Abstract
Purpose
To test whether patient's primary malignancy type and presence of FDG-avid cervical lymph node(s) are predictors of pathologic outcome of incidental focal FDG-avid parotid lesions.
Basic Procedures
Retrospective cohort study of pathologically proven incidental cases.
Main Findings
Focal parotid FDG uptake in the setting of head and neck cancer/melanoma(OR=24.6,p<0.01), lymphoma(OR=7.2,p=0.02), or FDG-avid cervical lymph node(s)(OR=3.6,p=0.07) has a higher odds of representing metastases. No malignant primary parotid tumors were incidentally discovered.
Principal Conclusions
In patients with head and neck cancer/melanoma, lymphoma, or FDG-avid cervical lymph node(s) there was a higher odds that focal parotid FDG uptake was a metastasis.
Keywords: Parotid, PET, PET/CT, FDG-PET, FDG, 18F-FDG
1.1 Introduction
Whole body F-18-fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging is commonly performed for the staging and characterization of many malignancies with an estimated 1.5 to 1.8 million studies performed annually in the United States.[1-3] One of the commonly encountered complexities of PET and PET/CT image interpretation is the occurrence of unexpected focal uptake in an organ other than the primary site of disease or common sites for metastases.[4, 5] Unexpected FDG uptake may potentially be non-malignant, an unusual metastasis, or a synchronous tumor which may warrant further investigation. The likely pathology of secondary tumors depends greatly upon the site of the FDG uptake.[4, 6-9] Due to relatively high rates of synchronous malignancies, patients with incidental FDG uptake in the thyroid have been recommended to undergo thyroid biopsy and patients with incidental FDG uptake in the colon have been recommended to undergo colonoscopy.[6, 7, 10-12]
The clinical significance of focal FDG uptake in the parotid gland identified on whole body PET imaging is less well established. Although there is variation in the literature, the prevalence of FDG avid parotid lesions identified on PET is estimated at 0.5-1% and the malignancy risk is estimated at 10-30%.[13-23] Our aim was to evaluate predictors of pathologic outcome of focal parotid FDG uptake identified on whole body PET imaging which may potentially help guide patient management. Our specific hypotheses were that both the type of primary malignancy and the presence or absence of FDG-avid cervical lymph nodes could serve as predictors of the pathologic outcome of focal parotid FDG uptake.
2.1 Methods
2.1.1 Design and Study Subjects
Following institutional review board approval, we performed a HIPAA compliant retrospective cohort study. We performed a database search of all whole body PET and PET/CT reports generated from 12/1999 to 12/2014 at our institution for the word “parotid” in the report impression. The medical records of identified cases were reviewed to determine if the following inclusion/exclusion criteria were met: Inclusion criteria were focal FDG-avid lesion within the parotid gland and documented pathologic follow-up for the identified lesion. Exclusion criteria were focal parotid FDG uptake that corresponded to a known parotid malignancy (not “incidental”) or diffuse bilateral parotid FDG uptake (considered inflammatory).
2.1.2 FDG- PET and PET/CT imaging protocol
All FDG-PET/CT examinations were performed on a Biograph 16 (Hi-Rez) PET/CT scanner (Siemens Medical Solutions) with an integrated PET and 16-MDCT scanner or a Discovery VCT PET/CT scanner (Siemens Medical Solutions) with an integrated PET and 64-MDCT scanner. All FDG-PET examinations were performed on a HR Plus PET scanner (Siemens Medical Solutions). Standard clinical protocol included: All patients fasted with hydration for at least 6 hours prior to PET/CT examinations. Patients had blood glucose levels <200 mg/dL prior to intravenous injection of 12.5 +/- 2.5 mCi of 18F-FDG was injected intravenously followed by a 10-mL normal saline flush. Patients rested for 60 +/- 15 minutes and voided before being positioned supine on the scanner table. CT examinations were performed after the injection of 150 mL of iohexol (Omnipaque 350, GE Healthcare) unless contraindicated due to allergy or renal impairment. CT images were reconstructed as contiguous 5-mm slices for the entire body and if there was a head and neck indication as a second set of contiguous 3-mm slices through the head and neck. No oral contrast media was administered. PET was performed immediately following CT, without patient repositioning. PET images were obtained at 7-10 bed positions per patient, with an acquisition time of 3 to 4 minutes per station, from the skull vertex through the mid thigh.
2.1.3 Data Collection
The medical records were reviewed to determine the primary malignancy/indication, gender, age, and pathology results from follow-up fine needle aspiration, core needle biopsy, or resection. Whole body PET studies were re-reviewed by both a radiology trainee with 4 years experience interpreting whole body PET imaging and a nuclear medicine/abdominal imaging attending radiologist with 9 years experience interpreting whole body PET imaging. PET and if applicable CT images were displayed in orthogonal planes and volumetric regions of interest were used to measure the maximum standardized uptake value (SUVmax) of the focal parotid lesion and of the cervical lymph nodes. CT features were not considered in order to focus on the PET appearance. Parotid lesions were considered focal if uptake was subjectively above parotid background uptake. There was agreement by both reviewers on all cases. Cervical lymph nodes were considered FDG-avid if they had a SUVmax ≥2.5.[24]
2.1.4 Data Analysis
Mean, standard deviation (SD), and range of patient age were calculated. Pathologic outcomes were categorized into three groups for analysis: 1) Manifestation of the patient's known primary malignancy (metastasis or lymphoma), 2) Synchronous/metachronous primary parotid neoplasm, 3) Non-neoplastic (benign lymphoid tissue/inflammation). The proportion and 95% confidence interval (CI) was calculated for each pathologic outcome category.
