Abstract
The pathophysiological processes underlying Alzheimer’s disease (AD) are hypothesized to begin years to decades before clinical symptom onset, while individuals are still cognitively normal. Although many studies have examined the effect of biomarkers of amyloid pathology on measures of cognitive performance, less is known about the effect of tau pathology on cognitive performance. The present study examined the association between cerebrospinal fluid (CSF) biomarkers of AD pathology (amyloid, total tau (t-tau), and phosphorylated tau (p-tau)) and cognition in a large sample of cognitively normal middle-aged and older adults. Associations were examined with multivariate regressions, in which either amyloid and t-tau or amyloid and p-tau were included as simultaneous predictors of cognitive performance. Cognitive performance was measured with three composite scores assessing working memory, verbal episodic memory, and visuospatial episodic memory. In their respective models, CSF measures of both t-tau and p-tau were associated with the visuospatial episodic memory composite score (p < .001 and p = .02, respectively), but not with the other measures of cognition. In contrast, CSF amyloid was not significantly associated with cognitive performance, raising the possibility that measures of tau pathology have a more direct relationship with cognition in cognitively normal individuals. These results also suggest that tau pathology may have effects on visuospatial episodic memory during preclinical AD that precede alterations in other cognitive domains.
Keywords: preclinical Alzheimer’s disease, cerebrospinal fluid, amyloid, tau, cognition, biomarkers
1. Introduction
Several lines of evidence suggest there is a preclinical phase of Alzheimer’s disease (AD) during which AD pathology is accumulating (i.e., amyloid plaques and tau neurofibrillary tangles), in the absence of clinical symptoms (Sperling et al., 2011). These pathophysiological processes are thought to begin years to decades before the onset of clinical symptoms of AD, when individuals are still cognitively normal. This conclusion is primarily based on evidence that a subset of older individuals who are cognitively normal have AD pathology in their brains, based on both autopsy findings (Bennett et al., 2006; Hulette et al., 1998; Knopman et al., 2003) and amyloid imaging studies (Morris et al., 2010; Rowe et al., 2010; Reiman et al., 2009).
Moreover, recent studies suggest that cognitively normal individuals with biomarker evidence of AD pathology are at increased risk for developing cognitive decline over time. For example, cerebrospinal fluid (CSF) biomarkers of AD pathology (e.g., decreased levels of amyloid-beta (Aβ1-42) and increased levels of total tau (t-tau) and phosphorylated tau (p-tau)) are associated with increased amyloid plaque burden and neurofibrillary tangle load at autopsy (Strozyk, Blennow, White, & Launer, 2003; Tapiola et al., 2009). Measured in cognitively normal individuals, these biomarkers are associated with increased risk for the development of clinical symptoms of AD (Fagan et al., 2007; Li et al., 2007; Roe et al., 2013; Moghekar et al., 2013). Cognitively normal individuals who subsequently develop clinical symptoms of AD also tend to perform more poorly on cognitive tests prior to symptom onset than individuals who remain cognitively normal (Albert et al., 2014; Howieson et al., 2008; for a discussion, see Sperling et al., 2011). This likely reflects the negative effect of AD pathology on cognition among those who subsequently progress, suggesting there should be a relationship between cognitive test performance and biomarker measures of AD pathology. While a number of previous studies have supported this hypothesis, and reported lower cross-sectional cognitive performance among cognitively normal individuals with higher biomarker levels of amyloid and tau pathology, findings are unusually mixed.
Most prior studies on this topic have evaluated biomarkers of amyloid pathology, measured either through CSF or positron emission tomography (PET). Several studies have reported cross-sectional associations between amyloid levels and episodic memory in cognitively normal older adults (e.g., using CSF amyloid: Stomrud et al., 2010; using amyloid imaging: Hedden et al., 2012; Kantarci et al., 2012; Pike et al., 2007, 2011; Rentz et al., 2011; Sperling et al., 2013; Villemagne et al., 2011), though others have not found these associations (e.g., using CSF amyloid: Li et al., 2014; Glodzik et al., 2011; Rami et al., 2011; Rolstad et al., 2011; Schott et al., 2010; Vemuri et al., 2011; using amyloid imaging: Aizenstein et al., 2008; Rodrigue et al., 2012; Rowe et al., 2010; Storandt et al., 2009). Additionally, while some studies have reported cross-sectional associations between amyloid levels and other domains of cognition, such as working memory, processing speed, and language (using CSF amyloid: Rolstad et al., 2011; Stomrud et al., 2010; using amyloid imaging: Kantarci et al., 2012; Rodrigue et al., 2012), findings from other groups have been negative (e.g., using CSF amyloid: Li et al., 2014; Rami et al., 2011; Sperling et al., 2013; Vemuri et al., 2011; using amyloid imaging: Aizenstein et al., 2008; Hedden et al., 2012; Pike et al., 2007, 2011; Rentz et al., 2011; Storandt et al., 2009). Despite these inconsistencies, a recent meta-analysis found small, but non-trivial, associations between biomarkers of amyloid pathology and cognition in cognitively normal older adults (Hedden et al., 2013).
Fewer studies have examined the relationship between biomarkers of tau pathology and cognition in cognitively normal adults, as the collection of cerebrospinal fluid biomarkers involves an invasive procedure and tau PET imaging has only recently become available. With one exception, the CSF studies have failed to find cross-sectional associations between biomarkers of tau pathology and cognitive performance (Glodzik et al., 2011; Rami et al., 2011; Rolstad et al., 2011; Stomrud et al., 2010; Vemuri et al., 2011). As the exception, Schott et al. (2010) reported an association between CSF t-tau and p-tau and performance on an individual task measuring executive function.
The variability of findings in these previous studies may be the result of several factors. First, the groups of cognitively normal individuals studied may have varied in the proportion of individuals who were destined to develop clinical symptoms over time, therefore varying in the amount of AD pathology present; this is a particular problem in studies with modest sample sizes. It is possible, therefore, that studies not finding associations between cognition and biomarkers of amyloid or tau pathology consisted of fewer individuals in the preclinical phase of AD, or with less advanced pathology. Second, differences in the genetic composition of the groups studied may also have contributed to variability of prior findings. For example, Kantarci et al. (2012) found that amyloid-cognition associations were stronger in ε4 allele carriers of the apolipoprotein E (APOE) gene (relative to non-carriers), a well-known genetic risk factor for AD (Farrer et al., 1997) that is associated with increased amyloid accumulation (e.g., Reiman et al., 2009; for a review, see Kim, Basak, & Holtzman, 2009). However, with a few exceptions (e.g., Kantarci et al., 2012; Li et al., 2014; Pike et al., 2011), previous studies have generally not included APOE carrier status in their analyses. Third, the cognitive measures have varied among prior studies, consisting of either individual cognitive scores or cognitive composite scores. Prior evidence suggests that cognitive composite scores may be more sensitive measures of cognition because they reduce type 1 error, variability attributable to idiosyncratic task demands, measurement error, or other sources of error (Gross et al., 2014; Nunnally, 1978). It is noteworthy that many of the studies that found significant amyloid-cognition associations used cognitive composite scores (e.g., Hedden et al., 2012; Kantarci et al., 2012; Pike et al., 2007, 2011; Rentz et al., 2011; Rodrigue et al., 2012; Rolstad et al., 2011; Villemagne et al., 2011), as opposed to individual task scores.
In addition, previous studies left unresolved the degree to which amyloid-cognition associations are independent of the effects of t-tau or p-tau pathology, as the effects of amyloid and tau biomarkers have rarely been examined together (see Li et al., 2014, as an exception). It is possible, for example, that in studies finding associations between amyloid burden and cognition, those individuals with the highest levels of amyloid burden also had high levels of tau biomarkers. The associations reported between amyloid and cognition, therefore, may reflect concomitant associations with tau pathology. Lastly, most previous studies have consisted of cognitively normal individuals in their mid-70s and 80s when first examined. Since evidence suggests that older individuals are more likely to have concomitant pathologies (Petersen et al., 2006; Schneider et al., 2009; Sonnen et al., 2007), it is possible that examination of a younger cohort will reveal associations obscured by the complexity of pathologies more common in older individuals.
