Abstract
Objectives
Evaluate ovarian morphology using 3-dimensional MRI in adolescent girls with and without PCOS. Compare the utility of MRI versus ultrasonography (US) for diagnosis of PCOS
Design
Cross-sectional
Setting
Urban academic tertiary-care children’s hospital
Patients
Thirty-nine adolescent girls with untreated PCOS and 22 age/BMI-matched controls.
Intervention
MRI and/or transvaginal/transabdominal US
Main Outcome Measure
Ovarian volume (OV); follicle number per section (FNPS); correlation between OV on MRI and US; proportion of subjects with features of polycystic ovaries on MRI and US.
Results
MRI demonstrated larger OV and higher FNPS in subjects with PCOS compared to controls. Within the PCOS group, median OV was 11.9 (7.7) cm3 by MRI, compared with 8.8 (7.8) cm3 by US. Correlation coefficient between OV by MRI and US was 0.701. Due to poor resolution, FNPS could not be determined by US or compared with MRI. ROC curve analysis for MRI demonstrated that increasing volume cut-offs for polycystic ovaries from 10cm3 to 14cm3, increased specificity from 77% to 95%. For FNPS on MRI, specificity increased from 82% to 98% by increasing cut-offs from ≥12 to ≥17. Using Rotterdam cut-offs, 91% of subjects with PCOS met polycystic ovary criteria on MRI, while only 52% met criteria by US.
Conclusions
US measures smaller OV than MRI, cannot accurately detect follicle number, and is a poor imaging modality for characterizing polycystic ovaries in adolescents with suspected PCOS. For adolescents in whom diagnosis of PCOS remains uncertain after clinical and laboratory evaluation, MRI should be considered as a diagnostic imaging modality.
Keywords: PCOS, Ovarian Imaging, MRI, Adolescent
INTRODUCTION
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders associated with overweight and obesity, affecting 5-10% of adolescent girls and women of reproductive age (1). However, there continues to be significant debate over the criteria for accurate diagnosis, specifically in adolescents, in whom no age-specific diagnostic criteria have been established (2). The 1990 National Institutes of Health (NIH) criteria defined PCOS as a disorder of hyperandrogenism and oligoovulation. In 2003, the Rotterdam criteria were proposed, requiring 2 of 3 features including: 1) oligoovulation or anovulation, 2) hyperandrogenism (clinical or biochemical), and 3) polycystic ovaries. (3). The 2006 Androgen Excess-PCOS (AES-PCOS) Society criteria, which encompass both the NIH and Rotterdam criteria, also include the assessment of ovaries for polycystic morphology (4).
Therefore, if the Rotterdam or AES-PCOS criteria are used, evaluation for polycystic ovaries should be performed. As stated by the Rotterdam consensus, polycystic ovaries are defined as including 12 or more follicles per ovary measuring 2-9 mm in diameter and/or ovarian volume (OV) greater than 10cm3, with features in one ovary being sufficient for diagnosis (3). These criteria were created based on studies evaluating ovarian morphology by transvaginal ultrasound (US) in adult women (5–7). However, in virginal adolescent girls, transvaginal US is contraindicated and transabdominal US is not optimal due to poor resolution and central adiposity. Therefore, transvaginal US data from adult women may be inappropriate to derive cut-off values to apply to adolescents, who not only differ in age, but also in method of ultrasound. Moreover, follicle number per ovary (FNPO) and follicle number per section (FNPS) are used interchangeably in the PCOS literature (8, 9). Due to these limitations, ovarian imaging by US, particularly in obese adolescent girls with PCOS, may preclude interpretation and diagnosis and should be challenged. An improved imaging modality for characterizing ovarian morphology is needed to assist with diagnosis of PCOS in this age group. In recent years, several small studies have used magnetic resonance imaging (MRI) to examine ovarian morphology in both adult women (10, 11) and adolescents (8, 12) with PCOS. Although these studies confirmed the presence of large ovaries and numerous small follicles, volumetric calculations were based on estimated volumes derived from 2-dimensional images and not true 3-dimensional volume rendering.
Thus, the aims for the present study were to perform a detailed comparison of ovarian ultra-structure including OV and follicle count in adolescents with and without PCOS using 3-dimensional volumetric analysis and to compare MRI versus US for identification of polycystic ovary features in a large group of adolescent girls with PCOS.