The mean focal parotid lesion SUVmax with 95%CI was calculated for each pathologic outcome category. One-way analysis of variance (ANOVA) was performed to test for differences in SUVmax and age between the groups. If ANOVA was overall statistically significant Tukey-Kramer pairwise post-hoc comparisons were performed. Gender was compared with a Fisher's exact test.
In order to test the hypothesis that the patient's primary malignancy was a predictor of pathologic outcome of focal parotid FDG uptake the cases were separated into the categories for analysis based upon the primary malignancy type: 1) Lymphoma (a systemic disease). 2) Head and neck cancer including skin cancer/melanoma (a regional disease). Ocular melanoma (n=1) was not included in this group, as ocular melanoma does not generally metastasize to regional lymph nodes, instead most commonly metastasizing to the liver.[25] 3) All other indications (not expected to have parotid involvement). The one case of ocular melanoma was included in this third group. The proportion and 95%CI was calculated for each category in the resulting contingency table. Nested contingency tables were used to evaluate for pairwise differences. Odds ratios (OR) and risk ratios (RR) with 95%CI for representing a manifestation of known malignancy were calculated for the primary malignancy categories of lymphoma and head and neck cancer/melanoma as compared to the “other indication” group.
To test the independent hypothesis that the presence or absence of FDG-avid cervical lymph node(s) was a predictor of pathologic outcome of parotid FDG uptake the cases were separated for analysis based upon the presence or absence of FDG-avid cervical lymph node(s). The proportion and 95%CI was calculated for each category in the resulting contingency table. The OR and RR with 95%CI for representing a manifestation of known malignancy was calculated for the presence of FDG-avid cervical lymph node(s).
To explore the effects and interactions of the multiple variables, multiple variable logistic regression was performed to predict focal parotid uptake representing a manifestation of the patients known malignancy. The variables shown to be individually statistically significant between the outcomes were included in this analysis, including primary malignancy type, presence of FDG-avid cervical lymph nodes, and age. Receiver operating characteristic (ROC) analysis was performed for each variable and the combined variable model with calculation and comparison of the area under the curve (AUC) using the ROC comparison function of MedCalc.[26]
Statistical analysis and line art production was performed using MedCalc for Windows, version 14.8.1 (MedCalc Software, Ostend, Belgium) and the R statistical software package (R Foundation for Statistical Computing, Vienna, Austria).[27] Contingency tables were tested for statistical significance with Fishers Exact tests. A p<0.05 was considered statistically significant.
3.1 Results
3.1.1 Patient and Lesion Characteristics
68 patients with 73 lesions meeting inclusion and exclusion criteria were identified out of 38,302 whole body FDG PET studies reported from 12/1999 to 12/2014 at our institution. There were 44 males and 24 females with a mean age of 60.7 years, (SD 17.2 years, range 2-90 years). Parotid pathologic results showed that 33/73 were manifestations of the patient's known malignancy (45%)(Figure 1), 25/73 benign primary parotid tumors (34%)(Figure 2), and 15/73 non-neoplastic (21%). There were no malignant primary parotid tumors identified in this series. Detailed pathology results with 95%CIs are presented in Table 1.
Figure 1.
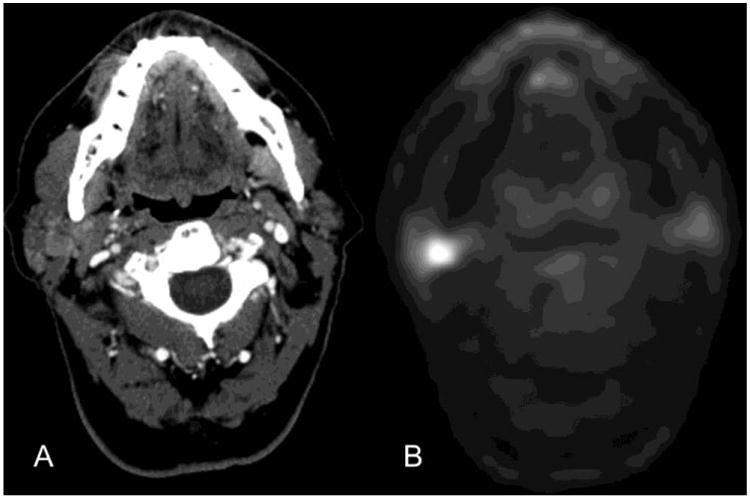
Parotid Metastasis. FDG PET/CT images from a 64-year old male patient with a right ear squamous cell carcinoma status post auriculectomy and flap reconstruction undergoing a restaging PET/CT. CT image (A) at the level of the right parotid demonstrates a relatively subtle lesion in the right parotid gland. FDG PET image (B) at the same level demonstrates FDG avidity with a standard uptake value (SUV) maximum of 8.1. There were additional right-sided FDG-avid cervical lymph nodes that are not pictured. Follow-up surgical pathology revealed that this was a metastasis. Our study suggests that this result was very likely.