The goal of the present study was to address some of the questions left open by previous reports, utilizing data from a large sample (N ≈ 200) of prospectively followed, middle-aged and older adults (mean age at baseline = 57 years), with both AD biomarker data and cognitive test scores, who have been followed for up to 19 years. These data allow us to test the hypothesis that higher baseline levels of AD pathology (as measured by CSF levels of Aβ1-42, t-tau, and p-tau) are associated with lower baseline cognitive performance (measured by composite test scores) among cognitively normal individuals. Importantly, we examined whether CSF amyloid, tau and p-tau levels confer independent effects on cognition, as would be predicted if AD pathology accumulates years prior to symptom onset. Additionally, our analyses examined whether associations between CSF measures of AD pathology and cognition are modified by APOE-4 genetic risk.
2. Materials and Methods
2.1. Participants and study design
The present study consists of individuals from the BIOCARD study, a prospectively followed cohort of 349 individuals. This study was designed to recruit and follow a cohort of cognitively normal individuals who were primarily middle aged at baseline (M = 57.2, SD = 10.3, range = 20-85). By design, approximately 75% of the cohort had a first degree relative with dementia of the Alzheimer’s type. The overall goal of the BIOCARD study was to identify variables among cognitively normal individuals that predict the subsequent development of mild to moderate symptoms of Alzheimer’s disease. This study was initiated in 1995 at the NIH, with recruitment occurring by the staff of the Geriatric Psychiatry Branch of the intramural program of the National Institute of Mental Health. Various sources were used for recruitment, including printed advertisements, informational lectures, articles in local or national media, and word-of-mouth. Individuals were excluded from participation if they were cognitively impaired, as determined by cognitive testing, or had significant medical problems such as severe cardiovascular disease, epilepsy, or drug or alcohol abuse. Participants were enrolled over time, beginning in 1995 and ending in 2005; all participants provided informed consent.
At baseline, participants completed a comprehensive evaluation that included a physical and neurological exam, an electrocardiogram, standard laboratory studies, neuropsychological testing, magnetic resonance imaging (MRI) scans, CSF from lumbar puncture, and blood specimens. APOE genotyping was established on all but one participant after enrollment. In 2005, this study was stopped for administrative reasons. In 2009, a research team from Johns Hopkins School of Medicine was funded to re-establish the cohort and continue annual clinical and cognitive assessments, collect blood, and evaluate previously acquired MRI scans, CSF, and blood specimens.
Details of this consensus diagnosis process have been described elsewhere (Albert et al., 2014); briefly, the diagnostic process can be summarized as follows: (1) clinical data were examined pertaining to the medical, neurologic and psychiatric status of the subject, (2) reports of changes in cognition by the subject and by collateral sources were examined, and (3) decline in cognitive performance was established on the basis of neuropsychological testing. Subjects received consensus diagnoses by the staff of the BIOCARD Clinical Core for each annual assessment, including those conducted at the NIH. For individuals with evidence of cognitive impairment, the age at which the clinical symptoms began was estimated (for details, see Albert et al., 2014).
Subjects included in the present study were cognitively normal at baseline, based on the consensus diagnosis procedures described above, and had appropriate cognitive, CSF and genetic data available, as outlined below. Of the 349 individuals in the BIOCARD cohort, data from 47 subjects were not considered for analysis (n = 33 have not yet re-enrolled in or withdrawn from the study and n = 14 had clinical symptom onset at or before baseline, as per their consensus diagnosis).
Of the 302 individuals who were cognitively normal at their baseline visit and have re-enrolled in the study (M follow-up = 11.8 years, SD = 3.9, range = 0 - 19 years) (Table 1), 62 have developed mild to moderate clinical symptoms of AD on follow-up, resulting in a diagnosis of either Mild Cognitive Impairment (MCI) or dementia due to AD (Albert et al., 2011; McKhann et al., 2011) (described here as ‘progressors’). Of the 240 individuals who have remained cognitively normal as of their last available consensus diagnosis (‘non-progressors’), a subset were excluded from the follow-up analyses (see below) due to the fact that some had no additional follow-up data since their last NIH visit (n = 28) and some had a diagnosis of Impaired not MCI (n = 35) (i.e., they had evidence of cognitive change as indicated by either self and/or informant reported complaints of worsening cognition OR slight changes on longitudinal neuropsychological testing, but not both) (Albert et al., 2011; Petersen et al., 2004). All living subjects included in the present study provided informed consent in accordance with the IRB at the Johns Hopkins University School of Medicine.
Table 1.
Baseline demographic and descriptive statistics for all subjects and by follow-up diagnosis. All means (standard deviations) are from raw data (i.e., not z-scored).
| All cognitively | ||||||
|---|---|---|---|---|---|---|
| normal subjects | Non-progressors | Progressors | ||||
| N (maximum) | N | 302 | n | 240 | n | 62 |
|
| ||||||
| Demographics | ||||||
|
| ||||||
| Age (years) | 302 | 56.6 (10.2) | 240 | 55.0 (9.5) | 62 | 62.6 (10.7)* |
| Education (years) | 302 | 17.0 (2.4) | 240 | 17.1 (2.3) | 62 | 16.6 (2.5) |
| Gender (% female) | 302 | 60% | 240 | 62% | 62 | 52% |
| MMSE # | 297 | 29.5 (0.8) | 237 | 29.6 (0.8) | 60 | 29.4 (1.0) |
| APOE-4 carriers (% total) ^ | 293 | 32% | 234 | 31% | 59 | 36% |
|
| ||||||
| Working memory tasks (with data on all 3 tasks) | ||||||
|
| ||||||
| Backwards Digit Span | 269 | 7.7 (2.3) | 212 | 8.0 (2.2) | 57 | 6.7 (2.2) * |
| Digit-symbol Substitution | 269 | 53.0 (11.9) | 212 | 55.1 (11.8) | 57 | 45.4 (8.6)* |
| Supermarket Fluency | 269 | 30.4 (7.3) | 212 | 31.2 (7.3) | 57 | 27.4 (6.8)* |
|
| ||||||
| Verbal episodic memory tasks (with data on all 3 tasks) | ||||||
|
| ||||||
| Logical Memory (immediate) | 290 | 14.8 (2.9) | 230 | 15.1 (2.9) | 60 | 13.7 (2.7)* |
| Logical Memory (delayed) | 290 | 12.9 (3.4) | 230 | 13.2 (3.4) | 60 | 11.7 (3.2)* |
| Paired Associates (immediate) | 290 | 20.5 (3.0) | 230 | 20.9 (2.9) | 60 | 19.2 (3.1)* |
|
| ||||||
| Visuospatial episodic memory tasks (with data on all 3 tasks) | ||||||
|
| ||||||
| Rey Recall | 284 | 18.2 (6.5) | 225 | 19.0 (6.3) | 59 | 15.1 (6.5)* |
| Figural Memory | 284 | 7.2 (1.4) | 225 | 7.4 (1.4) | 59 | 6.6 (1.4)* |
| Visual Reproduction (delayed) | 284 | 28.8 (7.1) | 225 | 29.7 (6.9) | 59 | 25.6 (7.0)* |
|
| ||||||
| Weighted composite scores | ||||||
|
| ||||||
| Working memory | 269 | 0.01 (0.99) | 212 | 0.21 (0.94) | 57 | −0.73 (0.80) * |
| Verbal episodic | 290 | 0.01 (1.4) | 230 | 0.18 (1.34) | 60 | −0.66 (1.35)* |
| Visuospatial episodic | 284 | −0.01 (1.39) | 225 | 0.20 (1.35) | 59 | −0.83 (1.23)* |
|
| ||||||
| CSF biomarkers (pg/ml) | ||||||
|
| ||||||
| AB1-42 | 225 | 400.8 (97.3) | 180 | 407.8 (92.7) | 45 | 372.9 (110.6) |
| Tau | 225 | 69.1 (31.1) | 180 | 65.2 (26.8) | 45 | 85.1 (40.9)* |
| P-tau | 225 | 35.8 (16.1) | 180 | 33.9 (13.6) | 45 | 43.4 (22.1)* |
MMSE = Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975).
