Abstract
Three experiments examined the relation between working memory capacity (WMC) and two different forms of cognitive conflict: stimulus-stimulus (S-S) and stimulus-response (SR) interference. Our goal was to test whether WMC’s relation to conflict-task performance is mediated by stimulus-identification processes (captured by S-S conflict), response-selection processes (captured by S-R conflict), or both. In Experiment 1, subjects completed a single task presenting both S-S and S-R conflict trials, plus trials that combined the two conflict types. We limited ostensible goal-maintenance contributions to performance by requiring the same goal for all trial types and by presenting frequent conflict trials that reinforced the goal. WMC predicted resolution of S-S conflict as expected: Higher-WMC subjects showed reduced response time interference. Although WMC also predicted S-R interference, here, higher-WMC subjects showed increased error interference. Experiment 2A replicated these results in a version of the conflict task without combined S-S/S-R trials. Experiment 2B increased the proportion of congruent (non-conflict) trials to promote reliance on goal-maintenance processes. Here, higher-WMC subjects resolved both S-S and S-R conflict more successfully than did lower-WMC subjects. The results were consistent with Kane and Engle’s (2003) two-factor theory of cognitive control, according to which WMC predicts executive-task performance through goal-maintenance and conflict-resolution processes. However, the present results add specificity to the account by suggesting that higher-WMC subjects better resolve cognitive conflict because they more efficiently select relevant stimulus features against irrelevant, distracting ones.
Keywords: working memory capacity, executive control, cognitive control, interference, individual differences
…our initial specification of the central executive was so vague as to serve as little more than a ragbag into which could be stuffed all the complex strategy selection, planning, and retrieval checking that clearly goes on when subjects perform even the apparently simple digit span task. This still seems a sensible way of starting to explore working memory, as it accepts the complexities and the ultimate need to explain them, while concentrating on analysing the simpler and presumably more tractable slave systems. (Baddeley, 1996, p. 6)
Working memory’s “executive” processes are typically characterized as general-purpose mechanisms that control and coordinate the activities of subordinate brain networks in the service of complex, goal-directed behavior (e.g., Baddeley, 1986; Norman & Shallice, 1986). As Baddeley (1996) recognized, however, such broad characterizations may offer little more than an ill-specified homunculus that masks the extent of our ignorance about the self-regulation of cognition and behavior (see also Burgess, 1997; Towse & Houston-Price, 2001). The present work takes a combined psychometric-experimental approach toward incrementally specifying the nature of executive control and its contribution to working memory capacity (WMC). Our logic follows Cronbach (1957) and Underwood (1975) in suggesting that psychological theories can be developed and tested by considering individual differences in the constructs of interest, especially in the ways that they interact with experimental treatments and elicit patterns of convergent and discriminant validity in their selective associations with other constructs (Campbell & Fiske, 1959; Cronbach & Meehl, 1955; Kosslyn et al., 2002; Vogel & Awh, 2008). In order to specify the central executive’s nomological network, then, we will seek its correlational boundary conditions. That is, we will explore whether individual differences in WMC are differentially associated with the control processes elicited by distinct forms of cognitive conflict.
Fractionating the Central Executive
Our approach has some parallels to the fruitful research program initiated by Miyake et al. (2000) into the “unity and diversity” of executive functions thought to be linked to the brain’s frontal lobes (for a review, see Miyake & Friedman, 2012). Their correlational studies have used latent-variable, factor-analytic techniques to indicate the individual-differences variance that is shared versus not shared among theoretically derived cognitive and neuropsychological tasks (i.e., to demonstrate both convergent and discriminant validity). Three broad domains, or factors, of executive functions have thus been identified: inhibiting, switching, and memory updating. These factors are partially dissociable in their correlations with one another and in their differential predictions of cognitive and behavioral outcomes (e.g., Friedman et al., 2006; 2007), indicating that the executive control construct is not monolithic. At the same time, all three factors also share considerable common variance, and this common executive factor is highly heritable and strongly predicts intelligence and externalizing behavior problems, indicating some domain general mechanisms of cognitive control (Friedman et al., 2008; Miyake & Friedman, 2012; Young et al., 2009). Of most importance here, this line of “unity and diversity” work began from relatively informal, descriptive conceptions of executive functions and, through individual-differences findings alone, has progressed to facilitate the preliminary development of formal theoretical models to instantiate different control processes (e.g. Chatham et al., 2011).
While similarly relying on individual-differences analyses, the present study more narrowly examines how normal variation in WMC, as assessed by complex memory span tasks, responds to different experimental manipulations of conflict within a single response-time (RT) task. Complex span tasks require subjects to immediately recall short lists of items that are interleaved with an unrelated processing task. These tasks are of theoretical interest because their performance predicts a variety of important cognitive capabilities, such as reading comprehension (e.g., Daneman & Carpenter, 1980; McVay & Kane, 2012a), learning (Kyllonen & Stephens, 1990; Shute, 1991), multitasking (e.g., Bühner, König, Pick, & Krumm, 2006; Hambrick, Oswald, Darowski, Rench, & Brou, 2010), and reasoning through novel intellectual problems (e.g., Kane, Hambrick, & Conway, 2005; Oberauer, Schulze, Wilhelm, & Süß, 2005). As well, normal variation in WMC predicts how well people perform seemingly simple attention-control tasks, such as dichotic listening and antisaccade (e.g., Colflesh & Conway, 2007; Conway, Cowan, & Bunting, 2001; Kane, Bleckley, Conway, & Engle, 2001; Unsworth, Schrock, & Engle, 2004). The apparent generality of WMC’s predictive power, and its association with lower-level attention capabilities, has led some theorists to propose that a small number of domain-free attention or executive functions largely drive WMC variation and its covariation with other abilities (e.g., Barrouillet & Camos, 2012; Engle & Kane, 2004; Hasher, Lustig, & Zacks, 2007).
Consistent with the Miyake-Friedman “unity and diversity” findings described above (e.g. Miyake et al., 2000; Miyake & Friedman, 2012), however, recent individual-differences research indicates that WMC variation and covariation may fundamentally reflect a number of core abilities beyond attention control (e.g., Shipstead, Lindsay, Marshall, & Engle, 2014; Unsworth & Engle, 2007a, 2007b; Unsworth & Spillers, 2010), and also that WMC correlates selectively with only some attentional-control processes and not others. Many studies have yielded associations between WMC measures and attention tasks that elicit some form of conflict between target versus distractor stimuli, or between habitual versus required responses (e.g., Ahmed & de Fockert, 2012; Bleckely, Durso, Crutchfield, Engle, & Khanna, 2003; Colzato, Spapé, Pannebakker, & Hommel, 2007; Conway et al., 2001; Heitz & Engle, 2007; Hutchinson, 2011; Kane & Engle, 2003; Long & Prat, 2002; McVay & Kane, 2009, 2012b; Meier & Kane, 2013; Morey et al., 2012; Redick & Engle, 2006; Unsworth et al., 2004; but see Keye, Wilhelm, Oberauer, & van Ravenzwaaij, 2009; Redick, Calvo, Gay, & Engle, 2011). In sharp contrast, WMC does not appear to correlate with performance of prototypical visual search tasks – even those thought to involve top-down influences (Kane, Poole, Tuholski, & Engle, 2006; Poole & Kane, 2009; Sobel, Gerrie, Poole, & Kane, 2007; but see Anderson, Vogel, & Awh, 2013) – unless those tasks require constraining search to only particular locations or subtle stimulus dimensions (Poole & Kane, 2009; Sobel et al., 2007). Similarly, in the auditory domain, a meta-analysis conducted by Sorqvist et al. (2013) suggests a dissociation between WMC variation and two types of auditory distraction. WMC appears to be uniquely related to oddball auditory distractions (with higher-WMC subjects showing less of a cost to the oddball distraction), but it does not correlate with susceptibility to more continuous forms of auditory distraction.
So, WMC does not predict performance in all varieties of attention-control tasks. We view findings like these, which point to the discriminant validity of WMC, as being especially important for specifying the executive-control construct. Through an inductive process of identifying boundary conditions to the association between WMC and attention control, we may eventually reverse-engineer the central executive (at least in part) to gain traction regarding the specific control processes it encompasses and how these processes relate to broader cognitive abilities.
Specifying the Executive Attention Account of WMC
In addition to the attentional control tasks mentioned above that are sensitive to WMC variation (i.e., dichotic listening, antisaccade, constrained search, and auditory distraction), research into the executive attention account of WMC has focused on the relation between WMC measures and executive-control tasks that elicit cognitive conflict, such as Stroop (1935) and flanker (Eriksen & Eriksen, 1974) tasks. These tasks demand control, in part, by requiring the flexible regulation of behavior in the pursuit of endogenous goals (Egner, 2008), typically by presenting subjects with multiple sources of information, some task-relevant and some irrelevant. When relevant and irrelevant task information elicit competing responses, cognitive conflict is generated. The general pattern of results from investigations of the relation between WMC and cognitive control is that the higher the subject’s WMC, the better he or she performs on cognitive control measures. According to the executive attention account (Engle & Kane, 2004; Kane & Engle, 2003; Kane et al., 2007; Unsworth, Spillers, Brewer, & McMillan, 2011), WMC counters interference in two ways: proactively keeping task goals accessible (i.e., resisting lapses of attention) and reactively resolving cognitive conflict (i.e., resolve interference online). Here, we are primarily focused on the latter factor of in-the-moment conflict resolution.
Executive control (or “cognitive control”) is a broad, arguably ambiguous construct (Braver, Gray, & Burgess, 2007; Funes, Lupianez, & Humphrey, 2010a) that refers to mental processes ostensibly operating from perception to action. Therefore, linking WMC to cognitive control allows WMC many possible entry points into information processing. To move WMC theory forward, we must further specify how WMC works to resolve cognitive conflict. To understand how WMC impacts performance (and to better understand cognitive control, itself), it should be helpful to decompose cognitive-control tasks into subcomponents that more clearly map onto aspects (or stages) of information processing, analogous to Baddeley’s (1996) call and effort to “fractionate” the central executive component of the working memory system.
The Dimensional Overlap Taxonomy
Kornblum’s dimensional overlap taxonomy (Kornblum, 1992; Kornblum, Hasbroucq, & Osman, 1992) provides a potential blueprint for decomposing cognitive-control tasks into separable processing elements. Tasks that provide conflicting information about which stimulus is the target or which response is to be made on a given trial are often called cognitive-control tasks because control is presumably needed to counter the interference elicited by the conflicting information. According to Kornblum, the strength of the conflict experienced by a subject is the result of the degree to which opposing task dimensions overlap. Here, overlap refers to how perceptually, conceptually, or structurally similar the sets (i.e., the entire stimulus and response sets) and the elements (i.e., individual items within the stimulus and response sets) are to one another (Kornblum & Lee, 1995). That is, the more overlap there is among conflicting task sets and elements, the more the irrelevant task element (i.e., the feature that provides information that will lead to an incorrect response) will disrupt performance.
Kornblum’s dimensional overlap taxonomy distinguishes choice-RT tasks based not on conflict strength, but rather on the task dimensions that provide the conflicting information. Conflict may arise between relevant and irrelevant stimulus dimensions (stimulus-stimulus, or S-S conflict), between irrelevant stimulus dimensions and relevant response dimensions (stimulus-response, or S-R conflict), or between irrelevant stimulus dimensions and both relevant stimulus and response dimensions (S-S plus S-R conflict). Information processing is often characterized by at least three broad (and further decomposable) stages: stimulus identification, response selection, and motor execution (e.g., Proctor & Vu, 2003). Accordingly, different types and combinations of dimensional overlap create different processing problems: S-S conflict impedes stimulus selection and/or identification, whereas S-R conflict interferes with response selection and/or motor execution.
To test whether S-S and S-R conflicts are independent of each other, Kornblum (1994) had subjects respond to stimuli that varied on relevant stimulus, irrelevant stimulus, and response dimensions. Color words or neutral words appeared in the center of a rectangle, which was divided into colored (red or green) and dark halves. The color of the rectangle was the relevant stimulus dimension, the words were irrelevant stimulus dimensions and the side of rectangle that was colored (left, right, top, and bottom) was also an irrelevant stimulus dimension. Subjects responded to color by pressing left or right keys (e.g., left key for a red patch), and completed four conflict-type blocks: pure S-S (color word was congruent or incongruent with the color patch; the color patch was presented on the top or bottom of the rectangle, making these neutral trials on the S-R dimension), pure S-R (color patch location was congruent or incongruent with the left-right response; the words were color-neutral), mixed S-S and S-R (presenting both S-S-only and S-R-only trials), and a combined-type S-S/S-R block where the irrelevant stimulus dimension overlapped with both the relevant stimulus dimension and the response (e.g., trials presenting a red color patch on right side of the rectangle [when a left response is called for] and the word GREEN in the rectangle’s center). Kornblum found that S-S and S-R effects were additive on trials that contained both and interpreted this as support for independence of S-S and S-R conflict (but see Hommel, 1997).
Additional behavioral evidence for the utility of the dimensional overlap taxonomy comes from investigations of congruency-sequence effects, in which the prior trial’s congruency modifies the magnitude of the present trial’s congruency effect (Funes, Lupianez, & Humphrey, 2010a, 2010b). Subjects performed series of mixed S-S and S-R trials (mixed with congruent trials presenting no conflict). Carry-over effects of control were conflict-specific: S-S interference was reduced after incongruent S-S trials but not after incongruent S-R trials (and vice versa), suggesting that the control processes initiated by the preceding incongruent trial were specialized for responding to that particular type of conflict (see also Verbruggen, Liefooghe, Notebaert, & Vandierendonck, 2005, Experiment 2). Time-course data also suggest distinct origins for S-S and S-R conflict, as S-R interference tends to decrease as RTs get longer, whereas S-S interference tends to increase with increasing RTs (Pratte, Rouder, Morey, & Feng, 2010). Finally, neuroimaging data further suggest S-S versus S-R dissociations, with hemodynamic responses in parietal cortex areas particularly associated with S-S conflict, and activity in the motor cortex more strongly associated with S-R conflict (Egner, Delano, & Hirsch, 2007; Liston, Matalon, Hare, Davidson, & Casey, 2006; Liu, Banich, Jacobsen, & Tanabe, 2004). Here, then, we propose that the dimensional overlap taxonomy may help specify the executive-control processes that are related to WMC.
Dimensional Overlap and WMC
Higher-WMC subjects resolve interference from Stroop color-words a bit more quickly than do lower-WMC subjects in tasks that present a high proportion of incongruent trials (Kane & Engle, 2003; Long & Prat, 2002; Meier & Kane, 2013). These findings suggest that WMC affects performance, in part, through conflict-resolution processes (the accuracy advantage that higher-WMC subjects in low-incongruency Stroop tasks is argued to reflect, in part, goal-maintenance abilities; e.g., Hutchison, 2011; Kane & Engle, 2003). According to the Dimensional Overlap taxonomy, the color-word Stroop task presents both S-S and S-R conflict: The irrelevant stimulus dimension (word identity) overlaps with both the relevant stimulus dimension (word color; S-S conflict) and the response dimension (naming the color aloud; S-R conflict). Given the Stroop task’s complex structure involving both S-S and S-R conflict, prior WMC-related findings have an ambiguous locus. That is, it is unclear if stimulus-selection and identification processes, or response-selection processes, or both, enable higher-WMC subjects to achieve superior performance to lower-WMC subjects.
Some light may be shed on the contributions of stimulus selection by examining WMC correlations with purer S-S interference tasks. In fact, higher-WMC subjects exhibit less interference from conflicting task elements in an Eriksen-type flanker task than do lower-WMC subjects (Heitz & Engle, 2007; Redick & Engle, 2006; but see Keye, Wilhelm, Oberauer, & van Ravenzwaaij, 2009). In the Kornblum taxonomy, the typical flanker task is an S-S conflict task, because conflict is generated when response-irrelevant stimulus information (i.e., flankers) provides information opposed to the information carried by the target representation. Here, it is argued that the conflict is generated by the overlap and conflicting nature of relevant and irrelevant stimulus features. The data thus suggest that higher-WMC subjects achieve better cognitive control, in part, because they are better able to resolve conflict between stimulus dimensions than are lower-WMC subjects. In this scenario higher-WMC subjects could either be inhibiting the irrelevant stimulus dimensions (Hasher et al., 2007) or amplifying the relevant stimulus dimensions early on in the information processing sequence (Egner & Hirsch, 2005).
However, we cannot yet be sure that higher-WMC subjects’ superior flanker performance is the result of better S-S interference resolution. It remains possible that, at stimulus identification and selection, both lower and higher-WMC subjects allow the same information (both task relevant and conflicting) into the processing stream, but then higher-WMC subjects are better able to resolve the conflict at the subsequent response-selection stage (perhaps by better suppressing conflict stimulus information through inhibition at a later processing stage; Ridderinkhof, van den Wildenberg, Wijnen, & Burle, 2004). Stated differently, although conflict originates in an S-S task from the overlapping stimulus dimensions between targets and distractors, performance differences can be achieved by either blocking this conflicting information from further processing (i.e., “early,” at stimulus identification and selection) or by more efficiently resolving the resulting conflict from the distractor information after it has entered the system (i.e., “late,” at response selection). This ambiguity is the product of the information-processing sequence where stimulus-identification processes must happen before response-selection processes. Therefore, by exclusively examining individual differences in a traditional flanker task, we still cannot determine what higher-WMC subjects do to achieve their superior performance. It should also be noted that recent work by Keye and colleagues (Keye et al., 2009) has reported null relations between WMC and interference in flanker tasks, and so earlier work on WMC-flanker task performance may have overestimated their association.
In order to most effectively discriminate between the contributions of stimulus-identification and response-selection processes to the associations between WMC and conflict tasks, we should compare conflict performance on S-S-type trials to performance on trials that only present S-R interference. Then, via subtractive logic (Donders, 1869), we may judge how WMC impacts performance. More precisely, if WMC-related differences were found in an S-S conflict task, but not in a task that presents interference only at the response selection phase (S-R task), it would suggest that WMC affects performance prior to the response-selection — most likely during the stimulus identification or selection processes.
