Abstract
Objectives
While primary treatment for high-grade serous ovarian cancer tends to be uniform – maximal debulking and platinum/taxane adjuvant chemotherapy – there is little standardization of treatment in the recurrent setting beyond the exhaustive use of platinum therapies. Using secondary data from multiple centers participating in the Cancer Genome Atlas study (TCGA), we seek to characterize clinical features, timing and serial response data to provide empirical evidence for treatment expectations in the recurrent setting.
Methods
We conducted a retrospective survival analysis of TCGA study primary and secondary patient chemotherapy regimens by characterizing the dynamics of 1119 lines of therapy comprising the treatment of 461 high-grade serous ovarian cancer patients. All patients with post-surgical drug therapy information from the TCGA database were included in this study.
Results
A complete response to adjuvant therapy led to longer overall survival, but did not affect treatment free intervals (TFI) after relapse of disease. A strong predictor of the TFI on the next treatment regimen was the previous TFI with a decaying effect. The number of previous treatments, of platinum treatments, and the length of time from surgery all have an exponentially decreasing effect on TFI. Re-treatment times appear to cluster at predictable times following surgery.
Conclusions
While patients experience a consistent reduction in TFI with increasing re-treatment, the initial adjuvant interval is unrelated to later interval lengths. Platinum re-treatment remained an effective option in patients typically thought to be platinum resistant and the timing of monitoring visits may drive overall re-treatment patterns.
Keywords: Chemotherapy, Ovarian cancer, Survival Analysis, Treatment-free Interval
Introduction
Nearly 75% of patients with advanced or high-grade ovarian cancer cases relapse and die of disease [1] implying that characterization of treatment dynamics in the post-recurrence setting is critically important. Relapsed patients have progressed following primary surgical debulking and treatment with systemic chemotherapy. While the standard first-line chemotherapy is platinum-based, the best treatment for recurrent disease after adaptation to platinum is unknown [2]. There is a plurality of available chemotherapies at first relapse [3], a paucity of biomarkers to guide treatment selection [4], and a highly heterogeneous response to relapse re-treatments [5]. Consequently, the oncologist and patient must experiment with potential regimens [6] to find a patient-specific therapeutic strategy.
Besides tolerability [7], response to therapy is generally quantified by two time-based variables: the time from the start of chemotherapy to the progression of disease -- progression-free survival (PFS) – and the time from the end of one chemotherapy regimen to the commencement of the next – the treatment-free interval (TFI) [8]. When patients are treated with platinum-based therapies, consideration of these times leads to the designation of patients as platinum sensitive, resistant or refractory [9]; this status is believed to be correlated with future response to platinum therapy. Noting that, in advanced cancers, the TFI decreases in subsequent treatments for patients treated serially with systemic therapies [10], response to platinum is expected to decay, perhaps due to drug selection forces [11].
For the 14% of patients who are initially refractory and to plan for the cancer’s eventual adaptation to platinum for those who are initially sensitive, other theories suggest that the modulation of different mechanisms of action may better attack heterogeneous disease [12] and may synergize under subclonal evolution hypotheses [11]. It is therefore of interest to evaluate the frequency of use of non-platinum therapies and their effectiveness. Further, recent targeted strategies like bevacizumab maintenance therapy and PARP inhibitors [13] and immunotherapies (cancer vaccines, anti-PDL1 therapies) are threatening to make the use of the platinum interval to determine treatments less relevant, prompting the search for new biomarkers for recurrent disease management [5] and more relevant clinical descriptions of expected survival [14].
One source of data is the drug and treatment file from the Cancer Genome Atlas’ (TCGA) study of high-grade serous ovarian cancer. While TCGA was initiated to conduct a multi-spectrum genomic analysis of around 500 serous carcinomas with clinical follow-up, the released data includes information on the timing and use of multiple chemotherapy lines in most of these patients.
In this article, we conduct an analysis of TCGA patient TFI times to determine empirically the dynamics of repeated treatments and the factors that accelerate or decelerate progression in the recurrent setting. We hypothesize that the course of ovarian cancer has a common trajectory. Furthermore, understanding the factors that influence that trajectory could inform clinicians on the best next therapy to administer at the time of relapse.