Figure 2.
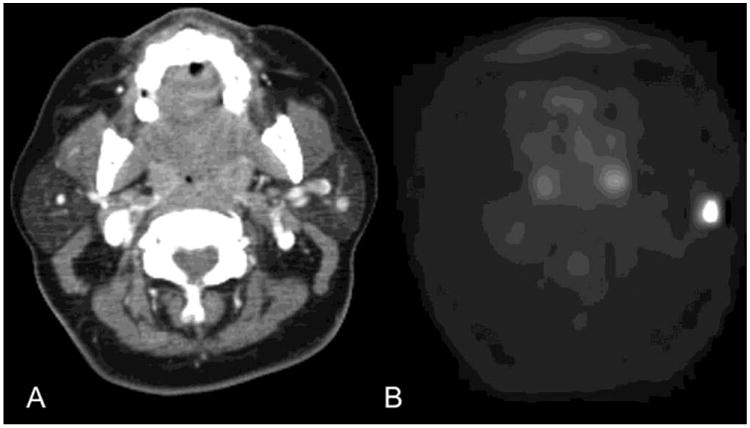
Benign Primary Parotid Tumor. FDG PET/CT images from a 57-year old female patient with breast cancer undergoing a staging PET/CT. CT image (A) at the level of the parotid glands demonstrates a small lesion in the left parotid gland. FDG PET image (B) at the same level demonstrates FDG avidity with a standard uptake value (SUV) maximum of 14.9. No FDG-avid cervical lymph nodes were present. Follow up fine needle aspiration revealed that this was a benign mixed tumor/pleomorphic adenoma. Our study suggests that this result was likely.
Table 1.
Pathology results. Pathology results of focal parotid FDG uptake identified on whole body PET imaging. BMT=Benign mixed tumor/pleomorphic adenoma. CI=Confidence interval.
| Pathology Results | ||||||
|---|---|---|---|---|---|---|
| Manifestation of Known Malignancy | Primary Parotid Tumor | Benign Lymphatic Tissue/Inflammation | ||||
| 33/73 (45%) 95% CI 34-57% |
25/73 (34%) 95% CI 24-46% |
15/73 (21%) 95% CI 13-31% |
||||
| 26 Metastasis | 7 Lymphoma | 14 Warthins | 7 BMTs | 4 Oncocytomas | 10 Lymphatic Tissue | 5 Inflammation |
Patient age differed between the outcome groups (one-way ANOVA F=4.43, p=0.017) with patients found to have benign primary parotid tumors (mean age 69.2 years, SD 10.9 years) being older (p<0.05) than patients found to have manifestations of their known malignancy (mean age 58.4 years, SD 19.1 years), or non-neoplastic parotid uptake (mean age 55.7, SD 16.8 years). Patient gender was not statistically significantly different between outcome groups (p=0.15).
3.1.2 SUVmax is not a Statistically Significant Predictor of Pathologic Outcome for Focal Parotid Uptake Identified on Whole Body PET Imaging
Mean SUVmax for lesions that were a manifestation of the patient's known malignancy was 8.4 (95%CI 6.6-10.2, range 2.4-33), for lesions that were benign primary parotid tumors was 10.3 (95%CI 5.5-15.1, range 2.9-25), and for lesions that were non-neoplastic was 5.51 (95%CI 3.9-7.1, range 2.1-15). One-way ANOVA showed no statistically significant difference in SUVmax between groups (F=1.82, p=0.17). These results are presented as box-plots in Figure 3.
Figure 3.
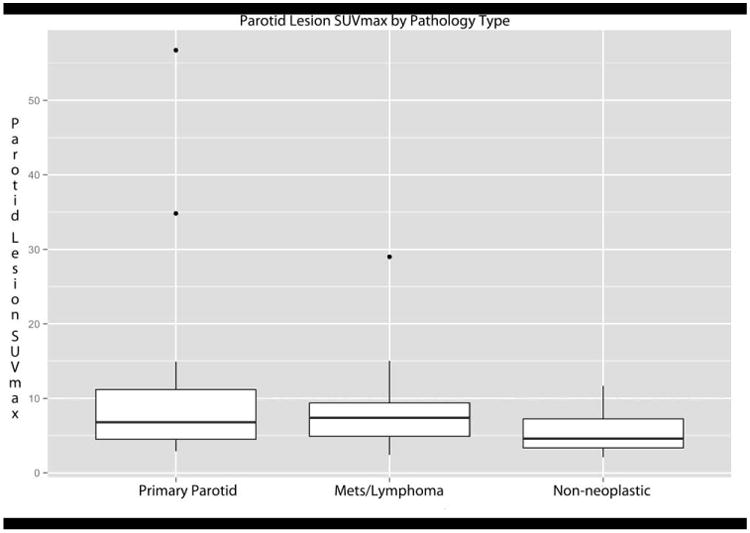
SUVmax Results. Box-plot of SUVmax for FDG-avid parotid lesions separated by pathology type. Mean SUVmax for lesions that were a manifestation of the patient's known malignancy was 8.4 (95%CI 6.6-10.2), for lesions that were benign primary parotid tumors was 10.3 (95%CI 5.5-15.1), and for lesions that were non-neoplastic was 5.51 (95%CI 3.9-7.1). One-way analysis of variance showed no statistically significant difference in SUVmax between groups (F=1.82, p=0.17).
