did not tolerate a pressure, it was decreased in 25mmHg–increments until tolerable.
Subacute assessments
Patients were followed through to hospital discharge. An NIHSS assessment and a non-contrast CT of the brain were performed at 24 hours after ECP treatment. Another NIHSS assessment was conducted at 7 days post-randomization, or at discharge, whichever was earlier. At 30 days, the patient underwent a general exam, a final NIHSS score, modified Rankin Scale (mRS)14 and Barthel Index (BI)15 determinations, and a blinding assessment. Adverse events (AEs) were monitored out to 48 hours, and serious adverse events (SAEs) were monitored out to 30 days.
Outcome measures
Primary outcome measures: Tolerance, feasibility and safety
The first primary outcome measure was tolerability and feasibility. Tolerance was defined as the absence of any indications to stop the procedure or reduce the pressure to a non-therapeutic level. Feasibility was defined in the full-pressure group as the sustained (at least 30 minutes) tolerance of any pressure capable of causing a 15% augmentation of MFV in 90% of subjects, and defined in the sham-pressure group as the sustained tolerance of the sham pressure in all subjects.
Safety was evaluated by the incidence of serious adverse events or acute neurological deterioration in relation to the study device and/or procedures at 30 days, the incidence of acute symptomatic hemorrhage on repeat imaging at 24 hours, the incidence of all adverse events in the first 48 hours, and mortality at 30 days. Acute neurological deterioration – which was captured as a serious adverse event - was defined as a ≥4-point increase on the NIHSS, or a ≥2-point decline in level of consciousness item 1a on the NIHSS, or a new neurological deficit, or clinically significant worsening of motor function lasting more than 8 hours and attributable to a neurological entity. Symptomatic intracranial hemorrhage was defined as new hemorrhage on CT that was associated with acute neurological deterioration16,17.
Secondary outcome measures
Several exploratory efficacy endpoints were also evaluated, including improvement in NIHSS during ECP, immediately post-ECP, at 7 days and 30 days post-randomization, as well as mRS and BI at 30 days post-stroke.
Statistical methods
All analyses were based on the intent-to-treat population and included all randomized participants. Baseline differences between the two treatment groups were assessed using Fisher’s Exact tests for categorical variables and Wilcoxon Rank-sum tests for continuous variables. Rates of adverse events (AEs), serious adverse events (SAEs), acute neurological deterioration, acute symptomatic hemorrhage and deaths between the two treatment groups were compared using Fisher Exact tests. Acute change in NIHSS from baseline, Barthel Index at 30 days post-stroke (categorized as a score of <95 vs 95–100) and Modified Rankin Scale at 30 days post-stroke (categorized as 0–1 vs 2–6) were compared between treatment groups using Fisher’s Exact tests. Acute ECP associated changes in vital signs and TCD flow velocities as well as ECP-associated effects on neurological deficit and secondary outcome measures at each time point were analyzed using Wilcoxon Rank-sum tests. Correlations between changes in acute-phase conditional NIHSS and augmentation of MFV on TCD were analyzed using Spearman’s correlation coefficients. The significance level for all comparisons was set at p<0.05 and all statistical testing were two-sided. Since this was a feasibility and tolerability study, no adjustments for multiple comparisons were made in these analyses. The statistical software R (http://www.r-project.org) was used for all statistical analyses.
Results
Participants and baseline characteristics
Twenty-three subjects were enrolled, 13 in the full-pressure arm and 10 in the sham-pressure arm, with no differences between full-pressure and sham-pressure groups in terms of age, sex, weight, cardiovascular and cerebrovascular co-morbidity and risk, vital signs, or baseline neurological deficit or neurological injury parameters (Table 1).
Table 1.
