Abstract
Background
Stroke is a common complication of extracorporeal membrane oxygenation (ECMO), and pediatric cardiac surgical patients may be at higher risk. Epidemiology and risk factors for stroke in these patients are not well-characterized.
Methods
We analyzed pediatric (<18 years-old) cardiac ECMO cases in the Extracorporeal Life Support Organization Registry from 2002-2013. Cardiac surgical patients were identified and procedures stratified by Society of Thoracic Surgeons (STS) morbidity categories. The primary outcome was any stroke (hemorrhagic or infarction) identified by neuroimaging. Risk factors were identified through multivariable logistic regression.
Results
We analyzed 3,517 cardiac surgical patients; 81% with cyanotic disease, and 57% in high-risk STS categories (4-5). Overall, 12% developed stroke on ECMO and those with stroke had greater in-hospital mortality (72% vs. 51%, p<0.0001). In multivariable analysis, neonatal status (adjusted odds ratio 1.8, 95% confidence interval 1.3-2.4), lower weight-for-age z-score (adjusted odds ratio 1.1 for each one-point decrease, 95% confidence interval 1.04-1.25), and longer ECMO duration (upper quartile [≥167 hours] adjusted odds ratio 1.4, 95% confidence interval 1.1-1.8) were independently associated with increased stroke risk, while cyanotic disease, STS category, and bypass time were not.
Conclusions
This multicenter analysis demonstrates that pediatric cardiac surgical patients on ECMO are at high risk of stroke; younger or underweight patients, and those with longer ECMO duration are at greatest risk, independent of procedural complexity. Future study is necessary to determine how anticoagulation or other clinical practices can be modified to reduce stroke incidence.
Keywords: Extracorporeal Membrane Oxygenation, Stroke, Congenital Heart Disease, Outcomes, Pediatric
Cardiothoracic surgeons increasingly use extracorporeal membrane oxygenation (ECMO) to support patients with post-cardiotomy myocardial depression after congenital cardiac repairs and in those with cardiomyopathy (1, 2). While ECMO remains a final option to salvage patients in need of temporary cardiopulmonary support after surgery, treatment can be limited by complications (3-6). ECMO exposure incites both bleeding, clotting cascades, and necessitates systemic anticoagulation during therapy, which places patients at high-risk for both hemorrhagic and ischemic strokes (7). Stroke remains a common and potentially devastating complication of ECMO with reported rates of intracranial hemorrhage or cerebral infarction in 7.6% and 5.8% of pediatric patients on ECMO, respectively (4).
Stroke occurs frequently after congenital cardiac surgery even in patients without the need for ECMO (8-9). Thus, pediatric patients undergoing cardiac surgery who then require ECMO support post-operatively may be at extremely high risk for stroke. Pro-coagulant factors related to cardiopulmonary bypass and ECMO circuits, decreased cardiac output preceding cannulation, and other factors likely increase the risk of stroke and may exacerbate neurologic injury in this population (10). We previously reported higher frequencies of hemorrhagic stroke in cardiac surgical patients compared to other cardiac ECMO patients (6).
Despite the scope of this problem, the epidemiology of and risk factors for stroke in pediatric cardiac surgical patients requiring ECMO remain unknown. Filling this knowledge gap is a necessary step to inform future efforts to reduce the frequency of stroke for these children. We aimed to examine the incidence of stroke in this population using a multi-institutional database and to identify patient, procedural, and clinical variables that could be targeted in future research and quality improvement efforts to improve outcomes for children requiring ECMO support after cardiac surgery. We hypothesized that cannulation techniques, thromboses in the ECMO circuit, and pump flows would associate with risk of stroke.
Material and Methods
Data Source
The Extracorporeal Life Support Organization (ELSO) maintains a registry of ECMO data from over 350 international ECMO centers and currently houses information from >58,000 ECMO runs since 1990. Each participating ECMO center collects and voluntarily reports standardized data on all patients undergoing ECMO including patient characteristics, details of the ECMO run, complications, and outcomes. Centers submit a cardiac addendum for patients who required ECMO for cardiac support with data on the underlying cardiac lesion, surgical information (if applicable), and physiologic data. The ELSO Registry data is de-identified prior to release, thus our study was deemed exempt after review by the University of Michigan Hospital and Health Systems Institutional Review Board.