MATERIALS AND METHODS
Subjects
Adolescent females, aged 13 to 18 years, with PCOS and control girls without PCOS were recruited for this study from the pediatric endocrine and adolescent medicine clinics at the Children’s Hospital at Montefiore in Bronx, NY from September 2010 through August 2014, as part of a larger study evaluating sleep disordered breathing, body composition, and metabolic parameters in adolescents. Subjects with PCOS were consecutively recruited during the study period after a new diagnosis of PCOS was established by the subject’s physician, based on biochemical hyperandrogenemia and irregular, infrequent menstrual bleeding, in accordance with NIH criteria (13). Hyperandrogenemia was defined as total testosterone >41ng/dL and/or free testosterone >3.9pg/mL, according to our clinical laboratory’s reference ranges. Control subjects, matched for age and BMI, had regular monthly menstrual cycles, defined as ≥10 menses per year (14), and no clinical evidence of hyperandrogenism. Subjects were excluded if they were taking any hormonally active medication including insulin sensitizers or oral contraceptive pills, were pregnant, or had other endocrine disorders. Control subjects were imaged during the early follicular phase of their menstrual cycle (days 2-10). Due to irregular menses, the timing of imaging in subjects with PCOS was unrelated to menses. MRI and US imaging studies were performed within a 24 hour time frame in the subjects with PCOS.
Informed consent was obtained from the legal guardian of each subject younger than 18 and from 18-year-old subjects themselves prior to participation in the study. The study was approved by the Institutional Review Board at Montefiore Medical Center and Albert Einstein College of Medicine.
Magnetic Resonance Imaging
Acquisition
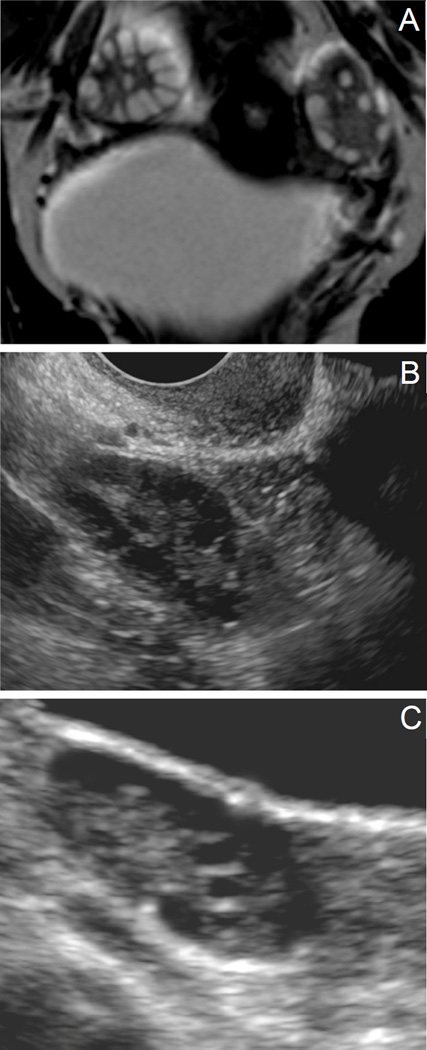
All subjects with PCOS and controls underwent a pelvic MRI using a 3T Philips Achieva system (Philips Medical Systems, Best, Netherlands). Axial and coronal T2-weighted spin-echo sequences were created for optimal visualization, characterization, and distribution of ovarian follicles. Axial T2-weighted images were obtained (TR- 4500 milliseconds, TE- 80 milliseconds) with slice thickness of 4mm and slice spacing of 5mm. The field of view during axial image acquisition was 210 × 229 mm (frequency encoding×phase encoding directions respectively). Coronal T2-weighted images were obtained (TR-4500 milliseconds, TE- 80 milliseconds) with a slice thickness of 3mm and slice spacing of 4mm. The field of view during coronal image acquisition was 250×231 mm (frequency encoding×phase encoding directions respectively) (Figure 1A). All images were archived on a picture archiving and communication system (PACS) network.
Figure 1.
Ovarian morphology. (A) MRI, (B) Transvaginal US, (C) Transabdominal US. Figure 1A displays a coronal view by MRI of an ovary in an adolescent subject with PCOS. Follicles (hyperintense) are clearly demarcated from stroma (hypointense). Figures 1B and 1C are ultrasound images from adolescent subjects with PCOS, with Figure 1B representing a transvaginal image and 1C representing a transabdominal image. Follicles are visualized in black (hypoechoic) with stroma appearing more hyperechoic. Distinguishing individual follicles by ultrasound is difficult, precluding a follicle count.
