Abstract
Tinnitus and hyperacusis are common, burdensome sources of morbidity with a high rate of co-occurrence. Knudson et al. (J Neurophysiol 112: 3197–3208, 2014) demonstrated that efferent suppression of cochlear activity by the medial olivocochlear system is enhanced in individuals with tinnitus and/or hyperacusis. Their findings stress that atypical activity in the efferent auditory pathway may represent a shared substrate, as well as a potential therapeutic target, in tinnitus and hyperacusis.
Keywords: tinnitus, hyperacusis, medial olivocochlear, efferent
tinnitus, the perception of sound in the absence of acoustic stimulation, and hyperacusis, markedly decreased sound-level tolerance (SLT) to moderate-intensity sounds, are prevalent and burdensome conditions with a high rate of comorbidity. The prevalence of hyperacusis in the tinnitus population, for example, has been estimated to be between 40 and 80% (Baguley 2003; Dauman and Bouscau-Faure 2005; Schenklmann et al. 2014), and the prevalence of tinnitus in the hyperacusis population has been reported to be as high as 86% (Anari et al. 1999). This high rate of co-occurrence is suggestive of shared risk factors and/or disease mechanisms involved in tinnitus and hyperacusis. Identifying and characterizing these shared mechanisms is clinically important, since it may facilitate the development of treatments that prove effective in both conditions.
A primary risk factor for both tinnitus and hyperacusis is acoustic overexposure, and both conditions are considered to emerge as maladaptive consequences of the brain's effort to compensate for the cochlear trauma that results from these exposures. Acoustic overexposure leads to cochlear damage as well as to reduced cochlear nerve activity, and as a result, the central auditory system is provided with reduced input. In response to this decrease in input, many structures in the central auditory pathway become hyperactive, including the dorsal (DCN) (but see Ma and Young 2006) and anteroventral cochlear nuclei (AVCN), the inferior colliculus (IC), and the auditory cortex (AC) (Noreña and Farley 2013). This hyperactivity manifests as a combination of elevated spontaneous firing rates (SFRs) (Brozoski et al. 2002; Kaltenbach et al. 1998), increased burst firing (Chang et al. 2002), steeper rate-level functions (RLFs) (Brozoski et al. 2002; Cai et al. 2009) and increased firing responses to acoustic stimulation (Finlayson and Kaltenbach 2009; Wang et al. 1996). Although the hyperactive state of the central auditory pathway after cochlear trauma may help to compensate for decreased cochlear activity reaching the brain, it also likely contributes to the perception of phantom sounds in tinnitus and to the reduced SLT in hyperacusis.
An ongoing debate in the field concerns whether noise-induced hyperactivity after acoustic overexposure predominantly arises from “bottom-up” (afferent) or “top-down” (efferent) sources. The afferent model hypothesizes that tinnitus/hyperacusis perception is driven by central hyperactivity that is generated intrinsically in the auditory brain stem and then relayed to higher thalamocortical centers (Noreña and Farley 2013). This hyperactivity may be generated intrinsically in the cochlear nuclei (CN) as a compensatory response to decreased afferent input (Li et al. 2013; Middleton et al. 2011). CN hyperactivity may also be driven, in part, by input from the increased proportion of low-threshold, high-SFR spiral ganglion afferent neurons that remains after the loss of high-threshold, low-SFR neurons that follows noise trauma (Furman et al. 2013). In contrast to the afferent model, the efferent model highlights top-down neuromodulation as a major driving force behind the emergence and maintenance of hyperactivity (Geven et al. 2014). Certainly, these models need not be mutually exclusive, and afferent and efferent processes may dominate different chronological stages of disease development (e.g., induction vs. maintenance).
One putative effector of top-down modulation in humans with tinnitus and hyperacusis is the medial olivocochlear (MOC) system. The cell bodies of MOC neurons are located in the medioventral periolivary region of the superior olivary complex (SOC) and the ventral nucleus of the trapezoid body (VNTB), and send their axons to either one or both cochleas to innervate and inhibit outer hair cells (OHCs) (Fig. 1A) (Guinan 2006). MOC neurons inhibit OHCs by releasing acetylcholine (ACh), which binds to postsynaptic α9α10 nicotinic ACh receptors, leading to intracellular calcium influx and the subsequent activation of both small-conductance (SK) and big-conductance (BK) calcium-dependent potassium channels (Fig. 1B) (Rohmann et al. 2015). Opening of these potassium channels, in turn, leads to potassium efflux and hyperpolarization of the OHC (Fig. 1B). Since OHC activity is a major determinant of basilar membrane motion (and by extension, acoustic sensitivity), MOC neurons are situated in an ideal location to modulate afferent auditory drive.