Study Patients
Under ELSO data sponsorship, registry data were queried on the initial ECMO run during a hospitalization from all children (<18 years-old at ECMO onset) cannulated to venoarterial ECMO for cardiac support between January 1, 2002 and April 30, 2013 (N=6,692). We identified the cohort with primary cardiac disease using codes available in the database, which include a combination of International Classification of Diseases, 9th Revision diagnostic codes, Current Procedural Terminology codes, and ELSO cardiac addendum procedure codes. Patients were included in the analysis if they had an ECMO run within six months following a cardiothoracic surgical procedure during the same hospitalization, including those on ECMO pre-operatively who continued on ECMO post-operatively. Patients who had pre-operative ECMO only were not considered to be at the same risk for ECMO complications as a postoperative patient, and therefore were excluded for a separate analysis. ECMO for pulmonary support (N=683) or extracorporeal cardiopulmonary resuscitation (N=1,717) were not included because the surgical data necessary for our analysis are not collected for these patients. Records with missing, implausible, or out of range values were also excluded (N=1,979).
Data collection
The primary outcome variable was any stroke (hemorrhagic or ischemic). Stroke in the ELSO database is defined as a positive finding identified by cranial ultrasound or computed tomography. Information on indications for neuroimaging and data on the clinical manifestations of stroke are not collected. Surgical procedures were classified into the Society of Thoracic Surgeons (STS) morbidity categories. The STS morbidity categories empirically group operations by associated morbidity risk (11). Examples of low morbidity procedures include atrial septal defect repair, bidirectional Glenn palliation, or coarctation repair (categories 1, 2, and 3, respectively) whereas an interrupted arch repair (category 4) or Norwood procedure (category 5) represent high morbidity operations. Each cardiac diagnosis was classified as cyanotic or acyanotic after review by the primary authors who are both pediatric cardiologists (DKW, MG).
Variables in the database considered to be biologically plausible risk factors for stroke were collected, including patient characteristics (race, age, weight-for-age, presence of structural heart disease, cyanotic heart lesions, presence of any known genetic anomalies, pre-ECMO mechanical support including ventricular assist device [VAD] or aortic balloon pump), ECMO factors (duration, high flow [relative to weight], low flow [independent from weight], greater than 2 cannulae, left atrial cannulation, neck cannulation, circuit component thrombosis), and surgical factors (STS morbidity category, cardiopulmonary bypass [CPB] time). Data on staffing, neuromonitoring, and anticoagulation therapy are not collected in the database, therefore these clinical practices could not be analyzed for association with stroke rates.
Statistical Analysis
Incidences of stroke were reported as the cumulative incidence proportion. Variables with > 10% missing data or those with a zero frequency were not included in analysis. Univariate comparisons of the characteristics selected were made between patients with and without stroke using Chi-square test for categorical variables and Wilcoxon rank sum or t-tests for continuous variables. Continuous variables were also evaluated categorically using quartiles and flow exposure variables were analyzed as upper or lower quartile versus normal. Risk factors found to be associated with stroke in univariate analyses at p < 0.15 were included in multivariable logistic regression to determine independence of the association. Multicollinearity for variables included in the multivariable analysis was examined using variance inflation factor, which is an indication of multicollinearity if greater than 10. Variance inflation factors for all variables in the model were less than 1.5, which was acceptable for inclusion. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA), with statistical significance set at p-values less than 0.05 using two-sided tests.
Results
Demographics
Our inclusion criteria identified 3,517 cardiac surgical patients requiring ECMO postoperatively. Characteristics of the cohort are presented in Table 1; 54% were neonates (age <30 days), 96% had structural heart disease (81% cyanotic), and 57% were in STS categories 4 or 5. ECMO duration varied considerably (median 104 hours, interquartile range 64-167).