Excludes n = 1 with no APOE genotyping and n = 8 with one ε2 and one ε4 allele.
Significant difference between non-progressors and progressors by univariate ANOVA for continuous variables or chi-square tests for dichotomous variables, p < .05. Cognition and CSF group comparisons included age as a covariate.
2.2. Neuropsychological tasks composing cognitive composite scores
Data from baseline cognitive tests were used to create three summary factor scores, referred to here as cognitive ‘composite scores’: working memory, verbal episodic memory, and visuospatial episodic memory. These domains were selected because they are hypothesized to be affected early in the course of AD. We selected nine tasks that were (a) hypothesized to load on these three cognitive constructs and (b) had data available from at least 250 subjects. Working memory/executive function was measured with the backwards digit span from the Wechsler Memory Scale-Revised (WMS-R) (n = 294; Wechsler, 1987), category fluency (number of supermarket items generated in 60 seconds; n = 278; Mattis, 1976), and digit-symbol substitution of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) (n = 289; Wechsler, 1981). Verbal episodic memory was measured with logical memory immediate recall (n = 292), logical memory delayed recall (n = 292), and paired associates immediate recall (n = 290) subtests of the WMS-R (Wechsler, 1987). Visuospatial episodic memory was measured with recall of the Rey-Osterreith Complex Figure (n = 298; Rey, 1941), the WMS-R figural memory subtest (n = 287; Wechsler, 1987), which assesses recognition memory for unfamiliar figures, and the WMS-R delayed visual reproduction subtest (n = 288; Wechsler, 1987), which assesses the accuracy of reproduction of unfamiliar figures.
2.3. Application of confirmatory factor analysis for composite scores
We used confirmatory factor analysis (CFA), a type of latent variable modeling, to (a) confirm that the nine neuropsychological tasks used to create the composite scores loaded on their hypothesized cognitive constructs and (b) establish task weights for creating composite scores (described below). Error variance of the immediate and delayed versions of the logical memory task were allowed to correlate given these variables reflect two measures from the same task. Model fit was evaluated with the chi-square goodness-of-fit statistic to assess the discrepancy between the sample and fitted covariance matrices (Hu & Bentler, 1998, p. 426); for this index, small, non-significant values indicate good fit. Model fit was also evaluated with Bentler’s comparative fit index (CFI), the root mean square error of approximation (RMSEA), and the standardized root-mean-square residual (SRMR). CFI is an incremental fit index that ranges from 0-1 and compares the fitted model to a restricted baseline model; values >.95 indicate good fit (Blunch, 2008). Both RMSEA and SRMR are absolute-fit indices based on residuals. For both, lower values (< .05) indicate good fit, and RMSEA should also be accompanied by a non-significant p-value. CFA models were estimated with the lavaan (latent variable analysis) package (Rosseel, 2012) in R.
The hypothesized three-factor model was compared to the nested two- and one-factor models to determine whether the nested models provided a more plausible fit to the data. Nested models were compared by the change in chi-square across models. The fuller, more complex model was accepted as having better fit if the change in chi-square was significant given the loss of degrees of freedom. The CFA analyses included all cognitively normal individuals who had data on the 9 tasks, regardless of whether they had CSF data (n = 262).
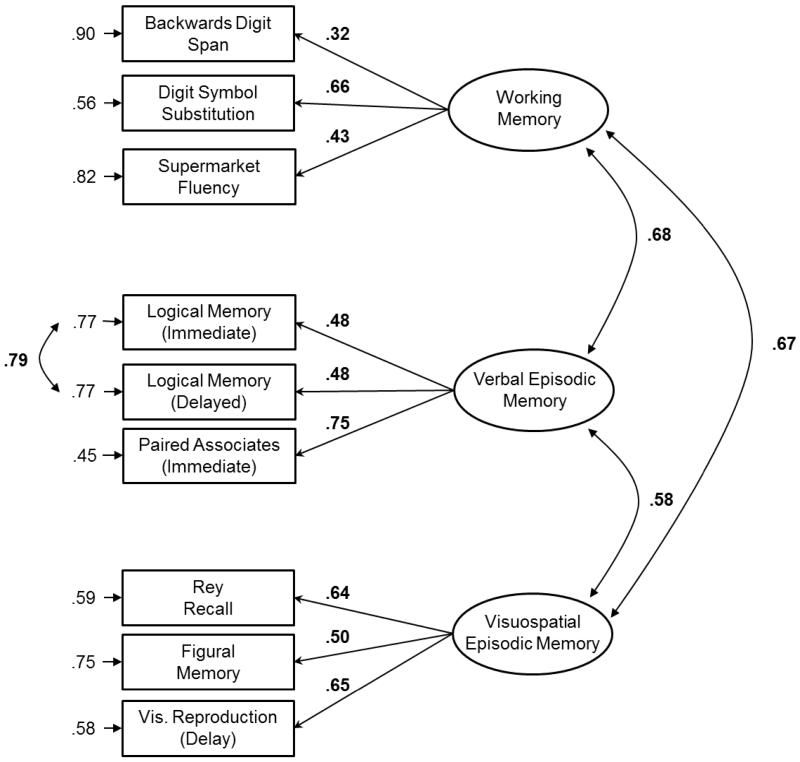
The factor loadings from the final CFA model were also used to create composite scores in which individual z-scored task scores were weighted by their standardized factor loadings (Figure 1). The weighted task scores within each cognitive domain were then summed to create the composite score for that domain. Composite scores were created for all individuals who had scores on the three tasks within an individual cognitive domain (even if they did not have data for all nine tasks), to ensure we had as much power as possible in the analyses.
Figure 1.
Three-factor cognitive model. Numbers next to curved arrows are correlations. Numbers above left-pointing arrows are standardized factor loadings. Numbers adjacent to right-pointing arrows are residual error variances. Significant values are indicated in bold.
2.4. CSF assessments
CSF measures were available for 225 participants who underwent lumbar puncture within 150 days of their baseline cognitive testing (M = 5.3 days between CSF draw and cognitive testing, SD = 17.8). CSF specimens were analyzed by the current group of investigators using the Alzheimer’s Disease Neuroimaging Initiative protocol. As reported in Moghekar et al. (2013), this protocol used the xMAP-based AlzBio3 kit (Innogenetics, Ghent, Belgium) run on the Bioplex 200 system. The kit contains monoclonal antibodies specific for Aβ1-42 (4D7A3), t-tau (AT120), and p-tau181p (AT270), each chemically bonded to unique sets of color-coded beads, and analyte-specific detector antibodies (HT7 and 3D6). Calibration curves were produced for each biomarker using aqueous buffered solutions that contained the combination of 3 biomarkers at concentrations ranging from 25 to 1555 pg/mL for recombinant tau, 54–1,799 pg/mL for synthetic Aβ1–42 peptide, and 15–258 pg/mL for a tau synthetic peptide phosphorylated at the threonine 181 position (i.e., the p-tau181p standard). All samples for each participant were analyzed on the same plate and run in triplicate. See Moghekar et al. (2012) for additional details regarding these procedures. Although p-tau is considered a more direct measure of AD pathology (i.e., neurofibrillary tangles), t-tau has commonly been used as a biomarker of neuronal injury (e.g., Rolstad et al., 2011; Stomrud et al., 2010; Vemuri et al., 2009, 2011). Given the role of tau pathology in preclinical AD is not well understood, both t-tau and p-tau were included in the present study.