According to the Kornblum taxonomy, one way to assess S-R conflict is via the Simon effect (Simon & Small, 1969), which reflects the overlap between the irrelevant stimulus dimension and the response. The Simon effect is typically demonstrated in tasks where subjects push a right- or left-positioned key depending on the identity of the stimulus (e.g., circle or square), and the stimulus is either presented on the left or right side of the screen. S-R conflict is produced because the stimulus location is coded as left or right and so is the response: If the stimulus is on left side of the screen, the “left” response is primed, which causes interference when the relevant dimension of the stimulus actually indicates a right response. The irrelevant stimulus location and the relevant stimulus dimension (usually the shape or color of a single object) do not overlap, so there is no opportunity for conflict between these stimulus elements (i.e., there is no S-S interference). Considerable behavioral evidence indicates that that the Simon effect involves response selection and not stimulus identification (Acosta & Simon, 1976; Mewaldt, Connelly, & Simon, 1980; Simon, 1982; Simon, Acosta, Mewalt, & Speidel, 1976; Van der Molen & Keuss, 1981). For example, degrading the quality of visual stimuli, which should affect stimulus identification, does not affect the magnitude of the Simon effect (Acosta & Simon, 1976), but the size of the to-be-executed response set executed does (Mewalt, Connelly, & Simon, 1980). Therefore, the Simon task seems to provide a relatively pure window into the response-selection stage of information processing.
The evidence for WMC predicting performance in Simon tasks is mixed and limited (and seemingly more limited than that described above connecting WMC to tasks involving S-S conflict). WMC is uncorrelated with Simon effects in RTs when trials are 50% congruent (Keye, Wilhelm, Oberauer, & van Ravenzwaaij, 2009; Weldon, Mushlin, Kim, & Sohn, 2013) and 75% congruent (Miller, Watson, & Strayer, 2012, who also found no WMC effect on Simon-task errors). In an 80% congruent task, however, Weldon et al. observed a modest correlation between WMC and the Simon RT effects (r = −.22). It is notable that Weldon et al. only observed a significant WMC association in a Simon task with a high proportion of congruent trials. Optimal performance on incongruent trials in high proportion-congruency conditions puts a premium on the ability to maintain the goal of the task because on the frequent congruent trials, correct responding can be achieved by either attending to the goal-mandated target or irrelevant information (Kane & Engle, 2003). In this context, the goal is infrequently reinforced by incongruent trials, which results in slowed or erroneous responding by subjects who have not adequately maintained the task goal. WMC effects in high-congruency contexts may therefore have little to do with in-the-moment conflict resolution.; WMC effects in high-congruency contexts may therefore have little to do with in-the-moment conflict resolution. In any case, the lack of a relation between WMC and Simon effects under normal task conditions suggests that WMC is not (or is only weakly) related to performance in tasks that provide only response-related conflict.
Experiment 1 tested whether WMC helps resolve S-S interference, S-R interference, or both, within a single task to avoid the ambiguities that arise when making across-task comparisons (e.g., differences in stimuli or task approach between Flanker and Simon tasks). We limited the influence of goal-maintenance abilities and task switching by using a task that, first, had the same goal for S-S and S-R conflict trials and, second, presented enough conflict trials (i.e., 50%) to reinforce task goals and minimize goal neglect. Subjects responded to upward or downward facing arrows by pressing left- or right-positioned keys. All that distinguished S-S from S-R trials was the location of the arrows on-screen. Therefore, any relations between WMC and conflict type should reflect differences in conflict-resolution processes.
Experiment 1
Method
Here, and in the Results section that follows, we report how we determined our sample size, all data exclusions, all manipulations, and all measures in the study (Simmons, Nelson, & Simonsohn, 2012).
Subjects
Two hundred and thirty six undergraduates (aged 18–30 years) from the University of North Carolina at Greensboro (UNCG) participated and received partial credit for a psychology course requirement. The goal was to test at least 200 subjects in the course of a single semester, and our stopping rule for data collection was the end of the semester.
General Procedure
Subjects volunteered to complete three testing sessions in groups of 1 – 4, with an experimenter present throughout each session to read instructions aloud and monitor subjects’ progress through the tasks. Only the relevant tasks from the first two sessions are described and analyzed here; the other tasks were all completed after the present ones, and were designed for other studies by different sets of authors (reflecting a mix of attention and long-term memory tasks, one of which included thought probes to assess mind-wandering). On average, these two sessions (for subjects who completed both) were completed 13 days apart (SD = 12). In the first session, we administered only the Operation Span task (OSPAN), the S-S/S-R task, and then the Reading Span task (RSPAN). In the second session, the Symmetry Span task (SSPAN) was administered as the first task, followed by the tasks for other studies. Each session was scheduled for 1.5 hr. Subjects were allowed to leave when everyone in their session finished the final task (for all sessions, no task was begun until all subjects in the session had completed the preceding task). Experimenters read on-screen instructions for each task aloud while subjects read along silently. Dell desktop computers, with 17 in cathode ray tube (CRT) monitors (85 MHz refresh rate) and running E-Prime (1.2) software, presented all task stimuli and recorded all responses.
WMC Tasks
We assessed WMC with three automated “complex span” tasks (OSPAN, SSPAN, and RSPAN; Redick et al. 2012; Unsworth, Heitz, Schrock, & Engle, 2005; Unsworth, Redick, Heitz, Broadway, & Engle, 2009). In these tasks, subjects attempt to maintain or recover mental access to memory items while intermittently completing an unrelated processing task. To prevent trading off between the processing and memorial portions of the task, an individualized response deadline (M + 2.5 SDs) was used for the processing portion, calculated during 15 processing-task-only items. For the processing portion of OSPAN, subjects verified (via mouse-clicking a “yes” or “no” onscreen) solutions to compound arithmetic equations (e.g., (3 * 2) − 1 = 4). In RSPAN, subjects judged (via “yes”/“no” mouse-click) whether or not sentences made sense (e.g., "I like to run in the sky."). In SSPAN, subjects assessed (via “yes”/“no” mouse-click) whether or not 8 × 8 black-and-white matrix patterns were vertically symmetrical.
The memory items in OSPAN and RSPAN were capital letters (randomly selected without replacement on each trial from these twelve: F, H, J, K, L, N, P, Q, R, S, T, Y). A letter followed 200 ms after the response to each processing item in the task and appeared for 250 ms. After 3–7 processing-letter pairs, all 12 letters appeared onscreen in a grid formation next to a check box. Subjects identified the letters presented in serial order, via mouse click in the check box. When a subject selected a letter, it appeared on the bottom of the screen in the order it was selected (arranged left to right). Subjects were instructed to click on a “blank” button on the screen for forgotten letters, to preserve item order. Pressing the blank button placed a dash in the array of letters on the bottom of the screen. OSPAN and RSPAN presented each set length (3–7) three times in random order.
In SSPAN, 200 ms after the symmetry judgment was made, one square of a 4 × 4 grid was shaded red for 650 ms. After 2–5 symmetry judgment-grid pairs, subjects recalled the locations of the shaded squares in serial order by mouse-clicking on the squares within an empty grid. When a square was clicked, it turned red with a number inside it to indicate its serial order. As with the OSPAN and RSPAN, there was an option to click on a “blank” button. The SSPAN presented each set length (2–5) three times in random order.
In all of the complex span tasks, subjects first completed four practice trials of just the recall portion (set sizes 2 and 3), then 15 processing-portion-only practice trials, and then three combined practice trials (set size 2) of both processing and recall. Task instructions warned subjects that they must achieve 85% accuracy on the processing portion for their data to be used in the study. Between sets, the programs provided subjects with accuracy feedback for the processing and memory portions of the task for the last set and also their cumulative accuracy for processing across all preceding sets.
S-S/S-R Task
Stimuli and trial types
We modeled the S-S/S-R task after Liu et al. (2004; see also Verbruggen et al., 2005). The stimuli were black arrows on a grey background. At a viewing distance of 60 cm, the arrows each occupied approximately 2° × 2° degrees of visual angle (although we did not physically restrict viewing distance, we placed tape on the floor, 60 cm from the screen on each side of each subject’s chair, and instructed them that the chair and their eyes should stay at this distance from the screen throughout the task). The target arrows pointed either upward or downward. We instructed subjects to press one key for an up arrow and another key for a down arrow, using the Q and P keys on a QWERTY keyboard, which were approximately 15.5 cm apart. We counterbalanced the mapping of arrows to keys between subjects (i.e., for some subjects, upward-pointing arrows required a left-handed response [Q key] and downward-pointing arrows required a right-handed response [P key], and for other subjects upward-pointing arrows required a right-handed response [P] and downward arrows required left-handed response [Q]).
S-R trials were located on the horizontal axis with congruency manipulated by changing the location of the up-down arrow stimulus with regard to the response to be made. For example, for a congruent S-R trial, the stimulus that called for a left-handed response was displayed to the left of the screen’s center; for an incongruent S-R trial a stimulus that called for a left-handed response was displayed to the right of the screen’s center. S-S trials were located on the vertical axis, with congruency manipulated between the stimulus’ location on the screen (i.e., up vs. down location) and the identity of the arrow (i.e., up arrow vs. down arrow).
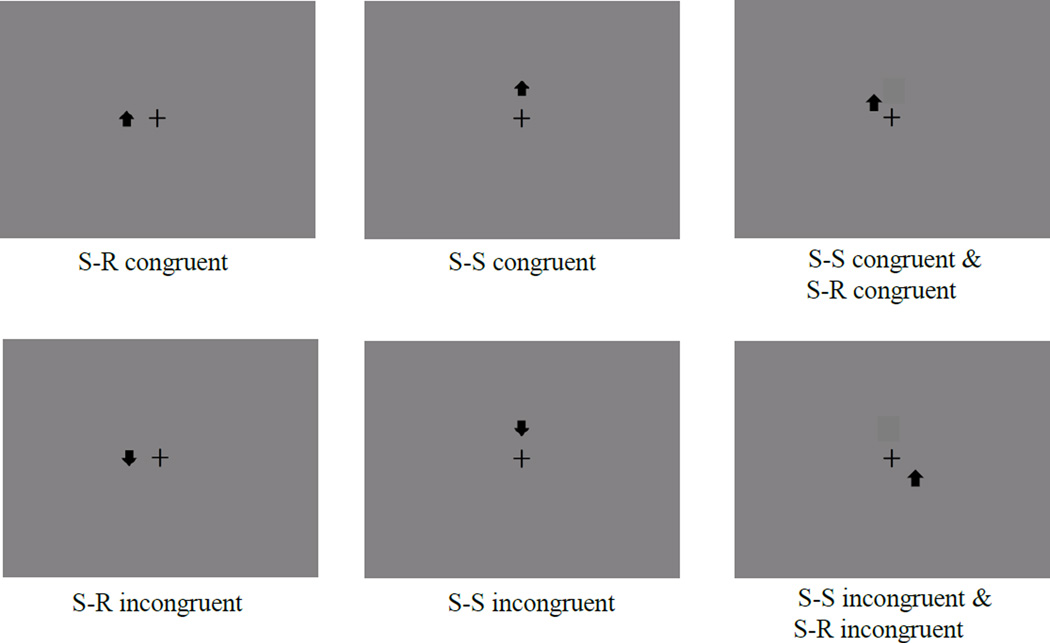
Response-Mapping practice trials presented arrows at the center of the CRT screen. For S-S/S-R task trials, the arrows appeared in eight different locations around the center of the screen. The arrows appeared approximately 2° (at a 60 cm viewing distance) from the center of the screen in one of eight locations: left, right, above, below, left-and-below, left-and-above, right-and-below, or right-and-above (see Figure 1). We randomized trial order in every block for every subject. Stimuli were congruent half of the time at each stimulus location (e.g., an upward-pointing arrow presented above the center of the screen; an arrow requiring a right-key response presented on the right side of the screen). When stimuli were presented in the corner positions (e.g. left-and-above, right-and-below), S-S and S-R conflict were factorially crossed.
Figure 1.
Depiction of some conflict trial types in Experiment 1for subjects who received the instructions to press the Q key (located on left of QWERTY keyboard) for up arrows and the P key (located on right of QWERTY keyboard) for down arrows. Combination trials in which one conflict type was incongruent and the other congruent are not pictured here (e.g., S-S congruent/S-R incongruent).
Procedure
Each S-S/S-R task trial presented subjects with one arrow pointing upward or downward in one of the eight locations, and the experimenter (and onscreen instructions) asked subjects to quickly and accurately press a key to indicate whether each arrow pointed upward or downward. Subjects first completed 10 trials of mapping practice with immediate feedback, by responding to arrows presented in the center of the screen. Following incorrect responses, the screen flashed red for 100 ms. Next, subjects completed 100 mapping-practice trials (again, with centered arrows) with accuracy and RT feedback provided only at the end of the block.
Following response-mapping practice, subjects completed 24 practice trials in which the arrows could appear in any of the eight positions around the center of the screen; subjects received trial-level error feedback (screen flashing red after errors). Next, for the actual task, subjects completed four blocks of 120 trials, with short breaks (< 30 seconds) permitted between blocks. The target arrow appeared directly above, below, to the left, or to the right of fixation on 80 trials per block (20 in each of those locations). On half of these trials the arrow direction was congruent with the arrow location. On the other 40 trials per block, the arrows appeared diagonally oriented to fixation (e.g., right-and-above; left-and-below) with left, right, up, and down locations being factorially crossed. That is, in a single block subjects were presented with 20 incongruent S-S trials, 20 congruent S-S trials, 20 incongruent S-R trials, 20 incongruent S-R trials. These trials were neutral in regard to the other dimension. In addition to these 80 trials, subjects also completed 10 mixed trials (appearing in the diagonal locations) that were congruent on both S-S and S-R dimensions, 10 that were incongruent on S-S and S-R dimensions, 10 that were congruent on S-S and incongruent S-R dimensions, and 10 that were incongruent on S-S and congruent on S-R dimensions. In Experiment 1, we distinguish “pure” trials that provided congruent or incongruent information on one conflict type (while being neutral in terms of the other conflict type) from “mixed” trials that present this information (i.e., congruent or incongruent) on both conflict types.
Every trial began with a central, black fixation cross, in 90 point bold Courier New font, for 500 ms, which remained on-screen during the 100 ms arrow presentation. After the target arrow, subjects saw a blank screen for 1250 ms. We recorded responses during the first 1150 ms of this screen. After each of the four blocks, subjects received RT and error feedback for that block. Finally, subjects completed another 100 trials of mapping (identical to the 100 mapping trials completed before the S-S/S-R task trials).
Inferential Analyses
We used the lme4 package (Bates, Maechler, & Bolker, 2013) in the R system for statistical computing (R Development Core Team, 2013) to compute inferential statistics. For RTs (from accurate trials only), we used linear mixed models (LMM). We interpreted parameters — determined by restricted maximum likelihood estimation — with t-values greater than 2 as significant; with a large number of observations the t-distribution becomes indistinguishable from the standard normal distribution, where absolute values over 2 reflect an alpha < .05. We used generalized linear mixed models (GLMM) for accuracy analyses, in order to account for the binomial distribution of trial-level accuracy (Dixon, 2008). In the raw data, correct trials were scored a 0 and incorrect trials were a 1. The betas for the GLMMS are log odds ratios with higher log odds ratios indicating that an error is more likely to be committed. In all models, we entered subjects as random effects (random intercept only) to account for the non-independence of the data, and WMC (centered on the grand mean of this experiment) was treated as a continuous variable.
Results
We dropped all data from fifteen subjects, eight who did not achieve 85% accuracy on the processing portion of at least two complex span tasks (e.g., Conway et al., 2005; Redick et al., 2012; two of these subjects never returned for the second session), and seven subjects — identified using box plots — who were further than three times the interquartile range from the mean accuracy for either the response-mapping or the congruent trials in the S-S/S-R task. Data remained from 221 subjects.
WMC Screening Results
The sum of items correctly recalled in serial position was the score for each complex span task (Conway et al., 2005); the theoretical maximum score was 75 for OSPAN and RSPAN and 42 for SSPAN. If a subject did not achieve 85% accuracy on the processing portion of a span task, the score from that task was not used in analyses (all data from subjects with fewer than two valid span scores were dropped from analyses). We converted span task scores (these raw Ms and SDs are reported with the correlations below) to Z scores (using the Ms and SDs from our laboratory database of more than 3000 UNCG students; for norms generated from this sample, see Redick et al., 2012) and averaged them into a WMC composite. Using a composite WMC measure is consistent with the evidence and theoretical perspective that WMC-related differences in cognitive performance are driven largely by domain-general processes (Kane et al., 2004). Moreover, it is preferable to draw conclusions at the level of hypothetical constructs rather than at the level of individual complex span tasks, each of which reflects not only WMC but also task-specific sources of measurement error. The raw span scores correlated with rs of .68 (RSPAN [M = 50, SD = 15] × OSPAN [M = 53, SD = 14]; N = 199), .51 (OSPAN × SSPAN [M = 26, SD = 7]; N = 178), and .43 (SSPAN × RSPAN; N = 184). Ns varied due to individually dropped tasks. The WMC composite was unimodal and symmetrically distributed (M = 0.08; SD = 0.81; skew = −0.72; kurtosis = 0.01).
Response-Mapping Trials
Response Times
On the pre-task mapping trials (M = 448 ms, SE = 4 ms), higher-WMC subjects responded faster than did lower-WMC subjects (b = −10 ms, SE = 5 ms, t = −2.2). We further divided the pre-task mapping trials to examine WMC’s relation to RTs for the first versus second half of mapping. Higher-WMC subjects responded significantly faster than did lower-WMC subjects on the first 50 mapping trials (b = −9 ms, SE = 5 ms, t = −2.0) and the second 50 mapping trials (b = −11 ms, SE = 5 ms, t = −2.3). When we examined only the last 25 trials of mapping, higher-WMC subjects were no longer significantly faster than lower-WMC subjects (b = −9 ms, SE = 5 ms, t = −1.8), but the modest WMC effect on RTs was of similar magnitude no matter how we analyzed it. In the post-task mapping trials (M = 464 ms, SE = 4 ms), WMC did not predict RTs (b = −5 ms, SE = 5 ms, t = −1.0).
Errors
We entered subjects as a random effect and WMC as fixed effect in a GLMM predicting errors. Higher-WMC subjects were less likely to commit errors than were lower-WMC subjects, overall (b = −0.26, SE = 0.06, Z = −4.2), on the first 50 trials (b = −0.31, SE = 0.07, Z = −4.2), and over the last 50 trials (b = −0.26, SE = 0.06, Z = −4.2). As in mapping RTs, when we examined only the last 25 trials, we did not find significant WMC-related differences (b = −0.09, SE = 0.08, Z = −1.2).