Analysis
DICOM files were analyzed utilizing a DICOM software reader, Amira 5.45 for image linear, area, and volumetric analysis. A board certified radiologist reviewed the images in blinded fashion. In both the axial and coronal planes, a region of interest (ROI) was determined around the ovary outer margin on each slice in order to calculate each slice area. OV was later determined by multiplying each area by slice thickness. Follicle count was determined on the axial slice encompassing the largest ovarian diameter, and the number of 2-9mm follicles was counted, giving a total FNPS for each subject as outlined by Lujan et al (9). Ovarian follicle distribution was noted to be peripheral, with follicles confined to the outermost margin of the ovary, or random. The ovarian stromal area was calculated as a percentage of total ovarian area from an axial slice encompassing the largest ovarian diameter.
Ultrasonography
Subjects with PCOS also had a pelvic US, either transvaginal (n=12; Figure 1B) if they were sexually active or transabdominal (n=21; Figure 1C) if they were virginal. No pelvic US imaging was done on control subjects as it was not indicated clinically and was not part of the larger study protocol.
Acquisition
Pelvic sonography was performed on a GE Logiq E9 unit, utilizing an IC5-9-D (3–10 MHz) or C1-5-RS (2–5 MHz) transducer for transvaginal or transabdominal imaging respectively. Image acquisitions were performed by a certified sonographer. The ovaries were imaged in the sagittal and transverse planes, and three orthogonal measurements were obtained in real time by the sonographer.
Analysis
OV was calculated using the ellipse formula of length (in cm)×width (in cm)×thickness (in cm)×0.523 (8). Follicle count was ascertained based on the clearest image frame, as determined by the radiologist, as is currently done in clinical practice, giving a total FNPS for each subject. Follicle distribution was categorized as peripheral or random. Stromal area was unable to be determined by US.
Literature Review
A literature review was conducted to describe current literature on ovarian morphology in adolescents with PCOS by a single investigator (LEK). A PubMed search was performed looking under Title/Abstract for the following search terms: polycystic ovary syndrome, adolescent(s), ultrasound, ultrasonography, sonographic, MRI, imaging, ovarian volume, and follicle number. Articles were excluded if the subjects did not have PCOS, no imaging data was included (OV or follicle count), or the article was not written in English.
Data Analysis
Descriptive analyses included calculation of the percentage distribution and medians with their associated interquartile range (IQR) as well as means with standard deviations (SD) for categorical and continuous variables. Prior to any analyses, variables were checked for normality. Differences in the percentage distribution of categorical variables were tested using chi-square tests for differences between categorical variables. When continuous variables were not normally distributed, we used non-parametric tests to examine associations. Group differences in means were tested using the Wilcoxon-Mann test. The concordance correlation coefficient was used to determine agreement between MRI and US in subjects with PCOS (15) and a Bland Altman was plotted. To assess the diagnostic performance of MRI we calculated the sensitivity and specificity using different thresholds for ovarian volume and follicle number, an overall performance was calculated by receiver operating characteristic (ROC) curve analysis. P-values <0.05 were considered statistically significant. Analyses were performed using STATA (STATA/SE 14.0, StataCorp LP, College Station, TX 77845, USA).
RESULTS
Subjects
Thirty-nine subjects with PCOS and 22 controls without PCOS were studied. Mean age of the PCOS group was similar to controls (16.68 ± 1.56 years versus 16.56 ± 1.77 years; p=0.97), as was mean BMI z-score for subjects with PCOS and controls (1.84 ± 0.92 versus 2.02 ± 0.64; p=0.78). Within the PCOS group, 80% were overweight or obese, as were 91% of controls.
Comparison of Ovarian Morphology by MRI in Subjects with PCOS vs. Controls
Thirty-nine subjects with PCOS and 22 controls had MRI performed and 78 and 44 ovaries were analyzed, respectively.