Table 1. Patient characteristics of pediatric cardiac surgical patients (N=3,517).
| Male sex | 1,976 (56.2) |
| Race | |
| Caucasian | 2,003 (57.0) |
| African American | 445 (12.7) |
| Asian | 254 (7.2) |
| Hispanic | 534 (15.2) |
| Other | 208 (5.9) |
| Unknown | 73 (2.1) |
| Current weight, kg | 3.6 (3.0-5.7) |
| Age group | |
| Neonate (< 30 days) | 1,902 (54.1) |
| Infant (31 –365 days) | 1,032 (29.3) |
| Child (366 days –18 years) | 583 (16.6) |
| Weight-for-Age z-score | -1.13 ± 1.38 |
| Underweight (weight-for-age z-score < -2) | 885 (25.2) |
| Normal (-2 ≤ weight-for-age z-score ≤ 2) | 2,287 (65.0) |
| Overweight (weight-for-age z-score > 2) | 43 (1.2) |
| Unknown | 302 (8.6) |
| Cardiac Diagnosis | |
| Structural heart disease | 3,363 (95.6) |
| Non-Structural heart disease | 97 (2.8) |
| Unknown | 57 (1.6) |
| Cyanotic Lesion | |
| Yes | 2,853 (81.1) |
| No | 607 (17.3) |
| Unknown | 57 (1.6) |
| Genetic anomaly | 238 (6.8) |
| Pre-ECMO | |
| Pre-ECMO Mechanical support | 64 (1.8) |
| On ECMO | |
| Duration of ECMO, hours | 104 (64-167) |
| Pump flow at either 4th hour or 24th hour, cc/min | |
| < 200 cc/min | 163 (4.6) |
| ≥ 200 cc/min | 3,282 (93.3) |
| Missing | 72 (2.0) |
| Pump flow at either 4th hour or 24th hour, cc/kg/min | |
| > 75th percentile | 1,056 (30.0) |
| ≤ 75th percentile | 2,380 (67.7) |
| Missing | 81 (2.3) |
| Additional cannulae | 272 (7.7) |
| Cannulation site | |
| Chest cannulation | 2,924 (83.1) |
| Neck cannulation | 411 (11.7) |
| Groin or other | 44 (1.3) |
| Unknown | 138 (3.9) |
| Presence of left atrial cannula | 350 (10.0) |
| Clots: Any | 919 (26.1) |
| Arterial limb | 558 (15.9) |
| Oxygenator | 415 (11.8) |
| Bridge | 126 (3.6) |
| Bladder | 193 (5.5) |
| Hemofilter | 160 (4.5) |
| Clinical Outcomes | |
| Recovery to decannulation | 2,519 (71.6) |
| Death at decannulation | 987 (28.1) |
| Alive at discharge | 1,646 (46.8) |
| Procedural Factors | |
| STS risk category | |
| 1 to 3 | 1,419 (40.3) |
| 4 or 5 | 2,005 (57.0) |
| Unknown | 93 (2.6) |
| CPB time, minutes | 193 (132-282) |
Abbreviations: CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; STS, Society of Thoracic Surgeons.
Data are presented as N (%) for categorical variables and Median (Interquartile range) or Mean ± Standard deviation, as appropriate, for continuous variables.
Stroke and Outcomes
Surgical patients had a 12.3% incidence of stroke with high rates of hemorrhagic stroke (10%), and low rates of ischemic stroke (3.3%). Patients with stroke experienced a greater rate of death at decannulation (52% vs. 25% without stroke, p<0.0001) and in-hospital mortality (72% vs. 51% without stroke, p<0.0001) (Figure 1); the overall in-hospital mortality rate for cardiac surgical patients requiring ECMO was 53%.
Figure 1.
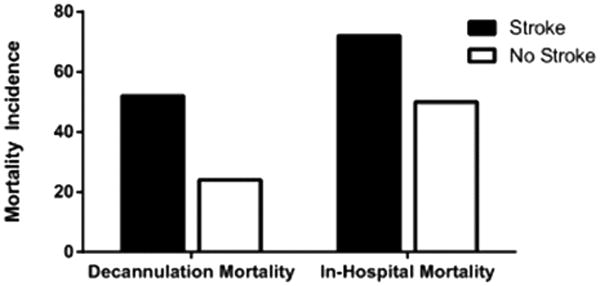
Mortality in cardiac surgical ECMO patients with and without stroke. Values are expressed as the percentage of patients with and without stroke who experienced mortality at the conclusion of ECMO and during their ECMO hospitalization (both p<0.0001). Dark bars represent patients with stroke (n=434), white bars represent patients without stroke (n=3,083).