Although WMC-related differences in mapping RTs and errors seemed to be resolving over the last 25 trials of practice, one might be concerned that overall differences in responding to arrow stimuli without conflict might affect any WMC-related differences in conflict resolution with these stimuli. Previous work has suggested that WMC is related to the ability to form stimulus-response mappings (Wilhelm & Oberauer, 2006), and so we wanted to examine WMC-related differences in conflict resolution while limiting the potential confound of WMC-related abilities to map stimuli with responses. We thus adopted a conservative strategy that used subjects’ centered mean RTs or error rates from the last 25 trials of pre-task mapping as a covariate in all of the following analyses (we also report all theoretically important effects that changed without the covariate, to eliminate concerns about researcher degrees of freedom; Simmons, Nelson, & Simonsohn, 2011). No interaction terms with this covariate were included our models. Unless otherwise noted, we entered subjects as a random effect and WMC and trial congruency as fixed effects. We coded trial congruency as a −.5/+.5 contrast, so this parameter represents the experimental effect.
S-S Trials
Response Times
Table 1 presents descriptive statistics for Experiment 1. We found significant S-S interference, such that responses to incongruent trials (e.g., downward pointing arrows presented above central fixation) were slower than responses to congruent trials (e.g., downward pointing arrows presented below fixation; b = 38 ms, SE = 1 ms, t = 32.8). WMC did not predict RTs, overall (b = −3 ms, SE = 4 ms, t = −0.7). However, higher-WMC subjects experienced less S-S interference than did lower-WMC subjects (b = −7 ms, SE = 1 ms, t = −5.1); that is, higher-WMC subjects showed a smaller RT difference between incongruent and congruent trials. To decompose this interaction between WMC and trial type, we analyzed congruent and incongruent trials separately: Higher-WMC subjects did not significantly differ from lower-WMC subjects on congruent-trial RTs (b = 1 ms, SE = 4 ms, t = 0.3) or on incongruent-trial RTs (b = −6 ms, SE = 4 ms, t = −1.7), but it appears that the significant reduction in S-S interference for higher-WMC subjects was due primarily to better performance on incongruent trials. (Parallel analyses without the mapping-RT covariate similarly indicated significant S-S interference [b = 38 ms, SE = 1 ms, t = 32.8] and a significant WMC × trial-type interaction [b = −7 ms, SE = 1 ms, t = −5.1], but additionally showed a main effect of WMC on RTs [b = −11 ms, SE = 5 ms, t = −2.2].)
Table 1.
Experiment 1 Descriptive Statistics
| Trial Category/DV | Trial Type | M | Std Err |
Min | Max | Skew | Kurtosis |
|---|---|---|---|---|---|---|---|
| Pure/RT | S-R congruent | 457 | 4 | 310 | 629 | 0.48 | −0.16 |
| S-R incongruent | 502 | 4 | 351 | 708 | 0.46 | −0.16 | |
| S-S congruent | 471 | 4 | 322 | 633 | 0.41 | −0.32 | |
| S-S incongruent | 509 | 4 | 376 | 742 | 0.63 | 0.24 | |
| Pure/ER | S-R congruent | 0.04 | 0.00 | 0.00 | 0.16 | 1.64 | 3.59 |
| S-R incongruent | 0.13 | 0.01 | 0.00 | 0.48 | 1.16 | 1.94 | |
| S-S congruent | 0.04 | 0.00 | 0.00 | 0.20 | 1.50 | 2.60 | |
| S-S incongruent | 0.10 | 0.00 | 0.00 | 0.49 | 1.53 | 4.68 | |
| Combination/RT | S-R con/ S-S con | 453 | 4 | 283 | 646 | 0.36 | −0.26 |
| S-R incon/S-S con | 480 | 4 | 342 | 666 | 0.58 | 0.08 | |
| S-R con/S-S incon | 490 | 5 | 324 | 749 | 0.66 | 0.51 | |
| S-R incon/S-S incon | 516 | 4 | 373 | 791 | 0.68 | 0.75 | |
| Combination/ER | S-R con/S-S con | 0.03 | 0.00 | 0.00 | 0.20 | 1.78 | 3.56 |
| S-R incon /S-S con | 0.06 | 0.00 | 0.00 | 0.25 | 1.31 | 1.41 | |
| S-R con/S-S incon | 0.09 | 0.00 | 0.00 | 0.38 | 1.31 | 2.04 | |
| S-R incon/S-S incon | 0.14 | 0.01 | 0.00 | 0.50 | 0.96 | 1.40 | |
Note: M = mean of subject means. ER = error rate. RT = response time. Std Err = standard error. S-S = stimulus-stimulus. S-R = stimulus-response. Con = congruent. Incon = incongruent.
Errors
Error rates also demonstrated significant S-S interference, with subjects making more errors on incongruent than on congruent trials (b = 0.87, SE = 0.05, Z = 19.2). WMC did not predict errors overall (b = 0.06, SE = 0.06, Z = 1.1), nor did it interact with trial congruency (b = −0.08, SE = 0.06, Z = −1.4). (Without the mapping-accuracy covariate, the only parameter to change was for the WMC main effect, which remained non-significant [b = 0.00, SE = 0.07, Z = 0.3].)
S-R Trials
Response Times
Subjects exhibited significant S-R interference, such that responses to incongruent stimuli (e.g., an arrow requiring a right-key response presented to the left of fixation) were slower than to congruent stimuli (e.g., an arrow requiring a right-key response presented to the right of fixation; b = 45 ms, SE = 1 ms, t = 39.8). WMC did not predict overall RT (b = −3 ms, SE = 3 ms, t = −0.9), nor did WMC interact with S-R interference (b = −1 ms, SE = 1 ms, t = −0.9); in contrast to S-S interference, then, S-R interference was insensitive to variation in WMC. (Analyses without the mapping-RT covariate showed no change to the parameters representing S-R interference [b = 45 ms, SE = 1 ms, t = 39.8] or the WMC × trial-type interaction [b = −1 ms, SE = 1 ms, t = −0.9], but the main effect of WMC on RTs was significant [b = −12 ms, SE = 5 ms, t = −2.3].)
Errors
Subjects made more errors on incongruent trials then on congruent trials, indicating significant S-R interference (b = 1.39, SE = 0.05, Z = 29.6). Overall, WMC did not predict errors (b = −.04, SE = 0.05, Z = −0.8), but WMC did interact with S-R congruency (b = 0.13, SE = 0.06, Z = 2.3). Here, higher-WMC subjects were non-significantly more likely to commit an error on incongruent trials than were lower-WMC subjects (b = 0.02, SE = 0.05, Z = 0.4), and non-significantly less likely to commit an error on congruent trials (b = −0.11, SE = 0.07, Z = −1.6), but the WMC effect seemed to reflect primarily greater facilitation on congruent trials for higher WMC subjects. (Without the mapping covariate, the only parameter that changed was for the main effect of WMC, which remained non-significant [b = −0.10, SE = 0.06, Z = −1.7].)
Comparison of S-S and S-R trials
For these analyses, conflict type was dummy-coded. S-R trials (arrows presented directly to the right or left of fixation) were set as the reference level and S-S trials (arrows presented directly above or below fixation) were the comparison level.
Response Times
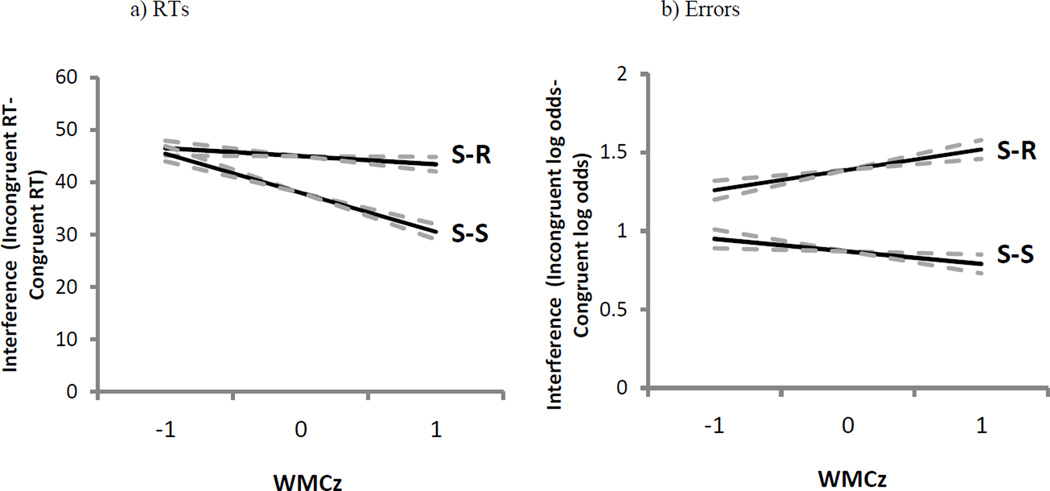
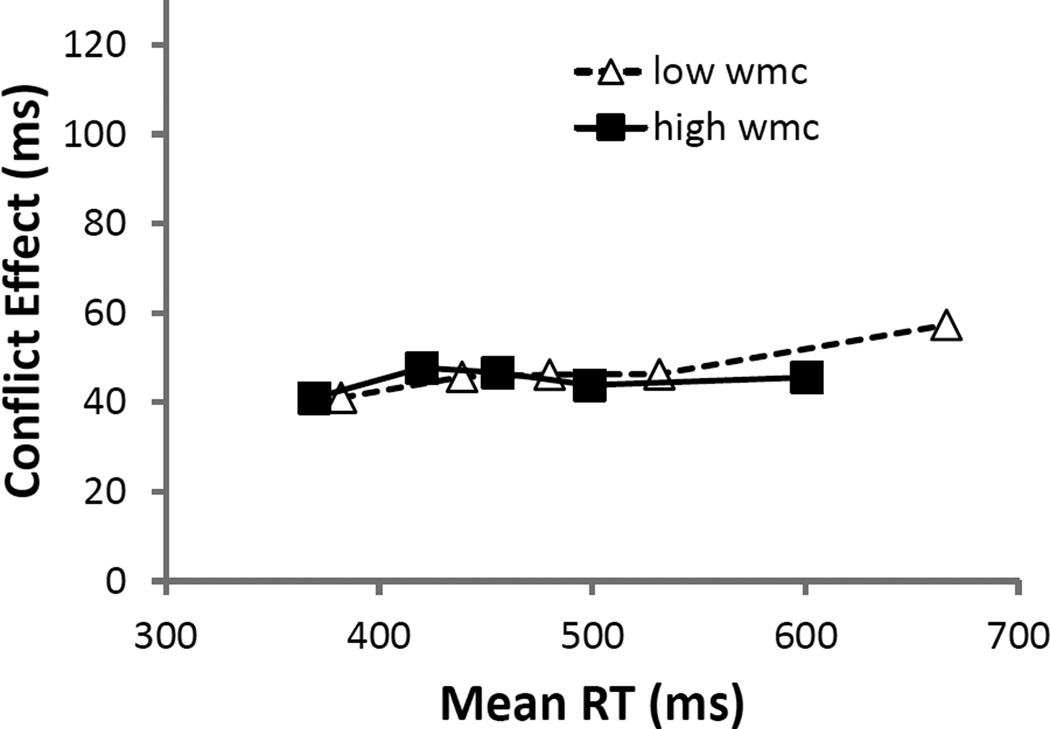
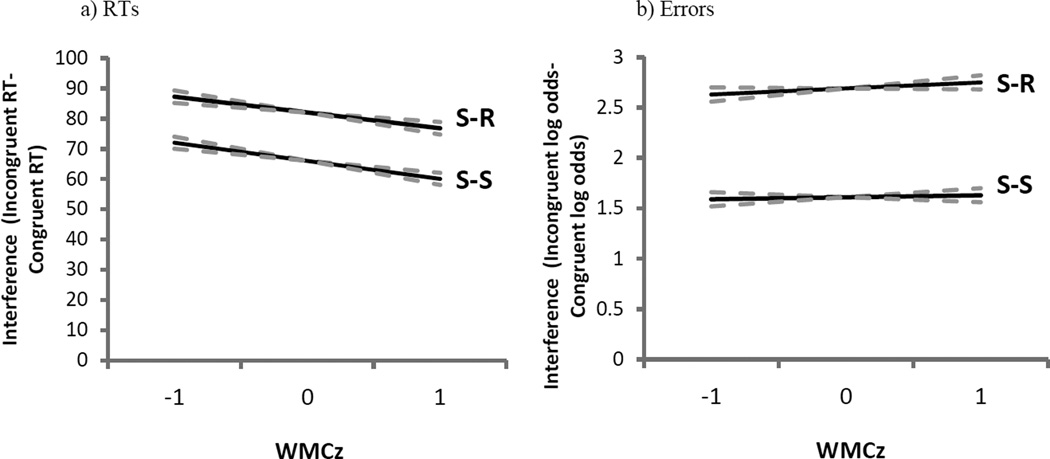
S-S trials yielded slower responses than did S-R trials (b = 11 ms, SE = 1 ms, t = 13.1) and subjects exhibited less S-S than S-R interference when contrasting incongruent to congruent trials (b = −7 ms, SE = 2 ms, t = −4.2). As evident in the significant interaction depicted in Figure 2a, WMC influenced the S-S interference difference score more than the S-R difference score (WMC × S-S × S-R interaction, b = −6 ms, SE = 2 ms, t = −3.0).1 That is, consistent with results reported above, higher-WMC showed a greater advantage over lower-WMC subjects in resolving S-S interference than in S-R interference. (None of these parameters changed in analyses without the mapping-RT covariate.)
Figure 2.
Experiment 1 model parameters of stimulus-response (S-R) and stimulus-stimulus (S-S) interference (incongruent RTs/errors – congruent RTs/errors) from pure trials as a function of composite working memory capacity Z-score (WMCz) in response times (RTs; panel A) and errors (panel B). The dotted lines represent the standard error of the estimate.
Errors
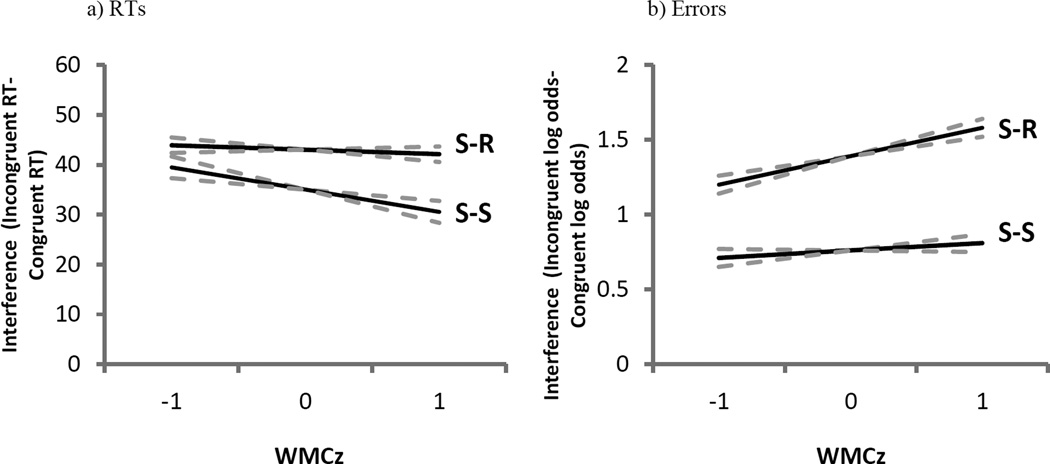
Error rates were statistically equivalent across S-S and S-R trials (b = −0.04, SE = 0.03, Z = −1.4), but subjects again showed less S-S than S-R interference when contrasting congruent to incongruent trials (b = −0.53, SE = 0.07, Z = −8.1). As depicted in Figure 2b, WMC negatively predicted interference in S-S trials and positively predicted interference in S-R trials, yielding a significant interaction among WMC, S-S, and S-R conflict types (b = −0.21, SE = 0.08, Z = −2.6). (None of these parameters changed in analyses without the mapping covariate.)
S-S/S-R Combination Trials
To analyze the trials that were composed of a factorial combination of congruency and S-S and S-R conflict types, we entered S-S and S-R conflict separately as fixed effects (entered as −.5/+.5 contrasts). Data from only the combination trials were included in these analyses.
Response Times
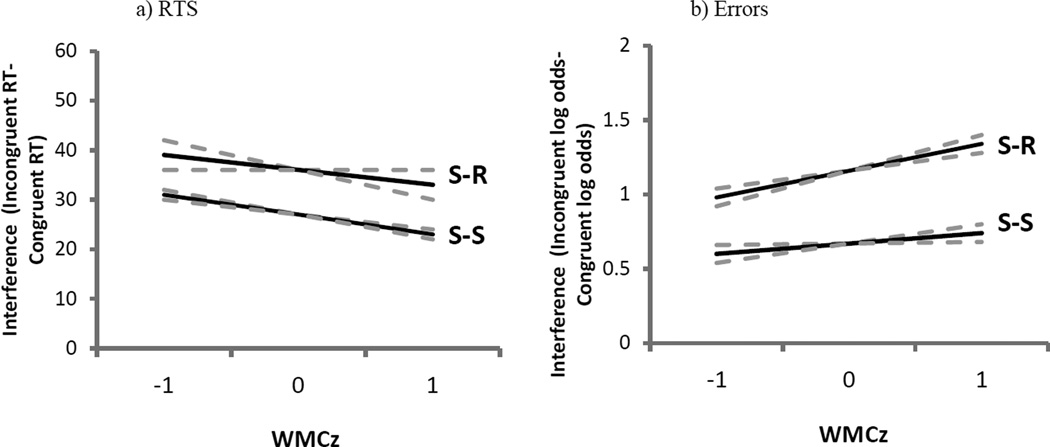
Subjects showed significant S-S interference (b = 27 ms, SE = 1 ms, t = 23.1) and S-R interference (b = 36 ms, SE = 1 ms, t = 31.6) and, consistent with prior research (e.g., Kornblum, 1994), these effects did not interact on trials that contained both conflict types (b = −1 ms, SE = 2 ms, t = −0.3). There was no main effect of WMC on RT, overall (b = −2 ms, SE = 4 ms, t = −0.6). Consistent with results from the pure trials analyzed above and, as shown in Figure 3a, higher-WMC subjects experienced less S-S interference than did lower-WMC subjects (b = −4 ms, SE = 1 ms, t = −2.7), but this pattern did not hold for S-R interference (b = −2 ms, SE = 1 ms, t = −1.1). However, the three-way interaction among WMC, S-S conflict, and S-R conflict was not significant (b = −3 ms, SE = 3 ms, t = −1.0). (In an analysis without the mapping-RT covariate, the only parameter that changed was the overall main effect of WMC on RT, which was now significant [b = −11 ms, SE = 5 ms, t = −2.1].)
Figure 3.