Baseline characteristics
| Sham-pressure | Full-pressure | p-value | |
|---|---|---|---|
| Demographics: | |||
| Age (median, IQR) | 57 (55,60) | 57 (50,66) | 0.926 |
| Gender (Female, n(%)) | 2 (20%) | 6 (46.2%) | 0.379 |
| Weight (median, IQR) | 81 (71, 84) | 88 (68,96) | 0.664 |
| Cardiovascular and cerebrovascular co-morbidity ((n (%))): | |||
| Hypertension | 9 (90%) | 10 (76.9%) | 0.604 |
| Diabetes | 3 (30%) | 4 (30.8%) | >0.999 |
| Hyperlipidemia | 5 (50%) | 10 (76.9%) | 0.221 |
| Cardiac Arrhythmias | 1 (10%) | 1 (7.7%) | >0.999 |
| EKG abnormal | 2 (20%) | 1 (7.7%) | 0.560 |
| Prior stroke or TIA | 3 (30%) | 6 (46.2%) | 0.669 |
| Pre-treatment vitals (median (IQR)): | |||
| Systolic Blood pressure | 148 (132, 160) | 155 (140, 168) | 0.515 |
| Diastolic Blood Pressure | 81 (75,86) | 74 (63,85) | 0.350 |
| Heart rate | 73 (60, 80) | 70 (67, 80) | 0.534 |
| Temperature | 37 (36,37) | 36 (36, 37) | 0.400 |
| Respiratory rate | 18 (17, 20) | 20 (18, 20) | 0.651 |
| Oxygen saturation | 98 (98,100) | 100 (97,100) | 0.792 |
| Neurological injury parameters: | |||
| NIHSS score (median (IQR)) | 7 (5,10) | 6 (3,8) | 0.639 |
| Pre-morbid mRS of 0 (n(%)) | 9 (90%) | 9 (70%) | NS |
| Stroke-onset-to-treatment time (min) (median (IQR)) | 2011 (1885, 2673) | 1700 (1426,2025) | 0.343 |
| Acute stroke evident on CT n(%) | 5 (50%) | 9 (69.2%) | 0.417 |
| Loss of grey-white distinction on CT n(%) | 3 (30%) | 3 (23.1%) | >0.999 |
| Hyperdense artery sign on CT n(%) | 2 (20%) | 1 (7.7%) | 0.560 |
Feasibility and tolerability
The study procedures during the acute phase of ECP treatment proved feasible to implement. Two of the 23 subjects (one patient each in each group) were unable to tolerate the procedure for the hour; one patient in the sham-pressure group asked to have the ECP procedure stopped due to the noise of the machine, and one patient in the full-pressure group asked to have it stopped due to discomfort. The remaining patients in both groups tolerated a full hour of ECP without significant issue. All the remaining 12 patients in the full-pressure group tolerated a 200mmHg starting pressure, and 11/12 subsequently tolerated a pressure that was either maximal at 300mmHg or high-enough to cause a ≥15% augmentation in MVF; 3/12 required the pressure to be increased early due to lack of MCA MFV augmentation at 10 minutes. While ECP was feasible to implement, our feasibility endpoint was affected by the fact that, despite use of maximal 300 mm Hg pressures, MCA MFV could not be augmented in 6/13 (46%) of full pressure patients.
Acute ECP treatment phase findings
There were no statistically significant differences between sham-pressure and full-pressure groups with regards to the median MAP immediately before treatment was started (105 mmHg [IQR, 101–111] versus 105 mmHg [IQR, 94–116], respectively), or immediately after ECP treatment was stopped (109 mmHg [IQR, 107–116] versus 100 mmHg [IQR, 94–113], respectively).
There were no statistically significant differences between groups in PSV and EDV at 30-minutes immediately prior to ECP, during ECP, or in the 20 minutes immediately after ECP. There was an unexpected trend in the direction of change in PSV and EDV (Table 2) that occurred in each group with ECP: In the full-pressure treatment group, the overall trend was a decrease in both PSV and EDV upon ECP initiation – a phenomenon more noticeable with higher pressures (Figure 1), whereas in the sham-pressure group, the overall trend was an increase in both PSV and EDV upon ECP initiation. Accordingly, calculated MFVs tended to decrease in full-pressure patients, even at maximal pressures, whereas they tended to increase in sham-pressure patients.
Table 2.
Flow velocities (cm/sec) in the two groups just before ECP was started (Time PRE-ECP), at 10 minutes into ECP (Time 10 min), 40 minutes into ECP (Time 40 min), and immediately after stopping ECP treatment (Time POST-ECP).