3.1.3 Primary Malignancy Type is a Statistically Significant Predictor of Pathologic Outcome
For patients with lymphoma, focal parotid FDG uptake pathologic outcome was: 7/13 lymphoma (54%), 4/13 benign primary parotid tumor (31%), and 2/13 non-neoplastic (15%). For patients with head and neck cancer/melanoma, pathologic results were: 23/29 metastasis (79%), 1/29 benign primary parotid tumor (3.4%), and 5/29 non-neoplastic (17%). For patients in the “other malignancy” group, pathologic results were: 3/31 metastasis (9.7%), 20/31 benign primary parotid tumor (65%), and 8/31 non-neoplastic (26%). These results were statistically significant (p<0.001) and are presented with 95%CIs in Table 2 and Figure 4. Differences in the rates of pathology follow-up were not statistically significant (p=0.28) between the three primary malignancy type groups. Pairwise comparisons of pathologic outcome were: Lymphoma compared to head and neck cancer/melanoma p=0.06, Lymphoma compared to other malignancy p=0.008, and head and neck cancer/melanoma compared to other malignancy p<0.001. These pairwise comparisons are presented in Figure 4. The primary malignancy category of lymphoma was associated with an OR of 10.9 and a RR of 5.6 (p=0.003) and the category of head and neck cancer/melanoma was associated with an OR of 35.7 and a RR of 8.2 (p<0.001) for focal FDG uptake in the parotid gland representing a manifestation of the patients known malignancy as compared to being in the “other malignancy” category. These results with 95%CIs are presented in Table 2.
Table 2.
Results of potential predictors of pathologic outcome. Differences in pathology outcome are statistically significantly different based upon the primary malignancy type (p<0.001). Differences in pathology outcome are statistically significantly different based upon the presence or absence of FDG-avid cervical lymph node(s) (p<0.001). Odds ratios and risk ratios for the primary malignancy categories of lymphoma and head and neck cancer/melanoma as compared to the “other malignancy” category and for the presence of FDG-avid cervical lymph node(s) are also presented. CI=Confidence interval. Ca=Cancer. FDG=Fluorodeoxyglucose.
| Pathology Results by Type of Primary Malignancy (p<0.001) | |||
|---|---|---|---|
| Primary Malignancy | Manifestation of Known Malignancy | Primary Parotid Tumor | Benign Lymphatic Tissue/Inflammation |
| Lymphoma | 7/13 (54%) 95% CI 29-77% |
4/13 (31%) 95% CI 13-58% |
2/13 (15%) 95% CI 4.3-42% |
| Head and Neck Ca/Melanoma | 23/29 (79%) 95% CI 62-90% |
1/29 (3.4%) 95% CI 0.6-17% |
5/29 (17%) 95% CI 7.6-35% |
| Other Malignancy | 3/31 (9.7%) 95% CI 3.4-25% |
20/31 (65%) 95% CI 47-79% |
8/31 (26%) 95% CI 14-43% |
| Pathology Results by Presence of FDG-Avid Cervical Lymph Node(s) (p<0.001) | |||
| FDG-Avid Cervical Lymph Node(s) | Manifestation of Known Malignancy | Primary Parotid Tumor | Benign Lymphatic Tissue/Inflammation |
| Yes | 18/23 (78%) 95% CI 58-90% |
2/23 (8.7%) 95% CI 2.4-27% |
3/23 (13%) 95% CI 4.5-32% |
| No | 15/50 (30%) 95% CI 19-44% |
23/50 (46%) 95% CI 33-60% |
12/50 (24%) 95% CI 14-37% |
| Odds Ratios and Risk Ratios for Focal Parotid FDG Uptake Representing a Manifestation of the Patient's Known Malignancy | |||
| Factor | Odds Ratio | Risk Ratio | Fishers Exact Test |
| Lymphoma as compared to “other malignancy” group | 10.9 (95% CI 2.2-55) | 5.6 (95% CI 1.7-18.2) | p=0.003 |
| Head and Neck Ca/Melanoma as compared to “other malignancy” group | 35.7 (95% CI 8.1-159) | 8.2 (95% CI 2.8-24) | p<0.001 |
| FDG-Avid Cervical Lymph Node(s) resent as compared to Absent | 8.4 (95% CI 2.6-26.8) | 2.6 (95% CI 1.6-4.2) | p<0.001 |
Figure 4.