Fig. 1.
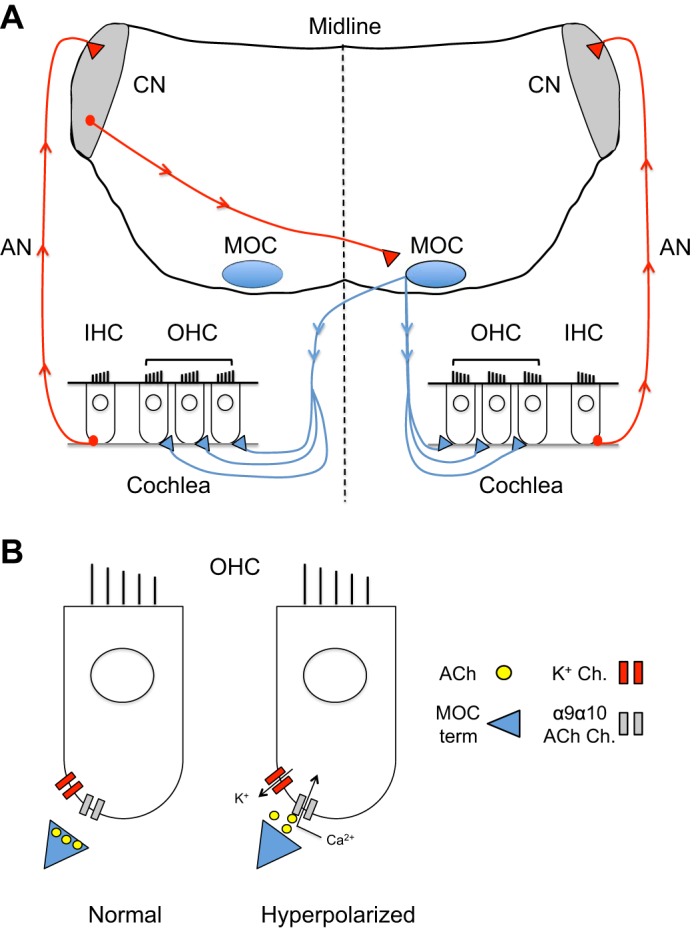
Inhibition of cochlear outer hair cells (OCHs) by the medial olivocochlear (MOC) system. A: cochlear inner hair cells (IHCs) send afferent excitatory input (red line) to the ipsilateral cochlear nuclei (CN) via the auditory nerve (AN). Neurons in the CN then send excitatory outputs across the midline to synapse in the contralateral MOC system. MOC neurons then send cholinergic projections (blue) to either one or both cochleas, where they have a net inhibitory effect on OHCs. B: OHCs are inhibited when acetylcholine (ACh) is released from MOC terminals and binds to α9α10 nicotinic ACh receptors on OHCs. Opening of these ACh receptors leads to a local influx of calcium, which activates calcium-dependent potassium channels (K+ Ch.) and leads to potassium efflux and membrane hyperpolarization.
Evidence for anomalous MOC function in tinnitus and/or hyperacusis, however, has been inconsistent, with some studies reporting impressive changes in MOC function (Attias et al. 1996) and others reporting no difference compared with controls (Geven et al. 2011). In a recent article, Knudson et al. (2014) hypothesized that this inconsistency of findings concerning MOC function in tinnitus was related to variations in SLT (hyperacusis) between participants. The basis of their argument was that since SLT varies widely between individuals, and MOC function likely plays a critical role in determining SLT, grouping participants with low and high SLT together might conceal meaningful differences in MOC function between individuals with and without tinnitus.
To test this hypothesis, Knudson et al. (2014) evaluated MOC function in patients with and without tinnitus, stratifying individual cases in each group further according to SLT levels (low vs. high). They evaluated MOC function in all participants by measuring the magnitude of distortion-product otoacoustic emissions (DPOAE) in the presence and absence of contralaterally presented sounds. DPOAEs are sounds generated by OHCs in response to acoustic stimulation. A common protocol for evoking and measuring DPOAEs involves presenting a pair of primary tones (f1 and f2) and measuring the distortion product (2f1-f2) of the emission that follows. DPOAE magnitude can be measured in the presence and absence of broadband noise presented to the contralateral ear, and the reduction in DPOAE magnitude that occurs in the presence of contralateral noise is interpreted as MOC-mediated inhibition of OHC activity (Puel and Rebillard 1990). Therefore, an increase in the magnitude of this DPOAE suppression is often interpreted as an increase in MOC-mediated inhibition of the OHCs that produce the DPOAE signal.