| PSV | EDV | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Min | Q1 | Median | Q3 | Max | p-value | Min | Q1 | Median | Q3 | Max | p-value | |
| Time PRE-ECP | |||||||||||||
| S | 10 | 30 | 38.25 | 45.2 | 80.5 | 171 | 0.4833 | 0 | 14.775 | 20 | 34.175 | 56 | 0.6864 |
| T | 13 | 20 | 43 | 56.6 | 83.9 | 130.3 | 10 | 17 | 24 | 37.1 | 56.4 | ||
| Overall | 23 | 20 | 41 | 55 | 85.05 | 171 | 0 | 16.5 | 20 | 38 | 56.4 | ||
| Time 10 min | |||||||||||||
| S | 9 | 26 | 44 | 60 | 81 | 202 | 0.8078 | 13 | 21 | 24 | 43.3 | 81 | 0.3549 |
| T | 12 | 20 | 43.9 | 55.6 | 70.3 | 153.4 | 10 | 17.275 | 21 | 34.375 | 55 | ||
| Overall | 21 | 20 | 44 | 59.2 | 77.2 | 202 | 10 | 20 | 22 | 37.3 | 81 | ||
| Time 40 min | |||||||||||||
| S | 10 | 35.7 | 45 | 55.5 | 82.85 | 216 | 0.5306 | 12 | 19.25 | 22.6 | 29.775 | 99 | 0.468 |
| T | 12 | 20 | 38.25 | 49.45 | 68.875 | 165 | 10 | 14.75 | 17.5 | 37.425 | 55 | ||
| Overall | 22 | 20 | 43.8 | 50.5 | 82.85 | 216 | 10 | 15.25 | 21 | 34.35 | 99 | ||
| Time POST-ECP | |||||||||||||
| S | 10 | 29 | 41.4 | 65.5 | 75.625 | 230 | 0.8951 | 11 | 21 | 30.5 | 43.4 | 160 | 0.3555 |
| T | 12 | 24 | 46.5 | 60.75 | 72.4 | 143.3 | 12 | 15.75 | 23.5 | 33.2 | 59.3 | ||
| Overall | 22 | 24 | 42 | 60.75 | 75.625 | 230 | 11 | 16.775 | 25.05 | 38.225 | 160 | ||
S = sham-pressure group; T = full-pressure group; PSV = peak systolic velocity; EDV = end-diastolic velocity.
Figure 1.
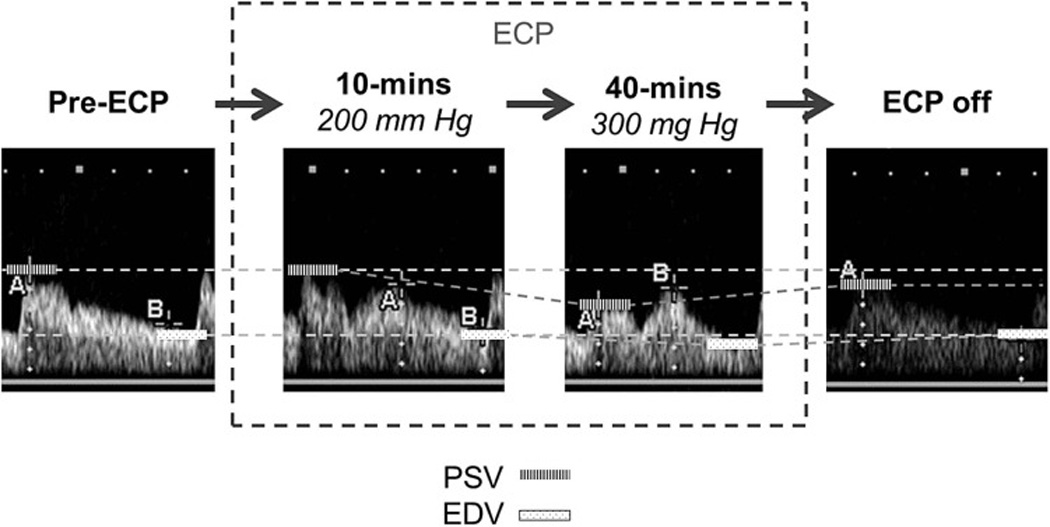
Representative TCD spectra from one of the full-pressure patients over time, revealing a tendency for peak systolic velocity (PSV) and end-diastolic velocity (EDV) to drift downward as a concomitant ECP-induced augmentation of flow velocity during diastole developed, and then reverse upon ECP being stopped.