Ovarian Volume
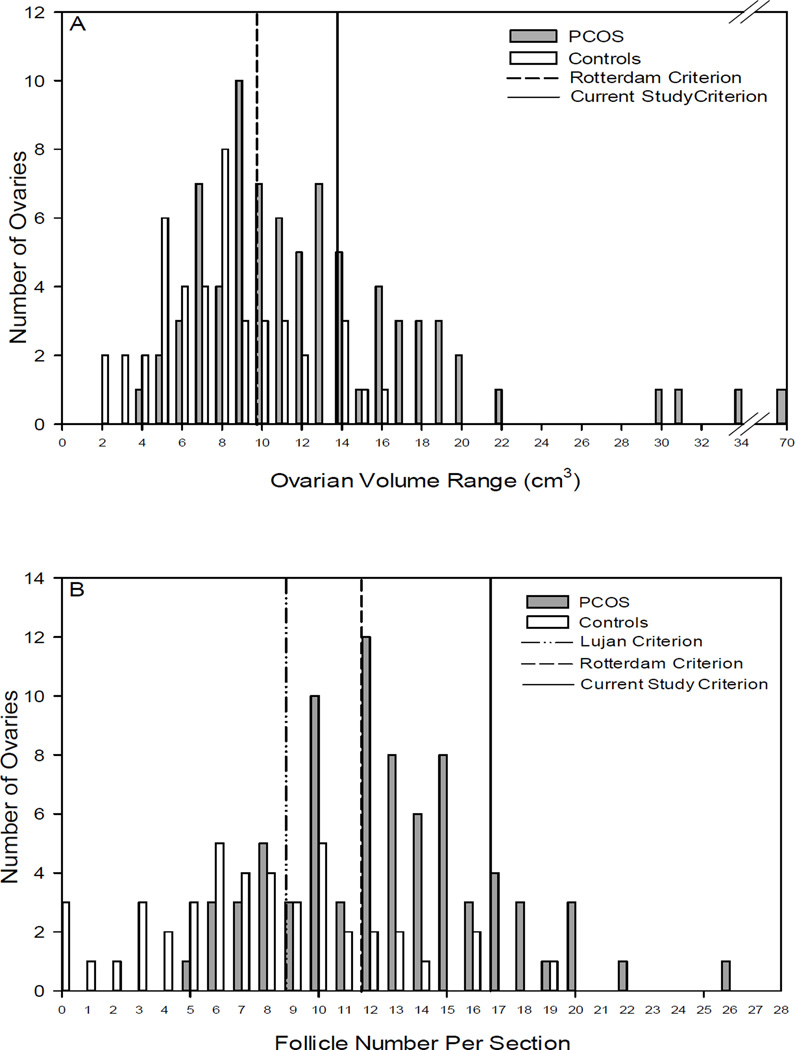
By MRI, median OV and interquartile range (IQR) was 11.8 (6.9) cm3 and 7.0 (4.4) cm3 in subjects with PCOS and controls, respectively (p<0.001) [Table 1]. Distribution of OV in subjects with PCOS and controls is shown in Figure 2A. There was no significant difference in mean stromal area between subjects with PCOS and controls [Table 1].
TABLE 1.
Comparison of Ovarian Morphology Measured by MRI in Adolescent Subjects with PCOS vs. Controls using Rotterdam, Lujan et al, and Current Study Criteria
| PCOS (N=39) | Controls (N=22) | p -value | |
|---|---|---|---|
| Number ovaries | 78 | 44 | |
| Median (IQR) OV(cm3) | 11.8 (6.9) | 7.0 (4.4) | <0.001 |
| Mean ± SD OV (cm3) | 13.6 ± 8.5 | 7.4 ± 3.4 | <0.001 |
| % (number) of ovaries with OV >10 cm3 (Rotterdam) | 65% (51) | 23% (10) | <0.001 |
| % (number) of ovaries with OV >14 cm3 (Kenigsberg et al) | 33% (26) | 5% (2) | <0.001 |
| Median (IQR) FNPS (2–9mm) | 12.0 (5.0) | 7.5 (5.0) | <0.001 |
| Mean±SD FNPS (2–9mm) | 12.7 ± 4.0 | 7.7 ± 4.4 | <0.001 |
| % (number) of ovaries with FNPS ≥12 (Rotterdam)a | 64% (50) | 18% (8) | <0.001 |
| % (number) of ovaries with FNPS ≥9 (Lujan et al) | 85% (66) | 41% (18) | <0.01 |
| % (number) of ovaries with FNPS ≥17 (Kenigsberg et al) | 17% (13) | 2% (1) | 0.01 |
| % (number) of ovaries with peripheral distribution of follicles | 62% (48) | 37% (15)b | 0.01 |
| % stromal area (stromal area/total area) | 51 ± 11.2 | 49 ± 14.3 | NS |
Data presented as median with interquartile range (IQR) or mean ± SD
Based on follicle count from Rotterdam criteria
Unable to evaluate on 3 ovaries thus denominator is 41 not 44
OV = Ovarian Volume; FNPS = Follicle Number Per Section
Figure 2.
Range of OV and FNPS by MRI in adolescents with PCOS versus controls without PCOS. Figure 2A shows the distribution of OV in subjects with PCOS and controls. The vertical dashed line delineates polycystic OV (>10cm3) from normal OV (≤10cm3) as per Rotterdam criteria and the solid line delineates polycystic OV (>14cm3) from normal OV (≤14cm3) as per the cut off calculated in this study (Kenigsberg et al.). Figure 2B shows the distribution of FNPS in subjects with PCOS and controls. The vertical dashed line delineates FNPS characterizing a polycystic ovary (≥12) versus normal (<12) per Rotterdam criteria, the dash/dot line per Lujan et al. criteria, and the solid line per the cut off calculated in this study (Kenigsberg et al.): polycystic ovary (≥17) versus normal (<17) .