Experiment 1 (E1) model parameters of stimulus-response (S-R) and stimulus-stimulus (S-S) interference from combination S-S/S-R trials as a function of composite working memory capacity Z-score (WMCz) in response times (RTs; panel A) and errors (panel B). The dotted lines represent the standard error of the estimate.
Errors
Subjects’ error rates showed both significant S-S (b = 0.67, SE = 0.5, Z = 13.5), and S-R interference (b = 1.14, SE = 0.05, Z = 23.0) but here, in contrast to the RT analyses, trials that presented both S-S and S-R conflict yielded an under-additive interaction approaching our significance criterion (b = −0.18, SE = 0.10, Z = −1.8). Again, there was no main effect of WMC on errors in these combination trials (b = 0.02, SE = 0.06, Z = 0.4). As in the pure conflict trials, WMC did not predict S-S interference (b = 0.07, SE = 0.6, Z = 1.2), but, as depicted in Figure 3b, higher-WMC subjects exhibited more S-R interference than lower-WMC subjects (b = 0.19, SE = 0.6, Z = 3.1). The three-way interaction among WMC, S-S conflict, and S-R conflict was not significant, however (b = −0.17, SE = 0.13, Z = −1.3). (Without the mapping-errors covariate in the analyses, only the main effect of WMC changed, becoming non-significantly negative [b = −0.03, SE = 0.06, Z = −0.5].)
Delta Plots
Next, we examined whether WMC-related differences in resolving interference were more pronounced in particular parts of the RT distribution. That is, we tested whether higher-WMC subjects showed an advantage over lower-WMC subjects because they experienced less interference on the fastest trials, on the slowest trials, or across the whole RT distribution. Previous work has suggested that different patterns of interference across the RT distribution reflect different conflict-resolution mechanisms. A pattern of decreasing interference with increasing RTs has been interpreted as reflecting a suppression mechanism with a fast automatic congruency effect being countered by a slow-to-arise inhibition (Ridderinkhof et al., 2004). In contrast, a pattern of increasing interference with increasing RTs may reflect selective attention processes, with irrelevant stimulus dimensions either affecting the rate of information accumulation or changing decision criteria on trials where attention is not focused on relevant stimulus dimensions from the trial onset (Pratte, Rouder, Morey, Feng, 2010).
Here, then, we tested whether WMC would differentially predict either pattern and thus provide evidence for the mechanism(s) through which WMC affects performance. We binned all subjects’ correct-trial RTs (from pure trials only), separately for congruent and incongruent trials, into five bins each. The bins were ordered so that each subject’s fastest 20% of trials represented the first bin, second fastest 20% of trials the second bin, etc., until we had five bins of approximately equal size for each subject for both conflict types for congruent and incongruent trials. We then subtracted each congruent-trial bin from its corresponding incongruent-trial bin, which left with five difference scores per subject for each conflict type.
S-S Trials
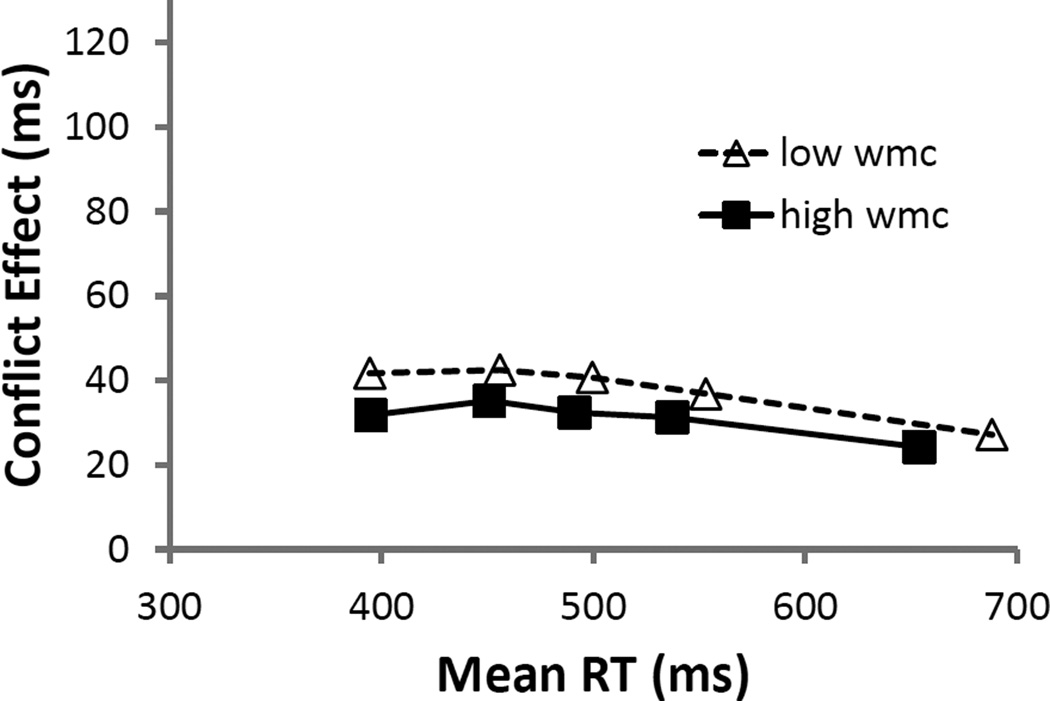
For illustrative purposes, we plot these bins collapsed across the highest and lowest third of WMC scores. As shown in Figure 4, the differences between higher and lower-WMC subjects were not localized to any specific portions of the RT distribution. This observation was confirmed by a LMM with subjects as a random effect and WMC (as a continuous variable) and bin (1–5) as fixed effects. Only bin (b = −2 ms, SE = 1 ms, t = −2.8) was a significant predictor of the S-S conflict difference score, with interference decreasing across bins. Typically, in tasks that present only S-S conflict in isolation, more interference is observed on the slowest trials (Pratte et al., 2010). However, when in tasks presenting both S-S and S-R conflict trials mixed within blocks, we know less about the expected delta-plot pattern. Here, we did not observe the main effect of WMC, possibly because of reduced power attending the loss of information in analyses of aggregated data (here, aggregating into 5 RT bins per participant going from 360 data points per participant to five). In any case, of most importance here is the null interaction between WMC and bin (b = −1 ms, SE = 1 ms, t = −1.6), suggesting that the significant effect of WMC we observed in our initial non-aggregated analysis above was relatively constant throughout the RT distribution.
Figure 4.
Delta plots of stimulus-stimulus (S-S) interference as a function of upper (high) and lower (low) tercile of composite working memory capacity Z-score (WMC) in Experiment 1.
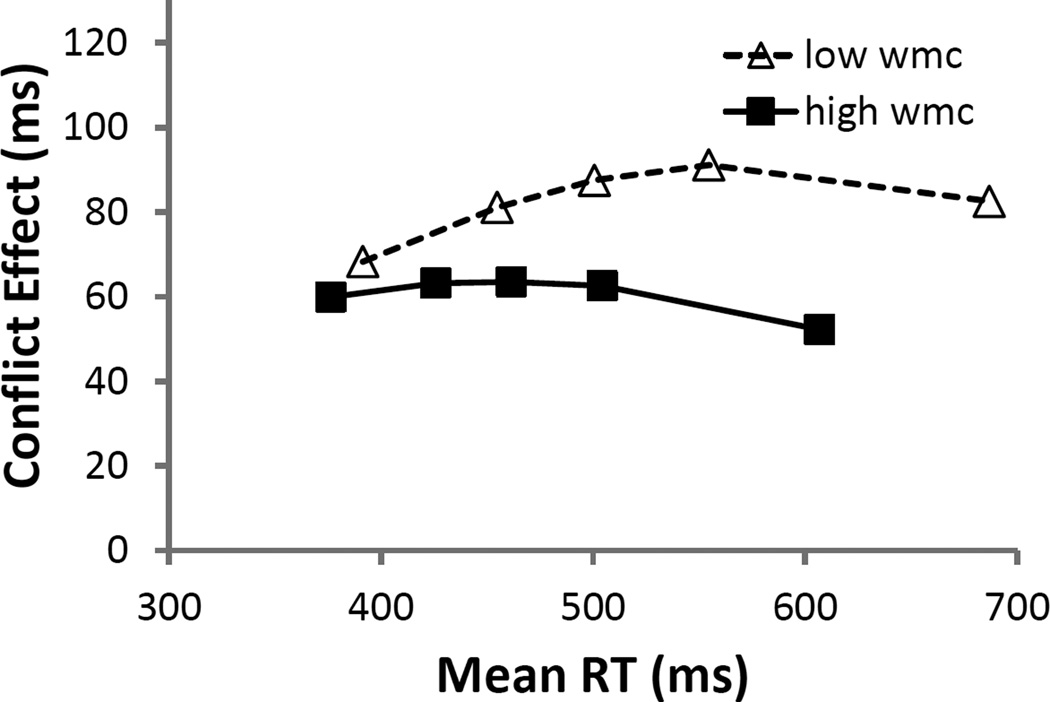
S-R Trials
Although our prior analyses showed that WMC did not predict resolution of S-R interference in RTs, for completeness we examined their delta plots, as well. On blocked S-R trials, where the irrelevant stimulus feature is stimulus location and the stimuli are presented horizontally, interference effects typically decrease with RTs (Pratte et al., 2010; Proctor, Miles, & Baroni, 2011). But again, we know less about what to expect when S-S and S-R trials are mixed. Figure 5 shows a good deal of overlap between the distributions, as well as some separation among WMC scores at the slowest RTs. The only statistically significant predictor in the LMM, however, was bin (b = 2 ms, SE = 1 ms, t = 2.9), with the difference scores increasing with RTs. As in the S-S trial analyses above (and consistent with the null WMC effect in S-R interference), WMC did not interact with bin (b = −1 ms, SE = 1 ms, t = −1.5).
Figure 5.
Delta plots of stimulus-response (S-R) interference as a function of upper (high) and lower (low) tercile of composite working memory capacity Z-score (WMC) Experiment 1.
Discussion
Here, in a task that presented 50% conflict trials as a means to control for WMC-related differences in goal maintenance (e.g., Kane & Engle, 2003), higher-WMC subjects showed less S-S interference in RTs than did lower-WMC subjects, but they showed equivalent S-R interference in RTs. Indeed, not only did higher-WMC subjects not experience less S-R RT interference than did lower-WMC subjects, they actually experienced greater S-R interference in errors than did lower-WMC subjects. This pattern of results was evident in both pure and combination trials. Clearly, Experiment 1 yielded a dissociation in the relation of WMC variation to performance on trials that present conflict between stimulus elements and trials that present conflict between stimulus and response elements.
Delta plots did not reveal any localized WMC-related differences in the experience of either S-S or S-R interference across the RT distribution. Differential slowing in the tail end of the RT distribution, with lower-WMC subjects experiencing greater slowing, has previously been interpreted as evidence for WMC-related differences in (lapses of) goal maintenance (Unsworth, Redick, Spillers, & Brewer, 2011). Because we saw no such localized differences here, we interpreted the dissociation of WMC with S-S versus S-R interference as consistent with the notion that higher WMC facilitates resolving conflict between relevant and irrelevant stimulus features, but is less effective (or even detrimental, with respect to errors) in resolving conflict between irrelevant stimulus dimensions and response dimensions. This dissociation indicates a conflict-specific WMC-related individual difference in identifying or selecting among competing visual stimuli.
We note, however, that our delta-plot findings, with a task that presented both S-S and S-R conflict, were inconsistent with many others from the literature with tasks presenting only S-S or S-R conflict. These discrepancies call into the question the generalizability of the effects and the processes implicated in single-conflict and dual-conflict contexts. If the characteristic delta plot pattern of ascending interference with S-S conflict and descending interference with S-R conflict only occur within single-conflict tasks, task approach at a global level may play an instrumental role. That is, the conflict types do not change between single and dual-conflict tasks, but the way the task is approached by the subject does. Therefore, S-S conflict, by itself, may not produce an ascending pattern of interference, but S-S conflict in a task that only presents S-S conflict may produce the ascending pattern because the single-task context encourages the subject to use a certain strategic approach. Task approach plus conflict type may produce the distinctive ascending and descending patterns previously seen.
We sought to replicate our novel WMC-related finding in Experiment 2A, which eliminated arrows in the diagonal positions so that we could determine whether the WMC-conflict dissociation would be found when arrows could appear only in four locations (e.g. above, below, left, and right). That is, we simplified the task environment as a way to rule out that WMC was somehow related only to performance on S-S trials because of the sheer number of stimulus-response pairings in the original version (16 stimulus-response pairs in Experiment 1 versus 8 stimulus-response pairs in Experiments 2A and 2B). In Experiment 2B, we additionally increased the proportion of congruent trials from 50% to 80% for both S-S and S-R trials, to bring goal maintenance processes into play; here we tested whether WMC would thus moderate performance across both conflict types. In a goal-supportive context (like that in Experiment 1 and Experiment 2A) where the task goal is reinforced by frequently occurring incongruent trials (forcing subjects to try to attend only to the relevant stimulus feature), WMC-related differences in goal maintenance are minimized. However, in a context where a majority of the trials are congruent, allowing subjects to respond correctly by either attending to the relevant or irrelevant stimulus features, lower-WMC subjects seem to lose access to the task goal periodically. They therefore show greater interference than do higher-WMC subjects when they encounter the infrequent incongruent trials (Kane & Engle, 2003). In addition, previous work using the Experiment 1 task has shown that increasing the proportion of congruent trials affects performance across conflict types (Funes et al., 2010a). In Experiment 2B, we tested whether WMC interacts with this generalized (i.e., not conflict-type specific) control.
In Experiments 2A and 2B, in addition to testing whether WMC was differently related to S-S and S-R conflict resolution, we examined a potential mediator of this differential relation: the effect of prior trial congruency on the performance of the current trial. Congruency sequence effects are often used as markers of in-the-moment cognitive control (Botvinick et al., 2001; for a review, see Egner, 2007). Using the same task as the present one, Funes et al. (2010a, 2010b) found a large reduction in interference following an incongruent trial only when the previous trial type was the same as the current trial type. For example, interference decreased for incongruent S-S trials that followed incongruent S-S trials and on incongruent S-R trials that followed incongruent S-R trials, but not on incongruent S-S trials following incongruent S-R trials (and vice versa). Prior findings indicate that WMC is unrelated to congruency sequence effects in Stroop (Meier & Kane, 2013; Unsworth et al., 2011) and flanker tasks (Keye et al., 2009; Unsworth et al., 2011), but lower-WMC subjects may show larger congruency sequence effects than do higher-WMC subjects in Simon tasks that present only S-R conflict (Keye et al., 2009; Weldon et al., 2013). Our aims for congruency-sequence analyses in Experiments 2A and 2B were to place our findings in the context of the prior work examining WMC and to test whether congruency sequence effects were responsible (at least in part) for any WMC-related differences in the resolution of S-S and S-R conflict.
Experiments 2A and 2B
Method
Subjects
We randomly assigned subjects to either Experiment 2A or 2B. One hundred and forty-four UNCG undergraduates participated in Experiment 2A and 151 participated in Experiment 2B (toward partial fulfillment of a psychology course requirement, as in Experiment 1). All were 18 – 35 years old and none had participated in Experiment 1.
General Procedure
Subjects volunteered to complete two testing sessions, in groups of 1 – 4, over the course of one semester. For subjects that completed both sessions, an average of 15 days (SD = 15) passed between sessions for both Experiments 2A and 2B. We administered the OSPAN task at the beginning of the first session, followed by the S-S/S-R task and the SSPAN task. In the second session, we administered the RSPAN after subjects had completed two tasks for another study, not reported here (again involving a mix of memory and attention tasks, conducted by different sets of investigators). We used the same computer hardware, software, and general procedures as in Experiment 1.
We intended to terminate data collection after one semester (with at least 100 subjects in each experiment), but our Ns after one semester were unambiguously insufficient for individual-differences analyses. In the first semester, we tested 57 subjects for Experiment 2A, and 47 subjects for Experiment 2B. We had to drop the data from five subjects in Experiment 2A and twenty-five subjects from 2B because of programming errors (details provided below), which left 52 usable subjects in Experiment 2A and 22 usable subjects in Experiment 2B. We therefore conducted data collection for a second full semester.
S-S/S-R task
Materials and design
For both Experiments 2A and 2B, we modified the task from Experiment 1 by not presenting arrows in the corner positions. That is, arrows only appeared directly above or below, or directly to the left or right, of fixation. We manipulated the proportion of congruent trials between Experiments 2A and 2B, with 50% congruent trials in Experiment 2A (as in Experiment 1) and 80% congruent trials in Experiment 2B. Like Experiment 1, all subjects completed 3 blocks of trials. In Experiment 2A, subjects completed 30 S-S congruent, S-S incongruent, S-R congruent, S-R incongruent trials per block. In Experiment 2B, subjects completed 12 incongruent S-S and S-R trials and 48 congruent S-S and S-R trials per block.
Procedure
The procedure was identical to that in Experiment 1.
Experiment 2A Results
We dropped all data from twenty-four subjects: 8 who did not meet the processing-accuracy criterion for the WMC composite (i.e., ≥ 2 complex span tasks with 85% accuracy), 11 who were further than three times the interquartile range from the mean accuracy rate for either mapping-practice or congruent trials, and 5 who, because of a programming error, received erroneous accuracy feedback during the mapping practice. We analyzed data from the remaining 120 subjects.
WMC Screening
We assessed WMC the same way as in Experiment 1. Complex span scores correlated with rs of .58 (RSPAN [M = 46, SD = 16] × OSPAN [M = 51, SD = 15]; N = 100), .40 (OSPAN × SSPAN [M = 26, SD = 8]; N = 104), and .50 (SSPAN × RSPAN; N = 98). The WMC composite mean was −0.07 (SD = 0.84), which did not differ from that in Experiment 1 (M = 0.08, SD = 0.81; t(339) = 1.6, p = .12). Also as in Experiment 1, the composite was unimodal and symmetrically distributed (skew = −0.44; kurtosis = −0.40).
Response-Mapping Trials
Response Times
Table 2 contains descriptive statistics for Experiment 2A. We entered subjects as a random effect and WMC as a fixed effect predicting RT. In the mapping trials that preceded the S-S/S-R task, WMC did not predict RT, overall (b = 2 ms, SE = 6 ms, t = 0.3), nor did it predict RT on the first half of trials (b = 1 ms, SE = 6 ms, t = 0.1), the second half (b = 3 ms, SE = 7 ms, t = 0.5), or the last 25 mapping trials (b = 1 ms, SE = 7 ms, t = 0.1). In the mapping trials following the S-S/S-R task, WMC again did not predict RTs (b = −10 ms, SE = 6 ms, t = −1.6).