2.5. APOE genetic status
APOE genotyping was determined by restriction endonuclease digestion of polymerase chain reaction amplified genomic DNA (Hixson & Vernier, 1990) (performed by Athena Diagnostics, Worcester, MA) and was unavailable for only 1 participant. The regression analyses described below excluded 8 individuals with an ε2/ε4 genotype, given the ε4 allele increases AD dementia risk (Corder et al., 1994), whereas the ε2 allele decreases AD dementia risk (Farrer et al., 1997). Of the 293 participants with eligible genotyping, 12.6% (n = 37) had at least one ε2 allele, 55.6% (n = 163) had two ε3 alleles, and 31.7% (n = 93) had at least one ε4 allele. Of those with at least one ε4 allele, 16.1% (n = 15) had two ε4 alleles. For all analyses, APOE-4 carrier status was denoted by an indicator variable coding the number of ε4 alleles (0, 1, 2), referred to below as APOE load.
2.6. Statistical analyses
Associations between baseline cognitive composite scores and baseline CSF measures were examined with multivariate linear regression. For each composite score, we ran two sets of linear regression models: the first set examined the association of Aβ1-42 and t-tau with cognition, and the second set examined the association of Aβ1-42 and p-tau with cognition. Aβ1-42 and t-tau (and similarly, Aβ1-42 and p-tau) values were simultaneously entered into the models to examine each variable’s association with cognition, independent of the other. For all models, cognitive composite scores served as the dependent variable, with CSF measures, age, years of education, gender (1 = male), APOE load, and the CSF measure by APOE load interactions (product) included as independent variables. CSF values by APOE interactions were included to determine whether the effect of a biomarker on cognition differed by APOE-4 carrier status. Given amyloid and tau pathology increase with age, the age variable was residually centered such that it reflected age orthogonalized for the CSF variables included in that model (i.e., the standardized residuals of regressing age on CSF Aβ1-42 and t-tau OR age on CSF Aβ1-42 and p-tau; Geldhof, Pornprasertmanit, Schoemann, & Little, 2013). All other continuous independent variables were standardized. Non-significant interaction terms were removed from the models and models were re-run without these terms. Analyses were corrected for multiple comparisons using false discovery rate (FDR) method with a q value of 0.05 (Benjamini & Hochberg, 1995). The number of subjects included in each regression analysis is shown in Table 4.
Table 4.
Regression results examining the association between CSF biomarkers (amyloid and t-tau; amyloid and p-tau) and cognitive composite scores (working memory, verbal episodic memory, visuospatial episodic memory). Significant values are indicated in bold and p-values are corrected for multiple comparisons.
| Set I: Relationship of CSF amyloid and t-tau with cognition | ||||
| Outcome: Working memory (n = 193) | ||||
| B | S.E. B | Beta | p | |
| CSF amyloid |
.08 | .07 | .09 | .31 |
| CSF t-tau | −.07 | .06 | −.08 | .27 |
| Outcome: Verbal Episodic Memory (n = 213) | ||||
| B | S.E. B | Beta | p | |
| CSF amyloid |
.15 | .10 | .10 | .37 |
| CSF t-tau | −.13 | .09 | −.09 | .24 |
| Outcome: Visuospatial Episodic Memory (n = 208) | ||||
| B | S.E. B | Beta | p | |
| CSF amyloid |
.20 | .09 | .14 | .17 |
| CSF t-tau | −.37 | .09 | −.26 | < .001 |
| Set II: Relationship of CSF amyloid and p-tau with cognition | ||||
| Outcome: Working memory (n = 193) | ||||
| B | S.E. B | Beta | p | |
| CSF amyloid |
.07 | .07 | .08 | .29 |
| CSF p-tau | −.05 | .07 | −.05 | .48 |
| Outcome: Verbal Episodic Memory (n = 213) | ||||
| B | S.E. B | Beta | p | |
| CSF amyloid |
.12 | .10 | .09 | .25 |
| CSF p-tau | −.10 | .10 | −.07 | .42 |
| Outcome: Visuospatial Episodic Memory (n = 208) | ||||
| B | S.E. B | Beta | p | |
| CSF amyloid |
.14 | .10 | .10 | .26 |
| CSF p-tau | −.24 | .09 | −.17 | .02 |
S.E. = standard error
3. Results
Baseline demographic and descriptive statistics are shown in Table 1, divided into three groups: (1) all cognitively normal subjects with baseline cognitive and CSF data, meeting the criteria outlined above, (2) the subset of individuals who remained cognitively normal over time (i.e., non-progressors, as defined above), and (3) those who were cognitively normal at baseline but have since progressed to clinical symptoms of MCI or dementia due to AD. Though all individuals were cognitively normal at baseline, those who have since progressed to clinical symptoms of MCI or AD dementia tended to be slightly older, have worse performance on the cognitive testing, and more abnormal CSF levels of AD pathology, including significantly higher levels of CSF t-tau and p-tau and numerically lower levels of CSF Aβ1-42 (see Table 1).
3.1. Evaluation of cognitive composite scores
Each z-scored cognitive variable was examined for distributional normality; all measures had skew and kurtosis values acceptable for psychometric purposes (largest skew = |0.89|; largest kurtosis = |0.73|). Correlations among cognitive tasks are shown in Table 2.
Table 2.
Pairwise correlations among standardized cognitive task scores (n = 262).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Backwards Digit Span | - | .18 ** | .05 | .15 * | .12 | .24 ** | .14 * | .17 ** | .19 ** |
| 2. Digit-symbol Substitution | - | .33 ** | .17 * | .18 ** | .32 ** | .25 ** | .23 ** | .34 ** | |
| 3. Supermarket Fluency | - | .11 | .07 | .26 ** | .13 * | .13 * | .14 * | ||
| 4. Logical Memory (immediate) | - | .84 ** | .35 ** | .23 ** | .21 ** | .17 * | |||
| 5. Logical Memory (delayed) | - | .36 ** | .23 ** | .19 ** | .22 ** | ||||
| 6. Paired Associates (immediate) | - | .22 ** | .26 ** | .27 ** | |||||
| 7. Rey Recall | - | .35 ** | .44 ** | ||||||
| 8. Figural Memory | - | .27 ** | |||||||
| 9. Visual Reproduction (delayed) | - |
p < .05,
p ≤ .005
We first tested the fit of the hypothesized three-factor model that consisted of three distinct cognitive domains: working memory, verbal episodic memory, and visuospatial episodic memory. This model (Model 1) provided a good fit to the data (χ2(23) = 29.09, p = .18; CFI = 0.989; RMSEA = 0.03, p = .80; SRMR = 0.04). The fit of Model 1 was compared to the two- and one-factor nested models (Models 2-5 in the Appendix; see Table A.1). Although these nested models tended to fit the data well, all provided a worse fit to the data than Model 1, as indicated by a significant change in chi-square (Table A.1, right). The three-factor model was therefore accepted as providing the best fit to the data (Figure 1).
3.2. Relationship between cognitive composite scores and CSF biomarkers
Correlations among demographic characteristics, CSF values, and cognitive composite scores are shown in Table 3. The results of the regression analyses are shown in Table 4. These results exclude the CSF biomarker by APOE interaction terms, as all interactions were non-significant (data not shown; all p’s > .29); these non-significant interactions suggest that CSF-cognition associations do not vary by APOE-4 allele carrier status.
Table 3.
