Abstract
Background
Both antenatal and postpartum depression have adverse, lasting effects on maternal and child well-being. Socio-economically disadvantaged women are at increased risk for perinatal depression and have experienced difficulty accessing evidence-based depression care. The authors evaluated whether “MOMCare,”a culturally relevant, collaborative care intervention,providing a choice of brief interpersonal psychotherapy and/or antidepressants,is associated with improved quality of care and depressive outcomes compared to public health Maternity Support Services(MSS-Plus).
Methods
A randomized multi-site controlled trial with blinded outcome assessment was conducted in the Seattle-King County Public Health System. From January 2010-July 2012, pregnant women were recruited who met criteria for probable major depression and/or dysthymia, English-speaking,had telephone access, and ≥18-years-old. The primary outcome was depression severity at 3-,6-,12-,18-month postbaseline assessments; secondary outcomes included functional improvement, PTSD severity, depression response and remission, and quality of depression care.
Results
All participants were on Medicaid and 27-years-old on average; 58% were non-white; 71% were unmarried; and 65% had probable PTSD. From before birth to 18-months-postbaseline, MOMC are (n=83) compared to MSS-Plus participants (n=85) attained significantly higher rates of depression remission (Wald'sχ2=3.67,df=1,p=.05), lower levels of depression severity (Wald's χ2=6.09, df=1,p=.01) and PTSD severity (Wald'sχ2=4.61,df=1,p=.04), and had a greater likelihood of receiving ≥4 mental health visits (Wald'sχ2=58.23,df=1,p<.0001) and of adhering to anti-depressants in the prior month (Wald'sχ2=10.00,df =1,p<.01).
Conclusion
Compared to MSS-Plus, MOMCare showed significant improvement in quality of care, depression severity and remission rates from before birth to 18-months-postbaseline for socioeconomically disadvantaged women. Findings suggest that evidence-based perinatal depression care can be integrated into the services of a county public health system in the US.
Keywords: perinatal depression, antenatal depression, interpersonal psychotherapy, collaborative care, antidepressants, PTSD
Introduction
A report from the Agency for Healthcare Research and Quality concluded that despite the profound negative impact of perinatal depression on public health, there is a paucity of high-quality research on the identification and management of perinatal depression in “real world” systems of care.1 Depression during pregnancy has been linked to low birthweight and prematurity, especially for socioeconomically disadvantaged women,2 and is strongly associated with postpartum depression.3 Both antenatal and postpartum depression have adverse, lasting effects on maternal, infant and child well-being.4,5,6 Antenatal depression alone has been found to be predictive of developmental adversity in childhood and adolescence. 5,6 For example, findings from a prospective study showed that depression during pregnancy increases the risk for depression in 16-year old offspring by 4.7 times compared to unexposed offspring, even when their mothers recover from depression after birth.6 Clearly, treating antenatal depression is critical in preventing deleterious consequences for women, children and families.
Poor and racially/ethnically diverse urban women are at least twice as likely as middle-class women to meet diagnostic criteria for major depression during pregnancy.7 Yet, they are difficult to engage and retain in a minimally adequate course of mental health treatment,8 due to numerous barriers to care at the system, provider, and patient levels.9,10 Pregnancy is an opportune time for initiating health interventions11 because pregnant women may be especially open to making changes to improve their mental health and reduce health risk behaviors before birth. To ameliorate antenatal depression and reduce the risk of postpartum depression, we conducted a randomized controlled trial of a collaborative care intervention, MOMCare, in a unique, progressive service environment--the 10-site Seattle-King County Public Health System(PHSKC),which routinely provides intensive Maternity Support Services (MSS-Plus) to women on Medicaid with perinatal depression. Despite improvements in perinatal depression screening, the leadership of PHSKC recognized that only a minority of depressed, pregnant women actually received evidence-based psychotherapy or pharmacotherapy in the community.
The development of the MOMCare intervention was based on positive findings from a previous randomized trial of brief interpersonal psychotherapy (Brief IPT) for perinatal depression, enhanced to address barriers to care and to be relevant to the cultures of poverty and race/ethnicity in socioeconomically disadvantaged women.12 MOMCare was also based on substantial, long-term evidence for the effectiveness of collaborative care models in treating depression in primary care13,14 and OB/Gyn clinics.15 To date, collaborative care for perinatal depression has not been evaluated. We hypothesized that compared to MSS-Plus, the MOMCare collaborative care intervention would be associated with greater engagement in depression treatment, improved quality of depression care, and lower levels of perinatal depression severity.
Materials and Methods
A multi-site randomized, controlled trial with blinded assessment was designed to evaluate the 18-month MOMCare collaborative care intervention for perinatal depression compared to intensive Maternity Support Services (MSS-Plus), provided in 10 PHSKC public health centers, with 3-,6-,12-,and 18-month follow-up assessments. The University of Washington Institutional Review Board approved the study and safety was evaluated by a Data Safety Monitoring Board (DSMB). Study interventions and methods are described elsewhere in detail.16
Study Participants
Recruitment for the study took place between January 2010 and July 2012. MSS social workers and nurses were the study's primary referral sources who routinely screened pregnant patients for depression on the Patient Health Questionnaire-9(PHQ-9)17 and referred patients scoring ≥10 to the study.
After referral, MSW depression care specialists (DCSs) consented participants and conducted a brief initial screening to quickly assess inclusion criteria and minimize participant burden:≥18 years, diagnosis of probable major depressive disorder (MDD; at least five symptoms scored as ≥2 with one cardinal symptom on the PHQ-9, plus a functional impairment item),17or diagnosis of probable dysthymia based on the MINI-International Neuropsychiatric Interview (MINI 5.0.018),12-32 weeks gestation, telephone access,and English-speaking. Inasmuch as MOMCare was designed as an effectiveness trial in 10 busy public health settings, we decided to use the PHQ-9 to assess major depression and MINI to assess dysthymia, by virtue of their excellent construct validity with structured clinical diagnostic interviews and because of their brevity and acceptability to patients. In addition, the PHQ-9 was already in use in the public health system. Throughout the paper, we refer to MDD and dysthymia as “probable” because we did not use a structured clinical interview to make these diagnoses.
For those who were deemed eligible on the initial screen, a longer secondary screening was conducted to rule out additional exclusion criteria, including acute suicidal behavior or multiple(≥2) prior suicide attempts (via self-report questions), schizophrenia(MINI18), bipolar disorder (MINI18), recent substance abuse/dependence(CAGE-AID19), severe intimate partner violence (via a one-item question about being hit, slapped, kicked, physically hurt or sexually abused, followed by questions on the whereabouts of the perpetrator), or currently seeing a psychiatrist or psychotherapist (via self-report question). We also collected data on gestational age and depression severity on the SCL-20,20 our primary outcome, for the purpose of stratification prior to randomization. Eligible participants received a description of the study, followed by written consent, randomization, and baseline assessment.
Randomization to the MOMCare intervention or MSS-Plus proceeded by means of an adaptive block randomization scheme, stratified on initial depression severity via the Hopkins Symptom Checklist-20 (SCL-20 ≤ 2.0 or>2.0) and gestational age(< 22 weeks or≥ 22 weeks).21,22 Within each of the 4 strata, random orders of block sizes of either 2 or 4 study arm assignments were created in order to insure balance of intervention participants within each strata. Each DCS randomized subjects via a computerized program. All study participants received a depression educational booklet provided by the study.23
Intensive Maternity Support Services (MSS-Plus)
Maternity Support Services(MSS) is the usual standard of care in the public health system of Seattle-King County for pregnant women on Medicaid, delivered by a multi-disciplinary team of public health social workers, nurses, and nutritionists, who routinely screen, at least once,for depression from pregnancy up to 2-months postpartum. Goals of “usual” MSS include offering services to promote healthy pregnancies and positive birth and parenting outcomes, providing case management services to meet basic needs, and facilitating regular contact with an OB provider. Pregnant women scoring PHQ-9 ≥10 were eligible for intensive MSS-Plus services, entailing more frequent, longer visits from their multi-disciplinary team. MSS-Plus providers referred depressed patients for mental health treatment in the community and/or from her OB provider. They did not provide evidence-based depression care, systematic outreach, measurement, or stepped care –all of which are key “active ingredients” of the MOMCare intervention.