Methods
Definitions of time-related events and quantities
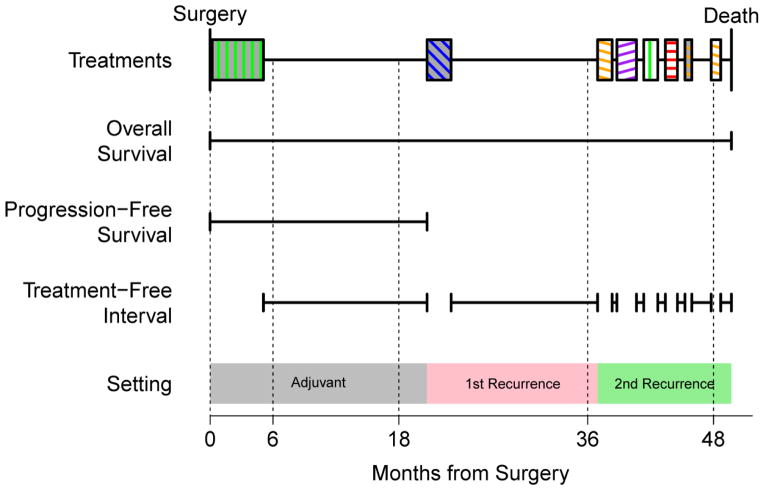
We defined event time-related quantities below with reference to Figure 1, a schematic representation of these times; and their relationship between each other, surgery time, times on treatment, and death or end of follow-up. For further reference, we graphed the treatment order and pattern for 49 heavily-treated patients who had at least 5 distinct treatment regimens (Figure 2).
Figure 1.
Schematic of a typical patient chemotherapy timeline. Overall survival spans the interval from surgery on the left to death on the right. Progression free survival is the time from surgery to the start of the first non-adjuvant chemotherapy. Chemotherapy regimens are filled blocks with colors encoding the type of chemotherapy (see Figure 2 for legend) and treatment free intervals are the spans in between them. We infer an increment in serial recurrences when a gap of longer than 30 days appears.
Figure 2.
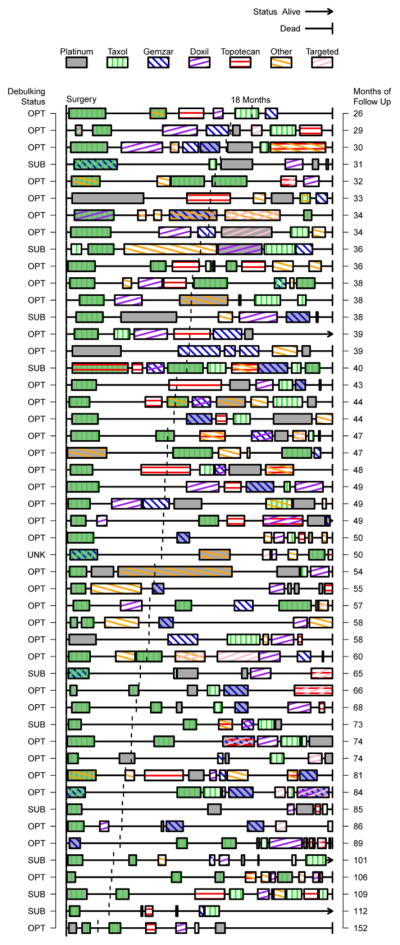
Treatment timelines for heavily treated (5+ regimens) patients ordered by total follow-up time. Debulking status is indicated on the left as optimal (OPT), suboptimal (SUB) or unknown (UNK). The 18 month mark is indicated by a vertical dashed line for each timeline.
Overall Survival (OS) was defined as the time from surgery to death.
We distinguished between patients who were disease-free (DF) following primary treatment and those who were never disease-free (NDF) due to residual disease, progressive or stable disease. DF patients may eventually recur while NDF patients will eventually progress. Evaluating recurrence and progression was determined solely by the participating TCGA sites and includes a mix of CA125 monitoring and radiological confirmation.
Progression-free survival (PFS) is the time from surgery to the first event: progression, recurrence, death or end of follow-up. This quantity is distinct from disease-free survival (which we did not study) that refers only to those patients who are DF after surgery and adjuvant chemotherapy.
The treatment-free interval (TFI) is the time between the end of one regimen and the start of the next regimen (death or the end of follow-up are censoring events). We sometimes use the term relapse to reference the end of a TFI. TFI associated with platinum chemotherapies are called platinum-free intervals (PFI).
In this article, PFS refers specifically to the first event after surgery which was defined by a specific field in the TCGA dataset. We emphasize that the later TFI are functionally defined using treatment times to determine when the interval starts and ends.
Patient population and Intervals
We downloaded the TCGA clinical data from cancergenome.nih.gov on July 24, 2014 (via the data matrix download option as all frozen sample data, excluding batch 409). The high-grade serous ovarian cancer TCGA population collected from 15 United States centers was described elsewhere [15]; the patients analyzed in this paper were on average 58.7 years old at diagnosis, half were diagnosed prior to 2004; they were predominantly FIGO stage IIIC/IV (87%), grade 3/4 (87%), with a majority optimally debulked (<1cm, 72%) (Table S1). TCGA inclusion criteria included primary treatment by maximal debulking surgery and adjuvant platinum chemotherapy; a small number of patients with neo-adjuvant chemotherapy were removed from our study. Following primary therapy, patients are typically evaluated with a combination of blood (CA125) and imaging (CT scan) tests to determine response to therapy. A complete response was defined as normalization of CA125 and no radiographic evidence of disease [16]. The average patient underwent 2.43 chemotherapy regimens (including adjuvant) spanning a median 47.6 months from surgery (44.4–52.3, 95%CI) until death (48% of patients have died) with 70% experiencing a first recurrence or progression by 17.8 months (16.4–19.0). A small fraction of patients survived beyond 60 months (15%).