Correlations among demographic characteristics, CSF measures, and cognitive composite scores (n = 194).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Age | - | .11 | −.17 * | .30 ** | .16 * | −.27 ** | −.15 * | −.34 ** |
| 2. Education | - | .07 | −.04 | −.03 | .17 * | .12 | .04 | |
| 3. CSF amyloid | - | −.004 | −.27 ** | .11 | .12 | .12 | ||
| 4. CSF tau | - | .67 ** | −.04 | −.04 | −.22 ** | |||
| 5. CSF p-tau | - | −.08 | −.09 | −.19 * | ||||
| 6. Working memory composite | - | .36 ** | .39 ** | |||||
| 7. Verbal episodic memory composite | - | .40 ** | ||||||
| 8. Visuospatial episodic memory composite | - |
p < .05,
p ≤ .005
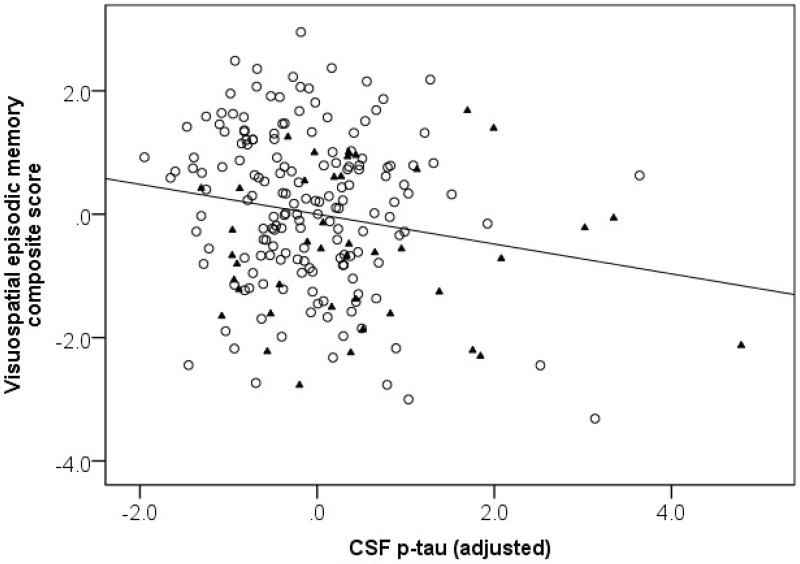
For the CSF biomarker-cognition associations, the first set of models included both CSF Aβ1-42 and t-tau. In these models, t-tau, but not Aβ1-42, was significantly associated with the composite score for visuospatial episodic memory (p < .001). In contrast, neither CSF t-tau nor Aβ1-42 was associated with the working memory or verbal episodic memory composite scores. The second set of models included both CSF Aβ1-42 and p-tau. In these models, p-tau was significantly associated with the composite score for visuospatial episodic memory (p = .02; Figure 2), though CSF Aβ1-42 was not. Again, neither CSF p-tau nor Aβ1-42 were associated with the composite scores for working memory or verbal episodic memory. The negative regression weights for both t-tau and p-tau indicate that individuals with higher CSF levels of tau pathology performed worse on measures of visuospatial episodic memory, independent of CSF levels of amyloid pathology.
Figure 2.
Partial correlation between the visuospatial episodic memory composite score and CSF p-tau in all subjects, adjusted for covariates. For visualization purposes, non-progressors are depicted by open circles and progressors by filled triangles.
Of the covariates, it is notable that years of education was positively associated with the composite scores of working memory and verbal episodic memory (βs = .24, ps < .005 and βs = .18, ps < .03, respectively), but not visuospatial episodic memory (βs < .01, ps > .90). Similarly, gender was negatively associated with the composites scores of working memory and verbal episodic memory (βs ≈ −.21, ps = .004 andβs = −.22, ps < .007, respectively) with females outperforming males, but gender was not associated with the composite score of visuospatial episodic memory (βs = −.01, ps > .85). The APOE indicator variable was not associated with any of the cognitive composite scores, suggesting little difference in cognitive performance between APOE-4 carriers and non-carriers (all βs < |.08|).
Since the participants in this study have been followed for many years, consensus diagnoses are now available regarding their current status. We were therefore able to conduct follow-up analyses on the significant visuospatial findings described above. We examined the main effects of CSF levels on cognition in the subset of individuals who have demonstrated no evidence of cognitive decline to date. The goal of this follow-up analysis was to determine whether associations between baseline CSF levels and visuospatial episodic memory were present in the subset of individuals who have remained cognitively normal over time and were therefore unlikely to have AD pathology at baseline (n = 127; M follow-up time = 12.5 years, SD = 2.9, range = 8 - 19 years). The absence of an association in this subgroup would support the hypothesis that the significant associations in the full sample reflect variability contributed by the preclinical AD group. These follow-up analyses excluded individuals with no follow-up since their last NIH visit (as we do not have a current clinical diagnosis for them) and individuals with a current diagnosis of ‘Impaired not MCI’. Age residuals were re-calculated to reflect the subset of individuals included. There were no significant main effects of CSF amyloid, CSF t-tau, or CSF p-tau on the visuospatial episodic memory composite score. As an additional follow-up, we tested whether the relationship between CSF and visuospatial episodic memory differed in those who have remained cognitively normal to date (as just described) relative to those who have since progressed. To do so, we re-ran our original models (described in Section 2.6), including interaction terms for CSF biomarker by follow-up diagnosis status (dichotomous: progressor vs. non-progressor) in place of the CSF biomarker by APOE interaction terms. There were no significant CSF biomarker by follow-up diagnosis interactions in either model (all ps > .30).
4. Conclusions
The present study examined the association between cerebrospinal fluid measures of amyloid and tau pathology and cognitive test scores in a large cohort of cognitively normal, middle-aged and older adults. Cognition was assessed by performance on three composite scores measuring working memory/executive function, verbal episodic memory, and visuospatial episodic memory. Our analyses included both CSF amyloid and CSF t-tau (first set of models), and CSF amyloid and CSF p-tau (second set of models), as simultaneous predictors of cognitive performance to determine whether the two sets of CSF measures incurred independent associations with cognition.
We found significant associations between CSF t-tau and CSF p-tau levels and visuospatial episodic memory, such that individuals with higher biomarker levels of tau pathology performed worse on the visuospatial episodic memory tests. In contrast, CSF Aβ1-42 was not significantly associated with cognition. These findings suggest that CSF levels of tau pathology have a more direct association with cognition than levels of amyloid, and raise the possibility that previously reported relationships between amyloid and cognition may be due to concomitant tau pathology. CSF biomarkers were not associated with measures of working or verbal episodic memory. Additionally, these associations did not vary as a function of APOE-4 carrier status.
These results extend previous work in three key ways. First, we examined CSF biomarker and cognition relationships in a longitudinally followed cohort of cognitively normal individuals, and included biomarkers of both amyloid and tau pathology as simultaneous predictors of cognitive performance. Second, this approach, in combination with our large sample size, allowed us to examine the effect of ApoE-4 carrier status in a more definitive manner. Third, we used composite scores to assess cognition, which may have provided more precise estimates of cognitive performance. In particular, the inclusion of a visuospatial episodic memory composite score permitted us to examine relationships with this cognitive domain more directly than has previously been done, either because such measures were not available in earlier studies or were included in composites not specific to memory.
Only one previous study to our knowledge has assessed whether associations between biomarkers of amyloid pathology were independent of biomarkers of tau pathology (Li et al., 2014), likely due to the fact that imaging studies have lacked measures of tau pathology until recently. In a lifespan sample of cognitively normal adults, Li and colleagues (2014) found no associations between biomarkers of tau pathology and cognition, though measures of visuospatial episodic memory were not included. Although a number of previous studies have found an association between biomarkers of amyloid pathology and episodic memory (e.g., Hedden et al., 2012; Kantarci et al., 2012; Pike et al., 2007; Villemagne et al., 2011), we found no significant amyloid-cognition associations with t-tau or p-tau in the regression models. We cannot rule out the possibility that the observed amyloid-cognition effect sizes, which are in the same range as previous observations (e.g., Hedden et al., 2013), may have been significant with a larger sample. However, our findings suggest biomarkers sensitive to alterations in tau pathology may be a primary influence on individual differences in cognition during preclinical AD. In line with this, a recent study found that [11C] Pittsburgh compound B (PiB) PET measures of amyloid are more strongly correlated with the ratio of CSF tau/Aβ1-42 (or p-tau/Aβ1-42), rather than CSF amyloid alone (Roe et al., 2013), also raising the possibility that alterations in tau levels contributed to previously reported amyloid imaging-cognition associations. Our findings are also consistent with preliminary data emerging from one of the first T807 tau PET imaging studies measuring both amyloid and tau levels in cognitively normal adults (Sperling et al., 2015). Consistent with our CSF results, this study reported an association between inferior temporal tau levels (but not amyloid) and episodic memory performance when both tau and amyloid served as simultaneous predictors of cognition.