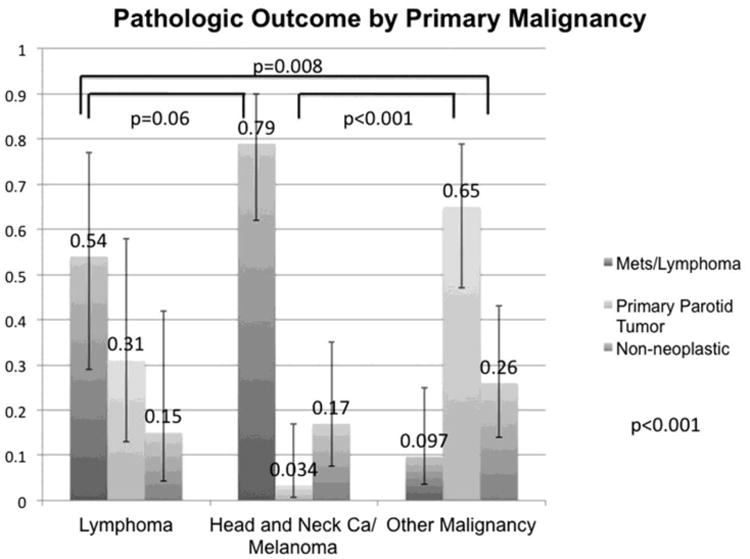
Results by Type of Primary Malignancy. Graphical representation of pathology results of focal parotid FDG uptake separated by type of primary malignancy. Proportions and 95% confidence intervals are represented. Overall Fishers Exact test p<0.001. Pairwise nested comparisons of pathologic outcome are statistically significant for the lymphoma group (p=0.008) and head and neck cancer/melanoma group (p<0.001) as compared to the “other malignancy” group.
3.1.4 The Presence of FDG Avid Cervical Lymph Node(s) is a Statistically Significant Predictor of Pathologic Outcome
For patients with FDG-avid cervical lymph node(s) pathologic results of focal parotid FDG uptake were: 18/23 manifestation of known malignancy (78%), 2/23 benign primary parotid tumor (8.7%), and 3/23 non-neoplastic (13%). For patients without FDG-avid cervical lymph node(s) pathologic results were: 15/50 manifestation of known malignancy (30%), 23/50 benign primary parotid tumor (46%), and 12/50 non-neoplastic (24%). These results of pathologic outcome by presence or absence of FDG-avid cervical lymph node(s) were statistically significant (p<0.001) and are presented with 95%CIs in Table 2 and Figure 5. The presence of FDG-avid cervical lymph node(s) was associated with an OR of 8.4 and a RR of 2.6 (p<0.001) for focal FDG uptake in the parotid gland representing a manifestation of the patients known malignancy as compared to the absence of FDG-avid cervical lymph node(s). These results with 95%CIs are presented in Table 2.
Figure 5.
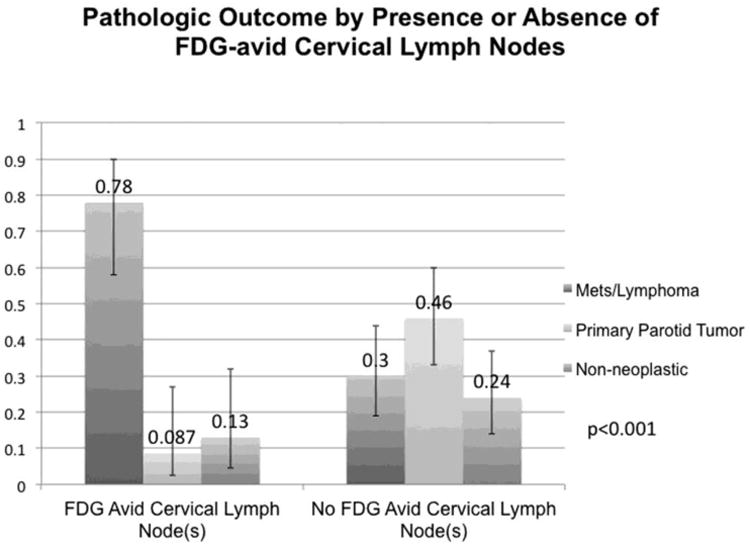
Results by Presence or Absence of FDG-avid Cervical Lymph Node(s). Graphical representation of pathology results of focal parotid FDG uptake separated by presence or absence of FDG-avid cervical lymph node(s) on the same PET study. Proportions and 95% confidence intervals are represented. Overall Fishers Exact test p<0.001 indicates that pathologic outcome is statistically significantly different based upon the presence or absence of FDG-avid cervical lymph node(s).
3.1.5 Multiple Variable Analysis
Multiple variable logistic regression using the individually statistically significant factors of primary malignancy type, presence of FDG-avid cervical lymph node(s), and patient age to predict that focal parotid FDG uptake was a manifestation of the patients known malignancy was overall statistically significant (p<0.001, intercept of -2.61). After accounting for the other variables, primary malignancy type remained a statistically significant predictor. Lymphoma (p=0.024) was associated with an OR of 7.2 and head and neck cancer/melanoma (p<0.001) was associated with an OR of 24.6 relative to the “other malignancy” group. The presence of FDG-avid cervical lymph node(s) contributed to the model performance but did not quite retain statistical significance after accounting for the other variables (p=0.073) and was associated with an OR of 3.6. Patient age was not a statistically significant factor after accounting for the other variables (p=0.84) and was associated with an OR of 1.0. ROC analysis demonstrated that the multiple variable model had an AUC of 0.873 (95% 0.774-0.939) and statistically significantly superior diagnostic performance as compared to primary malignancy type alone (AUC=0.768, 95% CI 0.655-0.859, p=0.032), FDG-avid lymph nodes alone (AUC=0.710, 95% CI 0.592-0.811, p<0.001), and patient age alone (AUC=0.622, 95% CI 0.501-0.733, p<0.001). Results are presented in Table 3 and Figure 6. Notably, in 13/15 (87%) of the patients with head and neck cancer/melanoma and FDG avid cervical lymph node(s) the pathologic outcome of focal parotid uptake was a metastasis.