Effects on neurological deficit and secondary outcome measures
There were no significant differences between full-pressure and sham-pressure groups with regards to acute change in NIHSS while on ECP, NIHSS at 7 days and 30 days, or modified Rankin Score and Barthel Index scores at 30 days. However, we frequently encountered acute improvements in NIHSS scores as well as subtle improvements in non-NIHSS item neurological deficits upon starting ECP (Figure 2). These effects were not exclusive to one study group; in fact, 70% of sham-pressure patients and 38% of full-pressure patients had an improvement in conditional NIHSS of ≥ 2 points within 20 or 50 minutes of ECP initiation (p = 0.2138). There were no statistically significant differences between the sham- and full-pressure groups with regards to overall changes in NIHSS. In sham-pressure patients, there was a strong correlation between the degree of MFV augmentation and the degree of change in conditional NIHSS between pre-ECP and NIHSS taken at 20-minutes into ECP (Spearman rho of 0.7059, p 0.0336), whereas there was no relationship found in full-pressure patients (Spearman rho of 0.2388, p 0.4548).
Figure 2.
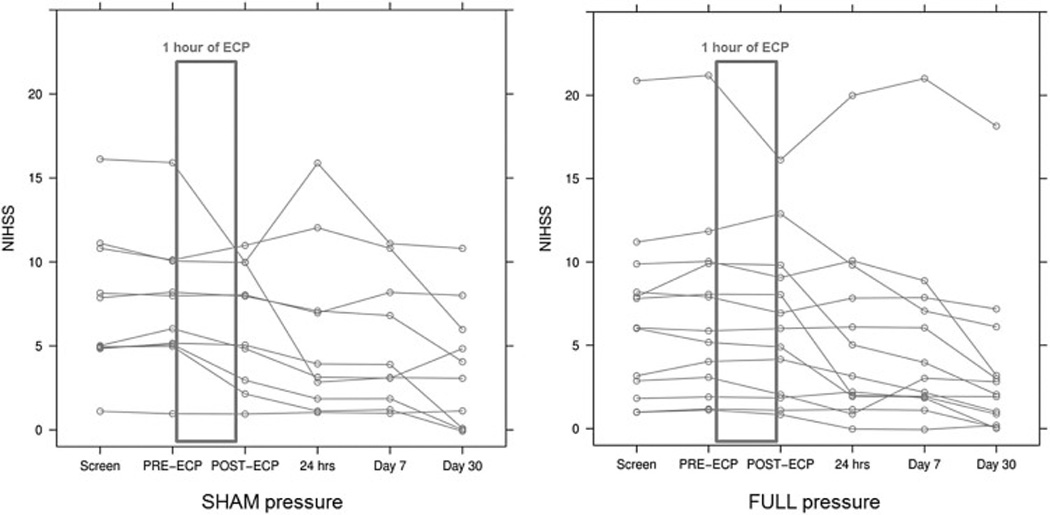
NIHSS (NIH Stroke Scale) score profiles of study subjects receiving ECP. ECP was frequently associated with an acute improvement in an NIHSS score that had remained relatively stable from screening to just before starting ECP (PRE-ECP). There was no statistically significant difference between groups with regards to this phenomenon. Each line represents an individual subject. SHAM pressure, sham-pressure patients; FULL pressure, full-pressure patients.
Three of the sham-pressure patients and one of the full-pressure patients were lost to follow up at 30 days. There were no statistically significant differences between the two groups in BI scores of 95–100 (5/7 [71%] versus 8/12 [67%]; p >0.9999), mRS of 0 or 1 (4/7 [57%] versus 6/12 [50%]; p >0.9999), or median NIHSS score (3.5 [IQR, 0.25–5.75] versus 2 [IQR, 1–3]; p = 0.7063).
Safety
No lower extremity DVTs were found on pre-randomization venous duplex exams. No participants had acute neuro-worsening while on ECP, acute treatment-related worsening, or symptomatic intracerebral hemorrhage on repeat imaging at 24 hours. There were no deaths reported out to 30 days.
There were three serious adverse events (SAEs) in the study, all of which occurred in the same patient. This patient was in the full-pressure ECP group and had no SAEs during the initial hospital stay, but subsequently had a one-day re-admission for a COPD exacerbation and two re-admissions for transient ischemic attacks within the 30 day follow-up period. None of these SAEs were felt to be device related.
There were 15 AEs noted in 8 (80%) of 10 sham patients, and 26 AEs noted in 12 (92%) of 13 individual patients in the full-pressure group (p= 0.5596). The majority of AEs were mild, and there was only one device-related AE, in a full-pressure patient, “urgency to urinate.” There were no reported events involving thigh or leg pain, back pain, bruising, or dermatological issues.