Table 2.
Experiment 2a Descriptive Statistics
| DV | Trial Type | M | Std Err |
Min | Max | Skew | Kurtosis |
|---|---|---|---|---|---|---|---|
| RT | S-R congruent | 477 | 5 | 351 | 662 | 0.44 | 0.28 |
| S-R incongruent | 520 | 6 | 384 | 733 | 0.51 | 0.36 | |
| S-S congruent | 493 | 6 | 343 | 683 | 0.27 | −0.14 | |
| S-S incongruent | 528 | 6 | 404 | 710 | 0.36 | −0.23 | |
| ER | S-R congruent | 0.04 | 0.00 | 0.00 | 0.14 | 1.18 | 0.78 |
| S-R incongruent | 0.13 | 0.01 | 0.01 | 0.38 | 0.74 | −0.10 | |
| S-S congruent | 0.05 | 0.00 | 0.00 | 0.18 | 1.14 | 0.79 | |
| S-S incongruent | 0.10 | 0.01 | 0.01 | 0.29 | 0.94 | 0.79 |
Note: M = mean of subject means. RT = response time. ER = error rate. Std Err = standard error. S-S = stimulus-stimulus. S-R = stimulus-response.
Errors
Higher-WMC subjects were less likely than lower-WMC subjects to make errors during the mapping trials that preceded the S-S/S-R task (b = −0.17, SE = 0.09, Z = −2.0). Higher-WMC subjects made significantly fewer errors on the first half of trials (b = −0.21, SE = 0.10, Z = −2.2), but not in the second half (b = −0.11, SE = 0.10, Z = −1.1), or in the last 25 trials of this block (b = −0.09, SE = 0.12, Z = −0.7). After the S-S/S-R task, WMC did not predict errors on mapping trials (b = 0.00, SE = 0.00, Z = −0.5).
To be consistent with the Experiment 1 analyses (and to facilitate cross-experiment comparisons), we entered the centered mean RT or error rate for the last 25 mapping trials as a covariate in the following analyses (again, we additionally report all key results without the covariate).
S-S Trials
Response Times
Subjects demonstrated significant S-S interference, with slower responses to incongruent than to congruent trials (b = 35 ms, SE = 1 ms, t = 26.5). WMC predicted RTs, overall, with higher-WMC subjects responding faster than did lower-WMC subjects (b = −12 ms, SE = 5 ms, t = −2.5). Most importantly, however, and replicating Experiment 1, higher-WMC subjects experienced less S-S interference than did lower-WMC subjects (b = −4 ms, SE = 2 ms, t = −2.4). We decomposed this interaction by examining congruent and incongruent trials separately. Here, in contrast to Experiment 1, higher-WMC subjects were significantly faster than were lower-WMC subjects on both congruent (b = −10 ms, SE = 5 ms, t = −2.0) and incongruent trials (b = −14 ms, SE = 5 ms, t = −2.9), but with a larger effect on incongruent trials. (In a model without the mapping-RT covariate, only the WMC main-effect parameter changed, [b = −10 ms, SE = 7 ms, t = −1.5].)
Errors
Subjects committed significantly more errors on incongruent than on congruent trials (b = 0.76, SE = 0.05, Z = 15.6). Overall, higher-WMC subjects were less likely to make an error (b = −0.14, SE = 0.07, Z = −2.1), but WMC did not predict the magnitude of S-S interference (b = 0.05, SE = 0.06, Z = 1.0). (Without the mapping covariate, only the parameter value for WMC changed, remaining significant [b = −0.17, SE = 0.07, Z = −2.3].)
S-R Trials
Response Times
Subjects showed S-R interference, responding significantly more slowly on incongruent trials than on congruent trials (b = 43 ms, SE = 1 ms, t = 33.1). Overall, higher-WMC subjects were faster than lower-WMC subjects on S-R trials (b = −11 ms, SE = 4 ms, t = −2.6). Again replicating Experiment 1, WMC did not predict the RT difference between incongruent and congruent trials (b = −1 ms, SE = 2 ms, t = −0.7). (Without the covariate, only the WMC main-effect parameter changed, and was no longer statistically significant, [b = −9 ms, SE = 6 ms, t = −1.5].)
Errors
Significant S-R interference was also evident in errors (b = 1.39, SE = 0.05, Z = 27.3). WMC did not predict errors overall (b = −0.08, SE = 0.07, Z = −1.2). As in Experiment 1, however, higher-WMC subjects experienced significantly more S-R interference in errors than did lower-WMC subjects (b = 0.19, SE = 0.06, Z = 3.3). Again, as in Experiment 1, the increase in error interference by higher-WMC subjects was the product of a negative slope on congruent trials (b = −0.19, SE = 0.09, Z = 2.0) and a non-significant positive slope on incongruent trials (b = 0.01, SE = 0.08, Z = 0.1). (Without the covariate, only the overall WMC parameter changed, remaining non-significant [b = −0.10 ms, SE = .07, Z = −1.4].)
Comparison of S-S and S-R trials
For these analyses, trial type was dummy-coded. S-R trials were set to the reference level and S-S trials the comparison level.
Response Times
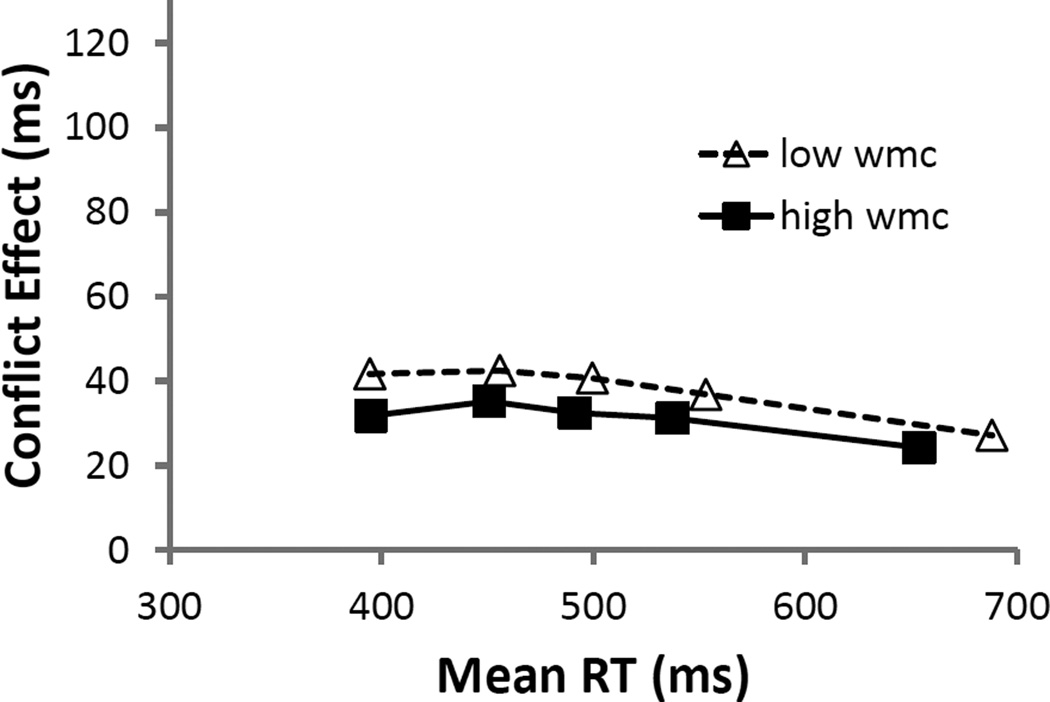
S-S trials were slower than S-R trials (b = 12 ms, SE = 1 ms, t = 13.5), and subjects experienced less interference on S-S trials than on S-R trials (b = −8 ms, SE = 2 ms, t = −4.2; see Figure 6a). Despite the significance of the WMC effect in S-S interference but not in S-R interference (see above) that replicated Experiment 1, here, the WMC slope for S-S interference was not significantly steeper than that for S-R interference (b = −3 ms, SE = 2 ms, t = −1.2),. (None of these parameters changed in a model without the mapping-RT covariate.)
Figure 6.
Experiment 2A model parameters of stimulus-response (S-R) and stimulus-stimulus (S-S) interference (incongruent RTs/errors – congruent RTs/errors) from pure trials as a function of composite working memory capacity Z-score (WMCz) in response times (RTs;panel A) and E\errors (panel B). The dotted lines represent the standard error of the estimate.
Errors
There were no overall differences in the amount of errors made on S-S and S-R trials (b = −0.06, SE = 0.04, Z = −1.7), but subjects exhibited less interference on S-S trials than on S-R trials (b = −0.63, SE = 0.07, Z = −8.9; see Figure 6b). Also inconsistent with Experiment 1, there was no significant difference in the relation between WMC and interference on S-S and S-R trials (b = −0.15, SE = 0.08, Z = −1.8), despite the significance of the WMC effect in S-R (favoring lower-WMC subjects) but not in S-S interference (see above) that replicated the pattern from Experiment 1. (None of these parameters changed in a model without the mapping covariate.)
Delta plots
S-S Trials
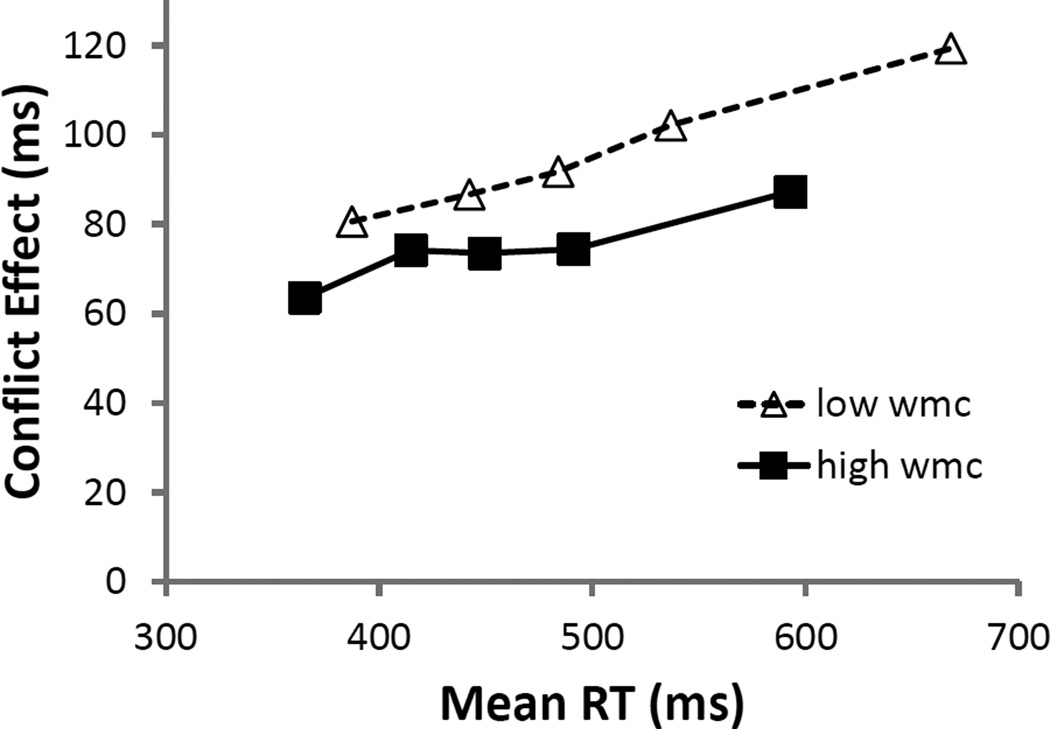
The delta plot for S-S trials in Experiment 2A (see Figure 7) looked strikingly like the one from Experiment 1, again showing a slight reduction in the amount of interference for longer RTs and a consistent WMC-related difference across the entire distribution of RTs. This visual assessment was confirmed by a LMM with subjects as a random effect and WMC and bin as fixed effects. Both bin (b = −2 ms, SE = 1 ms, t = −4.1) and WMC (b = −7 ms, SE = 3 ms, t = −2.1) were significant predictors of S-S interference, but of most importance, WMC and bin did not interact (b = 1 ms, SE = 1 ms, t = 1.3).
Figure 7.
Delta plots of stimulus-stimulus (S-S) interference as a function of upper (high) and lower (low) tercile of composite working memory capacity Z-score (WMC) in Experiment 2A.
S-R Trials
Also as seen in Experiment 1, S-R interference was relatively flat over RT bins and substantial WMC-related differences did not emerge over any part of the RT distribution (see Figure 8), as expected by the overall lack of WMC-related differences in S-R conflict. This visual observation was confirmed with a LMM in which neither WMC (b = 0 ms, SE = 4 ms, t = 0.1), bin (b = 0 ms, SE = 1 ms, t = 0.4), nor their interaction (b = −1 ms, SE = 1 ms, t = −0.6), significantly predicted S-R interference.
Figure 8.
Delta plots of stimulus-response (S-R) interference as a function of upper (high) and lower (low) tercile of composite working memory capacity Z-score (WMC) Experiment 2A.
Congruency sequence effects
First, we specified a LMM with subjects as a random effect and current trial congruency, previous trial congruency, WMC, and trial-type repetition as fixed effects (no covariates were included in this model). The trial-type repetition variable distinguished between trials where the conflict type repeated (e.g., an S-S trial preceded by an S-S trial) or alternated (e.g., an S-S trial preceded by an S-R trial). Current trial congruency and previous trial congruency were −.5/+.5 contrast coded; trial-type repetition was dummy coded with non-repetition trials (i.e., switch trials) as the reference level.
On trials where the trial type did not repeat, subjects showed slightly reduced interference on incongruent trials following incongruent trials versus incongruent trials following congruent trials (b = −6 ms, SE = 3 ms, t = −2.2), suggesting some generality of control processes. However, control-specific carry-over effects were considerably more impressive: Subjects showed an even greater reduction in interference on trials where the trial type repeated (b = −34 ms, SE = 4 ms, t = −8.4; note that this parameter reflects the additional reduction of interference over and above the parameter that was given for the non-repeat trials). WMC did not predict reductions in interference following incongruent trials, either on non-trial-type-repeat trials (b = −3 ms, SE = 3 ms, t = −1.0) or on repeat trials (b = −2 ms, SE = 5 ms, t = −0.4).
Next, to examine congruency sequence effects that might be specific to either S-S or S-R trials, and to assess their potential relations to WMC, we specified separate models for S-S and S-R repetition trials (i.e., consecutive S-S or S-R trials), with subjects as a random effect and current trial congruency, previous trial congruency, and WMC as fixed-effect predictors. In S-S trials, congruency sequence effects were found, with interference being reduced after an incongruent trial versus a congruent trial (b = −38 ms, SE = 4 ms, t = −8.8), but there was no interaction between these congruency sequence effects and WMC (b = 5 ms, SE = 5 ms, t = 1.0). S-R trials produced the same pattern, with substantial congruency sequence effects (b = −41 ms, SE = 4 ms, t = −9.5), but no interaction with WMC (b = −2 ms, SE = 5 ms, t = −0.3).
Experiment 2B Results
We dropped all data from forty-five subjects: 12 who did not meet the processing-accuracy criterion of at least two complex span tasks with ≥ 85% accuracy for the WMC composite (two of whom never returned for the second session), 8 who were further than three times the interquartile range from the mean accuracy for either pre-task mapping or congruent trials, and 25 who, because of a programming error during the first semester of data collection, only received 20% congruent S-R trials. We analyzed data from the remaining 106 subjects.
WMC Screening
Complex span scores correlated with rs of .69 (RSPAN [M = 44, SD = 15] × OSPAN [M = 51, SD = 16]; N = 84), .41 (OSPAN × SSPAN [M = 26, SD = 8]; N = 91), and .49 (SSPAN × RSPAN; N = 79). In Experiment 2B, the mean WMC composite was −0.11 (SD = 0.84) and was unimodal and symmetrically distributed (skew = −0.81; kurtosis = 0.15). This mean composite score was the lowest of the three experiments. However, a one-way ANOVA (with Experiment 2B as the reference level) indicated no main effect of Experiment, F(2, 444) = 2.36, p = .10. More specifically, the difference between Experiment 2B and Experiment 1 approached conventional significance (t = −1.95), whereas the difference between Experiment 2B and Experiment 2A did not (t = −0.41).
Response-Mapping Trials
Response Times
Table 3 presents descriptive statistics for Experiment 2B. In the pre-task mapping block, WMC significantly predicted RTs (b = −15 ms, SE = 6 ms, t = −2.5). Higher-WMC subjects were faster than lower-WMC subjects in the first half of the block (b = −15 ms, SE = 6 ms, t = −2.4), in the second half (b = −14 ms, SE = 6 ms, t = −2.3), and in the last 25 trials (b = −13 ms, SE = 7 ms, t = −1.9). There were no WMC-related differences in the post-task mapping RTs (b = −7 ms, SE = 7 ms, t = −1.0).
Table 3.
Experiment 2b Descriptive Statistics
| DV | Trial Type | M | Std Err |
Min | Max | Skew | Kurtosis |
|---|---|---|---|---|---|---|---|
| RT | S-R congruent | 435 | 5 | 338 | 602 | 0.69 | 0.23 |
| S-R incongruent | 518 | 7 | 376 | 711 | 0.44 | −0.13 | |
| S-S congruent | 455 | 6 | 350 | 661 | 0.71 | 0.46 | |
| S-S incongruent | 522 | 7 | 393 | 782 | 0.69 | 0.75 | |
| ER | S-R congruent | 0.02 | 0.00 | 0.00 | 0.11 | 1.59 | 2.93 |
| S-R incongruent | 0.26 | 0.01 | 0.04 | 0.60 | 0.56 | 0.10 | |
| S-S congruent | 0.04 | 0.00 | 0.00 | 0.14 | 0.99 | 0.68 | |
| S-S incongruent | 0.16 | 0.01 | 0.02 | 0.50 | 0.90 | 0.47 |
Note: M = mean of subject means. RT = response time. ER = error rate. Std Err = standard error. S-S = stimulus-stimulus. S-R = stimulus-response.
Errors
WMC did not predict errors in the pre-task mapping trials (b = −0.12, SE = 0.08, Z = −1.5). There were no WMC-related differences in the first half (b = −0.09, SE = 0.10, Z = −0.9), second half (b = −0.15, SE = 0.10, Z = −1.5), or last 25 trials of pre-task mapping (b = −0.01, SE = 0.14, Z = −0.1). In the post-task mapping, WMC also did not predict errors (b = 0.04, SE = 0.09, Z = 0.4).