Drug regimen collection and annotation
As of our review on April 22, 2015, the clinical_drug_ov.txt data table has remained static; it records that the most recent form completion is May 2, 2012. The raw file comprised 521 patients and 2482 records of individual drug treatments (each drug in a multi-drug regimen has its own record), the day of the start of treatment and the end of treatment. Discounting un-evaluable records due to missing times there were 461 patient records composed of 1119 treatment regimens (Table S2). The most common drugs were platinum-based (including carboplatin, cisplatin, etc.) (713/1119, 63.7%), taxanes (paclitaxel, docetaxel, etc.) (636/1119, 56.8%), doxorubicin (both liposomal doxorubicin and conventional) (133/1119, 11.9%), gemcitabine (128/1119, 11.4%), topotecan (110/1119, 9.8%); other therapies (119/1119, 10.6%) and targeted therapies (69/1119. 6.0%) were recorded as such and trial protocols were recorded generically as trials (7/1119, 0.6%) when the study drugs or randomization arms were not listed due to blinding.
We combined concurrent and overlapping treatments to form a single contemporaneous regimen attributable to a patient. Periods where patients transitioned between agents of similar classes (e.g., paclitaxel to docetaxel due to hypersensitivity) were considered a single regimen. Patients’ serial regimens were annotated so each patient’s history can be assessed individually (e.g., Figure 2). We recorded treatments with washout periods of less than 30 days as non-overlapping regimens but did not increase the “regimen number” as we argue this represents the failure of a one regimen and the search for another regimen (for example, the 2nd recurrence in Figure 1). These intervals are included in the analysis as “short intervals” where appropriate.
Statistical methods
Our analysis was performed entirely in the R 3.1.2 statistical language [17]. Our analysis relied heavily on standard Cox proportional hazards modeling [18] to estimate hazard ratios and their confidence intervals. Non-parametric k-nearest neighbor smoothing functions were implemented in the muhaz 1.2.6 package [19]. Smoothed hazards can be interpreted like a smoothed histogram or density plot for the event of interest. Multivariate Cox regression models were built using forward and backwards selection using the Aikaike’s Information Criterion.
RESULTS
Qualitative overview of heavily treated patients’ timelines and choice of therapy
To capture the scale and complexity of the post-recurrence treatment situation, we developed a plot summarizing treatments in order and their affiliated TFI. In Figure 2, we have considered only those patients with at least 5 distinct regimens. In this figure, each patient’s time course was scaled to align surgery times and the end of follow-up to emphasize relative, patient-specific benefit. Chemotherapy regimens were represented by filled boxes on the timeline with shading and patterns to denote combinations; platinum chemotherapies were represented as a full shaded box to highlight their unique importance. Timelines were ordered by total follow-up time, noted to the right of the plot. The surgical outcome (optimal debulking or otherwise) was noted on the left of the plot.
The patient in Figure 1 was representative of a typical platinum-based strategy [4] [6] [11]: an initial doublet followed by a second platinum doublet and then a period of searching for a replacement single treatment. This patient eventually expires while on their 6th attempted therapy in this period. In Figure 2, we noted a prevalence of patients who return to platinum treatments and experience small boosts versus non-platinum therapies. We also noted several terminal intervals (right-most) that appear longer than expected; this might have been due to under-reporting treatments or to a conscious decision to opt for palliative care avoiding the hazard of re-treatment.