Discussion
In this trial we found that external counterpulsation (ECP) was safe, well tolerated, and feasible to apply as an intervention in acute ischemic stroke, but was associated with unanticipated increases in flow velocity even at low inflation pressures, and with acute improvements in neurological deficits that did not correlate with the pressure used. Our study subjects were relatively younger and had milder deficits than those seen in other stroke trials, which may have reflected a known predisposition for younger and milder patients to show up later and outside the treatment windows for standard therapies18. The younger age and lower median NIHSS scores in this study may have an impacted an assessment of safety of ECP in older and more severely affected patients, but it reflects the practical reality of patients who show up outside the window for typical reperfusion therapies. Prior considerations for the use of ECP for stroke have mirrored the treatment regimen seen for chronic cardiac angina, in that it is typically administered in several times a week for up to seven weeks, incorporating therapeutic mechanisms of ECP-induced endovascular secondary messaging, upregulated angiogenesis and development of collaterals19. Lin and colleagues evaluated a multi-day ECP treatment regimen in subacute ischemic stroke patients with large cerebral artery occlusive disease, starting treatment a mean of 6.18 days after stroke onset in a rehabilitative paradigm, and found that it was feasible, safe, and resulted in augmentation of flow velocities and reduction in NIHSS scores20. In this study, we administered a treatment at a median of just under 19 hours from stroke onset, in a more acute time-frame. Our study is the first to evaluate safety, tolerability, and feasibility of ECP treatment in acute ischemic stroke patients this early from stroke onset.
We chose to evaluate the safety and feasibility of a one-time treatment to reflect a potential clinical application of ECP in stroke, as an acute “penumbral-rescue” treatment, pending spontaneous recanalization or reperfusion therapy. There are clinical data to suggest that a transient augmentation of perfusion has the potential to be beneficial. Alexandrov and colleagues reported that, in patients with acute proximal MCA ischemic strokes given a bolus of tissue plasminogen activator (t-PA), those who recanalized transiently but then re-occluded within two hours fared better that those who never re-canalized at all, with good outcomes at 3 months (defined as an mRS score of 0 to 1) in 33% and 8%, respectively (p < 0.05)21. A more direct inference as to the salutary effects of a transient augmentation of perfusion can be found in the experience published by Meier, in which a regimen of three, 1-hour long treatments of pressor therapy with boluses of epinephrine in acute stroke patients increased survival to 21 days (62.2% vs. 36.4%; p < 0.02)22. We chose to target a 15% augmentation of flow velocity because it was associated with a ≥2 point improvement in NIHSS in a majority (83%) of patients with symptomatic vasospasm from subarachnoid hemorrhage in which an intra-aortic device (Neuroflo™) was used to augment perfusion23.
We encountered counterintuitive but theoretically explainable effects from ECP on TCD MFVs. First, we could rarely attain the protocol-stipulated 15% increase in mean flow velocity in full-pressure patients, despite maximizing ECP pressures. This conundrum is reflected in findings from another study by Lin and colleagues published after our study had started, in which increasing pressures of ECP in stroke patients with large artery disease did not result in comparable increases in MCA TCD blood flow velocities24. A possible reason, given ECPs known effects on systemic vascular resistance, is a re-distributive reduction in arterial blood volume by ECP during diastole such that less blood was left available for forward flow during systole. Alternatively, there may have been downstream autoregulatory vasoconstriction during ECP that inhibited cerebral blood flow25. A second finding was that initiation of sham ECP at a low pressure that did not affect PDAV was associated with elevations in PSV and EDV (and therefore increased mean TCD flow velocity) in most of these patients. One consideration is elevated sympathetic tone and blood pressure from an acute stress reaction,26, 27, 28 due to the never-before-experienced noise and dynamism of ECP; however, this is made unlikely by the fact that blood pressures at screening and then before and after ECP were neither statistically nor clinically different from each other. An alternative explanation is that low sham pressures may have preferentially compressed more compliant, lower-pressure venous capacitance vessels in the legs without affecting the less compliant and higher-pressure arterial vasculature, causing an increased venous return and cardiac preload29, and ultimately improved stroke volume, cardiac output and cerebral blood flow during systole (all without discernable diastolic pressure effects). Due to the non-invasive nature of the trial, we did not capture continuous changes in blood pressure using an arterial line, which would have enabled a clearer understanding of both sham and active ECP affects during the treatment.