MOMCare Intervention
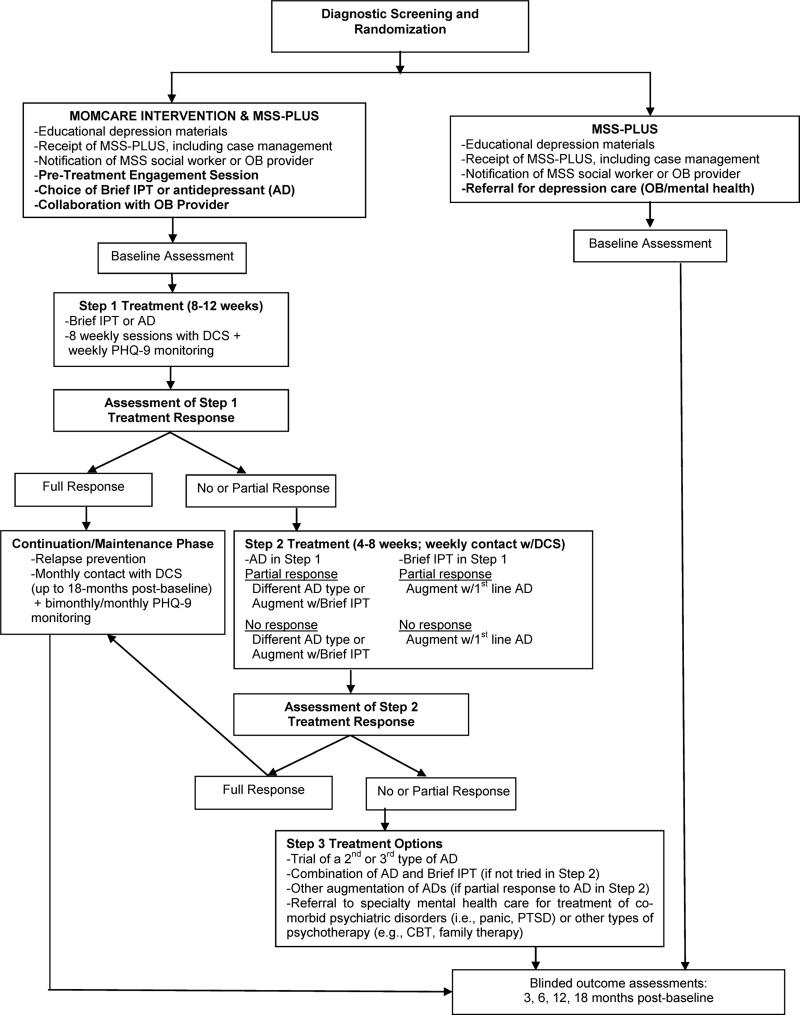
The MOMCare intervention was “added” onto MSS-Plus and delivered by three DCSs in consultation with the MOMCare study team(PI, and psychiatrist). The DCSs also routinely collaborated with their patients’ OB provider, informing the provider that the patient was receiving the MOMCare collaborative care intervention for antenatal depression, updating the provider on the patient's progress, and collaborating on medication management, if indicated. Intervention services were provided in the public health centers, by phone, in community settings, and infrequently at home. The DCSs also kept the MSS-Plus social worker apprised of the patient's progress. Except for the delivery of case management services, the roles of the DCSs and MSS-Plus providers were clearly differentiated from each other. The DCS specifically provided depression care management, whereas the MSS-Plus providers did not. The DCS spent an average of 10 minutes per acute treatment session on case management, if needed. Figure 1 shows the common and distinct features of MOMCare versus MSS-Plus.
Figure 1.
Study Stepped Care Treatment Algorithm
An innovative feature of MOMCare was that it was enhanced to decrease mental health treatment disparities in access to and quality of care.16 MOMCare included novel design components, including a pre-therapy engagement session to help resolve practical, psychological, and cultural barriers to care; patient choice of brief interpersonal psychotherapy (brief IPT) and/or pharmacotherapy from her OB provider for acute treatment; telephone sessions in addition to in-person visits;16 outreach for women missing sessions, including texting, telephone calls; and utilizing depression care specialists (DCSs) to deliver both the intervention and case management to meet basic needs (food, housing, job training, etc.).16
Brief IPT (8 sessions) was derived from Interpersonal Psychotherapy (IPT;16 sessions), which has demonstrated efficacy in treating acute and persistent depression,24,25 and antenatal and postpartum depression.26,27 Brief IPT is a multi-component model of care, and was adapted for MOMCare by including an engagement session followed by eight acute sessions of brief IPT before the birth and IPT maintenance sessions up to about one-year postpartum. 25 This brief version of IPT has received empirical support in studies of depressed pregnant and parenting women12,28 and was also enhanced to be relevant to the culture of race/ethnicity by incorporating the patient's cultural views of depression, treatment goals, and resources. Previous collaborative care studies13 using problem-solving therapy have typically reported 4-6 therapy sessions as adequate. In the case of our socio-economically disadvantaged public health population confronting the chronic stressors of living in poverty and at risk for recurrent depression, we thought that a model including at least 8 acute treatment sessions of brief IPT, followed by a lengthy period of maintenance sessions, would be (and had been) clinically effective for achieving or sustaining treatment response or remission.12 We also found that brief IPT and maintenance could be easily nested within a collaborative care model.
For women requesting antidepressants as an initial treatment, the DCS encouraged them to engage in a risk-benefit decision-making process29 with their OB provider to discuss the risks of both antidepressants30 and untreated depression during pregnancy and lactation.31,32 Once a woman made an informed decision, the study psychiatrist via the DCS made recommendations to her OB provider(usually for a selective serotonin reuptake inhibitor),based on a clinical algorithm, incorporating the patient's current medication and/or past response to antidepressants.
A stepped-care treatment approach was employed via weekly monitoring of depressive symptoms(see Figure 1). Medication and brief IPT recommendations were made at weekly team meetings attended by the DCSs, PI, and psychiatrist. Women with less than 50% improvement on the PHQ-9 by 6-8 weeks received a revised treatment plan. Women receiving Brief IPT alone could be augmented with a trial of antidepressant medication. Women on medication alone could receive an adjusted dosage to achieve optimal outcomes, medication change, and/or augmentation with brief IPT. Dosage was monitored, and sometimes increased over the course of pregnancy because of metabolic changes.33
The DCS followed participants every 1-2 weeks(in-person or by telephone) during the acute phase of treatment(about 3-6 months post-baseline) and monthly during the maintenance phase of treatment(up to 18-months follow-up) once a clinical response (≥50% decrease in PHQ-9 score from baseline) and/or remission(PHQ-9 <5) was achieved. At each contact the DCS monitored treatment response with the PHQ-9, utilizing a computerized tracking system. Patients ending the study without a full remission were referred to community providers serving Medicaid recipients.