Effects of complete response to treatment on overall survival, progression-free survival and treatment-free interval
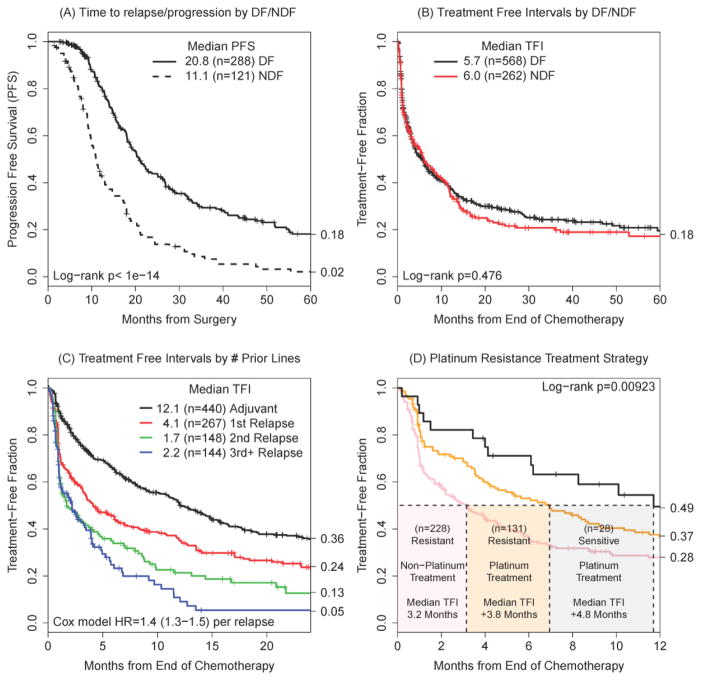
Following surgery and adjuvant platinum/taxane-based chemotherapy, patients either entered a disease-free interval or not. As expected, a complete response to primary treatment significantly increased the median time until death after relapse (30.0 vs 22.4 months, p<0.0001); the time from surgery to relapse was significantly longer than time to progression (20.8 to relapse following a DF state vs. 11.1 to progression following a NDF state, p<0.0001), (Figure 3A). The number of patients in the figure differs from Table S1 due to missing primary response data.
Figure 3.
Progression-free survival as a function of an initial disease-free (DF) state or never disease-free state (NDF) (A). The effect of DF or NDF states on later treatment-free intervals (TFI) (B); the sample size reflects the number of unique intervals with DF/NDF information. The duration of TFI as a function of the number of previous lines of therapy (C). Platinum treatment efficacy as a function of the previous platinum interval (D).
The median time to death after progression is 27.9 months (25.9–30.9) and longer initial PFS times were strongly associated with longer time to death in a continuous manner (per month of PFS: HR=0.968, 0.95–0.98, p=0.00015). More concretely, PFS times of at least 10 months or greater were required to see a significant difference in time to death after progression (29.0 vs. 24.0 months, p=0.00428); a woman with PFS greater than 18 months had a median of 36.5 vs. 24.8 months to death after progression (p=0.00044).
Patients who relapsed or progressed enter the disease-management phase -- characterized by serial treatment intervals -- where the duration of individual TFI did not appear to depend on whether the patient had had a complete response to primary therapy (log-rank p=0.476) (Figure 3B). Further, TFI decay with increasing re-treatment (Figure 3C, Cox model HR=1.4, 1.3–1.5, p<0.0001) and the number of treatment regimens was not associated with longer time to death, (log-rank p=0.241). Curiously, patients with initial complete responses had the same average number of treatments (1.61 versus 1.45, p=0.977). The increased time to death after relapse/progression could be traced to slightly longer average total time on treatment (11.2 vs 7.6 months, p=0.0339) and longer total time off treatment (20.7 versus 12.9 months, p=0.0050). In summary, individual TFI received no boost in complete response patients for a given interval, but were compounded when the entire treatment history was considered.
Re-treatment strategies based on previous treatment-free interval
The superior effect of re-treating platinum sensitive patients with platinum/taxane strategies was apparent (Figure 3D): patients who have most recently had a TFI of less than 18 months [11] treated with a non-platinum regimen were treatment-free for a median of 3.2 months. A platinum regimen for these patients doubled the time to 7.0 months. Patients with long TFI intervals (28/387 intervals, 7.2%) continued to do well with platinum (11.7 months). We excluded a minority of patients with long TFI treated with non-platinum regimens (n=13) as these patients uniformly transitioned to a different regimen within 4 months, experienced no deaths and returned to a platinum strategy afterwards; they were likely being treated with planned alternative therapies.