As in Experiments 1 and 2A, we entered the centered mean RT or error rate for the last 25 mapping trials as a covariate in the following analyses (and we additionally report key results without the covariate included).
S-S Trials
Response Times
Subjects showed significant S-S interference, responding more slowly on incongruent trials than on congruent trials (b = 66 ms, SE = 2 ms, t = 38.3). Although WMC did not predict RTs, overall (b = −5 ms, SE = 5 ms, t = −1.0), higher-WMC subjects experienced less S-S interference than did lower-WMC subjects (b = − 6 ms, SE = 2 ms, t = −3.1), just as in Experiments 1 and 2A; in decomposing this interaction, however, higher WMC subjects were not significantly faster than lower-WMC subjects on either congruent (b = − 2 ms, SE = 5 ms, t = −0.4) or incongruent trials (b = − 8 ms, SE = 5 ms, t = −1.5), but again the interaction seems most strongly driven by incongruent trial RTs. (In a model that did not include the mapping-RT covariate, only the overall WMC parameter changed, becoming significant [b = −18 ms, SE = 7 ms, t = −2.5].)
Errors
Subjects made significantly more errors on incongruent trials than congruent trials (b = 1.61, SE = 0.05, Z = 30.3). WMC did not predict errors, overall (b = 0.04, SE = 0.07, Z = 0.5), and WMC did not interact with S-S error interference (b = 0.02, SE = 0.07, Z = 0.3). (Again, without the covariate, only the WMC parameter changed, remaining non-significant [b = −0.03, SE = 0.08, Z = −0.4].)
S-R Trials
Response Times
Subjects showed significant S-R interference, with slower responding to incongruent than congruent trials (b = 82 ms, SE = 2 ms, t = 48.7). Overall, WMC did not predict RTs (b = −4 ms, SE = 4 ms, t = 1.0). In contrast to Experiments 1 and 2A, however, higher-WMC subjects experienced less S-R interference than did lower-WMC subjects (b = −5 ms, SE = 2 ms, t = −2.7). As for the S-S trials in this experiment, higher-WMC subjects were not statistically faster than lower-WMC subjects on either congruent (b = − 2 ms, SE = 4 ms, t = −0.5) or incongruent trials (b = − 6 ms, SE = 6 ms, t = −1.0). (Without the covariate, only the WMC main-effect parameter changed, becoming significant [b = −16 ms, SE = 6 ms, t = −2.5].)
Errors
Subjects committed more errors on incongruent than on congruent trials (b = 2.69, SE = 0.06, Z = 47.4), but WMC did not predict errors (b = 0.06, SE = 0.07, Z = 0.8). In contrast to Experiments 1 and 2A, here we did not detect a relation between error interference and WMC (b = 0.06, SE = 0.07, Z = 0.8). (Again, without the covariate, only the WMC parameter changed, remaining non-significant [b = − 0.00, SE = 0.08, Z = −0.0].)
Comparison of S-S and S-R trials
For these analyses, trial type was dummy-coded. S-R trials were the reference level and S-S trials the comparison level.
Response Times
S-S trials yielded longer RTs than did S-R trials (b = 12 ms, SE = 1 ms, t = 9.9) and, as can be seen in Figure 9a, subjects experienced more interference on S-R than on S-S trials (b = − 16 ms, SE = 2 ms, t = −6.7). The slope for WMC in S-S interference was not significantly steeper than in S-R interference (b = −1 ms, SE = 3 ms, t = −0.4).
Figure 9.
Experiment 2A model parameters of stimulus-response (S-R) and stimulus-stimulus (S-S) interference (incongruent RTs/errors – congruent RTs/errors) from pure trials as a function of composite working memory capacity Z-score (WMCz) in response times ( RTs;panel A) and E\errors (panel B). The dotted lines represent the standard error of the estimate.
Errors
There were no differences in error rate between S-S and S-R trials (b = −0.06, SE = 0.04, Z = −1.5) but, as can be seen in Figure 9b, subjects exhibited less interference on S-S trials than on S-R trials (b = −1.07, SE = 0.08, Z = −13.9). WMC did not predict interference on either S-S or S-R trials (b = −0.04, SE = 0.10, Z = −0.4).
Delta Plots
S-S trials
Figure 10 plots the difference in RTs between binned incongruent and congruent trials and shows that, in a high-congruency context, the difference grew between higher- and lower-WMC subjects as RTs got longer. This observation was confirmed by a LMM, in which neither the main effect of WMC (b = 0 ms, SE = 4 ms, t = −0.1), nor bin (b = 1 ms, SE = 1 ms, t = 1.2) was significant, but for the first time in this set of experiments, the interaction between WMC and bin was significant (b = − 2 ms, SE = 1 ms, t = −2.5). This interaction is consistent with that found by Unsworth et al. (2012), who interpreted it as evidence of WMC-related differences in goal maintenance.
Figure 10.
Delta plots of stimulus-stimulus (S-S) interference as a function of upper (high) and lower (low) tercile of composite working memory capacity Z-score (WMC) in Experiment 2B.
S-R trials
Figure 11 shows an ascending pattern of S-R interference, with higher-WMC subjects experiencing less interference than did lower-WMC subjects across all RT bins. In the LMM, only bin significantly predicted S-R interference (b = 7 ms, SE = 1 ms, t = 8.1). WMC did not (b = −6 ms, SE = 5 ms, t = −1.2), nor did the WMC-by-bin interaction (b = 0 ms, SE = 1 ms, t = −0.2). Although the LMM performed above on the non-aggregated RTs found that higher-WMC subjects experienced less interference than lower-WMC subjects, here in the delta plot analysis, WMC was not a statistically significant predictor. This discrepancy is most likely due to reduced power in using aggregated data in the delta plot analysis, but it does also suggest that the WMC effect in S-R interference, which we have observed for the first time across experiments here in the 80% congruent condition, may be less robust than the WMC effect in S-S interference.
Figure 11.
Delta plots of stimulus-stimulus (S-S) interference as a function of upper (high) and lower (low) tercile of composite working memory capacity Z-score (WMC) in Experiment 2B.
Congruency Sequence Effects
In contrast to Experiment 2A, subjects did not show a significant reduction in interference following an incongruent trial when the conflict type switched, from S-S to S-R, or vice versa (b = −1 ms, SE = 4 ms, t = −0.3). But, as in Experiment 2A, subjects showed a substantial reduction in interference following an incongruent trial when the conflict type repeated (b = −34 ms, SE = 6 ms, t = −5.3), again indicating relative specificity of control. WMC did not interact with congruency sequence effects on conflict-type-switch trials (b = 8 ms, SE = 5 ms, t = 1.6) or on repetition trials (b = −4 ms, SE = 8 ms, t = 0.5).
We again examined S-S and S-R repetitions trials (i.e., analyses for S-S and S-R trials that were preceded by the same trial type), and their associations with WMC, separately. In S-S trials, we found a significant congruency-sequence effect (b = −16 ms, SE = 4 ms, t = −3.7) and a null interaction with WMC (b = 1 ms, SE = 5 ms, t = 0.1). In S-R trials, we found the same pattern, with a significant congruency-sequence effect (b = −33 ms, SE = 7 ms, t = −4.7), but no WMC interaction (b = 9 ms, SE = 8 ms, t = 1.1).
Discussion
Experiment 2A replicated the critical finding from Experiment 1: Higher-WMC subjects showed less RT interference than did lower-WMC subjects on S-S trials but not on S-R trials; moreover, higher-WMC subjects showed greater interference in error rates than did lower-WMC subjects on S-R trials. Experiment 2A did not, however, replicate the significant three-way interaction among WMC, S-S interference, and S-R interference. We acknowledge this limitation, but argue that reproducing the overall dissociative pattern here, with consistent parameter estimates across experiments (Experiment 1, WMC × S-S = −7 ms and WMC × S-R = −3 ms; Experiment 2A WMC × S-S = −4 ms and WMC × S-R = −1 ms), constitutes a successful replication. In contrast, in Experiment 2B, with a greater proportion of congruent trials that put a premium on goal maintenance, higher-WMC subjects resolved interference better than did lower-WMC subjects on both S-S and S-R trials. These findings suggest that when cognitive conflict resolution processes can be isolated from goal maintenance abilities, having a higher WMC is beneficial for resolving interference close to the beginning of information-processing (i.e., at stimulus identification or selection), but less so when interference occurs with the action to be performed (i.e., at response selection). Moreover, in situations where goal maintenance abilities are especially important (Experiment 2B), higher WMC subjects show generalized performance benefits across conflict types.
Delta plots of the data from Experiment 2A data provided evidence that WMC-related differences in S-S interference were not localized to a specific portion of RT distribution; just as in Experiment 1, higher-WMC subjects did not perform better than lower-WMC subjects because of particular control abilities engaged when they were responding especially quickly or slowly. In contrast, in Experiment 2B, the S-S delta plots showed a pattern consistent with the ones observed by Unsworth et al. (2012), where WMC-related differences in performance increased with response times. Unsworth et al. interpreted this pattern to indicate that WMC variation is related to goal maintenance abilities, with lower-WMC subjects showing evidence of their lapses of goal maintenance in the slower trials (this finding is consistent with examinations of relations between WMC and ex-Gaussian RT distributions where WMC has been found to be more strongly related to performance on the slowest trials [McVay & Kane, 2012b; Schmiedek, Oberauer, Wilhelm, Süß, & Wittman, 2007; Unsworth, Redick, Lakey, & Young, 2010]) .
The S-R delta plot in Experiment 2A looked much like that from Experiment 1, with no significant separation between WMC terciles. In contrast, the S-R delta plot in Experiment 2B seemed to show consistent but modest separation between the higher and lower WMC terciles, with interference becoming greater for everyone as RTs increased. Our analysis of individual differences on these binned S-R difference scores confirmed that interference did increase as RTs got longer, but WMC did not significantly predict these difference scores, or their changes over bins. Therefore, on one hand, Experiment 2B showed that, in a high-congruency task, WMC predicted S-R interference in RTs. This is consistent with an interpretation of WMC-related differences in goal maintenance abilities and it is precisely what motivated our proportion-congruency manipulation in Experiment 2. On the other hand, the WMC effect on S-R interference was not confirmed by the LMM conducted on the binned data. To further assess the strength of the WMC by S-R interference interaction in Experiment 2B, we compared this interaction to the non-significant WMC × S-R interactions in Experiment 1 and Experiment 2A. In a LMM on all correct S-R trials, with experiment, WMC, and congruency as predictors, the Experiment 2B significant interaction between WMC and S-R was not significantly different from the non-significant interactions observed in Experiments 1 (t = 1.7) or 2A (t = 1.6). Therefore, although the significant WMC × S-R interference interaction is consistent with our a priori hypothesis (and dual-factor conceptions of cognitive control; e.g., Braver et al., 2007; Engle & Kane, 2004), we will interpret it cautiously in light of the delta plots and the cross-experiment analysis. Future replications of this effect will be needed to estimate its magnitude and generality.
Our examination of congruency sequence effects in both Experiment 2A and 2B yielded results mostly consistent with prior reports from this task (Funes et al., 2010a, 2010b), where the control processes initiated from a previous incongruent trial only affected performance on the current trial when the same conflict type repeated (although in Experiment 2A we found a small and significant general carryover effect [−6 ms] that was dwarfed by the effect when the conflict type repeated [−40 ms]). Indeed, most studies that have combined two conflict types have found that the cognitive control initiated by an incongruent trial is specific to the particular conflict elicited by that trial (Egner et al., 2007; Funes et al., 2010a, 2010b; Kiesel, Kunde, & Hoffmann, 2006; Notebaert & Verguts, 2008; Verbruggen et al., 2005; Wendt, Kluwe, & Peters, 2006; but see Freitas & Clark, 2014).
WMC did not moderate any of the congruency-sequence effects examined in Experiments 2A or 2B (or Experiment 1), indicating that WMC did not affect the reactive adjustments to control that subjects made following the processing of either S-S or S-R conflict trials. These null WMC effects confirm those reported by Meier and Kane (2013), who examined Stroop task performance (reflecting combined S-S and S-R conflict), by Unsworth et al. (2012), who examined both Stroop and flanker task performance (the latter reflecting primarily S-S conflict), and by Keye et al. (2009), who also did not detect a relationship in a flanker task. However, we note that our null WMC effects are inconsistent with two S-R-conflict-related findings: Keye et al. (2009) found a significant relation between WMC and congruency-sequence effects in a vertical Simon task (i.e., S-R trials) with higher WMC subjects showing less trial-to-trial adjustment, and Weldon et al. (2013) reported the same pattern in a horizontal Simon task. Although this collection of S-R findings varied with aspects of the design – null effects here with mixed S-R and S-S trials, and significant WMC effects in Keye et al. and Weldon et al. in pure S-R blocks, it is currently unclear how these designs would differentially affect WMC-related variation (or why design would interact with WMC in S-R but not in S-S conditions).
General Discussion
The primary goal of this project was to add specificity to accounts of WMC-related differences in conflict resolution – and our understanding of the executive control construct – by testing for WMC variation in a single attention-control task that provoked two, potentially dissociable, forms of interference: S-S conflict between relevant and irrelevant stimulus dimensions versus S-R conflict between stimulus and response dimensions. Depending on which forms of conflict were sensitive to WMC-related individual differences, the findings would suggest a role for WMC in either resolving interference relatively early in the information-processing stream, at stimulus identification or selection (indicated by S-S conflict), or relatively late, at response selection (indicated by S-R conflict), or perhaps at both relatively early and late stages. If WMC was predictive of both S-S and S-R conflict resolution, then it may have indicated that WMC affects online conflict resolution processes at a global level (e.g., superior goal maintenance or task approach). For example, higher-WMC subjects may better resolve all conflict (both S-S and S-R) because they have the task goal in a more activated state than lower-WMC subjects allowing for more efficient (or faster) conflict resolution processes downstream.
Using a task that minimized contributions of goal-maintenance and task-switching abilities by presenting a single goal (attend to arrow shape and ignore arrow location) that was frequently reinforced by conflict trials (Experiments 1 and 2A), we found that higher-WMC subjects experienced less S-S interference (in RTs) and more S-R interference (in errors) than did lower-WMC subjects. That is, in a scenario where the contribution of goal-neglect to performance was held consistent across the different conflict types by the experimental design, we found a dissociation in WMC’s prediction of S-S versus S-R conflict. In contrast, in a similar task that boosted goal-maintenance contributions by presenting 80% congruent trials (and thereby did not externally reinforce the goal of ignoring stimulus location), higher-WMC subjects in Experiment 2B were less vulnerable to both S-S and S-R interference than were lower-WMC subjects. These results support dual-factor theories of cognitive control (e.g., Braver et al., 2007; Engle & Kane, 2004) – with proactive goal maintenance and reactive conflict resolution representing two key control processes – and add specificity to the account of WMC–control relations by defining a boundary around the association.
WMC and S-S Interference
S-S interference occurred on trials where subjects pressed one horizontally aligned key for upward pointing arrows (e.g., the left-positioned key) and another for downward facing arrows (e.g., the right-positioned key). Horizontally-centered arrows located above or below the midline of the screen were either congruent (e.g., up arrow on the up portion of the screen) or incongruent (e.g., up arrow on the down side of the screen) between relevant (arrow direction) and irrelevant stimulus (arrow location) features. S-S interference represented the difference in RTs or errors on incongruent versus congruent trials. WMC did not significantly relate to errors caused by S-S conflict but, in all three experiments (and in both pure S-S trials and combined S-S/S-R trials in Experiment 1), higher-WMC subjects exhibited significantly less S-S interference in RTs than did lower-WMC subjects. WMC thus appears to affect information processing relatively early in the process, such as during stimulus identification or stimulus selection. It seems, then, that higher-WMC subjects are better able than lower-WMC subjects to selectively attend to the response-relevant stimulus dimension (here, arrow shape/direction) while limiting processing of the irrelevant stimulus dimension (here, arrow location). The finding that higher-WMC subjects are better able than lower-WMC subjects to resolve S-S conflict jibes well with previous work that has found performance benefits for higher-WMC subjects in other tasks that present S-S interference, for example, in Stroop tasks (Kane & Engle, 2003; Hutchison, 2011; Long & Prat, 2002; Meier & Kane, 2013; Unsworth et al., 2012) and flanker tasks (Heitz & Engle, 2007; Redick & Engle, 2006; Shipstead et al., 2012; but see Keye et al., 2009).
Research on the attentional blink (AB) also fits with our S-S conflict findings. In the AB paradigm, subjects attempt to identify two visual targets (defined by the experimenters) that are presented serially in rapid succession in the same spatial location amid non-targets. Regardless of intervening non-targets, subjects have difficulty reporting the second target when it appears within 200 – 500 ms of the first (e.g. Broadbent & Broadbent, 1987; Chun & Potter, 1995; Duncan, Ward, & Shapiro, 1994; Reeves & Sperling, 1986). This period of time is the AB. In their review, Dux and Marois (2009) provided evidence that the AB is the product of multiple processes including attentional selection of the target, encoding of the target into working memory, response selection, and the inhibition of distractors. Regarding WMC, Colzato et al. (2007) first documented that subjects who had higher OSPAN scores had smaller ABs (r = −.33), indicating that higher-WMC subjects were better able than lower-WMC subjects to identify a second target presented closer to the first. However, given the complexity of the AB, the Colzato et al. result did not provide much clarity regarding what higher-WMC subjects actually do to achieve this smaller AB.
Fortunately, Arnell and Stubitz (2010) further specified the relation between AB and WMC by having subjects complete a visual WMC task (change detection; Luck & Vogel, 1997) and a task designed to assess how effective subjects are at filtering information out of visual working memory (adapted from Vogel, McCollough, & Machizawa, 2005). Visual WMC was positively related to filtering efficiency, but only filtering efficiency was related to AB, with better filterers showing smaller ABs. The Arnell-Stubitz results suggest that WMC (as measured by complex span tasks) may be related to the AB because of selective attention processes at work during stimulus identification or selection. That is, lower-WMC subjects may permit at least some distractors (on some occasions) to progress through a relatively early stage of processing into a later stage, thereby interfering with the identification and reporting of the second target.