Follicle Count and Distribution
For ovaries in subjects with PCOS, the median FNPS was 12.0 (5.0) compared to 7.5 (5.0) in controls (p<0.001) [Table 1]. The FNPS distribution is shown in Figure 2B. When analyzing for follicle distribution, 62% of ovaries in the PCOS group, compared with 37% in the control group had a peripheral distribution of follicles (p=0.01) [Table 1].
Comparison of Ovarian Morphology in Subjects with PCOS by MRI vs. US
Of the 39 subjects with PCOS, 33 had both MRI and US imaging modalities performed and 66 ovaries were analyzed.
Ovarian Volume
The median OV (IQR) for the 66 ovaries in subjects with PCOS was 11.9 (7.7) cm3 by MRI, compared with 8.8 (7.8) cm3 by US imaging (p=0.05) [Table 2]. The concordance correlation coefficient (rho) in measuring OV by MRI and US imaging in subjects with PCOS was 0.701 (p<0.001). The Bland Altman plot illustrating the agreement between the two imaging modalities suggests moderate differences in ovary volume between tests, although a good agreement is maintained. This plot is presented in Supplemental Figure 1.
TABLE 2.
Ovarian Morphology in Adolescent Subjects with PCOS Measured by MRI vs. US Imaging Modalities
| MRI (N=33) |
US (N=33) |
p -value | |
|---|---|---|---|
| Number Ovaries | 66 | 66 | |
| Median (IQR) OV(cm3) | 11.9 (7.7) | 8.8 (7.8) | 0.05 |
| Mean ±SD OV(cm3) | 14.0 ± 9.1 | 11.0 ± 8.6 | 0.05 |
| % (number) of ovaries with OV >10 cm3 (Rotterdam)a | 65% (43) | 42% (28) | <0.001 |
| % (number) of ovaries with OV >14 cm3 (Kenigsberg et al) | 32% (21) | 24% (16) | NS |
| Median (IQR) FNPS (2–9mm) | 13 (5) | N/A | |
| Mean ± SD, FNPS (2–9mm) | 13.0 ± 4.0 | N/A | |
| % (number) of ovaries with FNPS ≥12 (Rotterdam) | 67% (44) | N/A | |
| % (number) of ovaries with FNPS ≥9 (Lujan et al) | 85% (56) | N/A | |
| % (number) of ovaries with FNPS ≥17 (Kenigsberg et al) | 18% (12) | N/A | |
| % (number) of ovaries with peripheral distribution of follicles | 61% (40) | 44% (28)b | NS |
| % (number) of subjects with at least 1 polycystic ovary based on Rotterdam criteria (OV >10 cm3 or FNPS ≥12) | 91% (30) | 52% (17) | 0.004 |
| % (number) of subjects with at least 1 polycystic ovary based on Lujan et al criteria (OV >10 cm3 or FNPS ≥9) | 94% (31) | 52% (17) | <0.001 |
| % (number) of subjects with at least 1 polycystic ovary based on Kenigsberg et al criteria (OV>14 cm3 or FNPS ≥17) | 64% (21) | 39% (13) | 0.04 |
Based on follicle count from Rotterdam criteria
Unable to evaluate on 3 ovaries thus denominator is 63 not 66
OV = Ovarian Volume; FNPS = Follicle Number Per Section; NS = Not Significant; N/A =cannot be determined. US= ultrasound, MRI =magnetic resonance imaging
Follicle Count and Distribution
Median FNPS was 13 (5.0) by MRI in subjects with PCOS. [Table 2] Poor images precluded the analysis of follicle count and distribution by US imaging in all subjects.
Application of Diagnostic Criteria
As no clear diagnostic criteria are established for ovarian morphology by MRI in adolescents with PCOS, we compared our MRI measurements of OV and FNPS with the Rotterdam criteria (OV >10cm3 and FNPO ≥12), as well as with the newly proposed Lujan et al criteria (OV 10cm3 and FNPS ≥9) (9). In addition, we established MRI cut-offs for characterization of an ovary as polycystic in adolescent subjects with PCOS (Kenigsberg et al.) by defining abnormal as greater than two standard deviations from the mean for our control subjects. We determined the cut-off for polycystic ovarian volume to be greater than 14cm3 (based on a mean for controls of 7.4 ± 3.4 cm3). For FNPS, this cut-off was established as ≥17 (based on a mean for controls of 7.7 ± 4.4). Based on our ROC analysis curve [Supplemental Figure 2], these new cut-offs allow for maximal specificity [Supplemental Table 1]. Images were evaluated both in terms of how many ovaries met criteria as well as how many individual subjects met criteria, as findings in one ovary are sufficient to qualify as a criterion for diagnosis of PCOS (3).