The stronger effects for biomarkers of tau pathology, rather than amyloid pathology, are likely related to findings indicating that increases in tau and p-tau in the CSF may be due to the combined effect of synaptic injury, neuronal loss, and the presence of neurofibrillary tangles (Holtzman, 2011). Numerous studies from different laboratories have demonstrated a significant decrease in synaptic density in neocortical association areas and the hippocampus in patients with AD (see reviews by Scheff et al., 2003; 2014), and these studies have shown that the strongest correlation with cognitive decline is with synaptic number and regional neuronal loss (DeKosky & Scheff, 1990; Terry et al. 1991; Sze et al. 1997; Masliah et al., 2001). Although the CSF tau measures used in the present study reflect aggregate (rather than regionally specific) measures of neuronal injury and tau pathology, future studies measuring tau accumulation with PET tracers should be able to address tau-cognition associations among cognitively normal older adults in a regionally specific manner.
The specificity of the relationship between CSF t-tau and p-tau and visuospatial – but not verbal – episodic memory is also of interest. Pathological studies in cognitively normal adults have suggested that the accumulation of AD-related tau pathology begins in medial temporal regions, with later dispersion to other brain regions (Braak & Braak, 1991, 1997; Price & Morris, 1999). These same medial temporal regions are also important for episodic memory (for a review, see, e.g., Burgess, Maguire, & O’Keefe, 2002; Squire, 1992). The visuospatial episodic memory tasks included in these analyses involved predominantly unfamiliar stimuli that were difficult to verbalize. In contrast, verbal episodic memory tasks may allow one to compensate for early medial temporal lobe pathology through the use of cortically mediated memory strategies, including verbal coding (e.g., the use of language/semantics) and the use of well-practiced heuristics in learning and retention (e.g., verbal associations). In line with this, level of education was associated with the composite score of verbal episodic memory (as well as working memory), but not visuospatial episodic memory. While prior studies have not reported a disproportional impairment on individual nonverbal memory tasks in preclinical AD (e.g., Albert et al., 2014), the present findings suggest a visuospatial episodic memory composite may be useful as a cognitive marker of preclinical AD. It should be noted that associations between CSF biomarkers and other domains of cognition (e.g., verbal episodic memory or working memory) may become apparent as the individuals age and accumulate additional AD pathology.
The finding that the tests of visuospatial episodic memory employed in the current study were not associated with level of education may also be of relevance to clinical trials. Such nonverbal tests may be less biased by educational and linguistic variables and thereby permit the selection of participants from a broad range of socioeconomic backgrounds with less adjustment for these cultural factors.
In the follow-up analyses, we found no significant interactions between CSF biomarkers and prospective clinical diagnosis. One possible interpretation for this finding is that the association between CSF biomarkers of AD pathology and measures of visuospatial episodic memory are the same for cognitively normal individuals who develop cognitive impairment over time and those who remain normal, reflecting age-related, rather than disease-related processes. However, we also found no significant biomarker-cognition associations in the subset of individuals who have remained cognitively normal to date. While this may simply reflect the reduction in power for the sub-group analyses, an alternative interpretation is that variability in cognitive scores and CSF protein levels across both groups of cognitively normal individuals is needed in order to detect associations between CSF biomarkers of AD and cognition. Supporting this view, the group who developed clinical symptoms of MCI or dementia at follow-up had higher baseline levels of both CSF t-tau and p-tau and lower cognitive test scores (Table 1). However, these follow-up analyses should be interpreted with caution. For example, the interaction terms do not account for time between baseline and clinical symptom onset, an important caveat given some subjects progressed to clinical symptoms within a few years of baseline while others progressed more than a decade later (mean time from baseline to clinical symptom onset = 7 years, range = 1 - 14). Nonetheless, our findings raise the possibility that the associations found in the entire sample of individuals who were cognitively normal at baseline were driven by variability contributed by individuals in the preclinical phase of AD (i.e., the progressors). Variability in the amount of AD pathology across samples of cognitively normal individuals may also help explain prior inconsistencies in the literature.
Additionally we found that APOE-4 carrier status was not directly associated with cognitive performance in the participants, in line with a number of previous studies finding no effect of APOE on cognition in cognitively normal adults (e.g., Li et al., 2014; Small et al., 2000; Smith et al., 1998). Furthermore, we found no differences in CSF biomarker-cognition associations between APOE-4 carriers and non-carriers (i.e., no biomarker by APOE interactions). This finding is in contrast to that of Kantarci et al. (2012), who reported stronger amyloid-cognition associations in APOE-4 carriers relative to non-carriers. However, the results of Kantarci et al. (2012) are difficult to compare to our own, given biomarkers of tau pathology were not included (and thus not controlled) and their participants were substantially older (mean age, 79 years) than those in the present study. Because amyloid deposition increases with age, with greater accumulation in APOE-4 carriers (Morris et al., 2010), the participants in the Kantarci et al. study may have had increased levels of amyloid deposition relative to our participants.
The present study has several limitations. The BIOCARD cohort is highly educated and primarily Caucasian, limiting the generalizability of these findings to more diverse, community populations. Additionally, many participants have a family history of dementia. These findings should be replicated in more diverse samples. Future studies could also examine whether CSF biomarkers of AD pathology are associated with other measures of verbal episodic memory, as our composite measure of verbal episodic memory consisted of both immediate and delayed recall measures. Although previous research has suggested that both types of episodic memory measures are sensitive predictors of clinical symptom onset among cognitively normal older adults (e.g., Albert et al., 2014), more challenging measures of verbal episodic memory may be more sensitive to preclinical levels of AD pathology cross-sectionally (e.g., Rentz et al., 2011). Lastly, we emphasize that the lack of a significant association among the non-progressors may be due to a reduction in sample size when compared with the first analysis; nevertheless, these results suggest that the inclusion of pre-symptomatic individuals may drive the associations between cognition and biomarkers of AD neuropathology in cognitively normal adults.
Hypothetical models of AD have described the order and pattern of biomarker accumulation over preclinical and clinical disease phases (Jack et al., 2013; Sperling et al., 2011). Though recent research has tried to address and validate these hypothetical models, the timing and consequences of preclinical AD pathology are not well understood. The present study suggests that biomarkers of tau pathology have early effects on cognition, as reflected by lower performance on measures of visuospatial episodic memory. As discussed above, this association demonstrates neuroanatomical consistency, given that visuospatial episodic memory utilizes medial temporal regions that are also some of the earliest regions affected by AD-related tau pathology.
HIGHLIGHTS.
CSF-cognition associations were examined in cognitively normal middle-aged sample.
CSF amyloid and total tau or amyloid and p-tau served as predictors of cognition.
CSF tau and p-tau (not amyloid) were associated with visuospatial episodic memory.