DCS training included instruction in the engagement session (manualized), motivational interviewing, culturally relevant brief IPT (manualized), case management, and general information on perinatal complications and pharmacotherapy. The study PI reviewed at least 75% of the audiotaped engagement and brief IPT sessions to minimize treatment drift, using the IPT Therapy Rating Scale.25
Blinded Outcomes
Baseline and 3-,6-,12-,and 18-month outcomes were collected via phone by an interviewer blinded to intervention status. At the 3-month follow-up, 88% of the sample was still pregnant. At the 6-,12-, and 18-month follow-ups, the baby was average of 3-months-old, 9-months-old, and 15-months old, respectively. The primary outcome was depression severity on the SCL-20.20 Secondary outcomes included: functional impairment on the Work and Social Adjustment Scale(WSAS),34 PTSD severity on the Post-Traumatic Stress Disorder Checklist-Civilian Version (PCL-C);35 treatment response(≥ 50% reduction in SCL-20 score from baseline); complete remission of depressive symptoms (SCL-20 score < 0.5);20 and probable generalized anxiety disorder (GAD) based on the PHQ.17 Quality of mental health care was assessed by standardized questions about attending an initial treatment session, ≥4 and ≥8 treatment sessions, and antidepressant medication use in each 3-or 6-month period.13 Intervention treatment costs were estimated, per our previously described model.36
Demographic and Mental Health Comorbidity Variables
Demographic information included age, gestational age, participant-designated race/ethnicity, marital status, education, employment status, annual income, and homelessness. Probable PTSD was determined by using a clinical algorithm from the PCL-C shown to have the highest sensitivity and specificity for a DSM-IV diagnosis of PTSD.35 Probable panic disorder was assessed on the PHQ.17 To ascertain history of child abuse and neglect, we employed the Childhood Trauma Questionnaire.37 Adult attachment orientations [i.e.,secure, anxious-ambivalent,avoidant, fearful] were measured via The Relationship Quality Questionnaire (RQ).38
Power and Analyses
With a sample of public health patients on Medicaid, we chose, conservatively, to assume that we would detect a small to medium effect size of 0.25-0.45 on our primary and secondary outcome variables – depressive symptoms (SCL-20), functioning capacity (WSAS), PTSD severity, depression remission and response rates. Thus, based on our previous work, 12 we estimated that we would need a sample of at least 156 to achieve 80% power to detect as modest an effect size as 0.25 on outcomes.
Analyses were conducted according to the intention-to-treat principle. Descriptive statistics were generated for all variables. Chi-square tests of proportions, relative risks, and 95% confidence intervals were used to determine group differences on the dichotomous depression remission rates, response rates, and quality of care variables at each assessment. Generalized estimating equation models are longitudinal analyses allowing for inclusion of all available data in the estimates of model parameters and were used to examine treatment group trends over time for the continuous and dichotomous outcome variables. Mixed-effects logistic regression models are useful for incomplete longitudinal dichotomous data. They can handle subjects measured incompletely or at different time points (missing data assumed MAR) and have been used extensively in randomized controlled trials.39
Baseline values and any significant demographic differences were used as covariates in the analyses. The outcomes were modelled longitudinally at 3, 6, 12 and 18 months. Unstructured covariance matrices with robust standard errors were used. A statistically significant treatment group-by-time interaction indicated differences in trends over time for the two groups. In the event of a non-significant interaction, the term was removed and the model was re-fit and the main effects of time and group were then assessed. Treatment group differences refer to a comparison of the mean of the 3-,6-,12-,and 18-month follow-up assessments, controlling for baseline values. In the event of a significant main effect for group, we report the pattern of means at each time point.
Effect sizes for the continuous outcome variables were computed using Cohen's d,40 while effect sizes for the dichotomous indices were calculated by converting the odds ratios to effect sizes.41
Results
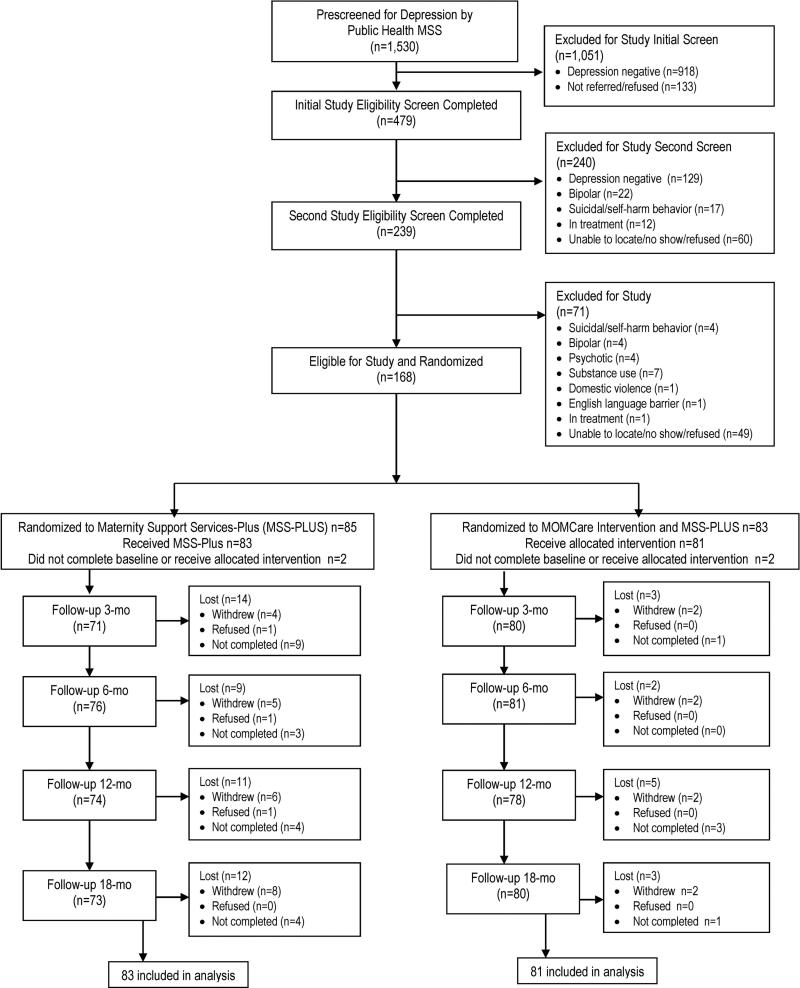
Figure 2 shows that of 1530 patients who were pre-screened on the PHQ-9 by their MSS provider, 612 (40%) screened positive for depression, 479(78%) completed the initial study eligibility screen, 239(50%) completed the second study eligibility screen, and 168(70%) were randomized, 83 to MOMCare, 85 to MSS-Plus. Overall, there were 8 patients who either did not initiate treatment or had data completely missing at all assessments – comprising a study attrition rate of 5%, equivalent across groups.
Figure 2.
CONSORT Flow Diagram
Participants (Table 1)
Table 1.