Factors Associated with Stroke
Factors associated with stroke in univariate analysis included: neonatal status, lower weight-for-age z-score, congenital heart disease, cyanotic heart disease, freedom from genetic anomalies, pre-ECMO other mechanical support, longer ECMO duration (Figure 2), higher ECMO pump flow, neck cannulation, circuit component thrombosis, higher STS morbidity category, and shorter CBP time (Table 2). In multivariable analysis, neonatal status, lower weight-for-age z-score, and longer ECMO duration (Table 2 and Figure 3) remained independent risk factors for stroke. The analysis also suggests that pre-ECMO mechanical support associates with subsequent stroke on ECMO, but because less than 2% of the patients in the cohort had this risk factor, the adjusted odds ratio did not reach statistical significance (adjusted odds ratio 2.17, 95% confidence interval 0.98-4.82; p=0.06).
Figure 2.
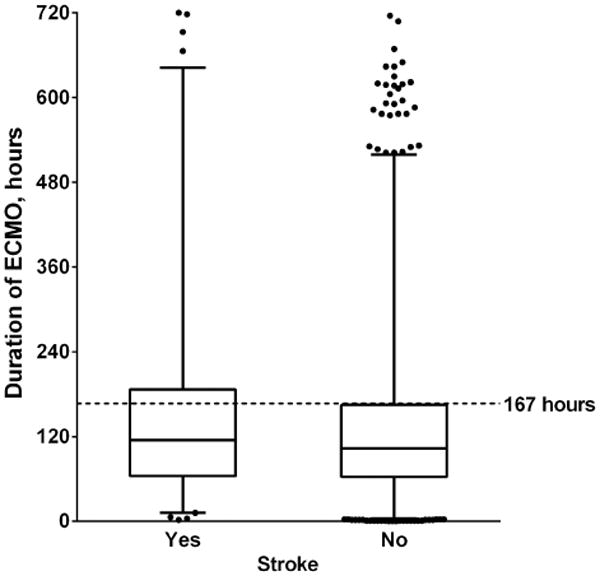
ECMO duration in patients with and without stroke. The middle line represents the median ECMO duration in each group; the bottom and top of the box represent the 25th and 75th percentiles, respectively; the whiskers represent the 1st and 99th percentiles. Scattered points represent outliers. The dotted line represents the 75th percentile for ECMO duration in the entire cohort (167 hours).
Table 2. Factors associated with Stroke in Cardiac Surgical patients (N=3,517).
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Characteristics | Odds Ratio | 95% CI | P-valuea | Odds Ratio | 95% CI | P-valueb |
| Neonate (< 30 days) | 1.73 | 1.41, 2.14 | <.0001 | 1.77 | 1.32, 2.36 | 0.0001 |
| Weight-for-age z-score (for each one-point decrease) | 1.06 | 0.98, 1.15 | 0.13 | 1.14 | 1.04, 1.25 | 0.004 |
| Cyanotic disease | 1.55 | 1.16, 2.11 | 0.004 | 1.26 | 0.86, 1.84 | 0.23 |
| Genetic anomaly | 0.71 | 0.44, 1.09 | 0.13 | 0.66 | 0.38, 1.15 | 0.14 |
| Pre-ECMO | ||||||
| Pre-ECMO mechanical support | 1.66 | 0.84, 3.02 | 0.12 | 2.17 | 0.98, 4.82 | 0.06 |
| On ECMO | ||||||
| Duration of ECMO ≥ 167 hours (upper quartile) | 1.45 | 1.16, 1.80 | 0.001 | 1.38 | 1.06, 1.78 | 0.02 |
| Pump flow at either 4th hour or 24th hour (> 75th %tile cc/kg/min) | 1.30 | 1.05, 1.60 | 0.02 | 1.10 | 0.85, 1.41 | 0.47 |
| Cannulation site: Neck | 1.38 | 1.03, 1.83 | 0.03 | 1.29 | 0.91, 1.81 | 0.15 |
| Clots: Any | 1.18 | 0.94, 1.47 | 0.14 | 1.00 | 0.77, 1.29 | 0.97 |
| Procedural | ||||||
| STS risk category: 4 or 5 | 1.32 | 1.07, 1.64 | 0.01 | 1.03 | 0.79, 1.34 | 0.83 |
| Cardiopulmonary bypass time < 132 min (lower quartile) | 1.34 | 1.06, 1.69 | 0.01 | 1.27 | 0.98, 1.64 | 0.07 |
Abbreviations: CI, confidence interval; ECMO, extracorporeal membrane oxygenation; STS, Society of Thoracic Surgeons.