Table 1 shows the application of the 3 criteria listed above to our MRI findings in ovaries from adolescent subjects with and without PCOS. Using an ovarian volume cut-off of greater than 10cm3 as an indication of a polycystic ovary, 65% of ovaries of subjects with PCOS met this criterion; however 23% of ovaries of subjects without PCOS also met the criterion. By increasing the cut-off to 14cm3, only 5% of ovaries from control subjects without PCOS qualify as polycystic, however in subjects known to have PCOS only 33% of ovaries now qualify as polycystic. When evaluating for FNPS meeting the criterion for a polycystic ovary, 85%, 64%, and 17% of ovaries in subjects with PCOS met the criteria of ≥9, ≥12, and ≥17 respectively. Ovaries in control subjects without PCOS have elevated FNPS in 41%, 18%, and 2%, using cut-offs of ≥9, ≥12, and ≥17 respectively, showing the increased specificity but decreased sensitivity of the more stringent criteria.
In our adolescent subjects with PCOS who had both MRI and US performed, ovarian morphology was compared by imaging modality [Table 2]. By MRI, 65% of ovaries had a volume greater than 10cm3, whereas only 42% of these same ovaries were greater than 10cm3 by US (p <0.001). When the volume cut-off for a polycystic ovary was increased to 14cm3, 32% of ovaries met this criterion by MRI and 24% by US; however this difference was not statistically significant. We were unable to determine individual follicle count by US in this study; therefore this feature could not contribute to a determination of whether or not an ovary was polycystic in our subjects.
Within subject analysis was performed. Using Rotterdam guidelines, 91% of subjects with PCOS met polycystic ovary criteria by MRI, having at least one ovary characterized as polycystic, and 52% met criteria by US imaging [Table 2]. Using Lujan et al. study’s cut-offs, 94% of subjects with PCOS met polycystic ovary criteria by MRI, yet only 52% met criteria by US. With our study’s more stringent cut-offs, 64% of subjects with PCOS met polycystic ovary criteria by MRI yet only 39% of the same subjects met criteria by US.
Literature Review
A Pub Med search of ovarian imaging in adolescents with PCOS revealed 54 articles. A total of nine studies (8, 12, 16–22) met the inclusion criteria, in addition to the present study. These studies were compared to the current study. Demographic data and pertinent results for each study are presented in Supplemental Table 2.
DISCUSSION
In the present study we have used MRI, a high precision radiographic modality, to characterize ovarian morphology in a large group of adolescent girls with PCOS, and compared it to morphology in girls without PCOS. Using MRI, we first confirmed that adolescents with PCOS had significantly larger ovarian volume than adolescents without PCOS and a higher number of follicles with a peripheral distribution. In our secondary analysis, we demonstrated that MRI as an important imaging modality that allows for improved characterization of polycystic ovarian morphology when compared to US. We showed that ovarian volumes are smaller when measured by US than when measured with 3-dimensional MRI. In addition, we found that US imaging did not allow for individual follicle counts in our predominantly overweight and obese adolescent girls, in contrast to MRI, in which follicle counts and distribution were easily obtained. Strikingly, in our adolescent subjects with known PCOS, we found that nearly 50% did not meet Rotterdam criteria for polycystic ovaries by US but over 90% had morphology consistent with polycystic ovaries by MRI. Based on our results as well as previous studies in adolescents illustrating the difficulty of obtaining individual follicle counts by US (19), it seems that US is a poor imaging modality for diagnosing polycystic ovaries in the adolescent age group by the current criteria.
Although MRI is more sensitive than US in characterizing polycystic ovaries using Rotterdam criteria, our study raises concern about the appropriateness of using the current diagnostic criteria that were developed based on transvaginal ultrasound imaging of adult women to apply to MRI findings in adolescent girls. For adolescents, imaging is often used as a confirmatory test for PCOS or in an adolescent whose diagnosis is uncertain. Thus we believe that it is important to maximize specificity over sensitivity as we found that, using Rotterdam criteria, nearly one quarter of our control girls without PCOS met criteria for polycystic ovaries. By using the new, more stringent cut-offs that we derived from MRI findings in our age and BMI matched adolescent control subjects without PCOS, an ovarian volume of 14 cm3 and FNPS of 17 maximizes specificity, albeit sacrificing sensitivity. However, future large scale studies are needed to justify these cut-offs.