Baseline Demographic and Clinical Variables of Study Participants
| Variable | Total Sample | MSS-Plus | MOMCare & MSS-Plus | Statistical p Value |
|---|---|---|---|---|
| (N=168) | (N=85) | (N=83) | ||
| Age- M (SD) Range: 18-44 yrs. old | 27.4 (6.1) | 27.2 (5.7) | 27.7 (6.5) | .59 |
| Race - % (N) | ||||
| White | 41.7 (70) | 41.2 (35) | 42.2 (35) | |
| African American | 23.2 (39) | 24.7 (21) | 21.7 (18) | |
| Latina | 22.6 (38) | 22.4 (19) | 22.9 (19) | .82 |
| Asian/Pacific Islander | 7.1 (12) | 8.2 (7) | 6.0 (5) | |
| American Indian/Alaska Native | 5.4 (9) | 3.5 (3) | 7.2 (6) | |
| Non-White - % (N) | 58.3 (98) | 58.8 (50) | 57.8 (48) | 1.00 |
| Marital Status - % (N) | ||||
| Married | 28.6 (48) | 22.4 (19) | 34.9 (29) | |
| Living with a partner | 33.3 (56) | 31.8 (27) | 34.9 (29) | |
| Partner (not living with) | 13.1 (22) | 12.9 (11) | 13.3 (11) | .08 |
| No partner | 25.0 (42) | 32.9 (28) | 16.9 (14) | |
| Education - % (N) | ||||
| Less than high school | 22.0 (37) | 22.4 (19) | 21.7 (18) | |
| High School degree/GED | 20.8 (35) | 18.8 (16) | 22.9 (19) | .93 |
| Some college/vocational | 46.5 (78) | 48.2 (41) | 44.6 (37) | |
| College degree or higher | 10.7 (18) | 10.6 (9) | 10.8 (9) | |
| Employment - % (N) | ||||
| Full-time/Part-time | 34.7 (57) | 44.6 (37) | 24.6 (20) | .02 |
| Unemployed | 65.3 (107) | 55.4 (46) | 75.3 (61) | |
| Income | ||||
| 10K or below - % (N) | 42.1 (69) | 41.2 (35) | 43.0 (34) | .88 |
| Homelessness - % (N) | 13.4 (22) | 15.7 (13) | 11.0 (9) | .49 |
| Past Pregnancy - % (N) | 71.4 (120) | 75.3 (64) | 67.5 (56) | .31 |
| Previous PPD -% (N) | 53.0 (62) | 47.6 (30) | 59.3 (32) | .26 |
| Gestational Age - M (SD) | 22.4 (6.1) | 22.5 (6.0) | 22.4 (6.3) | .94 |
| Unplanned Pregnancy- % (N) | 72.0 (118) | 78.3 (65) | 65.4 (53) | .08 |
| Quite a bit or Very Positive about Pregnancy - % (N) | 62.6 (102) | 62.7 (52) | 62.5 (50) | 1.00 |
| Depressive Disorders - % (N) | ||||
| PHQ Major Depression | 96.4 (162) | 98.8 (84) | 94.0 (78) | .11 |
| Dysthymia from MINI | 24.4 (41) | 25.9 (22) | 22.9 (19) | .72 |
| Depression and Dysthymia | 21.4 (36) | 24.7 (21) | 18.1 (15) | .35 |
| # Previous Depressive Episodes | ||||
| Any prior episode - % (N) | 80.4 (135) | 83.5 (71) | 77.1 (64) | .34 |
| Average # of episodes for those reporting at least 1 - M (SD) | 5.6 (5.5) | 5.1 (4.2) | 6.5 (7.1) | .37 |
| Anxiety Disorders - % (N) | ||||
| Panic Disorder | 21.3 (35) | 19.3 (16) | 23.5 (19) | .57 |
| GAD | 41.5 (68) | 41.0 (34) | 42.0 (34) | 1.00 |
| PTSD | 64.6 (106) | 69.9 (58) | 59.3 (48) | .19 |
| At least one anxiety disorder | 75.6 (127) | 76.5 (65) | 74.7 (62) | .86 |
| Baseline Functioning | ||||
| bSCL Depression Score -M (SD) | 1.8 (.6) | 1.8 (.6) | 1.8 (.6) | .56 |
| cPTSD Severity Score -Sum (SD) | 48.8 (11.3) | 49.2 (10.8) | 48.4 (11.9) | .67 |
| dWork & Social Functioning -M(SD) | 21.4 (8.7) | 21.4 (9.1) | 21.6 98.4) | .75 |
| Moderate to Severe Childhood Trauma (CTQ)e | ||||
| At least one type of trauma %(N) | 51.8 (87) | 55.3 (47) | 48.2 (40) | .44 |
| Attachment orientation - % (N) | ||||
| Secure | 16.5 (27) | 19.3 (16) | 13.6 (11) | |
| Preoccupied/Anxious | 25.0 (41) | 22.9 (19) | 27.2 (22) | .76 |
| Dismissing/Self-reliant | 11.5 (19) | 10.8 (9) | 12.3 (10) | |
| Fearful | 47.0 (77) | 47.0 (39) | 46.9 (38) | |
a PPD= postpartum depression.
SCL-20 score of ≥ 0.5 indicates possible depression, range 0-4.
PTSD Severity (PCL-C), range 17-85.
Work and Social Adjustment Scale (WSAS), range 0-45. Higher scores indicate more symptoms or greater impairment.
Childhood trauma = emotional abuse, physical abuse, sexual abuse, emotional neglect, physical neglect.
The study groups were well-balanced on socio-demographic and clinical variables at baseline, except that MOMCare participants were more likely to be unemployed, a difference for which we controlled in the analyses. Participants were 27 years old and at 22 weeks gestation, on average. A majority of them were non-White, unmarried, unemployed, and had an unplanned pregnancy. Over half had experienced childhood trauma and 47% endorsed a fearful attachment which may negatively affect treatment engagement.42
Of the women randomized to the MOMCare intervention, 81.0% started treatment with Brief IPT alone, 15.2% selected both Brief IPT and medication, and 3.8% preferred medication alone. Whenever necessary to achieve an optimal effect, anti-depressant medication was increased to the optimal dose or augmented with a different medication. Among the women initially treated with Brief IPT alone, 39.1% augmented with medication during the course of the intervention.
Outcome Analyses
Significant main effects were found for group and for time for our primary, secondary, and quality of care outcomes, reported below, controlling for baseline values and employment status. No significant group-by-time effects were found for any outcomes (with the exception of one quality of care measure). In Tables 2 and 3, we show the significant main effects for group for each variable, with the relevant p-values at each time point.
Table 2.
MOMCare Intervention versus Maternal Support Services-Plus (MSS-Plus) in Clinical Outcomes
| Variable | Total N of Patients | CONTINUOUS OUTCOMES |
p-Value | |||
|---|---|---|---|---|---|---|
| Estimated Mean (SD) |
ES | Average Differences between groups (MOMCare - MSS) Mean (95% CI) | ||||
| MOMCare Intervention & MSS-Plus | MSS-Plus | |||||
| Primary Outcome: | * 0.01 | |||||
| SCL-20 depression score | ||||||
| 3 Month | 151 | 1.08 (0.64) | 1.26 (0.64) | .28 | −0.18 (−0.38 – 0.01) | 0.07 |
| 6 Month | 157 | 0.84 (0.64) | 1.08 (0.74) | .35 | −0.24 (−0.46 - −0.03) | 0.03 |
| 12 Month | 152 | 0.93 (0.73) | 1.07 (0.74) | .19 | −0.14 (−0.37 – 0.08) | 0.22 |
| 18 Month | 152 | 0.79 (0.64) | 1.03 (0.74) | .35 | −0.25 (−0.45 - −0.04) | 0.02 |
| Secondary Outcomes: | ||||||
| WSAS Functional Impairment Score | *0.09 | |||||
| 3 Month | 151 | 14.0 (8.56) | 14.4 (9.86) | .04 | −0.45 (−3.32 – 2.43) | 0.76 |
| 6 Month | 157 | 11.6 (9.20) | 13.6 (8.76) | .22 | −2.00 (−4.80 - 0.81) | 0.16 |
| 12 Month | 152 | 11.9 (9.20) | 13.6 (8.76) | .19 | −2.07 (−5.21 – 1.06) | 0.20 |
| 18 Month | 152 | 9.4 (8.65) | 13.3 (9.96) | .42 | −3.89 (−6.79 - −1.00) | 0.008 |
| PTSD Severity Score | *0.04 | |||||
| 6 Month | 157 | 34.5 (11.49) | 37.9 (13.09) | .28 | −3.44 (−7.24 – 0.36) | 0.08 |
| 12 Month | 152 | 34.6 (13.57) | 37.2 (13.28) | .19 | −2.60 (−6.72 – 1.52) | 0.30 |
| 18 Month | 152 | 31.6 (12.75) | 36.7 (12.82) | .40 | −6.29 (−10.27 – 2.31) | 0.002 |
| Variable | Total N of Patients | DICHOTOMOUS OUTCOMES (secondary) |
p-Value | |||
|---|---|---|---|---|---|---|
| Patients % (N) |
ES | Odds Ratios (95% CI) | ||||
| MOMCare & MSS-Plus | MSS-Plus | |||||
| Response (at least 50% decrease in SCL-20 from baseline) | *0.32 | |||||
| 3 Month | 151 | 36.3 (29) | 33.8 (24) | .07 | 1.14 (0.57 – 2.18) | 0.86 |
| 6 Month | 157 | 58.0 (47) | 43.4 (33) | .32 | 1.80 (0.96 – 3.39) | 0.08 |
| 12 Month | 152 | 48.7 (38) | 47.3 (35) | .03 | 1.06 (0.56 – 2.00) | 0.87 |
| 18 Month | 152 | 53.8 (43) | 48.6 (35) | .14 | 1.23 (0.65 – 2.33) | 0.63 |
| Remission of depression symptoms (SCL-20 score<.05) | *0.05 | |||||
| 3 Month | 151 | 20.0 (16) | 15.5 (11) | .17 | 1.36 (0.59 – 3.17) | 0.53 |
| 6 Month | 157 | 37.0 (30) | 25.0 (19) | .31 | 1.76 (0.89 – 3.51) | 0.12 |
| 12 Month | 152 | 35.9 (28) | 27.0 (20) | .08 | 1.15 (0.76 – 3.01) | 0.30 |
| 18 Month | 152 | 48.3 (35) | 29.2 (21) | .36 | 1.90 (0.96 – 3.70) | 0.07 |
| GAD | *0.04 | |||||
| 6 Month | 157 | 18.5 (15) | 22.4 (17) | .13 | 0.79 (0.36 – 1.72) | 0.56 |
| 12 Month | 152 | 14.1 (11) | 27.0 (20) | .45 | 0.44 (0.20 – 1.00) | 0.07 |
| 18 Month | 152 | 10.0 (8) | 22.2 (16) | .52 | 0.39 (0.16 – 0.97) | 0.05 |
Main effect for group: 3-, 6-, 12-, and 18-months follow-ups, controlling for baseline values and employment status. Depression severity (SCL-20) range 0-422: Work and Social Adjustment Scale (WSAS) range 0 - 45 23; PTSD Severity (PCL-C) range 17-8524 Higher scores indicate more symptoms or greater impairment. 3-Month F/U = 88% still pregnant; 6-Month F/U = mean 3 months postpartum; 12-Month F/U = mean 9 months postpartum; 18-Month F/U = mean 15 months postpartum.