The primary finding of Knudson et al. (2014) was that DPOAE suppression by contralateral noise was increased in individuals with tinnitus and/or low SLT compared with individuals without tinnitus who also had high (normal) SLT. These results were interpreted to indicate an increase in MOC-mediated inhibition of OHC activity in individuals with tinnitus and/or hyperacusis compared with individuals without either condition. Importantly, the increased DPOAE suppression seen in individuals with tinnitus and/or low SLT could not be attributed to intergroup differences in a number of potentially confounding variables, including age, hearing threshold, baseline DPOAE magnitude, and stapedial reflex magnitude. Furthermore, there were no significant differences in DPOAE suppression between participants with tinnitus that had low SLT, participants with tinnitus that had high SLT, and participants without tinnitus that had low SLT, indicating that neither tinnitus nor hyperacusis (low SLT) alone could explain the enhancement of DPOAE suppression. Therefore, it may be that a common underlying process is driving enhanced MOC inhibition of OHCs in both conditions.
As Knudson et al. (2014) point out, the increased suppression of DPOAEs by contralateral noise in patients with tinnitus or low SLT could be indicative of a number of changes in the efferent MOC system. For instance, increased suppression of DPOAEs would be predicted if MOC neurons themselves became more excitable in response to incoming synaptic inputs. This might occur if MOC neurons in patients with tinnitus and/or low SLT exhibited altered intrinsic excitability because of changes in the expression and/or gating properties of voltage-gated potassium channels, such as have been described in the DCN of mice with behavioral evidence of tinnitus (Li et al. 2013). Increases in MOC neuron excitability could manifest as increased SFR, lower sound-evoked firing thresholds, altered dynamic range, and/or steepness of the input-output relation of sound vs. firing rate.
Additionally, increased MOC activity could be driven by changes in the presynaptic inputs to MOC neurons. MOC neurons receive excitatory synaptic inputs from T-stellate neurons in the contralateral VCN, via the cochlear nucleus commissure (Brown et al. 2003, 2013), and strengthening of these inputs could lead to increased MOC-mediated suppression of OHC activity. Consistent with this hypothesis, conductive hearing loss and cochlear ablation have been previously shown to increase the proportion of VCN neurons that receive sound-evoked excitatory commissural input from the unaffected ear (Bledsoe et al. 2009; Sumner et al. 2005), and this enhanced excitatory commissural input might extend to MOC neurons, as well. In addition, MOC fibers send collaterals to the VCN, which are thought to synapse onto T-stellate neurons and create a MOC-VCN feedback circuit (Fujino and Oertel 2001). Although functional studies in animal models with acoustic trauma are currently lacking, it is possible that alterations in this feedback circuit might also contribute to changes in DPOAE suppression in tinnitus and/or hyperacusis.
The intrinsic and synaptic changes that trigger increased MOC-mediated suppression of OHCs may themselves be driven by a combination of top-down and bottom-up processes. In the top-down framework, cochlear damage would drive “forebrain-mediated neuromodulation,” which in turn would produce a coordinated overactivation of many auditory brain stem structures, including the MOC and/or its presynaptic inputs. In the bottom-up framework, on the other hand, cochlear damage would first lead to increased spontaneous and/or evoked firing in the cochlear nuclei, which in turn would drive enhancement of MOC-mediated suppression of OHC activity.
Regardless of whether changes in MOC activity arise from bottom-up or top-down processes, increases in MOC activity could drive further hyperactivity. An enhancement of MOC-mediated suppression of OHC activity could decrease cochlear activity and inhibit cochlear nerve output to the brain, further depriving the central auditory pathways of input. In this way, MOC-mediated suppression of cochlear activity might itself lead to a homeostatic increase in evoked firing in certain neurons of the VCN, similar to what has been characterized after acoustic trauma (Cai et al. 2009). This homeostasis could involve increases in intrinsic neuronal excitability, such as have been demonstrated in the DCN (Li et al. 2013), and/or decreases in synaptic inhibition, such as have been seen in the DCN (Middleton et al. 2011), IC (Wang et al. 2009), and AC (Yang et al. 2011). These homeostatic increases in evoked firing in VCN neurons could create a positive-feedback loop, where MOC neuron-mediated suppression of cochlear function leads to increases in spontaneous and/or evoked firing in the ascending central auditory pathway, which in turn might lead to further enhancement of MOC-mediated suppression of cochlear activity.