P-value from univariate logistic regression.
P-value from multivariable logistic regression.
Figure 3.
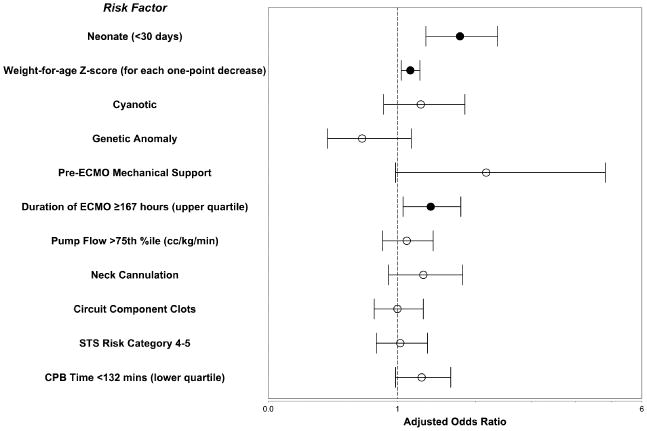
Factors associated with stroke in pediatric cardiac surgical patients on ECMO. The circle represents the adjusted odds ratio of each risk factor by multivariate analysis (closed circles are statistically significant, p<0.05); the whiskers represent the 95% confidence interval. CPB, Cardiopulmonary Bypass; STS, Society of Thoracic Surgeons.
Comment
This is the first multicenter analysis of stroke epidemiology in pediatric cardiac surgical patients on ECMO. We showed the incidence of hemorrhagic stroke is higher and the incidence of ischemic stroke is lower in cardiac surgical patients compared to previously published rates in mixed cohorts of children on ECMO (4), and that the risk of death is greatly increased in patients with stroke. Neonatal age, lower weight-for-age z-score, and longer ECMO duration were independently associated with stroke in our cohort.
These risk factors are not modifiable, and the findings cannot inform practice change at this time. This study does identify the population of pediatric cardiac surgical patients at highest risk of stroke, offering an important foundation for further investigation. Additionally, our epidemiologic findings suggest performance benchmarks for ECMO centers. Attention must now shift to collecting the necessary data on ECMO practices that may contribute to stroke incidence and outcomes in order to identify best practices. Since early detection and prevention of stroke could lead to improved outcomes, neuromonitoring strategies and anticoagulation management on ECMO represent two domains where practice change could significantly impact patients.
As many patients require deep sedation or neuromuscular blockade while on ECMO, signs of stroke may not be clinically apparent and neuromonitoring may be necessary to prevent impending strokes (12). ELSO recommends daily head ultrasounds for 5 days in neonates with respiratory failure and pre-discharge neuroimaging in all children who survive ECMO (13). A recent systematic review of neuromonitoring during ECMO shows vast heterogeneity in techniques including neuroimaging, electroencephalography, cranial Doppler ultrasound, cerebral oximetry, and plasma neuroinjury biomarkers (14). While these techniques show promise and may lead to earlier detection of neurologic injury, only cranial ultrasound is supported by available evidence. Identifying the neuromonitoring techniques at centers with the lowest incidence of stroke mortality may provide insight into best practices.
Another important knowledge gap relates to anticoagulation practices and blood product utilization – potentially modifiable processes related to stroke incidence – which vary by center (15-16). Previous studies suggest benefits from using direct thrombin inhibitors as first line anticoagulation in pediatric and adult patients on ECMO compared to unfractionated heparin (17-18). It remains unclear if regular use of these medications will lead to reduction in stroke incidence. Procoagulant medications represent another strategy for reducing hemorrhage in patients on ECMO, but may increase stroke risk. Debate persists regarding the value of adjunctive use of aminocaproic acid in patients on ECMO, and the literature reveals mixed results (19-20). Additional procoagulants, particularly recombinant activated factor VII have been used successfully to control surgical bleeding in pediatric ECMO. However, the risk of stroke associated with its use has not yet been defined (21). Determining the relative risks and benefits of anti- and procoagulants is a high-priority of the ECMO community, and may provide actionable data for centers looking to reduce stroke rates.