Table 3.
Quality of Care Differences for MOMCare Intervention vs. Maternity Support Services-Plus (MSS-Plus)
| Variable | Total N of Patients | Participants |
ES | OR (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| MOMCare Intervention & MSS-Plus | MSS-Plus | |||||
| MSS-Plus Visits in Unitsa M(SD) From Baseline to 2-Months Postpartum (MSS Administrative Data) | 160 | 12. 96 (13.35) | 11.35 (13.17) | .08 | 1.16 (−5.75 – 2.53) | .44 |
| Engagement in an initial specialty mental health session %N | 152 | 97.5 (79) | 35.2 (25) | 2.37 | 72.68 (16.45 – 321.02) | *< .0001 |
| 4 or more specialty mental health visits in prior 3 or 6 months %N | *<0.0001 | |||||
| 3-Month | 151 | 92.6 (75) | 22.5 (16) | 2.08 | 42.97 (15.80–116.88) | < .0001 |
| 6 -Month | 157 | 48.1 (39) | 13.2 (10) | 1.00 | 6.13 (2.71 - 13.57) | < .0001 |
| 12-Month (past 6 months) | 152 | 56.4 (44) | 14.9 (11) | 1.11 | 7.41 (3.39 – 16.19) | < .0001 |
| 18-Month (past 6 months) | 152 | 27.2 (22) | 6.8 (50) | .90 | 5.07 (1.81 – 14.23) | 0.001 |
| 8 or more acute mental health sessions by 18 months post baseline %N | 160 | 84.0 (68) | 24.1 (19) | 1.55 | 16.52 (7.52 – 36.26) | <.0001 |
| Any antidepressant for ≥ 25 days in the past month %N | *0.002 | |||||
| 3-Month | 151 | 32.5 (26) | 9.9 (7) | .82 | 4.40 (1.77 – 10.93) | 0.001 |
| 6-Month | 157 | 27.5 (22) | 14.5 (11) | .44 | 2.20 (0.98 – 13.57) | 0.08 |
| 12-Month | 152 | 28.2 (22) | 10.8 (8) | .65 | 3.24 (1.34 – 7.85) | 0.008 |
| 18-Month | 152 | 21.3 (17) | 10.8 (8) | .43 | 2.19 (0.88 – 5.44) | 0.12 |
| Satisfaction with all care b received during intervention period: %N Moderately or Very Satisfied | *0.004 | |||||
| 3 Month | 149 | 88.8 (71) | 70.4 (50) | .66 | 3.31 (1.40 – 7.84) | 0.007 |
| 6 Month | 149 | 88.8 (71) | 73.7 (56) | .52 | 2.54 (1.10 – 5.85) | 0.04 |
| 12 Month | 145 | 87.2 (68) | 71.6 (53) | .54 | 2.64 (1.17 – 6.21) | 0.03 |
| 18 Month | 143 | 79.5 (62) | 78.1 (57) | .05 | 1.09 (0.50 – 2.39) | 0.84 |
Main effect of group: at 3-,6-, 12-, and 18 months follow-ups, controlling for baseline values and employment status.
3-Month F/U = 88% still pregnant; 6-Month F/U = mean 3 months postpartum; 12-Month F/U = mean 9 months postpartum; 18-Month F/U = mean 15 months postpartum.
Each unit of public health MSS-Plus = 15 minutes.
Satisfaction with all care received for mood problems or stress during intervention period includes MSS-Plus services, community mental health provider, MOMCare depression care specialist, obstetrics provider.
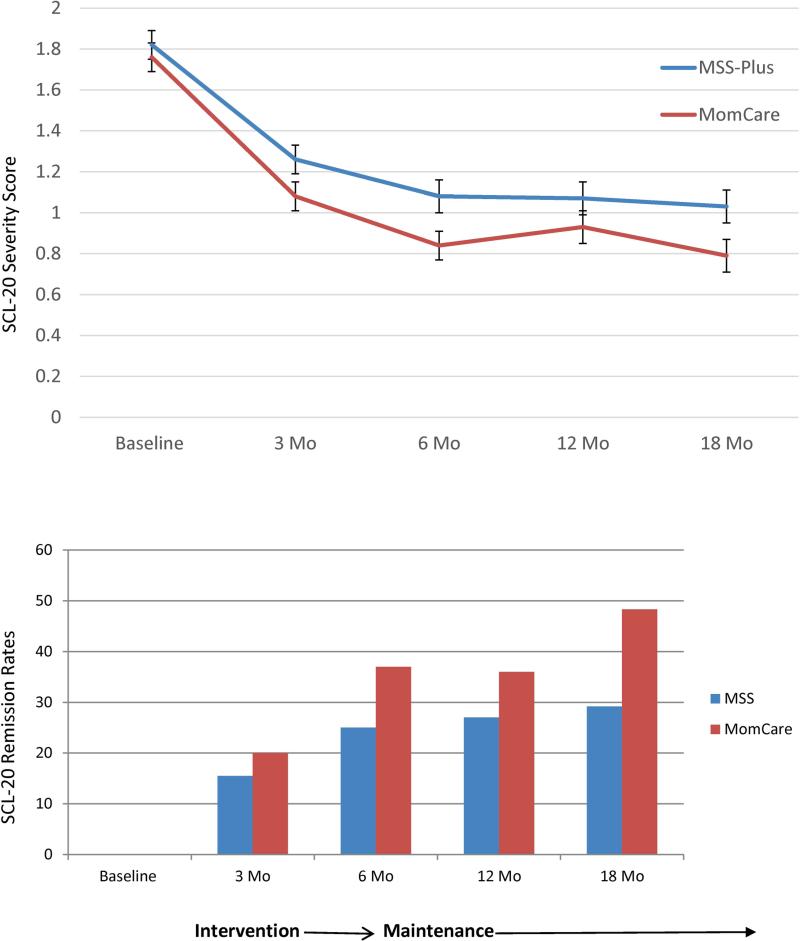
Primary Outcome (Table 2, Figure 3)
Figure 3.
Mean Depression Severity Scores and Remission Rates at Each Assessment for MOMCare Intervention versus MSS-Plus
The main effects model for our primary outcome, SCL-20 depression severity, showed significant main effects for group (Wald'sχ2=6.09,df=1,p=.01) and time (Wald'sχ2=25.13,df=3, p<.0001). Figure 2 shows that both conditions experienced a significant decline in depression severity over time. MOMCare, however, was significantly more effective than MSS-Plus in reducing depression severity than MSS-Plus, on average, across the 3-, 6-, 12-, and 18-month follow-ups, with an effect size of .35 at 18 months. The p-values for each time point indicate that the intervention showed a trend toward having a significant impact on depression severity at 3-months post-baseline for MOMCare participants and was significantly more effective than MSS-Plus in reducing depression severity at 6-months and 18-months post-baseline, by the end of the study.