Experiments in rodent models could help to differentiate between the top-down and bottom-up frameworks. For example, a comparison between the time courses of emerging hyperactivity following acoustic overexposure in the efferent MOC system and in afferent auditory brainstem nuclei such as the DCN and AVCN might help to distinguish top-down mechanisms from bottom-up processes. In these studies, tinnitus and hyperacusis behavior could be quantified with acoustic startle-based behavioral measures of gap detection (tinnitus) and sound responsiveness (hyperacusis) (Hayes et al. 2014; Hickox and Liberman 2014). If Knudson et al. (2014) are correct, and enhancement of MOC-mediated inhibition of OHCs is related to a top-down, forebrain-mediated overactivation of auditory brain stem structures, then one would predict that patterns of hyperactivity within the MOC and DCN would emerge along similar timescales. Alternatively, if enhancement of efferent MOC activity were a compensatory response to hyperactivity in the DCN and/or AVCN, then one would predict that this enhancement in the MOC would emerge after afferent hyperactivity was first established in the cochlear nuclei.
Regardless of the precise mechanistic basis for the enhancement of MOC-mediated suppression of OHCs in tinnitus and hyperacusis, it will be crucial to determine whether the role of this enhancement is predominately pathological or protective. In animal models, this could be accomplished by activating and inactivating efferent MOC fibers projecting to OHCs in vivo, either immediately following cochlear damage (to look at disease induction), or after behavioral evidence of tinnitus/hyperacusis has been established (to look at disease maintenance).
Taken together, the data presented by Knudson et al. (2014) suggest that efferent suppression of cochlear output by the MOC is enhanced in patients with tinnitus and/or hyperacusis. These findings are intriguing, and they raise many exciting scientific questions, which are experimentally tractable and may have important clinical implications. For example, if enhanced MOC-mediated suppression of cochlear activity contributes to tinnitus and/or hyperacusis, then selective reductions of MOC pathway activity may prove useful in ameliorating patients' symptoms. Furthermore, the data presented by Knudson et al. also help to explain why the role of the MOC in tinnitus has remained so unclear in the scientific community. By incorporating SLT information, as well a number of additional crucial controls, they were able to clearly illustrate an enhancement of efferent MOC activity in patients with either tinnitus or low SLT while also providing a parsimonious explanation for how these differences could have been overlooked in previously utilized study designs.
In conclusion, the medial olivocochlear system appears to be more strongly engaged by acoustic stimulation in humans with tinnitus and/or hyperacusis, suggesting a shared role for enhanced efferent modulation of cochlear activity in both conditions. The findings presented by Knudson et al. (2104) significantly advance the field by highlighting the importance of taking hyperacusis into account in all future human studies of tinnitus to avoid concealing meaningful differences between individuals. Although it remains to be determined whether enhancement of MOC activity plays a pathological or protective role, the evidence presented by Knudson et al. opens up new and exciting avenues for the development of therapies that could prove useful in treating tinnitus and hyperacusis simultaneously.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grants 1F30DCO14177 (to J. J. Sturm) and 1F32DC013207 (to C. J. C. Weisz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J.S. and C.J.C.W. prepared figures; J.J.S. and C.J.C.W. drafted manuscript; J.J.S. and C.J.C.W. edited and revised manuscript; J.J.S. and C.J.C.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Karl Kandler for scholarly support and valuable comments on a previous version of the manuscript.