Re-treatment times cluster at predictable times
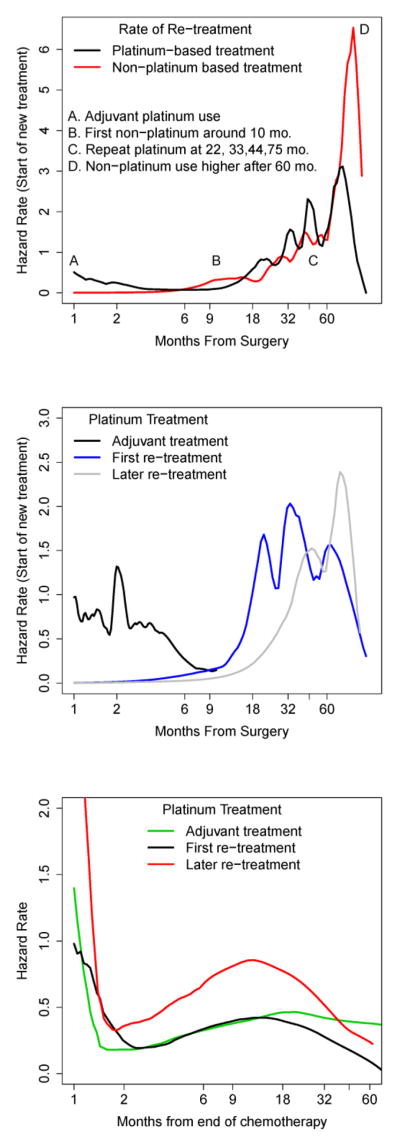
Following the smoothed hazard rate plots, platinum treatments clustered around 22, 33, 44 and 75 months after surgery (Figure 4A). The first non-platinum treatments appeared 10 months after surgery and spike in frequency for patients who survive to 60 months. Focusing on the timing of platinum treatments by order of recurrence (Figure 4B), we noted a cluster of patients starting adjuvant therapy 2 months after recovering from their surgery; times to first re-treatment with platinum cluster at 18, 32 and 60 months after surgery. Focusing on a given interval, the time to re-treatment appeared to have a similar-shaped hazard regardless of line of treatment (Figure 4C) supporting the use of proportional hazards models: there was an early hazard of treatment failure that tapered off by month 2. The hazard of retreatment picked up again around 6 months and peaked between 10 and 18 months after which it remained constant for adjuvant therapies.
Figure 4.
Smoothed hazard rate estimates for time to start of next treatment (A), the timing of platinum treatments by chemotherapy line (B), and the hazard of retreatment given a platinum regimen (C).
Serial treatment-free intervals are correlated across centers and multiple recurrences
On the log scale, there was significant correlation between one TFI and the next (Pearson’s r=0.2204, p<0.0001) which persisted regardless of scale (Spearman’s ρ=0.233, p<0.0001). Reflecting different practices and their different sample sizes, the effects varied by treatment center but nearly every effect was positive, so longer previous intervals were associated with longer TFI (Table S3). This correlation was robust to the number of prior treatments (Table S4), but the effect weakened from adjuvant TFI and first recurrence (n=267 pairs, ρ=0.245, p<0.0001), first to second (n=148, ρ=0.214, p=0.0089), and second to third (n=74, ρ=0.173, p=0.142).
Prognostic Factors affecting treatment-free intervals
Recurrent TFI were not associated with most baseline clinical differences (Table 1): complete response to primary therapy (Cox model p=0.482), year of diagnosis (p=0.7481), age at diagnosis (p=0.0946), high stage (p=0.8200) or optimal debulking (p=0.0685); pathological grades G3 and G4 were weakly associated with shorter TFI (HR=1.9, p=0.0349). While stage is typically known as a strong prognostic factor, the lack of association may be due to low variation in stage (87% are FIGO Stages IIIC, IV, Table S1). With respect to timing and ordering, interval-specific effects were highly significant: the duration of the last TFI reduces hazard of re-treatment (HR=0.90, p=0.0445), and longer time from surgery (HR=1.4, p=0.0003), later recurrences (HR=1.1, p=0.007) and increasing use of platinum (HR=1.4, p=0.0002) all increase the hazard associated with transitioning to a new chemotherapy regimen.
Table 1.
TFI Cox regression: univariate effects and multivariate models.