Secondary Outcomes (Table 2, Figure 3)
Regarding remission (SCL-20<0.5), the main effects model demonstrated significant effects for group (Wald'sχ2=3.67,df =1,p=.05) and time (Wald'sχ2= 25.99,df=3,p<.0001). In general, both groups showed improvement over time in approaching remission. By 18-months post-baseline, 48% of MOMCare patients had achieved or sustained remission compared to 29% of MSS-Plus patients, with an effect size of .36. It took a mean of 8.6 (SD=3.8) acute brief IPT/medication management sessions and a mean of 6.4 (SD=5.3) maintenance sessions over 18 months for those in MOMCare to achieve or sustain remission. With respect to a treatment response(≥ 50% decrease in SCL-20 scores from baseline), the main effects model showed that both groups achieved a significant effect for time (Wald'sχ2=17.65,df=3,p=.001), but the effect for group was non-significant (Wald's χ2=1.18,df =1,p=.28).
The main effects model for the WSAS functional improvement based on the four assessments demonstrated a trend-level effect for group (Wald'sχ2=2.83,df=1,p=.09) and a significant main effect for time (Wald'sχ2=15.28,df=3,p<.01), indicating that both conditions improved in work and social functioning over time. For PTSD severity, the main effects model was significant for group (Wald'sχ2=4.38, df=1,p<.05), and time (Wald'sχ2=7.48,df=2,p<.05). Both groups experienced a reduction in PTSD severity over time, but MOMCare was significantly more effective than MSS-Plus in reducing PTSD severity, on average, across the study period, with an effect size of .40 at 18 months. In addition, with respect to probable generalized anxiety disorder, the main effects model was significant, not for time, but for group (Wald'sχ2=4.14,df=1,p<.04). On average, MOMCare participants experienced a reduction in generalized anxiety over the study period, with a medium effect size of .52 at 18 months.
In sum, although both groups experienced significant improvements in primary and secondary outcomes over time, the MOMCare intervention, relative to MSS-PLUS, yielded a small, but significant benefit, on average, in improving depression severity, remission rates, PTSD severity, and generalized anxiety across the study period – outcomes that were particularly salient by 18 months post-baseline.
Quality of Care Variables (Table 3)
Table 3 shows that most women in the MOMCare intervention group (97.5%) completed an initial brief IPT or medication management treatment session (effect size=2.37), 84% had ≥8 acute brief IPT or medication management sessions (effect size=1.55), and 93% had ≥4 acute brief IPT or medication management sessions (effect sizes ranged from 2.08 to .90). Effect sizes for quality of control variable represent the difference in percentage of participants who met a certain criterion. The analysis for receiving ≥4 mental health visits showed a group-by-time interaction indicating that the trends over time were different for the study groups (Wald'sχ2=14.43,df=3,p<.01), with MOMCare participants more likely to receive at least 4 or more mental health visits over time.
Across the study period, MOMCare patients had a mean of 4.7 (SD=4.1) acute in-person sessions and a mean of 4.8 (SD=4.3) acute telephone sessions, making a total of 9.5 (SD=4.0) acute treatment sessions. Almost all (95%) of the acute treatment sessions were completed within the 3-month (75.5%) or 6-month (19.1%) treatment window. Regarding maintenance, MOMCare patients received a mean total of 7.3 (SD=6.1) IPT maintenance and/or medication management sessions [2.4 (SD=3.8) in person and 4.9 (SD=4.7) by phone]. Seventy-nine percent of MOMCare participants had at least one maintenance session through the 18-months follow-up. None of the reported MOMCare treatment or maintenance sessions included the separate MSS-Plus visits. Table 3 shows that MOMCare and MSS-Plus participants received an equivalent number of MSS-Plus visits (11-13) in 15-minute units.
With respect to any anti-depressant use, the groups did not differ at baseline, but across the follow-up period intervention patients generally had higher rates of antidepressant use than MSS-Plus, with the main effects model showing significant effects for group (Wald'sχ2=8.10,df=1,p<.01), and time (Wald's χ2=18.67,df=3,p<.0001). Table 3 displaying the main effects model for any antidepressant adherence for 25 days in the past month showed a significant group effect (Wald'sχ2=10.00,df=1,p=.002), with higher adherence rates in the intervention group than in MSS-Plus across the 4 follow-ups (effect sizes ranged from .82 to .43), and no significant time effect (Wald'sχ2 =2.26,df =3,p=.51). MOMCare participants reported greater satisfaction, on average, with all depression care received from all sources across the follow-up period than MSS-Plus participants, with the main effects model showing non-significance for time (Wald'sχ2=0.36,df=3,p=.95), but significance for group (Wald'sχ2=8.28, df=1,p=0.004). Effect sizes ranged from .66 at 3 months, to .52 at 6 months, to .54 at 12 months, and to .05 at 18 months, when most providers (OB, MSS-Plus, DCS) were no longer being seen regularly.
Cost Analyses
The estimated cost per patient, based on our algorithm36 including all DCS contacts, supervision and information system support was $1,117. Costs for intervention services provided by study staff, which included caseload supervision, were calculated using actual salary and fringe benefit rates plus a 30% overhead rate (e.g. space, administrative support). The resulting unit costs were $80 for each depression care specialist (DCS) visit (typically 45-60 minutes) and $31 for each DCS telephone contact (typically 20-30 minutes). These estimates included the time required for outreach efforts and record-keeping. Intervention costs over the study period also included a fixed $247 cost per patient for caseload supervision and information support.
Study groups did not differ on medical co-morbidities or complications during pregnancy or adverse birth outcomes.
Discussion
Socio-economically disadvantaged women are twice as likely as middle-class women to meet diagnostic criterial for antenatal major depression and have more persistent depression, but lack access to high quality mental health care and have proven difficult to engage and retain in a minimally adequate course of treatment. Given the public health importance of treating antenatal depression to prevent postpartum depression3, and the adverse consequences of both antenatal and postpartum depression for maternal and child health and mental health,2,4,5,6 the study findings are hopeful. The MOMCare intervention, compared to intensive Maternity Support Services(MSS-Plus), improved access and adherence to evidence-based depression care, depression severity and remission outcomes, and satisfaction with depression care in a public health population of pregnant, depressed women with high rates of childhood trauma, PTSD, and chronic social stressors. Intervention effects were apparent, on average, by the 6-month follow-up, when the baby was about 3-months-old, and maintained at the 18-month follow-up, when the baby was about 15-months-old. Notably, the timing of rapid, sustained improvement in depressive symptoms for intervention participants occurring shortly after birth and beyond is a critically important precondition for favorable mother-infant bonding experiences.4
The MOMCare intervention was developed in partnership with the administrative leadership and staff of the PHSKC MSS program and was well-accepted and feasible to provide in this 10-site public health setting. The effect size (0.35) for depressive outcomes in the current study at 18 months, though modest, is comparable in range to that (0.34) observed in a recent meta-analysis of 79 randomized, controlled collaborative depression care trials of over 24,000 primary care patient clinics worldwide.43 On the other hand, an important question to consider is why we did not find a significant group-by-time time interaction for the depressive outcomes in this trial. First, it may be that the MOMCare collaborative care intervention was less effective in reducing depression severity than that of previous collaborative care studies of low-income, minority women receiving county health or welfare services 44 or medical care in Ob/Gyn clinics.45 A comparison of response rates between MOMCare(54%) and these two studies (range of 51%-56%), however, does not support this possibility.