For adolescents who meet PCOS diagnostic criteria based on clinical and laboratory findings, an evaluation of ovarian morphology may not be required. However for adolescents who require radiologic evaluation due to uncertain diagnosis after a clinical evaluation, this study suggests that once appropriate diagnostic criteria are established for identifying polycystic ovaries in adolescent girls, MRI may be a useful imaging modality. In addition, some sources now advocate the need for adolescents to meet all 3 criteria of hyperandrogenism, polycystic ovaries, and irregular menses in order to be diagnosed with PCOS (23). If these criteria are adapted, the traditionally used modality of US imaging will lead to under-diagnosis of PCOS in a significant number of adolescent girls, thereby precluding appropriate early treatment and prevention of cardiovascular and other morbidities associated with PCOS.
Not only does MRI provide clearer, more precise images which allows for identification of individual ovarian follicles, this methodology is not altered by obesity. In fact, MRI has the added advantage of allowing the clinician to evaluate body composition simultaneously, which may provide additional information to improve clinical care. Furthermore, ovarian volume calculated by MRI is computer generated based on volumes in continuous axial segments, as opposed to volume estimation by US, which relies on an assumption of a standardized ellipse formula.
A review of the literature supported the findings in this study that although ovarian volume may measure differently by MRI versus US, there is a correlation between the two modalities (8), and in all studies OV was significantly larger in adolescents with PCOS compared with adolescents without PCOS, regardless of imaging modality (12, 16, 19, 20, 22) [Supplemental Table 2]. However our study is unique in that the OV is based on true 3-dimensional volume rendering, as opposed to estimated volume derived from 2-dimensional images. The lack of literature on follicle number in adolescents with PCOS emphasizes the difficulty of obtaining this measurement by US. In general, data on ovarian morphology in adolescents with and without PCOS remains inconsistent and scarce, making it difficult to reach a consensus using the currently available literature.
Only two studies have compared MRI and US findings in adolescents with PCOS, including the current study and Yoo et al (8), and findings between these two studies are inconsistent. However, both studies found a significant correlation between OV by MRI and OV by US. Only Yoo et al compared follicle count by different imaging modalities in adolescents with PCOS, finding a significantly larger number of follicles identified on MRI when compared with ultrasound.
Limitations of this study include a relatively small sample size and inability to count follicles by US, preventing a comparison of follicle number between MRI and US. As recommended by Dewailly et al, maximal resolution can be obtained by US if a transducer frequency of ≥8 MHz is used (24) and this may allow for counting of individual follicles. Moreover, we evaluated ovarian morphology in adolescents with known PCOS. Additional studies are needed to confirm our findings in girls who are undergoing evaluation for PCOS but have not yet been diagnosed. Our study did not assess reproducibility, as only one radiologist reviewed the images. Future studies with multiple radiologists will allow investigation of the reproducibility of MRI versus US.
Overall, more research is needed to define MRI characteristics of ovarian morphology in adolescents with PCOS and to establish normal data. However, the current study demonstrated that MRI allows for precise imaging of ovaries and that US, due to underestimation of ovarian volume and inability to assess individual follicle number, may be insufficient to properly characterize polycystic ovaries in a predominately obese adolescent population. Although MRI is more expensive and may require more resources, once normal values and cut-offs are established, MRI will allow for improved detection of ovaries with polycystic features.
Supplementary Material
Acknowledgments
Supported by NIH funding: R01 HL-105212
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have nothing to disclose.