An important question remains: What about complex span tasks allows them to predict the resolution of S-S interference? Do complex span tasks, themselves, present a form of S-S conflict? The interference generated between memory items and the processing task (e.g., letters and equations) does seem more akin to S-S than S-R interference, with the equation mapping onto the irrelevant stimulus dimension and letter onto the relevant stimulus dimension. In the computerized complex span tasks that are popular today (and used here), responses are usually made via a mouse click, therefore there is no overlap – or potential for interference – between distractors and responses, suggesting that complex span tasks may present a form of S-S interference. Formal modeling work by Oberauer et al. (2012) provides evidence that complex span task performance is determined by an interference-resolution control process that removes interfering information from working memory. Perhaps, here, this same control process is active in the complex span tasks and in the S-S/S-R task, but is only effective at either identifying or removing items that interfere with stimulus representations.
WMC and S-R Interference
S-R interference was measured on trials where irrelevant stimulus location was either congruent or incongruent with the response to be made. For example, if a vertically-centered upward arrow appeared on the left side of the screen and a left-key response was assigned to upward arrows, the stimulus location and response were congruent to each other. On trials where an upward arrow appeared on the right side of the screen, in contrast, the stimulus location (right) was incongruent with the response (left). In Experiments 1 and 2A, higher and lower-WMC subjects showed equivalent RT interference on S-R trials (with −3 ms per WMC standard deviation being the largest parameter value), but higher-WMC subjects showed significantly greater error interference than did lower-WMC subjects.2 Although the irrelevant stimulus dimension was arrow location for both S-S and S-R trials, whatever higher-WMC subjects were able to do to combat S-S interference was not helpful in resolving S-R interference. Experiment 2B, with its high proportion of congruent trials (80%), yielded a different pattern: Higher-WMC subjects showed less RT interference than did lower-WMC subjects (−5 ms per WMC standard deviation) and equivalent error interference (but note in a cross-experiment analysis this effect was not significantly different from the WMC-SR effects in Experiments 1 and 2A). The zero-order correlation between WMC and an aggregated score for S-R interference in Experiment 2B (r = −.17) was of similar magnitude to those for the S-S trials from Experiments 1, 2A, and 2b (rs = −.24, −.16, and −.23, respectively) while being substantially larger than the correlations between WMC and S-R interference from Experiments 1 and 2A (both rs = −.04). The Experiment 2B results suggest that when goal-maintenance processes are made relevant to task success by the context (here, via a high congruency proportion), WMC will again influence performance in more typical ways, with higher-WMC subjects performing best regardless of the conflict type, because they are better able to maintain ready access to task goals.
Two recent studies may shed light on why higher-WMC subjects do not outperform lower-WMC subjects on S-R conflict trials presented in low-congruency contexts (Wuhr & Biebl, 2011; Zhao, Chen, & West, 2010). Both studies examined the effects of a memory load on S-R task performance and reported that memory load reduced the magnitude of S-R conflict effects, supporting a response discrimination account of S-R conflict (Ansorge & Wuhr, 2004). This account suggests that S-R conflict is the product of interference between S-R rules (e.g., if the arrow is pointing up, press the left key) held in working memory, and the stimulus-location codes (left or right) that enter working memory during stimulus processing. (In S-S trials, there is no conflict between left and right response codes and either the location [up or down] and the arrow direction [up or down], making S-S trials fundamentally different from S-R trials; indeed, memory loads tend to increase S-S interference effects [de Fockert, Rees, Frith, & Lavie, 2001; Lavie, Hirst, de Fockert, & Viding, 2004]). So, by the response-discrimination account, loading WMC prevents the stimulus location codes from accessing working memory and the S-R conflict effect is thereby reduced or eliminated. Perhaps here, in the 50% congruency conditions, higher-WMC subjects’ performance was not superior to that of lower-WMC subjects’ on incongruent S-R trials because the higher-WMC subjects have additional capacity that allows them to grant stimulus location codes access to their working memory, thereby causing greater interference. As another way of looking at this, lower-WMC subjects can be thought of working under a constant working memory load (e.g., Kane & Engle, 2000; Rosen & Engle 1997) and thus experience less conflict because they permit less access to stimulus location codes than do higher-WMC subjects. In the 80% congruency condition, however, higher-WMC subjects constricted their greater capacity to maintain the task goal thereby occupying the capacity that stimulus location codes entered in the 50% congruency conditions.
Alternatively, the theory of event coding (TEC; e.g., Hommel, 2009; Hommel, Müsseler, Aschersleben, & Prinz, 2001), provides another approach to the dissociation between WMC and S-S/S-R conflict types. Following from selection-for-action theories of attention (e.g., Allport, 1987; Allport, Tipper, & Chmiel, 1985; Neumann, 1987), the TEC framework proposes that perception and action planning rely on a common representational system that: (1) codes the features of both external objects and intended actions and; (2) temporally binds them into event files (with those features weighted by goal relevance). Moreover, because the targets and effects of actions are external to the actor, action codes (and resulting event files) reflect their distal properties, including outcomes and spatial locations. In our experimental preparation, then, only location along the vertical plane was truly irrelevant to task completion, because it did not define targets and it was not associated with pressing horizontally arranged response keys. Only controlling S-S conflict thus involved the regulation of processing a truly irrelevant stimulus feature, and only controlling S-S conflict showed evidence of a higher-WMC advantage.
Attempts to control S-R conflict, in contrast, which was produced by stimuli presented along the horizontal plane, could not target an “irrelevant” dimension of the stimulus because subjects represented features of the intended action (e.g., press right or left) bound with features of the stimulus for action (e.g., an arrow on the right or left). From this perspective, WMC isn’t particularly linked to control over S-S conflict, per se, but rather to limiting the processing of task-irrelevant stimulus features. Because S-R conflict arises, at least in our task, from a stimulus dimension that is relevant – if not integral – to action planning (and so, presumably, cannot be blocked without disrupting that very planning), the “distraction control” processes linked to WMC are ineffectual. Indeed, our finding that higher-WMC subjects tended to be more accurate than lower-WMC subjects on S-R compatible trials suggests that WMC may actually have a slightly positive effect on binding perception-action representations (see Oberauer, 2005, 2009; Oberauer et al., 2007). If correct, this view predicts that WMC-related control over S-S conflict would be disrupted if the stimulus distractor dimension (i.e., vertical-plane location in our task) was made relevant to target selection and therefore was integral to successful performance. One way to implement this would be to present a distractor arrow just above or below fixation on each S-S trial (where the target is presented further from fixation), so that location features are now required to select the target from the distractor; here we might find that higher-WMC subjects no longer demonstrate weaker S-S interference than do lower-WMC subjects because there is no longer an irrelevant dimension to ignore.
WMC and Congruency Sequence Effects
Limited prior work has assessed the trial-to-trial dynamics of WMC’s association with cognitive control by testing for its moderation of congruency sequence effects (Keye et al., 2009; Meier & Kane, 2013; Unsworth et al., 2012; Weldon et al., 2013). The results have been mixed. Using Stroop tasks, flanker tasks, or both (with both involving S-S conflict), Meier and Kane (2013) and Unsworth et al. (2012) found no association between WMC and congruency sequence effects. In Simon tasks (which only elicit S-R conflict), however, Keye et al. (2009) and Weldon et al. (2013) found that lower-WMC subjects exhibited more reactivity to the congruence of the prior trial when processing the current trial. The present Experiments 2A and 2B found no link between WMC and congruency-sequence-effects in either S-S or S-R trials, or in analyses that focused exclusively on trial sequences that repeated or switched conflict types. The discrepancy between the S-R sequence findings here and those in Keye et al. (2009) and Weldon et al. (2013) may be the product of different task approaches cultivated by the mixed S-S/S-R trial presentation we used versus the blocked S-R presentations used previously. In any case, we think it most important that, in the experiments conducted here, there is nothing to suggest that trial-by-trial cognitive-control adjustments in response to previously experienced conflict are responsible for the WMC-related dissociations we found regarding S-S versus S-R conflict.3
Our findings complement recent work that has established boundary conditions for the association between WMC and executive control. Whereas prior research suggested that WMC influenced executive-task performance through goal-maintenance abilities and on-line conflict resolution processes (e.g., Kane & Engle, 2003), the present study adds specificity by suggesting that WMC impacts conflict-resolution processes by resolving conflict on the front-end of information processing (i.e., at stimulus identification or selection), or, according to the TEC theory, by resolving conflict produced by distractors that are wholly task-irrelevant. Considering the work presented here, along with previous boundary-establishing research examining visual search (Kane et al., 2006; Poole & Kane, 2009; Sobel et al., 2007), congruency-sequence effects (Meier & Kane, 2013; Unsworth et al., 2012) and auditory distraction (Sorqvist et al., 2013), we propose that the relation between WMC and executive control is a complex and nuanced one. The developing nomological network surrounding WMC and executive-control constructs suggests a system of control processes that enhance information processing in conditions that present interference by either gating irrelevant information from being processed, or by efficiently removing the conflicting information from working memory before it impairs response selection – but only for some forms of interference. A front-end, early-selection view of WMC-related interference reduction suggests that the association between WMC and ostensible response-inhibition tasks, such as go/no-go and antisaccade, is driven by variation in proactive goal-maintenance processes rather than inhibitory ones that operate at response selection (see also McVay & Kane, 2009; Unsworth et al., 2004). Clearly, the evidence presented here does not support a relation between WMC and the resolution of overlap between stimulus and response elements, except under conditions that put a premium on goal maintenance, and thus promote goal neglect in lower-WMC subjects.
Conclusions
We examined how individual differences in WMC related to two dissociable types of conflict in an attempt to seek out boundary conditions for the association between WMC and executive control. We were successful at dissociating WMC’s influence on the processing of conflict among stimulus and response elements. Our results provide evidence that WMC affects performance on tasks that demand attentional control (or executive attention) early in information processing to encode information into working memory by using control processes that filter out irrelevant information that can interfere with relevant stimulus identification and selection. The control processes used by WMC appear to be less effective in resolving interference that occurs among candidate responses to be made (or among stimulus dimensions that are integral to action planning). In fact, the experiments detailed above provide some evidence that WMC’s control processes can actually hinder performance when interference is generated between an overlap between irrelevant stimulus features and their response coding (e.g. as left or right). By revealing the boundaries between WMC and online-conflict resolution, we have arguably pruned the central executive’s nomological network.
Future work should determine the generality and robustness of the findings presented here. That is, will S-S/S-R dissociations with WMC be evident in other tasks that present separable S-S and S-R conflict? A limitation of the present study is that all S-S interference was generated by stimuli along the vertical axis and all S-R interference was generated by stimuli along the horizontal axis, and so it is possible (if unlikely) that WMC is uniquely unrelated to managing responses to stimuli on the horizontal plane (Pratte et al., 2010; Vallesi, Mapelli, Schiff, Amodio, & Umilta, 2005; Wiegand & Wascher, 2005). Future work might assess, for example, whether having subjects respond to right- or left-pointing arrows with upward or downward thrusts of a joystick would similarly produce WMC-related differences in S-S interference (here produced along the horizontal axis, when right-pointing arrows appear to the left of fixation, and vice-versa) but not in S-R interference (here produced along the vertical axis, when an arrow direction (e.g., right-pointing) that is assigned to an upward response motion appears below fixation, and vice versa). To assess the generalizability of our findings, then, future work should dissociate S-S and S-R trials from particular spatial locations and orientations.
The work here highlights that all interference is not the same — a point understood by many but nonetheless worth repeating — and consideration must be given to what processes are impacted by specific interference manipulations, especially when examining individual differences. Future investigations that seek to test empirically whether executive attention mediates the relation between WMC and higher-order cognition should select at least some cognitive-control tasks that present S-S conflict. We hope that by establishing boundaries around when and where WMC impacts performance, we will eventually be able to explain the WMC and executive control constructs with even sharper resolution.
Acknowledgments
We are grateful to Tracy Aumon, Jason Bostic, Laura Cook, Paul Grey, Caitlin Grindall, Nick Hiatt, Jeff Love, Lauren McCarthy, Lauren Richardson, Bridget Smeekens, James Smith, and Luz Toribio for their assistance with data collection for this project.
Portions of this work were supported by an award from the National Institutes of Mental Health (Award Number R15MH09377101). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH.
Footnotes
We note that the parameter estimate and t value for the 3-way interaction of WMC × S-S × S-R did not change between the dummy-coded model reported here and an effect-coded model, because both estimate the change in slope between WMC and interference when contrasting S-R to S-S trial types.
Here, the zero-order correlations between WMC and an S-R interference score computed from aggregated congruent and incongruent error rates were small and non-significant (.03, .09, and .05 for the Experiments 1, 2A, and 2B pure trials). This highlights the utility of modeling error rates as dichotomous outcomes with a logistic function. The logistic function removes the boundaries and compression that is experienced when error rates are very high or low (Dixon, 2008).
The exact stimulus (i.e., arrow direction and screen location) repeated on 1.4% of trials in Experiment 2A and on 13% of trials in Experiment 2B. We re-ran all congruency-sequence analyses without these exact-repetition trials. Given the rarity of repetition trials in Experiment 2A, all parameter estimates were almost exactly the same as reported in the original analyses. In Experiment 2B, although some parameter estimates were slightly smaller than those in the initial analyses, there were no changes in statistical significance, including for the (null) associations of congruency-sequence effects with WMC.