While this study was neither designed nor powered as an efficacy study, we did find that initiating ECP was associated with acute, concurrent improvements in neurological deficit in both sham and active treatment patients, and across study sites. Investigator bias was unlikely, since these findings were documented in both groups; additionally, placebo effect is also unlikely, because a blinding assessment done at 30 days revealed no correlation between what patients actually received and what they thought they received. It is possible that ECP had a physiological effect in both arms of the study, through different mechanisms. While the exact cause of these findings remains unclear, they inspire future work to uncover the hemodynamics of ECP therapy in acute ischemic stroke.
This study has several limitations that primarily affect the secondary outcome measures used. First, it was a safety and feasibility study, and the small sample size may have precluded our ability to detect more subtle physiological effects. Secondly, there were some patients lost to 30-day follow up, which may have obscured our ability to fully detect all safety issues; however, the majority of physiologically-relevant safety data were captured prior to hospital discharge. A third limitation was the use of TCD to measure blood flow augmentation, which we chose for the capability to measure real-time changes in cerebral blood flow during a dynamic intervention. TCD is only a surrogate measure for CBF. This being said, some more definitive assessments of CBF – such as perfusion-weighted MRI or xenon-CT – would have essentially been impossible to do while ECP was ongoing. In addition, since emergent vascular imaging was not routine for delayed stroke patients not undergoing reperfusion therapy at all of our sites at the time of the study, and because we wanted to evaluate the implementation of ECP as an empirically-administered therapy (even something that might one day be administered in an ambulance), we did not mandate vascular imaging prior to ECP. Vascular imaging might have lent additional insight into to potential ECP-induced alterations in blood flow that were in play.
Conclusions
Use of ECP is safe and feasible to implement in patients presenting within 48 hours of onset of acute ischemic stroke. Our study brings up the possibility that ECP even at low pressures might have a physiological effect in stroke. Future work to the evaluate the utility of ECP for acute stroke should explore development of sham methods that are unlikely to contribute to flow augmentation, the use of optimized of real-time cerebral blood flow and arterial blood pressure monitoring methods, and – as investigation migrates towards an evaluation of efficacy - the use of vascular and perfusion imaging to potentially select patients with augmentable collateral blood flow and salvageable perfusion deficits, and to evaluate treatment effects.
Acknowledgments
This study was funded by National Institute of Neurological Diseases and Stroke (NIH/NINDS) grants (P50NS044148 and P50NS044378).
The authors would like to acknowledge the support of the National Institute of Neurological Diseases and Stroke, and the efforts of the clinicians and nurses whose efforts contributed to the implementation of this trial, including Wendy Brown, MD; Gilda Tafreshi, MD; Teresa Rzesiewicz, RN; Nancy Kelly, RN; Judy Guzy, RN; Andrew Stemer, MD; Branko Huisa-Garate, MD; Thilo Hoeschler, MD; Nhu Bruce, MD; William Neil, MD; Amy Guzik, MD; Ajeet Sodhi, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kama Z. Guluma, Department of Emergency Medicine, University of California, San Diego, San Diego, California.
David S. Liebeskind, Associate Nurology Director, UCLA Stroke Center, Department of Neurology, University of California, Los Angeles, Los Angeles, California.
Rema Raman, Family Medicine and Public Health and Neurosciences, University of California at San Diego, La Jolla, California.
Karen A. Rapp, UCSD Stroke and Coordinating Center, University of California, San Diego, La Jolla, California.
Karin B. Ernstrom, Family Medicine & Public Health, University of California, San Diego, La Jolla, CA 92093-0725, kernstrom@ucsd.edu, (858) 822-0617.
Andrei V. Alexandrov, Department of Neurology, University of Tennessee Health Science Center, Memphis, Tennessee.
Reza B. Shahripour, Neurosonology, Comprehensive Stroke Center, University of Alabama at Birmingham, Birmingham, Alabama.
Kristian Barlinn, Department of Neurology, Dresden University Stroke Center, Carl Gustav Carus University Hospital Dresden, Dresden, Germany.
Sidney Starkman, UCLA Stroke Center and Departments of Emergency Medicine and Neurology, University of California, Los Angeles, Los Angeles, California.
Ileana D. Grunberg, UCLA Stroke Network, University of California, Los Angeles, Los Angeles, California.
Thomas M. Hemmen, Department of Neurosciences, UCSD Stroke Program, University of California, San Diego, La Jolla, California.