Table 3.
Results of multiple variable logistic regression to predict that focal parotid FDG uptake represented a manifestation of the known malignancy. After accounting for multiple variables primary malignancy type remains a statistically significant predictive variable. The presence of FDG-avid cervical lymph node(s) contributed to the overall diagnostic model performance but did not quite retain individual statistical significance after accounting for the other variables. Age was not a statistically significant factor after the other variables had been considered. FDG=Fluorodeoxyglucose. Ca=Cancer. CI-Confidence Interval. AUC=Area under the curve.
| Multiple Variable Logistic Regression Overall Model p<0.001, intercept -2.61, AUC=0.873 | ||
|---|---|---|
| Variable | p-value | Odds Ratio |
| Primary Malignancy Lymphoma | p=0.024 | 7.2 (95% CI 1.3-39.5) |
| Primary Malignancy Head and Neck Ca/Melanoma | p<0.001 | 24.6 (95% CI 5.1-119) |
| Presence of FDG-Avid Cervical Lymph Node(s) | p=0.073 | 3.6 (95% CI 0.89-14.4) |
| Patient Age in Years | p=0.84 | 1.0 (95% CI 0.97-1.04). |
Figure 6.
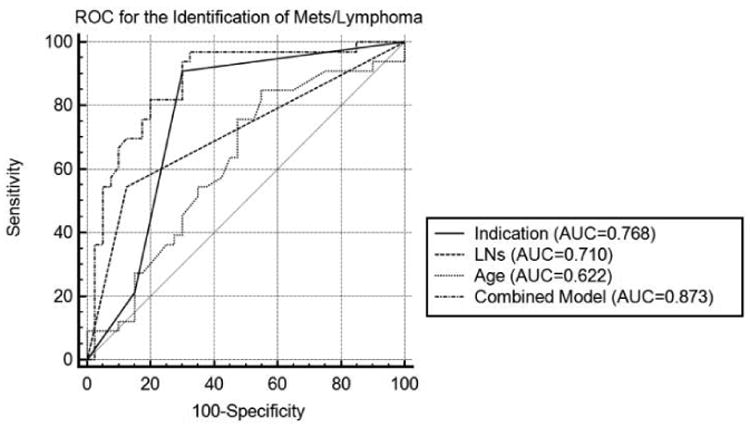
Receiver Operating Characteristic Curves. ROC curves for the identification of focal parotid FDG uptake representing a manifestation of known malignancy. The multiple variable model had an AUC of 0.873 (95% 0.774-0.939) and statistically significantly superior diagnostic performance as compared to primary malignancy type alone (AUC=0.768, 95% CI 0.655-0.859, p=0.032), FDG-avid lymph nodes alone (AUC=0.710, 95% CI 0.592-0.811, p<0.001), and patient age alone (AUC=0.622, 95% CI 0.501-0.733, p<0.001).
4.1 Discussion
In our study we have found that the pathology results of focal FDG were statistically significantly different when independently sorted based upon either the clinical indication for PET imaging or the presence or absence of FDG-avid cervical lymph node(s). Multiple variable analysis demonstrated that clinical indication was the dominant variable but that both factors contributed to diagnostic performance (combined model AUC=0.873). Our data suggests that focal parotid FDG uptake is very likely to represent a manifestation of the patient's known malignancy in the setting of head and neck cancer/melanoma (OR=24.6), lymphoma (OR=7.2), or FDG-avid cervical lymph node(s) (OR=3.6). It is thus not truly incidental, as it would likely be managed per the patient's primary malignancy. Furthermore, no malignant primary parotid lesions were identified first on PET or PET/CT imaging. The patient's primary malignancy type and the presence or absence of FDG-avid cervical lymph node(s) are factors that affect the likely pathologic outcome of focal parotid FDG uptake and should be taken into consideration when interpreting this imaging finding and when considering the need for biopsy.
Multiple prior studies have evaluated the topic of FDG-avid parotid lesions, predominantly exploring the prevalence, overall malignancy risk, and utility of SUV measurements.[13-23] Overall our approach was quite different, instead focusing on the role of the potential predictive factors of primary malignancy type and presence or absence of FDG-avid cervical lymph nodes. The lack of utility of SUVmax for differentiating between pathologic outcomes of parotid lesions found on PET seems to be due to the fact that many benign primary parotid tumors demonstrate high uptake values in the range of malignancies. A recent meta-analysis reported that the pooled prevalence of focal parotid incidental uptake on PET was 0.6%, that the pooled risk of malignancy was 9.6% overall (range 0-28%) and 20.4% (range 0-50%) for lesions that were pathologically proven, and that SUV overlapped for malignant and benign lesions.[22] Our overall rate of malignancy was higher at 45%, which could potentially represent differences in inclusion/exclusion criteria, patient population, and practice patterns. The largest single study evaluating the unexpected finding of focal parotid FDG uptake on PET imaging (by Wang et al) also has some significant differences as compared to our study.[17] That study consisted of 58 cases (51 with pathology follow-up) of focal FDG uptake in the parotid gland. Their results included a larger number of primary parotid tumors (74% vs. our 34%) including malignant primary parotid tumors (12% vs. 0%) and lower number of metastasis/lymphoma (5% vs. 45%). That study was performed in a patient population including healthy patients undergoing whole body PET/CT for cancer screening whereas our study was performed at a large cancer center with diagnostic studies in patients with cancer, a factor that may account for some of the differences in findings.