| Baseline Factors | Risk Factor | HR | p |
|---|---|---|---|
| Clinical Response | no DFI | 1.1 (0.8–1.5) | 0.4821 |
| Year of Diagnosis | per year | 1.0 (1.0–1.0) | 0.7481 |
| Age at Diagnosis | per decade | 0.9 (0.8–1.0) | 0.0946 |
| Stage | IIIC/IV | 1.0 (0.7–1.5) | 0.8200 |
| Grade | 3/4 | 1.9 (1.0–3.5) | 0.0349 |
| Debulking | optimal | 1.3 (1.0–1.7) | 0.0685 |
|
| |||
| Interval-specific factors | Risk level | HR | p |
|
| |||
| Last TFI | per month | 0.9 (0.8–1.0) | 0.0445 |
| Months from Surgery | per month | 1.4 (1.2–1.8) | 0.0003 |
| Recurrences | per previous | 1.1 (1.0–1.3) | 0.0070 |
| 0 vs. 1 | First recurrence | 1.3 (1.1–1.5) | 0.0089 |
| 1 vs. 2 | Second | 1.5 (1.2–1.9) | 0.0009 |
| 2 vs. 3 | Third | 1.4 (1.0–1.9) | 0.0580 |
| 3 vs. 4+ | Later | 0.7 (0.5–1.1) | 0.1500 |
| Platinum treatments | per previous use | 1.4 (1.2–1.6) | 0.0002 |
| Number of Cycles* | Per cycle | 0.8 (0.8–0.9) | <0.0001 |
| Adj. for # Recurrences | Per cycle | 0.9 (0.9–1.0) | 0.1755 |
| Days on Treatment* | Observed/(28 x # Cycles) | 1.0 (0.8–1.4) | 0.8274 |
| Adj. for # Recurrences | Observed/(28 x # Cycles) | 1.1 (0.8–1.4) | 0.7224 |
|
| |||
| Multivariate (Timing Model) | HR | p | |
|
| |||
| Any gemcitabine, taxane, topotecan, doxorubicin or other | 1.7 (1.1–2.5) | 0.0100 | |
| Months from Surgery (log scale) | 1.5 (1.2–1.8) | 0.0001 | |
| Months last TFI (log scale) | 0.9 (0.8–1.0) | 0.0087 | |
| Likelihood ratio test=27.2 on 3 df, p=5.26e-06 | |||
|
| |||
| Multivariate (Regimen Model) | HR | p | |
|
| |||
| Any gemcitabine, taxane, topotecan, doxorubicin or other | 1.7 (1.1–2.5) | 0.0130 | |
| # Previous Regimens | 1.4 (1.2–1.6) | 0.0001 | |
| Likelihood ratio test=22.8 on 2 df, p=1.15e-05 | |||
Restricted to cycles (2–8)
The number of treatment cycles was available for 93% of the intervals. Alone, more cycles were not associated with better prognosis (p=0.16), but when restricted to 2–8 cycles to eliminate aborted treatments and maintenance therapies, more cycles appear to be associated with longer TFI (HR=0.8, p<0.001). Accounting for the order of recurrences eliminated the effect (p=0.1755) and we noted that there was no association between recurrence and cycles (in the 2–8 cycle range, ANOVA p=0.3468). To approximate treatment delays, we compared the observed treatment duration to the number of cycles assuming a 28-day cycle, but found no significant association (p=0.8274) even after accounting for recurrences (Table 1).
Considering the interval-specific factors in a multivariate context, we developed two models: one based on the timing of the re-treatments and one based on the number of previous re-treatments (Table 1). Increasing months from surgery, the length of the last TFI, and the use of any combination of gemcitabine, taxane, topotecan or doxorubicin appear to add together to form a strong prognostic model. The prognostic effect associated with the use of any of these drugs likely does not reflect inferior treatment; it may simply signal that the oncologist felt this patient would not tolerate or respond to platinum therapy. The hazard ratios in the multivariate model were roughly the same size as in the univariate analysis suggesting that the drug, time and last response have independent effects. We developed an alternative model based on the use of any of the non-platinum drugs and the number of previous regimens. Each additional line of chemotherapy increased the hazard by a factor of 1.4 (95% CI: 1.1–2.5) which accelerated the time to the start of a new therapy (Table 1). If we examine the recurrence order in detail, we see that the hazard ratio increases: HR=1.3 for the first re-treatment, HR=1.5 for the second, HR = 1.4 for the third, and stays constant for fourth and further recurrences (p=0.150).
We also investigated whether the treatment on the previous interval interacts with the current choice of chemotherapy treatment (Table S5) and found little significant impact on TFI beyond the repeated use of platinum.
Platinum treatment timing and the platinum-free interval
Considering the use of platinum (alone or in combination), we found that platinum regimens appeared to perform uniformly better than non-platinum regimens regardless of the previous response to platinum (Table 2); patients were stratified into 3 equally sized groups based on previous PFI. For first re-treatments, the difference is small (3+ months, median) but significant regardless of PFI given the small sample sizes. In this situation, we removed patients who did not have at least one more follow-up interval – we noted that under-reporting errors here made those intervals appear unusually large. Due to this restriction, the intervals were robust to reporting errors.
Table 2.
Average treatment-free interval (TFI) given platinum treatment and the most recent TFI.
Median (95% CI) treatment free interval (PFI) given platinum treatment and the previous PFI.
| First Re-treatment Months previous PFI | <7.4 | 7.4–15.7 | >15.7 |
|---|---|---|---|
| # Intervals | |||
| Platinum | 19 | 19 | 25 |
| Non-platinum | 59 | 12 | 9 |
|
| |||
| Median (95% CI) | |||
| Platinum | 4.1 (1.9–12.2) | 4.6 (3.5–13.5) | 6.1 (3.4–12.6) |
| Non-platinum | 1.1 (0.9–2.3) | 1.2 (0.8-NA) | 1.0 (0.7-NA) |
| Log-rank Test | p=0.0263 | p=0.0003 | p=0.0018 |
|
| |||
| Later Re-treatment Months previous PFI | <7.4 | 7.4–15.7 | >15.7 |
|
| |||
| # Intervals | |||
| Platinum | 8 | 9 | 3 |
| Non-platinum | 46 | 15 | 5 |
|
| |||
| Median (95% CI) | |||
| Platinum | 3.6 (1.2-NA) | 2.6 (0.9-NA) | 3.4 (3.2-NA) |
| Non-platinum* | 1.0 (0.9–1.2) | 2.2 (1.0–8.8) | 0.7 (0.7-NA) |
| Log-rank test | p=0.1450 | p=0.7640 | p=0.0136 |
Log-rank p-values test platinum vs. non-platinum TFI in a given stratum.