REFERENCES
- Acosta E, Simon JR. The effect of irrelevant information on the stages of processing. Journal of Motor Behavior. 1976;8(3):181–187. doi: 10.1080/00222895.1976.10735070. [DOI] [PubMed] [Google Scholar]
- Ahmed L, de Fockert JW. Focusing on attention: The effects of working memory capacity and load on selective attention. PLoS ONE. 2012;7:e43101. doi: 10.1371/journal.pone.0043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport DA. Selection for action: Some behavioural and neurophysiological considerations of attention and action. In: Heuer H, Sanders AF, editors. Perspective on perception and action. Hillsdale, NJ: Erblaum; 1987. pp. 395–419. [Google Scholar]
- Allport DA, Tipper SP, Chmiel NRJ. Perceptional integration and postcategorical filtering. In: Posner MI, editor. Attention and performance. XI. Hillsdale, NJ: Erlbaum; 1985. pp. 107–132. [Google Scholar]
- Anderson DE, Vogel EK, Awh E. A common discrete resource for visual working memory and visual search. Psychological Science. 2013;24:929–938. doi: 10.1177/0956797612464380. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ansorge U, Wuhr P. A response-discrimination account of the Simon effect. Journal of Experimental Psychology: Human Perception and Performance. 2004;30(2):365–377. doi: 10.1037/0096-1523.30.2.365. [DOI] [PubMed] [Google Scholar]
- Arnell KM, Stubitz SM. Attentional blink magnitude is predicted by the ability to keep irrelevant material out of working memory. Psychological Research/Psychologische Forschung. 2010;74(5):457–467. doi: 10.1007/s00426-009-0265-8. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Exploring the central executive. Quarterly Journal of Experimental Psychology. 1996;49A:5–28. [Google Scholar]
- Barrouillet P, Camos V. As time goes by: Temporal constraints in working memory. Current Directions in Psychological Science. 2012;21:413–419. [Google Scholar]
- Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. R package version 0.999999-2. 2013 http://CRAN.R-project.org/package=lme4. [Google Scholar]
- Bleckley MK, Durso FT, Crutchfield JM, Engle RW, Khanna MM. Individual differences in working memory capacity predict visual attention allocation. Psychonomic Bulletin & Review. 2003;10(4):884–889. doi: 10.3758/bf03196548. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–624. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. Variation in working memory. 2007:76–106. [Google Scholar]
- Broadbent DE, Broadbent MH. From detection to identification: Response to multiple targets in rapid serial visual presentation. Perception & Psychophysics. 1987;42(2):105–113. doi: 10.3758/bf03210498. [DOI] [PubMed] [Google Scholar]
- Bühner M, König C, Pick M, Krumm S. Working Memory Dimensions as Differential Predictors of the Speed and Error Aspect of Multitasking Performance. Human Performance. 2006;19(3):253–275. [Google Scholar]
- Burgess PW. Theory and methodology in executive function research. In: Rabbitt P, editor. Methodology of frontal and executive function. East Sussex, UK: Psychology Press; 1997. pp. 81–116. [Google Scholar]
- Campbell DT, Fiske DW. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychological bulletin. 1959;56(2):81–105. [PubMed] [Google Scholar]
- Chatham CH, Herd SA, Brant AM, Hazy TE, Miyake A, O'Reilly R, Friedman NP. From an executive network to executive control: A computational model of the n-back task. Journal of cognitive neuroscience. 2011;23(11):3598–3619. doi: 10.1162/jocn_a_00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. Journal Of Experimental Psychology: Human Perception And Performance. 1995;21(1):109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Colflesh JGH, Conway ARA. Individual differences in working memory capacity and divided attention in dichotic listening. Psychonomic Bulletin & Review. 2007;14:699–703. doi: 10.3758/bf03196824. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Spapé MMA, Pannebakker MM, Hommel B. Working memory and the attentional blink: Blink size is predicted by individual differences in operation span. Psychonomic Bulletin & Review. 2007;14:1051–1057. doi: 10.3758/bf03193090. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Cowan N, Bunting MF. The cocktail party phenomenon revisited: The importance of working memory capacity. Psychonomic Bulletin & Review. 2001;8:331–335. doi: 10.3758/bf03196169. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user's guide. Psychonomic Bulletin & Review. 2005;12(5):769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ. The two disciplines of scientific psychology. American psychologist. 1957;12(11):671–684. [Google Scholar]
- Cronbach LJ, Meehl PE. Construct validity in psychological tests. Psychological bulletin. 1955;52(4):281–302. doi: 10.1037/h0040957. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19:450–466. [Google Scholar]
- de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291(5509):1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Dixon P. Models of accuracy in repeated-measures designs. Journal of Memory and Language. 2008;59(4):447–456. [Google Scholar]
- Donders FC. On the speed of mental processes. In: Koster WG, editor. Attention and Performance II. Acta Psychologica. Vol. 30. 1869. pp. 412–431. (Original work published in 1868.) [DOI] [PubMed] [Google Scholar]
- Duncan J, Ward R, Shapiro KL. Direct measurement of attentional dwell time in human vision. Nature. 1994;369(6478):313–315. doi: 10.1038/369313a0. [DOI] [PubMed] [Google Scholar]
- Dux PE, Marois R. The attentional blink: A review of data and theory. Attention, Perception, & Psychophysics. 2009;71(8):1683–1700. doi: 10.3758/APP.71.8.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T. Congruency sequence effects and cognitive control. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):380–390. doi: 10.3758/cabn.7.4.380. [DOI] [PubMed] [Google Scholar]
- Egner T. Multiple conflict-driven control mechanisms in the human brain. Trends in Cognitive Sciences. 2008;12(10):374–380. doi: 10.1016/j.tics.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Egner T, Delano M, Hirsch J. Separate conflict-specific cognitive control mechanisms in the human brain. Neuroimage. 2007;35(2):940–948. doi: 10.1016/j.neuroimage.2006.11.061. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature neuroscience. 2005;8(12):1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Current Directions in Psychological Science. 2002;11:19–23. [Google Scholar]
- Engle RW, Kane MJ. Executive attention, working memory capacity, and a two-factor theory of cognitive control. Psychology of Learning and Motivation. 2004;44:145–199. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target in a non-search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Freitas AL, Clark SL. Generality and specificity in cognitive control: conflict adaptation within and across selective-attention tasks but not across selective-attention and Simon tasks. Psychological research. 2014:1–20. doi: 10.1007/s00426-014-0540-1. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Haberstick BC, Willcutt EG, Miyake A, Young SE, Corley RP, Hewitt JK. Greater attention problems during childhood predict poorer executive functioning in late adolescence. Psychological Science. 2007;18:893–900. doi: 10.1111/j.1467-9280.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Corley RP, Young SE, DeFries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychological Science. 2006;17:172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Robinson JL, Hewitt JK. Developmental trajectories in toddlers’ self-restraint predict individual differences in executive functions 14 years later: A behavioral genetic analysis. Developmental Psychology. 2011;47:1410–1430. doi: 10.1037/a0023750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes MJ, Lupiáñez J, Humphreys G. Sustained vs. transient cognitive control: evidence of a behavioral dissociation. Cognition. 2010a;114(3):338–347. doi: 10.1016/j.cognition.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Funes MJ, Lupiáñez J, Humphreys G. Analyzing the generality of conflict adaptation effects. Journal of Experimental Psychology: Human Perception and Performance. 2010b;36(1):147–161. doi: 10.1037/a0017598. [DOI] [PubMed] [Google Scholar]
- Hambrick DZ, Oswald FL, Darowski ES, Rench TA, Brou R. Predictors of multitasking performance in a synthetic work paradigm. Applied Cognitive Psychology. 2010;24(8):1149–1167. [Google Scholar]
- Hasher L, Lustig C, Zacks RT. Inhibitory mechanisms and the control of attention. Variation in working memory. 2007:227–249. [Google Scholar]
- Heitz RP, Engle RW. Focusing the spotlight: Individual differences in visual attention control. Journal of Experimental Psychology: General. 2007;136:217–240. doi: 10.1037/0096-3445.136.2.217. [DOI] [PubMed] [Google Scholar]
- Hommel B. Interactions between stimulus-stimulus congruence and stimulus-response compatibility. Psychological Research. 1997;59(4):248–260. [Google Scholar]
- Hommel B. Action control according to TEC (theory of event coding) Psychological Research. 2009;73(4):512–526. doi: 10.1007/s00426-009-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B, Müsseler J, Aschersleben G, Prinz W. The theory of event coding (TEC): A framework for perception and action planning. Behavioral and Brain Sciences. 2001;24:849–937. doi: 10.1017/s0140525x01000103. [DOI] [PubMed] [Google Scholar]
- Hutchison KA. The interactive effects of listwide control, item-based control, and working memory capacity on Stroop interference. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2011;37:851–860. doi: 10.1037/a0023437. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Bleckley MK, Conway ARA, Engle RW. A controlled-attention view of working-memory capacity. Journal of Experimental Psychology: General. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Conway ARA, Hambrick DZ, Engle RW. Variation in working memory as variation in executive attention and control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in Working Memory. New York: Oxford University Press; 2007. pp. 21–48. [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity, proactive interference, and divided attention: limits on long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(2):336–358. doi: 10.1037//0278-7393.26.2.336. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General. 2003;132(1):47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: A latent-variable approach to verbal and visuospatial memory span and reasoning. Journal of Experimental Psychology-General. 2004;133(2):189–217. doi: 10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Poole BJ, Tuholski SW, Engle RW. Working memory capacity and the top-down control of visual search: Exploring the boundaries of "executive attention". Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32(4):749–777. doi: 10.1037/0278-7393.32.4.749. [DOI] [PubMed] [Google Scholar]
- Keye D, Wilhelm O, Oberauer K, van Ravenzwaaij D. Individual differences in conflict-monitoring: testing means and covariance hypothesis about the Simon and the Eriksen Flanker task. Psychological research. 2009;73(6):762–776. doi: 10.1007/s00426-008-0188-9. [DOI] [PubMed] [Google Scholar]
- Kiesel A, Kunde W, Hoffmann J. Evidence for task-specific resolution of response conflict. Psychonomic Bulletin & Review. 2006;13(5):800–806. doi: 10.3758/bf03194000. [DOI] [PubMed] [Google Scholar]
- Kornblum S. Dimensional overlap and dimensional relevance in stimulus-response and stimulus-stimulus compatibility; Portions of this paper were presented at the Annual Meeting of the Psychonomic Society, Nov 1990, New Orleans, LA; North-Holland. 1992. [Google Scholar]
- Kornblum S. The way irrelevant dimensions are processed depends on what they overlap with: The case of Stroop-and Simon-like stimuli. Psychological Research. 1994;56(3):130–135. doi: 10.1007/BF00419699. [DOI] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A. Dimensional overlap: Cognitive basis for stimulus-response compatibility – A model and taxonomy. Psychological Review. 1990;97:253–270. doi: 10.1037/0033-295x.97.2.253. [DOI] [PubMed] [Google Scholar]
- Kornblum S, Lee JW. Stimulus-response compatibility with relevant and irrelevant stimulus dimensions that do and do not overlap with the response. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:855–875. doi: 10.1037//0096-1523.21.4.855. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Cacioppo JT, Davidson RJ, Hugdahl K, Lovallo WR, Spiegel D, Rose R. Bridging psychology and biology: the analysis of individuals in groups. American Psychologist. 2002;57(5):341–351. [PubMed] [Google Scholar]
- Kyllonen PC, Stephens DL. Cognitive abilities as determinants of success in acquiring logic skill. Learning and Individual Differences. 1990;2:129–160. [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General. 2004;133(3):339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006;50(4):643–653. doi: 10.1016/j.neuron.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. NeuroImage. 2004;22:1097–1106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Long DL, Prat CS. Working memory and stroop interference: an individual differences investigation. Memory & cognition. 2002;30(2):294–301. doi: 10.3758/bf03195290. [DOI] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Conducting the train of thought: working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35(1):196–204. doi: 10.1037/a0014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Drifting from slow to “d'oh!”: Working memory capacity and mind wandering predict extreme reaction times and executive control errors. Journal Of Experimental Psychology: Learning, Memory, And Cognition. 2012;38(3):525–549. doi: 10.1037/a0025896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Why does working memory capacity predict variation in reading comprehension? On the influence of mind wandering and executive attention. Journal Of Experimental Psychology: General. 2012;141:302–320. doi: 10.1037/a0025250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier ME, Kane MJ. Working memory capacity and Stroop interference: Global versus local indices of executive control. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2013;39(3):748–759. doi: 10.1037/a0029200. [DOI] [PubMed] [Google Scholar]
- Mewaldt SP, Connelly CL, Simon JR. Response selection in choice reaction time: Test of a buffer model. Memory & Cognition. 1980;8(6):606–611. doi: 10.3758/bf03213780. [DOI] [PubMed] [Google Scholar]
- Miller AE, Watson JM, Strayer DL. Individual differences in working memory capacity predict action monitoring and the error-related negativity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2012;38(3):757–763. doi: 10.1037/a0026595. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Morey CC, Elliott EM, Wiggers J, Eaves SD, Shelton JT, Mall JT. Goal-neglect links Stroop interference with working memory capacity. Acta Psychologica. 2012;141:250–260. doi: 10.1016/j.actpsy.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Neumann O. Beyond capacity: A functional view of attention. In: Heuer H, Sanders AF, editors. Perspectives on perception and action. Hillsdale, NJ: Erlbaum; 1987. pp. 361–394. [Google Scholar]
- Notebaert W, Verguts T. Cognitive control acts locally. Cognition. 2008;106(2):1071–1080. doi: 10.1016/j.cognition.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Oberauer K. Binding and inhibition in working memory – Individual and age differences in short-term recognition. Journal of Experimental Psychology: General. 2005;134:368–387. doi: 10.1037/0096-3445.134.3.368. [DOI] [PubMed] [Google Scholar]
- Oberauer K. Design for a working memory. Psychology of Learning and Motivation. 2009;51:45–100. [Google Scholar]
- Oberauer K, Lewandowsky S, Farrell S, Jarrold C, Greaves M. Modeling working memory: An interference model of complex span. Psychonomic bulletin & review. 2012;19(5):779–819. doi: 10.3758/s13423-012-0272-4. [DOI] [PubMed] [Google Scholar]
- Oberauer K, Schulze R, Wilhelm O, Süß H-M. Working memory and intelligence – their correlation and their relation: Comment on Ackerman, Beier, and Boyle (2005) Psychological Bulletin. 2005;131:61–65. doi: 10.1037/0033-2909.131.1.61. [DOI] [PubMed] [Google Scholar]
- Oberauer K, Süβ H-M, Wilhelm O, Sander N. Individual differences in working memory capacity and reasoning ability. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse J, editors. Variation in working memory. New York: Oxford University Press; 2007. pp. 49–75. [Google Scholar]
- Poole BJ, Kane MJ. Working-memory capacity predicts the executive control of visual search among distractors: The influences of sustained and selective attention. The Quarterly Journal of Experimental Psychology. 2009;62(7):1430–1454. doi: 10.1080/17470210802479329. [DOI] [PubMed] [Google Scholar]
- Pratte MS, Rouder JN, Morey RD, Feng C. Exploring the differences in distributional properties between Stroop and Simon effects using delta plots. Attention, Perception, & Psychophysics. 2010;72(7):2013–2025. doi: 10.3758/APP.72.7.2013. [DOI] [PubMed] [Google Scholar]
- Proctor RW, Miles JD, Baroni G. Reaction time distribution analysis of spatial correspondence effects. Psychonomic Bulletin & Review. 2011;18(2):242–266. doi: 10.3758/s13423-011-0053-5. [DOI] [PubMed] [Google Scholar]
- Proctor RW, Vu KL. Human information processing: An overview for human-computer interaction. In: Asko JA, Sears A, editors. The human-computer interaction handbook: Fundamentals, evolving techniques, and emerging applications. Mahwah, NJ: Erlbaum; 2003. pp. 35–51. [Google Scholar]
- R Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2013. R: A language and environment for statistical computing. URL http://www.R-project.org/. [Google Scholar]
- Redick TS, Broadway JM, Meier ME, Kuriakose PS, Unsworth N, Kane MJ, Engle RW. Measuring working memory capacity with automated complex span tasks. European Journal of Psychological Assessment. 2012;28(3):164–171. [Google Scholar]
- Redick TS, Calvo A, Gay CE, Engle RW. Working memory capacity and go/no-go task performance: Selective effects of updating, maintenance, and inhibition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2011;37(2):308–324. doi: 10.1037/a0022216. [DOI] [PubMed] [Google Scholar]
- Redick TS, Engle RW. Working memory capacity and attention network test performance. Applied Cognitive Psychology. 2006;20:713–721. [Google Scholar]
- Reeves A, Sperling G. Attention gating in short-term visual memory. Psychological Review. 1986;93(2):180–206. [PubMed] [Google Scholar]
- Rosen VM, Engle RW. The role of working memory capacity in retrieval. Journal of Experimental Psychology: General. 1997;126(3):211–227. doi: 10.1037//0096-3445.126.3.211. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Oberauer K, Wilhelm O, Süß H, Wittmann WW. Individual differences in components of reaction time distributions and their relations to working memory and intelligence. Journal Of Experimental Psychology: General. 2007;136(3):414–429. doi: 10.1037/0096-3445.136.3.414. [DOI] [PubMed] [Google Scholar]
- Shipstead Z, Lindsey DR, Marshall RL, Engle RW. The mechanisms of working memory capacity: Primary memory, secondary memory, and attention control. Journal of Memory and Language. 2014;72:116–141. [Google Scholar]
- Shin YK, Proctor RW, Capaldi EJ. A review of contemporary ideomotor theory. Psychological Bulletin. 2010;136(6):943–974. doi: 10.1037/a0020541. [DOI] [PubMed] [Google Scholar]
- Shute VJ. Who is likely to acquire programming skills? Journal of Educational Computing Research. 1991;7:1–24. [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U. False-positive psychology: Undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological Science. 2011;22:1359–1366. doi: 10.1177/0956797611417632. [DOI] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U. A 21 word solution. Dialogue: The Official Newsletter of the Society for Personality and Social Psychology. 2012;26:4–7. [Google Scholar]
- Simon JR. Effect of an auditory stimulus on the processing of a visual stimulus under single-and dual-tasks conditions. Acta Psychologica. 1982;51(1):61–73. doi: 10.1016/0001-6918(82)90019-1. [DOI] [PubMed] [Google Scholar]
- Simon JR, Acosta E, Mewaldt SP, Speidel CR. The effect of an irrelevant directional cue on choice reaction time: Duration of the phenomenon and its relation to stages of processing. Attention, Perception, & Psychophysics. 1976;19(1):16–22. [Google Scholar]
- Simon J, Small AR. Processing auditory information: Interference from an irrelevant cue. Journal of Applied Psychology. 1969;53(5):433–435. doi: 10.1037/h0028034. [DOI] [PubMed] [Google Scholar]
- Sobel KV, Gerrie MP, Poole BJ, Kane MJ. Individual differences in working memory capacity and visual search: The roles of top-down and bottom-up processing. Psychonomic Bulletin & Review. 2007;14(5):840–845. doi: 10.3758/bf03194109. [DOI] [PubMed] [Google Scholar]
- Sörqvist P, Marsh JE, Nöstl A. High working memory capacity does not always attenuate distraction: Bayesian evidence in support of the null hypothesis. Psychonomic Bulletin & Review. 2013:1–8. doi: 10.3758/s13423-013-0419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Towse JN, Houston-Price CMT. Reflections on the concept of the central executive. In: Andrade J, editor. Working memory in perspective. East Sussex, UK: Psychology Press; 2001. pp. 240–260. [Google Scholar]
- Underwood BJ. Individual differences as a crucible in theory construction. American Psychologist. 1975;30(2):128–134. [Google Scholar]
- Unsworth N, Engle RW. On the division of short-term and working memory: an examination of simple and complex span and their relation to higher order abilities. Psychological bulletin. 2007a;133(6):1038–1066. doi: 10.1037/0033-2909.133.6.1038. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. The nature of individual differences in working memory capacity: active maintenance in primary memory and controlled search from secondary memory. Psychological review. 2007b;114(1):104–132. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Heitz RP, Schrock JC, Engle RW. An automated version of the operation span task. Behavior research methods. 2005;37(3):498–505. doi: 10.3758/bf03192720. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Redick TS, Heitz RP, Broadway JM, Engle RW. Complex working memory span tasks and higher-order cognition: A latent-variable analysis of the relationship between processing and storage. Memory. 2009;17(6):635–654. doi: 10.1080/09658210902998047. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Redick TS, Lakey CE, Young DL. Lapses in sustained attention and their relation to executive control and fluid abilities: An individual differences investigation. Intelligence. 2010;38(1):111–122. [Google Scholar]
- Unsworth N, Redick TS, Spillers GJ, Brewer GA. Variation in working memory capacity and cognitive control: goal maintenance and microadjustments of control. The Quarterly Journal of Experimental Psychology. 2012;65(2):326–355. doi: 10.1080/17470218.2011.597865. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Schrock JC, Engle RW. Working memory capacity and the antisaccade task: Individual differences in voluntary saccade control. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30:1302–1321. doi: 10.1037/0278-7393.30.6.1302. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Spillers GJ. Working memory capacity: Attention control, secondary memory, or both? A direct test of the dual-component model. Journal of Memory and Language. 2010;62(4):392–406. [Google Scholar]
- Unsworth N, Spillers GJ, Brewer GA, McMillan B. Attention control and the antisaccade task: A response time distribution analysis. Acta psychologica. 2011;137(1):90–100. doi: 10.1016/j.actpsy.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Mapelli D, Schiff S, Amodio P, Umiltà C. Horizontal and vertical Simon effect: Different underlying mechanisms? Cognition. 2005;96(1):B33–B43. doi: 10.1016/j.cognition.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Van der Molen MW, Keuss PJG. Response selection and the processing of auditory intensity. The Quarterly Journal of Experimental Psychology. 1981;33(2):177–184. [Google Scholar]
- Verbruggen F, Liefooghe B, Notebaert W, Vandierendonck A. Effects of stimulus–stimulus compatibility and stimulus–response compatibility on response inhibition. Acta Psychologica. 2005;120(3):307–326. doi: 10.1016/j.actpsy.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Awh E. How to Exploit Diversity for Scientific Gain Using Individual Differences to Constrain Cognitive Theory. Current Directions in Psychological Science. 2008;17(2):171–176. [Google Scholar]
- Vogel EK, McCullough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438(7067):500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Weldon RB, Mushlin H, Kim B, Sohn MH. The effect of working memory capacity on conflict monitoring. Acta Psychologica. 2013;142(1):6–14. doi: 10.1016/j.actpsy.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Wendt M, Kluwe RH, Peters A. Sequential modulations of interference evoked by processing task-irrelevant stimulus features. Journal of Experimental Psychology: Human Perception and Performance. 2006;32(3):644–667. doi: 10.1037/0096-1523.32.3.644. [DOI] [PubMed] [Google Scholar]
- Wiegand K, Wascher E. Dynamic aspects of stimulus-response correspondence: evidence for two mechanisms involved in the Simon effect. Journal of Experimental Psychology: Human Perception and Performance. 2005;31(3):453–464. doi: 10.1037/0096-1523.31.3.453. [DOI] [PubMed] [Google Scholar]
- Wilhelm O, Oberauer K. Why are reasoning ability and working memory capacity related to mental speed? An investigation of stimulus–response compatibility in choice reaction time tasks. European Journal of Cognitive Psychology. 2006;18(1):18–50. [Google Scholar]
- Wühr P, Biebl R. The role of working memory in spatial SR correspondence effects. Journal of Experimental Psychology-Human Perception and Performance. 2011;37(2):442–454. doi: 10.1037/a0020563. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Chen A, West R. The influence of working memory load on the Simon effect. Psychonomic Bulletin & Review. 2010;17(5):687–692. doi: 10.3758/PBR.17.5.687. [DOI] [PubMed] [Google Scholar]