A relatively large proportion (45%) of the cases of focal parotid FDG uptake in our study were found to represent a manifestation of the patients' known malignancy. Our analysis indicated that focal parotid FDG uptake in head and neck cancer/melanoma (OR=24.6), lymphoma (OR=7.2), or when FDG-avid cervical lymph node(s) were present (OR=3.6) was even more likely to represent a manifestation of the patients' known malignancy. While the presence of FDG- avid lymph nodes did not retain individual statistical significance after the other variables were considered (p=0.073) it did contribute overall to the diagnostic model. Statistically superior diagnostic performance as evident by larger AUC on ROC analysis in the combined variable model demonstrates that there is value to considering both lymph node status and primary malignancy type. Without a history of lymphoma or head and neck cancer/melanoma (65%) or FDG-avid cervical lymph node(s) (46%), benign primary parotid tumors were commonly encountered. Overall, we encountered no synchronous primary malignant parotid tumors in our study, suggesting that a malignant primary parotid tumor is very unlikely to be the pathologic result of a focal parotid FDG uptake identified on PET imaging. This is dissimilar from focal thyroid FDG uptake where the main consideration driving the recommendation for biopsy is the likelihood of a synchronous primary thyroid malignancy.
Our study contains several limitations to consider, mostly related to the retrospective technique. The retrospective technique allowed us to efficiently study this relatively uncommon condition with our search spanning 38,302 studies from 12/1999 to 12/2014. This caused a reliance on imaging reports to identity cases, a technique that may have missed cases in which the radiologist did not draw attention to the focal parotid FDG uptake. Also due to the retrospective technique we had little control over the decision of whether or not to pursue a biopsy in the patients with focal parotid FDG uptake. There may thus be a selection/referral bias regarding the decision to refer for biopsy and preselection that occurred prior to our patients receiving a pathology diagnosis. This bias could potentially have significantly altered our results, however we did find that the pathology follow up rate was not statistically significantly different between our three primary malignancy categories. We considered clinical follow-up as a proxy for pathology but found that this added significant uncertainty as to which category a patient belonged in and only added a modest number of cases. The results of our modest retrospective study should thus be interpreted with caution and should hopefully be used as the basis for designing a prospective investigation. These limitations would all be best addressed by a prospective study in which all cases of focal parotid FDG uptake were identified and subsequently biopsied.
5.1 Conclusion
When encountering incidental focal parotid FDG uptake on PET imaging the patient's primary malignancy type and the presence of FDG-avid cervical lymph node(s) are factors that should be taken into consideration. In the setting of head and neck cancer/melanoma, lymphoma, or FDG-avid cervical lymph node(s), focal parotid FDG uptake had a higher odds of representing a manifestation of the patient's known malignancy. In the absence of these factors benign primary parotid tumors were more commonly encountered. No incidental synchronous malignant primary parotid lesions were encountered in this study.
Acknowledgments
MCM and RRF are supported by an NIH T32 training grant (5T32EB001631-10).
Footnotes
Authors report no relevant financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Podoloff DA, et al. NCCN task force report: positron emission tomography (PET)/computed tomography (CT) scanning in cancer. J Natl Compr Canc Netw. 2007;5 Suppl 1:S1–22. quiz S23-2. [PubMed] [Google Scholar]
- 2.Fletcher JW, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49(3):480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 3.Weber WA, Grosu AL, Czernin J. Technology Insight: advances in molecular imaging and an appraisal of PET/CT scanning. Nat Clin Pract Oncol. 2008;5(3):160–70. doi: 10.1038/ncponc1041. [DOI] [PubMed] [Google Scholar]
- 4.Ishimori T, Patel PV, Wahl RL. Detection of unexpected additional primary malignancies with PET/CT. J Nucl Med. 2005;46(5):752–7. [PubMed] [Google Scholar]
- 5.Beatty JS, et al. Incidental PET/CT findings in the cancer patient: how should they be managed? Surgery. 2009;146(2):274–81. doi: 10.1016/j.surg.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Eloy JA, et al. The significance and management of incidental [18F]fluorodeoxyglucose-positron-emission tomography uptake in the thyroid gland in patients with cancer. AJNR Am J Neuroradiol. 2009;30(7):1431–4. doi: 10.3174/ajnr.A1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treglia G, et al. Clinical significance of incidental focal colorectal (18)F-fluorodeoxyglucose uptake: our experience and a review of the literature. Colorectal Dis. 2012;14(2):174–80. doi: 10.1111/j.1463-1318.2011.02588.x. [DOI] [PubMed] [Google Scholar]