Second, it is also possible that the study comparison condition(MSS-Plus) offered more psychotherapeutic components than did usual care in the aforementioned studies. 44,45 In the latter studies, usual care participants received depression screening, psychoeducation about depression,and a referral to community mental health services. In MSS-Plus, participants received all of the features of usual care, plus an array of maternity support services, including parenting and nutrition classes, case management, and an average of six 30-minute individual sessions with a public health social worker or nurse. 48% of participants achieved a depression treatment response in the MSS-Plus condition, compared to 37% of participants in usual care in the other two studies.44,45 This pattern of treatment response suggests that MSS-Plus may have been more potent than the “usual care.” In line with this explanation, a recent meta-analysis showed that “usual care” in randomized trials consists of heterogeneous conditions, ranging from minimal to strong routine psychotherapeutic services,46 and group-by-time interaction effects (and effect sizes) may be reduced when the “usual care” group receives stronger supportive services
The study population was unique in that all of the women suffered significant economic disadvantage and 65% had both MDD and PTSD, a combination that constitutes a high-risk factor for preterm birth.47 Although MOMCare participants relative to MSS-Plus showed a trend toward experiencing more relief from depression by the 3-months follow-up before the birth, the significant improvement in depression severity for MOMCare did not become salient until the 6-months follow-up. Both socio-economic deprivation48 and PTSD49 are associated with higher prevalence and persistence of depression, and PTSD co-morbidity may delay or diminish treatment response for those with MDD.50 Of note is that by the 18-month follow-up, MOMCare participants showed significantly improved depression severity, higher remission rates, and reduced PTSD severity and anxiety. A full 18-month intervention for difficult-to-treat perinatal depression that includes an initial engagement session, proactive outreach, and case management may be needed to improve clinical outcomes in settings serving depressed women with high poverty and anxiety symptoms. Although MSS-Plus providers currently provide services to public health patients up to 2-months postpartum, it is possible that with Medicaid expansion, MSS-Plus services, including maintenance sessions by phone, may be able to be expanded up to one-year postpartum, particularly for high-risk women with major depression and PTSD. Considering that PTSD may complicate treatment response for those with major depression,50 our next inquiry will be to examine whether comorbid PTSD severity moderates the impact of MOMCare and MSS-Plus on depression severity (Grote et al., under review).
The present study's strengths include the randomized design, the strong public health comparison condition, patient diversity, perinatal depression heterogeneity, low study attrition (about 5%), high rates of intervention adherence, implementation in ten public health settings, and culturally relevant adaptations to engage and retain socio-economically disadvantaged women in care. The ability of the DCSs, in consultation with the MOMCare team, to collaborate with over 40 OB providers in Seattle-King County is an additional strength. Improving depression care for patients of OB providers is important because they rate their confidence in treating depression and skills with counseling and antidepressant medication as less than internists or family physicians.51
Limitations of the study include the lack of a significant group-by-time-interaction effect, possibly due to having a strong comparison group in the study design, and the self-report of antidepressant use, although previous studies found high rates of agreement between self-reported antidepressant use and pharmacy data-base prescription data.10 Study results may not be generalizable to non-English-speaking populations, to other US populations on Medicaid, or to potentially eligible women who refused to be screened or to eligible women who did not consent to being in the study. In addition, due to the design of the intervention and the trial, it is impossible to know which specific components of the MOMCare intervention produced the significant outcomes. In other words, to what extent did the engagement session, choice of treatment, active outreach, brief IPT, medication, stepped care, or collaborative care contribute to the significant effects observed in the study? This is a question for future research.
It is also possible that between-group differences were due to MOMCare participants having received more attention than those in MSS-Plus. Indeed, they did receive more attention from their DCSs, but MOMCare participants did not differ from their MSS-Plus counterparts in number of visits received from their MSS-Plus providers. Given the research demonstrating the therapeutic benefits of case management, 52 it may be that part of the effectiveness of MOMCare, independent of its “active ingredients,” was due to its integrated case management component (which typically took about 10 minutes of each 60-minute acute intervention session). However, because MSS-plus participants also received regular case management services from their MSS-Plus providers, this is unlikely.
In summary, a collaborative stepped care model for treating perinatal depression can be feasibly integrated into a county public health system, is significantly, though modestly, more effective than intensive Maternity Support Services in improving quality of mental health care and depressive outcomes, and can be provided at modest cost. Improving mental health care provision for perinatal depression in public health care settings has important implications for the well-being of US women, children and families, particularly with the anticipated expansion in demand for mental health services associated with health care reform.
Acknowledgements
Study support was provided by NIMHR01-MH084897. All authors gave final approval of this manuscript and made substantive contributions: study design (NG, WK, JR, MJL, KC), data acquisition (NG, MJL, WK, MC, EG), analysis and interpretation of the data (NG, WK, JR, MJL, KC, MC, EG), drafting the manuscript (NG, JR, WK, MJL). Nancy Grote, Ph.D, School of Social Work, University of Washington, and Joan Russo, Ph.D., Department of Psychiatry and Behavioral Sciences, University of Washington Medical School, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. None of the authors had conflicts of interest to report. We acknowledge Evette Ludman, Ph.D. and Greg Simon, M.D., of the Group Health Research Institute for their consultation on study implementation and guidance on the interpretation of the data and Jennifer Melville, M.D., Department of Obstetrics and Gynecology, University of Washington, for her contribution in training the depression care specialists. We thank our study team, Chelsea Clark, MSW, for depression care management, and Marilyn Gregory, MSW, Elaine Howell, BA, Anthippy Petras, MSW, and Verl Canizzaro, BASW, for assistance with recruiting, data collecting and tracking, and administrative support. They were all paid staff for the study. We thank Paul Pilkonis, Ph.D., Scott Stuart, M.D., and Brenda Kurland, Ph.D. for serving on the DSMB. We also acknowledge Ms. Patricia Kennedy, MSW, and Ms. Phala Chea, MSW, of the Maternity Support Services (MSS) of Seattle-King County Public Health and MSS social workers, nurses, and nutritionists for their unfailing efforts in collaborating with the MOMCare team. Brief portions of this paper were presented at the 5th International Conference of the International Society of Interpersonal Psychotherapy. Iowa City, IA., June 2013; the Society for Social Work Research 17th Annual Conference, San Diego, CA, January 2013; and the 4th International Conference of the International Society for Interpersonal Psychotherapy. Amsterdam, the Netherlands, June 2011 and the 6th International Conference of the International Society for Interpersonal Psychotherapy, London, England, June 2015.