Biomarkers of tau pathology are associated with memory during preclinical AD.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health (U19-AG03365, P50-AG005146, and T32-AG027668). The BIOCARD Study consists of 7 Cores with the following members: (1) the Administrative Core (Marilyn Albert, Barbara Rodzon); (2) the Clinical Core (Ola Selnes, Marilyn Albert, Anja Soldan, Rebecca Gottesman, Ned Sacktor, Guy McKhann, Scott Turner, Leonie Farrington, Maura Grega, Gay Rudow, Daniel D’Agostino, Sydney Feagen, David Dolan); (3) the Imaging Core (Michael Miller, Susumu Mori, Tilak Ratnanather, Timothy Brown, Hayan Chi, Anthony Kolasny, Kenichi Oishi, Thomas Reigel, William Schneider, Laurent Younes); (4) the Biospecimen Core (Abhay Moghekar, Richard O’Brien, Abby Spangler); (5) the Informatics Core (Roberta Scherer, David Shade, Ann Ervin, Jennifer Jones, Matt Toepfner, Lauren Parlett, April Patterson, Aisha Mohammed); (6) the Biostatistics Core (Mei-Cheng Wang, Shanshan Li, Qing Cai, Daisy Lu); and (7) the Neuropathology Core (Juan Troncoso, Barbara Crain, Olga Pletnikova, Gay Rudow, and Karen Fisher). The authors are grateful to the members of the BIOCARD Scientific Advisory Board who provide continued oversight and guidance regarding the conduct of the study including: Drs John Cernansky, David Holtzman, David Knopman, Walter Kukull, and John McArdle, and Drs Neil Buckholtz, John Hsiao, Laurie Ryan, and Jovier Evans, who provide oversight on behalf of the National Institute on Aging and the National Institute of Mental Health (NIMH), respectively. The authors thank the members of the BIOCARD Resource Allocation Committee who provide ongoing guidance regarding the use of the biospecimens collected as part of the study, including: Drs Constantine Lyketsos, Carlos Pardo, Gerard Schellenberg, Leslie Shaw, Madhav Thambisetty, and John Trojanowski.
The authors acknowledge the contributions of the Geriatric Psychiatry Branch of the intramural program of NIMH who initiated the study (Principle investigator: Dr. Trey Sunderland). The authors are particularly indebted to Dr. Karen Putnam, who has provided ongoing documentation of the Geriatric Psychiatry Branch study procedures and the data files received from NIMH.
Appendix
Table A.1.
Confirmatory factor analysis model fits indices and change in chi-square across models (relative to Model 1).
| χ2 | CFI | RMSEA | SRMR | Δχ2 | Δ df | p-value | |
|---|---|---|---|---|---|---|---|
| Hypothesized factor structure | |||||||
| (1) 3-factor model | χ2(23) = 29.09, p = . 18 | 0.989 | 0.03 (p = .80) | 0.04 | - | - | - |
| Nested models | |||||||
| (2) 2-factors (vsEM = vEM) | χ2(25) = 48.46, p = .003 | 0.959 | 0.06 (p =.24) | 0.05 | 19.37 | 2 | < .001 |
| (3) 2-factors (vsEM = WM) | χ2(25) = 42.92, p = .01 | 0.969 | 0.05 (p =.41) | 0.04 | 13.83 | 2 | 0.001 |
| (4) 2-factors (vEM = WM) | χ2(25) = 37.48, p = .052 | 0.978 | 0.04 (p =.62) | 0.05 | 8.39 | 2 | 0.02 |
| (5) 1-factor | χ2(26) = 54.12, p = .001 | 0.951 | 0.06 (p =.16) | 0.05 | 25.03 | 3 | < .001 |
CFI = comparative fit index. RMSEA = root mean square error of approximation. SRMR = standardized root-mean-square residual, df = degrees of freedom. WM = working memory. vEM = verbal episodic memory. vsEM = visuospatial episodic memory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of Neurology. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. doi:10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M, Soldan A, Gottesman R, McKhann G, Sacktor N, Farrington L, Grega M, Turner RS, Lu Y, Li S, Wang MC, Selnes O, the BIOCARD Research Team Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Current Alzheimer Research. 2014;11:773–784. doi: 10.2174/156720501108140910121920. doi: 10.2174/156720501108140910121920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B. 1995;57:289–300. [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Blunch NJ. Introduction to structural equation modeling using SPSS and AMOS. Sage Publications Ltd.; London: 2008. [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiology of Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. doi: 10.1016/S0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. doi: 10.1016/S0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmedhel DE, Gaskell PC, Pericak-Vance MA. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nature Genetics. 1994;7:180–184. doi: 10.1038/ng0694-180. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- DeKosky S, Scheff S. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Annals of Neurology. 1990;27:457–464. doi: 10.1002/ana.410270502. dpi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Archives of Neurology. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. The Journal of the American Medical Association. 1997;278:1349–1356. dpi: 10.1001/jama.1997.03550160069041. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geldhof GJ, Pornprasertmanit S, Schoemann a. M., Little TD. Orthogonalizing through residual centering: Extended applications and caveats. Educational and Psychological Measurement. 2013;73:27–46. doi: 10.1177/0013164412445473. [Google Scholar]
- Glodzik L, de Santi S, Tsui WH, Mosconi L, Zinkowski R, Pirraglia E, de Leon MJ. Phosphorylated tau 231, memory decline and medial temporal atrophy in normal elders. Neurobiology of Aging. 2011;32:2131–2141. doi: 10.1016/j.neurobiolaging.2009.12.026. doi: 016/j.neurobiolaging.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AL, Sherva R, Mukherjee S, Newhouse S, Kauwe JSK, Munsie LM, Crane PK. Calibrating longitudinal cognition in Alzheimer’s disease across diverse test batteries and datasets. Neuroepidemiology. 2014;43:194–205. doi: 10.1159/000367970. doi: 10.1159/000367970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Mormino EC, Amariglio RE, Younger AP, Schultz AP, Becker JA, Rentz DM. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. The Journal of Neuroscience. 2012;32:16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. doi:10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–1348. doi: 10.1212/WNL.0b013e31828ab35d. doi:10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. The Journal of Lipid Research. 1990;31:545–548. [PubMed] [Google Scholar]
- Holtzman DM. CSF biomarkers for Alzheimer’s disease: Current utility and potential future use. Neurobiology of Aging. 2011;32:S4–S9. doi: 10.1016/j.neurobiolaging.2011.09.003. doi: 10.1016/j.neurobiolaging.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howieson DB, Carlson NE, Moore MM, Wasserman D, Abendroth CD, Payne-Murphy J, Kaye JA. Trajectory of mild cognitive impairment onset. Journal of the International Neuropsychological Society. 2008;14:192–198. doi: 10.1017/S1355617708080375. doi: 10.1017/S1355617708080375. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Fit Indices in Covariance Structure Modeling: Sensitivity to Underparameterized Model Misspecification. Psychological Methods. 1998;3:424–453. doi: 10.1037/1082-989X.3.4.424. [Google Scholar]
- Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in“ normal” aging: Evidence for preclinical Alzheimer disease in cognitively normal individuals. Journal of Neuropathology and Experimental Neurology. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurology. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Lowe V, Przybelski SA, Weigand SD, Senjem ML, Ivnik RJ, Jack CR. APOE modifies the association between AB load and cognition in cognitively normal adults. Neurology. 2012;78:232–240. doi: 10.1212/WNL.0b013e31824365ab. doi: 10.1212/WNL.0b013e31824365ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D, Parisi J. Neuropathology of cognitively normal elderly. Journal of Neuropathology and Experimental Neurology. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- Li G, Millard SP, Peskind ER, Zhang J, Yu C-E, Leverenz JB, Montine TJ. Cross-sectional and longitudinal relationships between cerebrospinal fluid biomarkers and cognitive function in people without cognitive impairment from across the adult life span. Alzheimer’s & Dementia. 2014;71:742–51. doi: 10.1001/jamaneurol.2014.445. doi: 10.1001/jamaneurol.2014.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Montine TJ. CSF tau/Aß42 ratio for increased risk of mild cognitive impairment: A follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer ’s disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellack L, Karasu TG, editors. Geriatrics Psychiatry: A Handbook for Psychiatrists and Primary Care Physicians. Grune & Stratton; New York: NY: 1976. pp. 77–120. [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghekar A, Goh J, Li M, Albert M, O’Brien RJ. Cerebrospinal fluid Aβ and tau level fluctuation in an older clinical cohort. Archives of Neurology. 2012;69:246–250. doi: 10.1001/archneurol.2011.732. doi: 10.1001/archneurol.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghekar A, Li S, Lu Y, Li M, Wang M-C, Albert M, O’Brien R. CSF biomarker changes precede symptom onset of mild cognitive impairment. Neurology. 2013;81:1753–1758. doi: 10.1212/01.wnl.0000435558.98447.17. doi: 1212/01.wnl.0000435558.98447.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts AB but not tau Alzheimer’s pathology in cognitively normal aging. Annals of Neurology. 2010;67:122–131. doi: 10.1002/ana.21843. doi: doi.org/10.1002/ana.21843.APOE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnally JC. Psychometric theory. 2nd ed. McGraw-Hill; New York: 1978. [Google Scholar]