In light of our findings that stroke risk increases significantly with longer ECMO duration, alternative forms of mechanical support (VAD vs. continued ECMO) must be considered when myocardial recovery and weaning from ECMO appears unlikely. The decision to transition from ECMO to VAD is complicated and varies by clinician and center, but should be informed by an understanding of complication risks with both modes of therapy, the likelihood of myocardial recovery, and the relative benefits of VAD therapy including weaning from mechanical ventilation and sedation, and initiating rehabilitation. Stroke risk factors in pediatric VAD patients include female gender, pump replacement for thrombus, and the risk of stroke decreases incrementally as time on support progresses (22-23). As both VAD and ECMO therapies evolve, the risks for neurologic complications over time using either mode of mechanical circulatory support should be re-examined and compared in order to guide decision-making to select the optimal approach at different time points in a patient's illness (24).
Contrary to our hypothesis, circuit flow, ECMO cannulation techniques, and presence of thromboses within the circuit were not associated with stroke. Neonates who have undergone CPB may be prone to increased fluctuations in cerebral blood flow during high and low circuit flow (10, 25), therefore we suspected that flow rates may be associated with stroke. However, we found no relationship between ECMO flow and stroke, though this may be due to limitations in measuring the exposure variable; we were limited to assessing flow rates as maximum and minimum within defined time periods, thus could not account for the factor of time on this variable.
Neck cannulation has been shown in other cohorts to be highly associated with neurologic injury in ECMO patients (6), though we found no association in our cohort. The link between neck cannulation and stroke has been hypothesized to be due to carotid artery ligation and increased jugular venous pressures (26-27). However, our highest risk patients for stroke (neonates) were more often cannulated through the chest in the post-operative period (86%). The distribution of this exposure and collinearity with other factors complicates the analysis and cannulation site is largely dictated by clinical circumstances; there is likely confounding by indication. A study of neonates with congenital heart disease requiring ECMO support showed a similar null association between neck cannulation and stroke (28).
Our analysis revealed no independent association between circuit component thromboses and stroke in our cohort. To our knowledge, we are the first to evaluate this exposure. Prior analysis of the ELSO registry demonstrates decreasing rates of ECMO circuit component failures over time, and this complication is not related to center volume (3, 5). However, increased experience at a center may lead to proficiency with recognizing and preventing ECMO complications. Higher volume centers have better outcomes (29-30) despite similar rates of circuit component failures. It is possible that high-volume centers have well-defined practices regarding the management of circuit thrombosis, which in-turn decrease the likelihood of clot embolization. Data on the management of circuit thrombosis are not collected within the database and we did not have access to center volume, thus we could not evaluate these relationships in this study.
Limitations
Limitations of our study include those common to observational studies: limited data availability and confounding by indication are notable examples. Even considering those limitations, the ELSO registry is the foremost data repository for outcomes analysis in patients requiring ECMO, and is the best source for estimating the clinical epidemiology of complications. Stroke detection in this patient population is complicated, as routine neuroimaging is not universal and clinical assessment is complicated by use of continuous sedation and neuromuscular blockade. Thus, stroke incidence may be underreported in the database. Conversely, each stroke reported in the database is defined by radiological findings, making it difficult to ascertain the clinical and functional implications of these events. Also, as noted earlier, the indication for neuroimaging and presence of symptoms are not reported and centers have varied practices on routine neuroimaging and monitoring in ECMO patients.
As noted previously, data on specific clinical practices, important modifiable risk factors to understand in this population, are not collected in the ELSO registry. Once these data begin to be collected, hierarchical analyses that assess both center- and patient-level exposures may shed light on the best strategies for preventing stroke. We tried to assess variables existing in the database that might be related to modifiable practices – pump flow rates, cannulation site, and circuit thromboses – but none were associated with the primary outcome.