REFERENCES
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Roe AH, Dokras A. The diagnosis of polycystic ovary syndrome in adolescents. Rev Obstet Gynecol. 2011;4:45–51. [PMC free article] [PubMed] [Google Scholar]
- 3.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 4.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 5.Pache TD, Wladimiroff JW, Hop WC, Fauser BC. How to discriminate between normal and polycystic ovaries: transvaginal US study. Radiology. 1992;183:421–423. doi: 10.1148/radiology.183.2.1561343. [DOI] [PubMed] [Google Scholar]
- 6.van Santbrink EJ, Hop WC, Fauser BC. Classification of normogonadotropic infertility: polycystic ovaries diagnosed by ultrasound versus endocrine characteristics of polycystic ovary syndrome. Fertil Steril. 1997;67:452–458. doi: 10.1016/s0015-0282(97)80068-4. [DOI] [PubMed] [Google Scholar]
- 7.Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod. 2003;18:598–603. doi: 10.1093/humrep/deg115. [DOI] [PubMed] [Google Scholar]
- 8.Yoo RY, Sirlin CB, Gottschalk M, Chang RJ. Ovarian imaging by magnetic resonance in obese adolescent girls with polycystic ovary syndrome: a pilot study. Fertil Steril. 2005;84:985–995. doi: 10.1016/j.fertnstert.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 9.Lujan ME, Jarrett BY, Brooks ED, Reines JK, Peppin AK, Muhn N, et al. Updated ultrasound criteria for polycystic ovary syndrome: reliable thresholds for elevated follicle population and ovarian volume. Hum Reprod. 2013;28:1361–1368. doi: 10.1093/humrep/det062. [DOI] [PubMed] [Google Scholar]
- 10.Barber TM, Alvey C, Greenslade T, Gooding M, Barber D, Smith R, et al. Patterns of ovarian morphology in polycystic ovary syndrome: a study utilising magnetic resonance imaging. Eur Radiol. 2010;20:1207–1213. doi: 10.1007/s00330-009-1643-8. [DOI] [PubMed] [Google Scholar]
- 11.Hauth EA, Umutlu L, Libera H, Kimmig R, Forsting M. Magnetic resonance imaging of the pelvis in patients with polycystic ovary syndrome. Rofo. 2009;181:543–548. doi: 10.1055/s-0028-1109179. [DOI] [PubMed] [Google Scholar]
- 12.Brown M, Park AS, Shayya RF, Wolfson T, Su HI, Chang RJ. Ovarian imaging by magnetic resonance in adolescent girls with polycystic ovary syndrome and age-matched controls. J Magn Reson Imaging. 2013;38:689–693. doi: 10.1002/jmri.23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.JK Zawadski AD. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. Boston, Mass: Blackwell Scientific Publications; 1992. pp. 377–384. [Google Scholar]
- 14.Rieder J, Santoro N, Cohen HW, Marantz P, Coupey SM. Body shape and size and insulin resistance as early clinical predictors of hyperandrogenic anovulation in ethnic minority adolescent girls. J Adolesc Health. 2008;43:115–124. doi: 10.1016/j.jadohealth.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 16.Chen Y, Yang D, Li L, Chen X. The role of ovarian volume as a diagnostic criterion for Chinese adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2008;21:347–350. doi: 10.1016/j.jpag.2008.01.081. [DOI] [PubMed] [Google Scholar]
- 17.Dramusic V, Goh VH, Rajan U, Wong YC, Ratnam SS. Clinical, endocrinologic, and ultrasonographic features of polycystic ovary syndrome in Singaporean adolescents. J Pediatr Adolesc Gynecol. 1997;10:125–132. doi: 10.1016/s1083-3188(97)70072-x. [DOI] [PubMed] [Google Scholar]
- 18.Pawelczak M, Kenigsberg L, Milla S, Liu YH, Shah B. Elevated serum anti-Mullerian hormone in adolescents with polycystic ovary syndrome: relationship to ultrasound features. J Pediatr Endocrinol Metab. 2012;25:983–989. doi: 10.1515/jpem-2012-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi B, Sukalich S, Droz J, Griffin A, Cook S, Blumkin A, et al. Prevalence of metabolic syndrome and related characteristics in obese adolescents with and without polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4780–4786. doi: 10.1210/jc.2008-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villa P, Rossodivita A, Sagnella F, Moruzzi MC, Mariano N, Lassandro AP, et al. Ovarian volume and gluco-insulinaemic markers in the diagnosis of PCOS during adolescence. Clin Endocrinol (Oxf) 2013;78:285–290. doi: 10.1111/j.1365-2265.2012.04475.x. [DOI] [PubMed] [Google Scholar]
- 21.Silfen ME, Denburg MR, Manibo AM, Lobo RA, Jaffe R, Ferin M, et al. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab. 2003;88:4682–4688. doi: 10.1210/jc.2003-030617. [DOI] [PubMed] [Google Scholar]
- 22.Youngster M, Ward VL, Blood EA, Barnewolt CE, Emans SJ, Divasta AD. Utility of ultrasound in the diagnosis of polycystic ovary syndrome in adolescents. Fertil Steril. 2014;102:1432–1438. doi: 10.1016/j.fertnstert.2014.07.1241. [DOI] [PubMed] [Google Scholar]
- 23.Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol. 2010;203:201 e1–201 e5. doi: 10.1016/j.ajog.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Dewailly D, Lujan ME, Carmina E, Cedars MI, Laven J, Norman RJ, et al. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Human reproduction update. 2014;20:334–352. doi: 10.1093/humupd/dmt061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.