- Petersen R. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, Kokmen E. Neuropathologic features of amnestic mild cognitive impairment. Archives of Neurology. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- Pike KE, Ellis K. a, Villemagne VL, Good N, Chételat G, Ames D, Rowe CC. Cognition and beta-amyloid in preclinical Alzheimer’s disease: Data from the AIBL study. Neuropsychologia. 2011;49:2384–2390. doi: 10.1016/j.neuropsychologia.2011.04.012. doi: 10.1016/j.neuropsychologia.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Rowe CC. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Annals of Neurology. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. doi: 10.1002/1531-8249(199903)45:3<358::AID-ANA12>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Rami L, Fortea J, Bosch B, Solé-Padullés C, Lladó A, Iranzo A, Molinuevo JL. Cerebrospinal fluid biomarkers and memory present distinct associations along the continuum from healthy subjects to AD patients. Journal of Alzheimer’s Disease. 2011;23:319–326. doi: 10.3233/JAD-2010-101422. doi: 10.3233/JAD-2010-101422. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Caselli RJ. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. PNAS. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Amariglio RE, Becker JA, Frey M, Olson LE, Frishe K, Sperling RA. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia. 2011;49:2776–2783. doi: 10.1016/j.neuropsychologia.2011.06.006. doi: 10.1016/j.neuropsychologia.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie. 1941;28:286–340. [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, Rieck JR, Hebrank AC, Diaz-Arrastia R, Park DC. β-amyloid burden in healthy aging: Regional distribution and cognitive consequences. Neurology. 2012;78:387–395. doi: 10.1212/WNL.0b013e318245d295. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe C, Fagan A, Grant E, Hassenstab J, Moulder K, Dreyfus D, Morris J. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–1791. doi: 10.1212/WNL.0b013e3182918ca6. doi: 10.1016/j.jalz.2013.05.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolstad S, Berg AI, Bjerke M, Blennow K, Johansson B, Zetterberg H, Wallin A. Amyloid-β42 is associated with cognitive impairment in healthy elderly and subjective cognitive impairment. Journal of Alzheimer’s Disease. 2011;26:135–42. doi: 10.3233/JAD-2011-110038. doi: 10.3233/JAD-2011-110038. [DOI] [PubMed] [Google Scholar]
- Rosseel Y. lavaan: An R package for Structural Equation Modeling. Journal of Statistical Software. 2012;48:1–36. Retrieved from: http://www.jstatsoft.org/v48/i02. [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Neltner JH, Nelson PT. Is synaptic loss a unique hallmark of Alzheimer’s disease? Biochemical Pharmacology. 2014;88:517–28. doi: 10.1016/j.bcp.2013.12.028. doi: 10.1016/j.bcp.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff S, Price DA. Synaptic pathology in Alzheimer’s disease: A review of ultrastructural studies. Neurobiology of Aging. 2003;24:1029–1046. doi: 10.1016/j.neurobiolaging.2003.08.002. doi: 10.1016/j.neurobiolaging.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Schneider J, Arvanitakis Z, Leurgans S, Bennett D. The Neuropathology of Probable Alzheimer’s Disease and Mild Cognitive Impairment. Annals of Neurology. 2009;66:200–208. doi: 10.1002/ana.21706. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott JM, Bartlett JW, Fox NC, Barnes J. Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal guild Aβ1-42. Annals of Neurology. 2010;68:825–834. doi: 10.1002/ana.22315. doi: 10.1002/ana.22315. [DOI] [PubMed] [Google Scholar]
- Small BJ, Graves AB, McEvoy CL, Crawford FC, Mullan M, Mortimer JA. Is APOE-ε4 a risk factor for cognitive impairment in normal aging? Neurology. 2000;54:2082–2088. doi: 10.1212/wnl.54.11.2082. doi: 10.1212/WNL.54.11.2082. [DOI] [PubMed] [Google Scholar]
- Smith GE, Bohac DL, Waring SC, Kokmen E, Tangalos EG, Ivnik RJ, Petersen RC. Apolipoprotein E genotype influences cognitive ‘phenotype’ in patients with Alzheimer’s disease but not in healthy control subjects. Neurology. 1998;50:355–362. doi: 10.1212/wnl.50.2.355. doi: 10.1212/WNL.50.2.355. [DOI] [PubMed] [Google Scholar]
- Sonnen J. a., Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Montine TJ. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Annals of Neurology. 2007;62:406–413. doi: 10.1002/ana.21208. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. doi:10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Johnson KA, Doraiswamy PM, Reiman EM, Fleisher AS, Sabbagh MN, Pontecorvo MJ. Amyloid deposition detected with florbetapir F 18 ((18)F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiology of Aging. 2013;34:822–31. doi: 10.1016/j.neurobiolaging.2012.06.014. doi: 10.1016/j.neurobiolaging.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Mormino EC, Rentz DM, Schultz AP, Sepulcre J, Hedden T, Johnson KA. Regional tau PET measures associated with memory performance in clinically normal older individuals; Paper presented at the Alzheimer’s Association International Conference; Washington, DC. 2015, July. [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. doi: 10.1037/0033-295X.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stomrud E, Hansson O, Zetterberg H, Blennow K, Minthon L, Londos E. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Archives of Neurology. 2010;67:217–223. doi: 10.1001/archneurol.2009.316. doi:10.1001/archneurol.2009.316. [DOI] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive Decline and Brain Volume Loss as Signatures of Cerebral Amyloid-β Peptide Deposition Identified With Pittsburgh Compound B: Cognitive Decline Associated With Aβ Deposition. Archives of Neurology. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strozyk D, Blennow K, White LR, Launer LJ. CSF Aß 42 levels correlate with amyloid-neuropathology in a population-based. Neurology. 2003;60:652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- Sze C-I, Troncoso JC, Kawas C, Moution P, Price DL, Martin LJ. Loss of the presynaptic vesicle protein synaptophys in hippocampus correlates with cognitive decline in Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 1997;56:933–944. doi: 10.1097/00005072-199708000-00011. doi: 10.1097/00005072-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Tapiola T, Alafuzoff I, Herukka S-K, Parkkinen L, Hartikainen P, Soininen H, Pirttila T. Cerebrospinal fluid ß-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Archives of Neurology. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Annals of Neurology. 1991;30:572–580. doi: 10.1002/ana.410300410. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Weigand SD, Przybelski SA, Knopman DS, Smith GE, Trojanowski JQ, Jack CR. Cognitive reserve and Alzheimer’s disease biomarkers are independent determinants of cognition. Brain. 2011;134:1479–1492. doi: 10.1093/brain/awr049. doi: 10.1093/brain/awr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Chételat G, Ellis K. a, Mulligan RS, Bourgeat P, Rowe CC. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Annals of Neurology. 2011;69:181–192. doi: 10.1002/ana.22248. doi:10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Revised Manual. The Psychological Corporation; New York: 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale - Revised Manual. Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]