Conclusions
Stroke remains one of the most clinically important complications of ECMO leading to cessation of support and long-term sequelae for survivors. We described the epidemiology of stroke in pediatric cardiac surgical patients on ECMO and identified patients at highest risk for stroke after cardiac surgery. Several questions remain regarding the optimal approach to anticoagulation and neuromonitoring in children who require ECMO after cardiac surgery. Multicenter efforts to collect data on modifiable clinical practices are necessary to improve outcomes in these critically-ill children.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haines NM, Rycus PT, Zwischenberger JB, Bartlett RH, Ündar A. Extracorporeal life support registry report 2008: Neonatal and pediatric cardiac cases. ASAIO Journal. 2009;55(1):111–116. doi: 10.1097/MAT.0b013e318190b6f7. [DOI] [PubMed] [Google Scholar]
- 2.Brown KL, Ichord R, Marino BS, Thiagarajan RR. Outcomes following extracorporeal membrane oxygenation in children with cardiac disease. Pediatric Critical Care Medicine. 2013;14:S73–S83. doi: 10.1097/PCC.0b013e318292e3fc. [DOI] [PubMed] [Google Scholar]
- 3.Fleming GM, Gurney JG, Donohue JE, Remenapp RT, Annich GM. Mechanical component failures in 28,171 neonatal and pediatric extracorporeal membrane oxygenation courses from 1987 to 2006. Pediatric Critical Care Medicine. 2009;10(4):439–444. doi: 10.1097/PCC.0b013e318198b275. [DOI] [PubMed] [Google Scholar]
- 4.Hervey-Jumper SL, Annich GM, Yancon AR, Garton HJ, Muraszko KM, Maher CO. Neurological complications of extracorporeal membrane oxygenation in children. Journal of Neurosurgery: Pediatrics. 2011;7(4):338–344. doi: 10.3171/2011.1.PEDS10443. [DOI] [PubMed] [Google Scholar]
- 5.Zwischenberger JB, Nguyen TT, Upp JR, Jr, et al. Complications of neonatal extracorporeal membrane oxygenation Collective experience from the extracorporeal life support organization. J Thorac Cardiovasc Surg. 1994;107(3):838–849. [PubMed] [Google Scholar]
- 6.Werho D, Pasquali S, Yu S, et al. Hemorrhagic complications in pediatric cardiac patients on extracorporeal membrane oxygenation: An analysis of the extracorporeal life support organization registry. Pediatric Critical Care Medicine. 2015;16(3):276–288. doi: 10.1097/PCC.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliver WC. Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth. 2009;13(3):154–175. doi: 10.1177/1089253209347384. [DOI] [PubMed] [Google Scholar]
- 8.Domi T, Edgell DS, McCrindle BW, et al. Frequency, predictors, and neurologic outcomes of vaso-occlusive strokes associated with cardiac surgery in children. Pediatrics. 2008;122(6):1292–1298. doi: 10.1542/peds.2007-1459. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Zimmerman RA, Jarvik GP, et al. Perioperative stroke in infants undergoing open heart operations for congenital heart disease. Ann Thorac Surg. 2009;88(3):823–829. doi: 10.1016/j.athoracsur.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominguez TE, Wernovsky G, Gaynor JW. Cause and prevention of central nervous system injury in neonates undergoing cardiac surgery. Semin Thorac Cardiovasc Surg. 2007;19(3):269–277. doi: 10.1053/j.semtcvs.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs ML, O'Brien SM, Jacobs JP, et al. An empirically based tool for analyzing morbidity associated with operations for congenital heart disease. J Thorac Cardiovasc Surg. 2013;145(4):1046–1057.e1. doi: 10.1016/j.jtcvs.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bembea MM. Neuromonitoring of neonatal extracorporeal membrane oxygenation patients using serial cranial ultrasounds. Pediatr Crit Care Med. 2013;14(9):903–904. doi: 10.1097/PCC.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.2013 ELSO Guidelines for Neonatal Respiratory Failure. [Accessed April 12, 2015]; Available at https://www.elso.org/Portals/0/IGD/Archive/FileManager/8588d1a580cusersshyerdocumentselsoguidelinesforneonatalrespiratoryfailure13.pdf.