NA: indicates n upper bound that cannot be evaluated.
significant difference across PFI levels, p=0.0363.
For later re-treatments, we noted that non-platinum usage was strongest with shorter PFI (reflecting the common use of PFI to select treatments). Again, platinum regimens appear to be uniformly superior although the difference was not significant in the intermediate interval (previous PFI, 7.4–15.7 months). The lack of significance may have been due to small sample sizes but may also hint at window of non-platinum treatment effect (across PFI categories, log-rank p=0.0363): this might be a range where there is still some non-platinum benefit to be had.
DISCUSSION
We summarized the patterns related to serial chemotherapy treatments in patients with recurrent high-grade serous ovarian cancer. These patients were selected for TCGA’s retrospective biobank study and reflect a population of patients treated at centers across the United States initially diagnosed between 1996 and 2009. Most patients were treated with standard chemotherapy agents -- a small number reported clinical trial interventions and the use of bevacizumab during this time period.
We see strong evidence that TFI decrease with successive chemotherapies up to a futility limit of about one month of treatment-free response. This represents a functional limit: one month was our selected resolution for distinguishing between regimens because it reflects the practical amount of time it would take to evaluate the success of one regimen and to let the patient recover before starting the next.
An interesting finding is that individual TFI do not appear to depend on a history of complete response to primary therapy which implies that, when considering her at a single moment in time, a single patient’s relevant history only depends on the number of previous treatments and the use of platinum chemotherapy. In contrast, when considering the patient’s whole history, we see a trend that patients with a compete response to primary therapy (disease-free after chemotherapy) received more chemotherapy and survived longer after recurrence.
However, we have recorded evidence that the first recurrence or progression is a prognostic reset with respect to complete response to primary therapy; the recurrent clinical scenario is similar for all women regardless of the time it takes to get there. We noted that patients who are not disease-free after primary treatment still experience a median of 11 months from surgery to death or progression. We made the prognostic observation that at least 10 months of PFS time after primary treatment was required to see a significant change in time to death after relapse.
We observed that treatment times cluster at predictable intervals relative to surgery. We suspect these are treatments initiated in response to scheduled follow-up visits: 10 months corresponds to the rough amount of time to recover from surgery, undergo adjuvant chemotherapy and to determine primary response (non-responders immediately progress to non-platinum treatment). An annual or semi-annual visit lines up with the 22 and 33 and 44 month spikes in treatment.
Regarding treatments, because of decaying effects, more treatment does not seem to improve time to death on average and we record evidence that platinum still holds a significant benefit for patients classically labeled platinum refractory (<12 months PFI). Conversely, we saw a lack of significant platinum effect versus non-platinum treatments for PFI in the intermediate range, likely due to smaller sample sizes. However, the difference admits the possibility that the wrong patients are being selected for non-platinum therapies.
In prospective, interventional clinical trials, investigators have studied recurrent disease for superiority and tolerability of different regimens; our approach to studying more than one or two serial recurrences adds information about the natural history of the selection and response to therapy. We have learned that there is significant correlation between serial intervals supporting patient specific effects. That is, consistent with subclonal resistance evolutionary theories, we might hypothesize that patients presenting at baseline could be sorted into sensitive or resistant trajectories that set the pace of second and third relapses.
Because of the dominance of platinum treatments with early treatment intervals, it is difficult to estimate whether early beneficial effects are due to less decay of effect, the superior effect of platinum, or the oncologist assigning healthier patients to platinum regimens; we postulate that estimating the opportunity cost of platinum treatment (or non-platinum treatment) may be a useful way to cast this question.
The study conclusions are limited by the observational nature of the data. If it were feasible, a randomized study among all candidate treatment regimens would have been preferable, however the observational study is representative of the clinical situation and a model developed from our analysis might serve as a benchmark for “physician’s choice” standard of care in recent decades. A more planned retrospective study will be required to model crossover between multiple sets of treatments in the same patient.