Brett C. Meyer, Department of Neurosciences, UCSD Stroke Program, University of California, San Diego, La Jolla, California.
Anne W. Alexandrov, College of Nursing, University of Tennessee Health Science Center, Memphis, Tennessee.
REFERENCES
- 1.Applebaum RM, Kasliwal R, Tunick PA, et al. Sequential external counterpulsation increases cerebral and renal blood flow. Am Heart J. 1997;133:611–615. doi: 10.1016/s0002-8703(97)70161-3. [DOI] [PubMed] [Google Scholar]
- 2.Werner D, Schneider M, Weise M, Nonnast-Daniel B, Daniel WG. Pneumatic external counterpulsation: a new noninvasive method to improve organ perfusion. Am J Cardiol. 1999;84:950–952. doi: 10.1016/s0002-9149(99)00477-4. [DOI] [PubMed] [Google Scholar]
- 3.Hilz MJ, Werner D, Marthol H, Flachskampf FA, Daniel WG. Enhanced external counterpulsation improves skin oxygenation and perfusion. Eur J Clin Invest. 2004;34(6):385–391. doi: 10.1111/j.1365-2362.2004.01352.x. [DOI] [PubMed] [Google Scholar]
- 4.Werner D, Tragner P, Wawer A, Porst H, Daniel WG, Gross P. Enhanced external counterpulsation: a new technique to augment renal function in liver cirrhosis. Nephrol Dial Transplant. 2005;20(5):920–926. doi: 10.1093/ndt/gfh755. [DOI] [PubMed] [Google Scholar]
- 5.Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, Nesto RW. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and angina episodes. J Am Coll Cardiol. 1999;33:1833–1840. doi: 10.1016/s0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 6.Cohn PF. Enhanced external counterpulsation for the treatment of angina pectoris. Prog in Cardiovasc Dis. 2006;49(2):88–97. doi: 10.1016/j.pcad.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Soran O, Kennard ED, Kfoury AG, Kelsey SF. Two-year clinical outcomes after enhanced external counterpulsation (EECP) therapy in patients with refractory angina pectoris and left ventricular dysfunction (Report from the International EECP Patient Registry) Am J Cardiol. 2006;97:17–20. doi: 10.1016/j.amjcard.2005.07.122. [DOI] [PubMed] [Google Scholar]
- 8.Taguchi I, Ogawa K, Oida A, Abe S, Kaneko N, Sakio H. Comparison of hemodynamic effects of enhanced external counterpulsation and intra-aortic balloon pumping in patients with acute myocardial infarction. Am J Cardiol. 2000;86:1139–1141. doi: 10.1016/s0002-9149(00)01175-9. [DOI] [PubMed] [Google Scholar]
- 9.Werner D, Marthol H, Brown CM, Daniel WG, Hilz MJ. Changes of cerebral blood flow velocities during enhanced external counterpulsation. Acta Neurol Scand. 2003;107:405–411. doi: 10.1034/j.1600-0404.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 10.Taguchi I, Ogawa K, Kanaya T, Matsuda R, Kuga H, Nakatsugawa M. Effects of enhanced external counterpulsation on hemodynamics and its mechanism. Circ J. 2004;68(11):1030–1034. doi: 10.1253/circj.68.1030. [DOI] [PubMed] [Google Scholar]
- 11.Alexandrov AW, Ribo M, Wong KS, Sugg RM, Garami Z, Jesurum JT, Montgomery B, Alexandrov AV. Perfusion augmentation in acute stroke using mechanical counterpulsation-phase IIa: effect of external counterpulsation on middle cerebral artery mean flow velocity in five healthy subjects. Stroke. 2008;39(10):2760–2764. doi: 10.1161/STROKEAHA.107.512418. [DOI] [PubMed] [Google Scholar]
- 12.Han JH, Leung TW, Lam WW, Soo YO, Alexandrov AW, Mok V, Leung YF, Lo R, Wong KS. Preliminary findings of external counterpulsation for ischemic stroke patient with large artery occlusive disease. Stroke. 2008;39(4):1340–1343. doi: 10.1161/STROKEAHA.107.500132. [DOI] [PubMed] [Google Scholar]
- 13.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 14.Wilson JT, Hareendran A, Grant M, Baird T, Schulz UG, Muir KW, Bone I. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33(9):2243–2246. doi: 10.1161/01.str.0000027437.22450.bd. [DOI] [PubMed] [Google Scholar]