- 14.Bembea MM, Felling R, Anton B, Salorio CF, Johnston MV. Neuromonitoring during extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2015 doi: 10.1097/PCC.0000000000000415. Online Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 15.Annich G, Adachi I. Anticoagulation for pediatric mechanical circulatory support. Pediatric Critical Care Medicine. 2013;14:S37–S42. doi: 10.1097/PCC.0b013e318292dfa7. [DOI] [PubMed] [Google Scholar]
- 16.Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation. Pediatric Critical Care Medicine. 2013;14(2):e77–e84. doi: 10.1097/PCC.0b013e31827127e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pieri M, Agracheva N, Bonaveglio E, et al. Bivalirudin versus heparin as an anticoagulant during extracorporeal membrane oxygenation: A case-control study. J Cardiothorac Vasc Anesth. 2013;27(1):30–34. doi: 10.1053/j.jvca.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Pappalardo F, Agracheva N, Covello RD, et al. Anticoagulation for critically ill cardiac surgery patients: Is primary bivalirudin the next step? J Cardiothorac Vasc Anesth. 2014;28(4):1025–1029. doi: 10.1053/j.jvca.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Downard CD, Betit P, Chang RW, Garza JJ, Arnold JH, Wilson JM. Impact of amicar on hemorrhagic complications of ecmo: A ten-year review. J Pediatr Surg. 2003;38(8):1212–1216. doi: 10.1016/s0022-3468(03)00270-7. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JM, Bower LK, Fackler JC, Beals DA, Bergus BO, Kevy SV. Aminocaproic acid decreases the incidence of intracranial hemorrhage and other hemorrhagic complications of ecmo. J Pediatr Surg. 1993;28(4):536–540. doi: 10.1016/0022-3468(93)90612-o. [DOI] [PubMed] [Google Scholar]
- 21.Veldman A, Neuhaeuser C, Akintuerk H, et al. RFVIIa in the treatment of persistent hemorrhage in pediatric patients on ecmo following surgery for congenital heart disease. Paediatr Anaesth. 2007;17(12):1176–1181. doi: 10.1111/j.1460-9592.2007.02328.x. [DOI] [PubMed] [Google Scholar]
- 22.Jordan LC, Ichord RN, Reinhartz O, et al. Neurological complications and outcomes in the berlin heart excor(r) pediatric investigational device exemption trial. Journal of the American Heart Association. 2015;4(1):e001429–e001429. doi: 10.1161/JAHA.114.001429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrnes JW, Prodhan P, Williams BA, et al. Incremental reduction in the incidence of stroke in children supported with the berlin excor ventricular assist device. Ann Thorac Surg. 2013;96(5):1727–1733. doi: 10.1016/j.athoracsur.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Mohite PN, Sabashnikov A, Patil NP, et al. Short-term ventricular assist device in post-cardiotomy cardiogenic shock: Factors influencing survival. Journal of Artificial Organs. 2014;17(3):228–235. doi: 10.1007/s10047-014-0773-1. [DOI] [PubMed] [Google Scholar]
- 25.Polito A, Barrett CS, Wypij D, et al. Neurologic complications in neonates supported with extracorporeal membrane oxygenation. An analysis of elso registry data. Intensive Care Med. 2013;39(9):1594–1601. doi: 10.1007/s00134-013-2985-x. [DOI] [PubMed] [Google Scholar]
- 26.O'Connor TA, Haney BM, Grist GE, Egelhoff JC, Snyder CL, Ashcraft KW. Decreased incidence of intracranial hemorrhage using cephalic jugular venous drainage during neonatal extracorporeal membrane oxygenation. J Pediatr Surg. 1993;28(10):1332–1335. doi: 10.1016/s0022-3468(05)80323-9. [DOI] [PubMed] [Google Scholar]
- 27.Teele SA, Salvin JW, Barrett CS, et al. The association of carotid artery cannulation and neurologic injury in pediatric patients supported with venoarterial extracorporeal membrane oxygenation. Pediatric Critical Care Medicine. 2014;15(4):355–361. doi: 10.1097/PCC.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 28.Polito A, Barrett CS, Rycus PT, Favia I, Cogo PE, Thiagarajan RR. Neurologic injury in neonates with congenital heart disease during extracorporeal membrane oxygenation. ASAIO Journal. 2015;61(1):43–48. doi: 10.1097/MAT.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 29.Freeman CL, Bennett TD, Casper TC, et al. Pediatric and neonatal extracorporeal membrane oxygenation: Does center volume affect mortality. Crit Care Med. 2014;42(3):512–519. doi: 10.1097/01.ccm.0000435674.83682.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karamlou T, Vafaeezadeh M, Parrish AM, et al. Increased extracorporeal membrane oxygenation center case volume is associated with improved extracorporeal membrane oxygenation survival among pediatric patients. J Thorac Cardiovasc Surg. 2013;145(2):470–475. doi: 10.1016/j.jtcvs.2012.11.037. [DOI] [PubMed] [Google Scholar]