Funding Sources:
National Institute of Mental Health - R01-MH084897 (Principal Investigator: Grote) Horizons Foundation, Seattle, WA
Footnotes
Clinical Trial Registration: ClinicalTrials.govNCT01045655
References
- 1.Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, et al. AHRQ Publication 05-E006-2. Agency for Healthcare Research and Quality; Rockville, MD: 2005. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess No. 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grote NK, Bridge J, Gavin AF, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Hara M, Swain A. Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry. 1996;8:37–54. [Google Scholar]
- 4.Murray L, Cooper P. The role of infant and maternal factors in postpartum depression, mother-infant interactions, and infant outcome. In: Murray L, Cooper P, editors. Postpartum depression and child development. Guilford; New York: 1997. pp. 111–135. [Google Scholar]
- 5.Tegethoff M, Greene N, Olsen J, et al. Stress during pregnancy and offspring pediatric disease: a national cohort study. Environ Health Perspect. 2011;119:1647–1652. doi: 10.1289/ehp.1003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawlby S, Hay DF, Sharp D, et al. Antenatal depression predicts depression in adolescent offspring: prospective longitudinal community-based study. J Affect Disord. 2009;113:236–243. doi: 10.1016/j.jad.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Hobfoll S, Ritter C, Lavin J, et al. Depression prevalence and incidence among inner-city pregnant and postpartum women. J Consult Clin Psychol. 1995;63:445–53. doi: 10.1037//0022-006x.63.3.445. [DOI] [PubMed] [Google Scholar]
- 8.Miranda J, Chung JY, Green BL, et al. Treating depression in predominantly low-income young minority women: a randomized controlled trial. JAMA. 2003;290:57–65. doi: 10.1001/jama.290.1.57. [DOI] [PubMed] [Google Scholar]
- 9.Katon WJ. The Institute of Medicine “Chasm” report: implications for depression collaborative care models. Gen Hosp Psychiatry. 2003;25:222–229. doi: 10.1016/s0163-8343(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 10.Grote NK, Zuckoff A, Swartz HA, et al. Engaging women who are depressed and economically disadvantaged in mental health treatment. Soc Work. 2007;52:295–308. doi: 10.1093/sw/52.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute of Medicine (IOM) Fetal alcohol syndrome: diagnosis, epidemiology, prevention, and treatment. National Academy Press; Washington, DC: 1996. [Google Scholar]
- 12.Grote NK, Swartz HA, Geibel S, et al. A randomized controlled trial of culturally relevant, brief interpersonal psychotherapy for perinatal depression. Psychiatr Serv. 2009;60:313–321. doi: 10.1176/appi.ps.60.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katon W, Robinson P, Von Korff M, et al. A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry. 1996;53:924–932. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- 14.Richardson LP, Ludman E, McCauley E, et al. Collaborative care for adolescents with depression in primary care: a randomized clinical trial. JAMA. 2014;312:809–816. doi: 10.1001/jama.2014.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melville JL, Reed SD, Russo J, et al. Improving care for depression in obstetrics and gynecology. Obstet Gynecol. 2014;123:1237–1246. doi: 10.1097/AOG.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grote NK, Katon WJ, Lohr MJ, et al. Culturally relevant treatment services for perinatal depression in socio-economically disadvantaged women: the design of the MOMCare study. Contemp Clin Trials. 2014;39:34–49. doi: 10.1016/j.cct.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spitzer RL, Williams JB, Kroenke K, et al. Validity and utility of the PRIME-MD patient health questionnaire in assessment of 3000 obstetric-gynecologic patients: the PRIME-MD patient health questionnaire obstetrics-gynecology study. Am J Obstet Gynecol. 2000;183:759–769. doi: 10.1067/mob.2000.106580. [DOI] [PubMed] [Google Scholar]
- 18.Sheehan DV, Lecrubier Y, Harnett Sheehan K, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 19.Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 20.Derogatis L. SCL-90-R administration, scoring and procedures manual II for the revised version. Clinical Psychometric Research; Towson (MD): 1983. [Google Scholar]
- 21.Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58:403–417. [Google Scholar]
- 22.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 23.Katon W, Ludman E, Simon G. The depression helpbook. Bull Publishing Co; Boulder (CO): 2002. [Google Scholar]
- 24.Weissman M, Klerman G, Prusoff B, et al. Depressed outpatients: results after one year of treatment with drugs and/or interpersonal psychotherapy. Arch Gen Psychiatry. 1981;38:51–55. doi: 10.1001/archpsyc.1981.01780260053005. [DOI] [PubMed] [Google Scholar]
- 25.Frank E, Kupfer DJ, Wagner EF, et al. Efficacy of interpersonal psychotherapy as a maintenance treatment of recurrent depression: contributing factors. Arch Gen Psychiatry. 1991;48:1053–1059. doi: 10.1001/archpsyc.1991.01810360017002. [DOI] [PubMed] [Google Scholar]
- 26.Spinelli MG, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry. 2003;160:555–562. doi: 10.1176/appi.ajp.160.3.555. [DOI] [PubMed] [Google Scholar]
- 27.O'Hara MW, Stuart S, Gorman LL, Wenzel A. Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry. 2000;57:1039–1045. doi: 10.1001/archpsyc.57.11.1039. [DOI] [PubMed] [Google Scholar]
- 28.Swartz HA, Frank E, Shear MK, et al. A pilot study of brief interpersonal psychotherapy for depression among women. Psychiatr Serv. 2004;55:448–450. doi: 10.1176/appi.ps.55.4.448. [DOI] [PubMed] [Google Scholar]
- 29.Wisner KL, Zarin DA, Holmboe ES, et al. Risk-benefit decision-making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157:1933–1940. doi: 10.1176/appi.ajp.157.12.1933. [DOI] [PubMed] [Google Scholar]
- 30.Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166:557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. J Amer Med Assoc. 2006;295:499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- 32.American Academy of Obstetricians and Gynecologists ACOG practice bulletin no. 92: use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1000–1020. [Google Scholar]
- 33.Sit DK, Perel JM, Helsel JC, Wisner KL. Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry. 2008;69:652–658. doi: 10.4088/jcp.v69n0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mundt JC, Marks IM, Shear MK, Greist JH. The work and social adjustment scale: a simple accurate measure of impairment in functioning. Br J Psychiatry. 2002;180:461–464. doi: 10.1192/bjp.180.5.461. [DOI] [PubMed] [Google Scholar]
- 35.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL). Behav Res Ther. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 36.Katon WJ, Russo JE, VonKorff M, Lin EH, et al. Long-term effects on medical costs of improving depression outcomes in patients with depression and diabetes. Diabetes Care. 2008;31:1155–1159. doi: 10.2337/dc08-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernstein D, Fink L. Childhood trauma questionnaire: a retrospective self-report. Psychological Corporation; San Antonio (TX): 1998. [Google Scholar]
- 38.Griffin D, Bartholomew K. The metaphysics of measurement: the case of adult attachment. In: Bartholomew K, Perlman D, editors. Advances in personal relationships vol 5: attachment processes in adulthood. Jessica Kingsley; London: 1994. pp. 17–52. [Google Scholar]
- 39.Hedeker D. Generalized linear models. In: Everitt B, Howell D, editors. Encyclopedia of statistics in behavioral science. Wiley; New York: 2005. [Google Scholar]
- 40.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence Earlbaum Associates; Hillsdale (NJ): 1988. [Google Scholar]
- 41.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Statist Med. 2000;19:3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 42.Ciechanowski PS, Russo JE, Katon WJ, et al. The association of patient relationship style and outcomes in collaborative care treatment for depression in patients with diabetes. Med Care. 2006;44:283–291. doi: 10.1097/01.mlr.0000199695.03840.0d. [DOI] [PubMed] [Google Scholar]
- 43.Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. doi: 10.1002/14651858.CD006525.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Miranda J, Green BL, Krupnick JL, et al. One-year outcomes of a randomized clinical trial treating depression in low-income minority women. J Consult Clin Psychol. 2006;74:99–111. doi: 10.1037/0022-006X.74.1.99. [DOI] [PubMed] [Google Scholar]
- 45.Katon W, Russo J, Reed SD, et al. A randomized trial of collaborative depression care in obstetrics and gynecology clinics: Socioeconomic disadvantage and treatment response. Am J Psychiatry. 2015;172:32–40. doi: 10.1176/appi.ajp.2014.14020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wampold BE, Budge SE, Laska KM, et al. Evidence-based treatments for depression and anxiety versus tretment-as-usual: a meta-analysis of direct comparisons. Clin Psychol Rev. 2011;3:1304–1312. doi: 10.1016/j.cpr.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Yonkers KA, Smith MV, Forray A, et al. Pregnant women with posttraumatic stress disorder and risk of preterm birth. JAMA Psychiatry. 2014;71:897–904. doi: 10.1001/jamapsychiatry.2014.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostler K, Thompson C, Kinmonth AL, et al. Influence of socio-economic deprivation on the prevalence and outcome of depression in primary care: the Hampshire Depression Project. Br J Psychiatry. 2001;178:12–17. doi: 10.1192/bjp.178.1.12. [DOI] [PubMed] [Google Scholar]
- 49.Tanskanen A, Hintikka J, Honkalampi K, et al. Impact of multiple traumatic experiences on the persistence of depressive symptoms—a population-based study. Nord J Psychiatry. 2004;58:459–464. doi: 10.1080/08039480410011687. [DOI] [PubMed] [Google Scholar]
- 50.Hegel MT, Unutzer J, Tang L, et al. Impact of comorbid panic and posttraumatic stress disorder on outcomes of collaborative care for late-life depression in primary care. Am J Geriatr Psychiatry. 2005;13:48–58. doi: 10.1176/appi.ajgp.13.1.48. [DOI] [PubMed] [Google Scholar]
- 51.Williams JW, Jr, Rost K, Dietrich AJ, et al. Primary care physicians' approach to depressive disorders: effects of physician specialty and practice structure. Arch Fam Med. 1999;8:58–67. doi: 10.1001/archfami.8.1.58. [DOI] [PubMed] [Google Scholar]
- 52.Miranda J, Azocar F, Organista K, et al. Treatment of depression among impoverished primary care patients from ethnic minority groups. Psychiatr Serv. 2003;54:219–225. doi: 10.1176/appi.ps.54.2.219. [DOI] [PubMed] [Google Scholar]