- 8.Hyun SH, et al. Incidental focal 18F-FDG uptake in the pituitary gland: clinical significance and differential diagnostic criteria. J Nucl Med. 2011;52(4):547–50. doi: 10.2967/jnumed.110.083733. [DOI] [PubMed] [Google Scholar]
- 9.Agress H, Jr, Cooper BZ. Detection of clinically unexpected malignant and premalignant tumors with whole-body FDG PET: histopathologic comparison. Radiology. 2004;230(2):417–22. doi: 10.1148/radiol.2302021685. [DOI] [PubMed] [Google Scholar]
- 10.Tatlidil R, et al. Incidental colonic fluorodeoxyglucose uptake: correlation with colonoscopic and histopathologic findings. Radiology. 2002;224(3):783–7. doi: 10.1148/radiol.2243011214. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, et al. Evaluation of thyroid FDG uptake incidentally identified on FDG-PET/CT imaging. Nucl Med Commun. 2009;30(3):240–4. doi: 10.1097/MNM.0b013e328324b431. [DOI] [PubMed] [Google Scholar]
- 12.Nayan S, Ramakrishna J, Gupta MK. The Proportion of Malignancy in Incidental Thyroid Lesions on 18-FDG PET Study: A Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg. 2014;151(2):190–200. doi: 10.1177/0194599814530861. [DOI] [PubMed] [Google Scholar]
- 13.Basu S, Houseni M, Alavi A. Significance of incidental fluorodeoxyglucose uptake in the parotid glands and its impact on patient management. Nucl Med Commun. 2008;29(4):367–73. doi: 10.1097/MNM.0b013e3282f8147a. [DOI] [PubMed] [Google Scholar]
- 14.Lee SK, Rho BH, Won KS. Parotid incidentaloma identified by combined 18F-fluorodeoxyglucose whole-body positron emission tomography and computed tomography: findings at grayscale and power Doppler ultrasonography and ultrasound-guided fine-needle aspiration biopsy or core-needle biopsy. Eur Radiol. 2009;19(9):2268–74. doi: 10.1007/s00330-009-1407-5. [DOI] [PubMed] [Google Scholar]
- 15.Rubello D, et al. Does 18F-FDG PET/CT play a role in the differential diagnosis of parotid masses. Panminerva Med. 2005;47(3):187–9. [PubMed] [Google Scholar]
- 16.Uchida Y, et al. Diagnostic value of FDG PET and salivary gland scintigraphy for parotid tumors. Clin Nucl Med. 2005;30(3):170–6. doi: 10.1097/00003072-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Wang HC, et al. Efficacy of conventional whole-body (1)(8)F-FDG PET/CT in the incidental findings of parotid masses. Ann Nucl Med. 2010;24(8):571–7. doi: 10.1007/s12149-010-0394-6. [DOI] [PubMed] [Google Scholar]
- 18.Hadiprodjo D, et al. Parotid gland tumors: preliminary data for the value of FDG PET/CT diagnostic parameters. AJR Am J Roentgenol. 2012;198(2):W185–90. doi: 10.2214/AJR.11.7172. [DOI] [PubMed] [Google Scholar]
- 19.Adams HL, Jaunoo SS. Clinical significance of incidental findings on staging positron emission tomography for oesophagogastric malignancies. Ann R Coll Surg Engl. 2014;96(3):207–10. doi: 10.1308/003588414X13814021678871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SB, et al. Diagnostic Criteria on (18)F-FDG PET/CT for Differentiating Benign from Malignant Focal Hypermetabolic Lesions of Parotid Gland. Nucl Med Mol Imaging. 2012;46(2):95–101. doi: 10.1007/s13139-012-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebro R, Aparici CM, Pampaloni MH. Frequency and clinical implications of incidental new primary cancers detected on true whole-body 18F-FDG PET/CT studies. Nucl Med Commun. 2013;34(4):333–9. doi: 10.1097/MNM.0b013e32835f163f. [DOI] [PubMed] [Google Scholar]
- 22.Treglia G, et al. Prevalence and risk of malignancy of focal incidental uptake detected by fluorine-18-fluorodeoxyglucose positron emission tomography in the parotid gland: a meta-analysis. Eur Arch Otorhinolaryngol. 2014 doi: 10.1007/s00405-014-3308-8. [DOI] [PubMed] [Google Scholar]
- 23.Seo YL, et al. Incidental focal FDG uptake in the parotid glands on PET/CT in patients with head and neck malignancy. Eur Radiol. 2015;25(1):171–7. doi: 10.1007/s00330-014-3397-1. [DOI] [PubMed] [Google Scholar]
- 24.Joo YH, et al. The value of preoperative 18F-FDG PET/CT for the assessing contralateral neck in head and neck cancer patients with unilateral node metastasis (N1-3) Clin Otolaryngol. 2014;39(6):338–44. doi: 10.1111/coa.12295. [DOI] [PubMed] [Google Scholar]
- 25.Lorigan JG, Wallace S, Mavligit GM. The prevalence and location of metastases from ocular melanoma: imaging study in 110 patients. AJR Am J Roentgenol. 1991;157(6):1279–81. doi: 10.2214/ajr.157.6.1950883. [DOI] [PubMed] [Google Scholar]
- 26.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 27.Team R.C. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]