- 15.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 16.Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G. Thrombolysis with Alteplase for Acute Ischaemic Stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (Sits-Most): An Observational Study. Lancet. 2007;369(9558):275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 17.Wahlgren N, Ahmed N, Eriksson N, Aichner F, Bluhmki E, Davalos A, Erila T, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kohrmann M, Larrue V, Lees KR, Machnig T, Roine RO, Toni D, Vanhooren G. Multivariable Analysis of Outcome Predictors and Adjustment of Main Outcome Results to Baseline Data Profile in Randomized Controlled Trials: Safe Implementation of Thrombolysis in Stroke-Monitoring Study (Sits-Most) Stroke. 2008;39(12):3316–3322. doi: 10.1161/STROKEAHA.107.510768. [DOI] [PubMed] [Google Scholar]
- 18.Lacy CR, Suh DC, Bueno M, Kostis JB. Delay in presentation and evaluation for acute stroke: Stroke Time Registry for Outcomes Knowledge and Epidemiology (S.T.R.O.K.E.) Stroke. 2001;32(1):63–69. doi: 10.1161/01.str.32.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Han JH, Wong KS. Is counterpulsation a potential therapy for ischemic stroke? Cerebrovasc Dis. 2008;26(2):97–105. doi: 10.1159/000139655. Epub 2008 Jun 17. [DOI] [PubMed] [Google Scholar]
- 20.Lin W, Xiong L, Han J, Leung TW, Soo YO, Chen X, Wong KS. External counterpulsation augments blood pressure and cerebral flow velocities in ischemic stroke patients with cerebral intracranial large artery occlusive disease. Stroke. 2012;43(11):3007–3011. doi: 10.1161/STROKEAHA.112.659144. [DOI] [PubMed] [Google Scholar]
- 21.Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59(6):862–867. doi: 10.1212/wnl.59.6.862. [DOI] [PubMed] [Google Scholar]
- 22.Meier F, Wessel G, Thiele R, Gottschild D, Brandstatt H. Induced hypertension as an approach to treating acute cerebrovascular ischaemia: possibilities and limitations. Experimental Pathology. 1991;42:257–263. doi: 10.1016/s0232-1513(11)80079-4. [DOI] [PubMed] [Google Scholar]
- 23.Lylyk P, Vila JF, Miranda C, Ferrario A, Romero R, Cohen JE. Partial aortic obstruction improves cerebral perfusion and clinical symptoms in patients with symptomatic vasospasm. Neurol Res. 2005;27(Suppl 1):S129–S135. doi: 10.1179/016164105X35512. [DOI] [PubMed] [Google Scholar]
- 24.Lin W, Xiong L, Han J, Leung H, Leung T, Soo Y, Chen X, Wong KS. Increasing pressure of external counterpulsation augments blood pressure but not cerebral blood flow velocity in ischemic stroke. J Clin Neurosci. 2013 doi: 10.1016/j.jocn.2013.09.023. pii: S0967-5868(13)00638-3. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Zhu YS, Hill C, Armstrong K, Tarumi T, Hodics T, Hynan LS, Zhang R. Cerebral autoregulation of blood velocity and volumetric flow during steady-state changes in arterial pressure. Hypertension. 2013;62(5):973–979. doi: 10.1161/HYPERTENSIONAHA.113.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pomidossi G, Parati G, Casadei R, Mancia G. Absence of alarm reactions with use of non-invasive blood pressure monitoring devices. Clin Exp Hypertens A. 1985;7(2–3):429–436. doi: 10.3109/10641968509073569. [DOI] [PubMed] [Google Scholar]
- 27.Mancia G, Parati G, Pomidossi G, Grassi G, Casadei R, Zanchetti A. Alerting reaction and rise in blood pressure during measurement by physician and nurse. Hypertension. 1987;9(2):209–215. doi: 10.1161/01.hyp.9.2.209. [DOI] [PubMed] [Google Scholar]
- 28.McEwen BS. Stressed or stressed out: what is the difference? J Psychiatry Neurosci. 2005;30(5):315–318. [PMC free article] [PubMed] [Google Scholar]
- 29.Michaels AD, Tacy T, Teitel D, Shapiro M, Grossman W. Invasive left ventricular energetics during enhanced external counterpulsation. Am J Ther. 2009;16(3):239–246. doi: 10.1097/MJT.0b013e318175d116. [DOI] [PubMed] [Google Scholar]