There is a natural weakness in that not all centers submitted drug information data and the quality varies with some missing times affecting our total sample sizes and precluding analyses of dose level, cure rate and adherence to guidelines. Our work here has prompted us to initiate an all-comers type retrospective study of the experience at our own center that will capture a representative sample of other stages and histologies; with better control of the chemotherapy workflow data, we anticipate a data quality sufficient to validate or invalidate our findings in this study.
Supplementary Material
HIGHLIGHTS.
Complete response to primary therapy is associated with improved progression-free survival and overall survival, but not subsequent treatment-free intervals.
Treatment-free interval decreases with successive courses of chemotherapy, time from surgery and shorter previous intervals.
Evidence suggests that platinum treatments are superior on average regardless of previous 50 response.
Acknowledgments
This work was funded by the Roswell Park Cancer Institute, Career Development Program funds from RPCI-UPCI SPORE P50CA159981, NIH grants P30CA016056 (RPCI), K01LM012100 (KHE), R01HG007377 (BMH) T32CA108456 (JBS) and a grant from the Roswell Park Alliance Foundation (KHE).
Footnotes
Conflict of Interest Statement
The authors declare no financial conflict of interest.
The results published here are based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhoola S, Hoskins WJ. Diagnosis and management of epithelial ovarian cancer. Obstetrics & Gynecology. 2006;107(6):1399–410. doi: 10.1097/01.AOG.0000220516.34053.48. [DOI] [PubMed] [Google Scholar]
- 2.Morgan RJ, Alvarez RD, Armstrong DK, et al. Ovarian cancer, version 3.2012. Journal of the National Comprehensive Cancer Network. 2012;10:1339–1349. doi: 10.6004/jnccn.2012.0140. [DOI] [PubMed] [Google Scholar]
- 3.Ushijima K. Treatment for recurrent ovarian cancer at first relapse. 2010 Journal of Oncology. 2010:497429. doi: 10.1155/2010/497429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaughan S, Coward J, Bast R, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nature Reviews Cancer. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledermann JA, Kristeleit RS. Optimal treatment for relapsing ovarian cancer. Ann Oncol. 2010;21(S7):vii218–vii222. doi: 10.1093/annonc/mdq377. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong DK. Relapsed ovarian cancer: challenges and management for a chronic disease. The Oncologist. 2002;7:20–28. doi: 10.1634/theoncologist.7-suppl_5-20. [DOI] [PubMed] [Google Scholar]
- 7.Herzog TJ. Recurrent ovarian cancer: how important is it to treat disease progrsesion? Clin Cancer Res. 2004;10:7439–7449. doi: 10.1158/1078-0432.CCR-04-0683. [DOI] [PubMed] [Google Scholar]
- 8.Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. Journal of Clinical Oncology. 1991;9:389–393. doi: 10.1200/JCO.1991.9.3.389. [DOI] [PubMed] [Google Scholar]
- 9.Markman M, Hoskins W. Responses to salvage chemotherapy in ovarian cancer: a critical need for precise definitions of the treated population. J Clin Oncol. 1992;10:520–28. doi: 10.1200/JCO.1992.10.4.513. [DOI] [PubMed] [Google Scholar]
- 10.Bailey CH, Jameson G, Sima C, et al. Progression-free survival decreases with each subsequent therapy in patients presenting for phase I clinical trials. J Cancer. 2012;3:7–13. doi: 10.7150/jca.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke S, Brenton J. Evolution of platinum resistance in high-grade serous ovarian cancer. The Lancet Oncology. 2011;12:1169–1174. doi: 10.1016/S1470-2045(11)70123-1. [DOI] [PubMed] [Google Scholar]
- 12.Bookman MA. Extending the platinum-free interval in recurrent ovarian cancer: the role of topotecan in second-line chemotherapy. Oncologist. 1999;4:87–94. [PubMed] [Google Scholar]
- 13.Campos SM, Ghosh S. A current review of targeted Therapeutics for ovarian cancer. Journal of Oncology. 2010:149362. doi: 10.1155/2010/149362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurta ML, Edwards RP, Moysich KB, et al. Prognosis and conditional disease-free survival among patients with ovarian cancer. 2014;22(36):4102–4112. doi: 10.1200/JCO.2014.55.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rustin GJS, Vergote I, Eisenhauer E, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA125 agreed by the Gynecological Cancer Intergroup. Int J of Gynecological Cancer. 21(2):419–423. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 17.R Core Team. R: A Language and Evironment for Statistical Computing. Vienna, Austria: 2014. [Google Scholar]
- 18.Hosmer DW, Lemeshow S, May S. Applied Survival Analysis. 2. Wiley; 2008. [Google Scholar]
- 19.Hess K, Gentleman R. muhaz: Hazard Function Estimation in Survival Analysis. 2014 http://CRAN.R-project.org/package=muhaz.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.