REFERENCES
- Anari M, Axelsson A, Eliasson A, Magnusson L. Hypersensitivity to sound. Questionnaire data, audiometry and classification. Scand Audiol 28: 219–230, 1999. [DOI] [PubMed] [Google Scholar]
- Attias J, Bresloff I, Furman V. The influence of the efferent auditory system on otoacoustic emissions in noise induced tinnitus: clinical relevance. Acta Otolaryngol 116: 534–539, 1996. [DOI] [PubMed] [Google Scholar]
- Baguley DM. Hyperacusis. J R Soc Med 96: 582–585, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe SC, Koehler S, Tucci DL, Zhou J, Le Prell C, Shore SE. Ventral cochlear nucleus responses to contralateral sound are mediated by commissural and olivocochlear pathways. J Neurophysiol 102: 886–900, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, De Venecia RK, Guinan JJ Jr. Responses of medial olivocochlear neurons: specifying the central pathways of the medical olivocochlear reflex. Exp Brain Res 153: 491–498, 2003. [DOI] [PubMed] [Google Scholar]
- Brown MC, Mukerji S, Drottar M, Windsor AM, Lee DJ. Identification of inputs to olivocochlear neurons using transneuronal pseudorabies virus (PRV). J Assoc Res Otolaryngol 5: 703–717, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski J, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci 22: 2383–2390, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Wei-Li MD, Young ED. Encoding intensity in ventral cochlear nucleus following acoustic trauma: implications for loudness recruitment. J Assoc Res Otolaryngol 10: 5–22, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Cehn K, Kaltenbach JA, Zhang J, Godfrey DA. Effects of acoustic trauma on dorsal cochlear nucleus neuron activity in slices. Hear Res 164: 59–68, 2002. [DOI] [PubMed] [Google Scholar]
- Dauman R, Bouscau-Faure F. Assessment and amelioration of hyperacusis in tinnitus patients. Acta Otolaryngol 5: 503–509, 2010. [DOI] [PubMed] [Google Scholar]
- Finlayson PG, Kaltenbach JA. Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hear Res 256: 104–117, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Cholinergic modulation of stellate cells in the mammalian ventral cochlear nucleus. J Neurosci 21: 7372–7383, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol 110: 577–586, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geven L, de Kleine E, Free RH, van Dijk P. Contralateral suppression of otoacoustic emissions in tinnitus patients. Otol Neurotol 32: 315–321, 2011. [DOI] [PubMed] [Google Scholar]
- Geven L, Koppl C, de Kleine E, van Dijk P. Plasticity in tinnitus patients: a role for the efferent auditory system? Otol Neurotol 35: 796–802, 2014. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear 27: 589–607, 2006. [DOI] [PubMed] [Google Scholar]
- Hayes SH, Radziwon KE, Stolzberg DJ, Salvi RJ. Behavioral models of tinnitus and hyperacusis in animals. Front Neurol 5: 179, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol 111: 552–564, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA, Neumann JB, McCaslin DL, Afman CE, Zhang J. Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: relation to threshold shift. Hear Res 124: 78–84, 1998. [DOI] [PubMed] [Google Scholar]
- Knudson IM, Shera CA, Melcher JR. Increased contralateral suppression of otoacoustic emissions indicates a hyper-responsive medical olivocochlear system in humans with tinnitus and hyperacusis. J Neurophysiol 112: 3197–3208, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Choi V, Tzounopoulos T. Pathogenic plasticity of Kv7.2/3 channel activity is essential for the induction of tinnitus. Proc Natl Acad Sci USA 110: 9980–9985, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WL, Young ED. Dorsal cochlear nucleus response properties following acoustic trauma: response maps and spontaneous activity. Hear Res 216: 176–188, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pederson C, Turner J, Shepherd G. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci USA 108: 7601–7606, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreña AJ, Farley BJ. Tinnitus related neural activity: theories of generation, propagation and centralization. Hear Res 295: 161–171, 2013. [DOI] [PubMed] [Google Scholar]
- Puel JL, Rebillard G. Effects of contralateral sound stimulation on the distortion product 2F1-F2: evidence that the medial efferent system is involved. J Acoust Soc Am 87: 1630–1635, 1990. [DOI] [PubMed] [Google Scholar]
- Rohmann KN, Wersinger E, Braude JP, Pyott SJ, Fuchs PA. Activation of BK and SK channels by efferent synapses on outer hair cells in high-frequency regions of the rodent cochlea. J Neurosci 35: 1821–1830, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M, Landgrebe M, Langguth T; the TRI Database Study Group. Phenotypic characteristics of hyperacusis in tinnitus. PLoS One 9: e86944, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner CJ, Tucci DL, Shore SE. Responses of ventral cochlear nucleus neurons to contralateral sound after conductive hearing loss. J Neurophysiol 94: 4234–4243, 2005. [DOI] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage. Hear Res 168: 238–249, 2009. [DOI] [PubMed] [Google Scholar]
- Wang J, Salvi RJ, Powers N. Plasticity of response properties of inferior colliculus neurons following acute cochlear damage. J Neurophysiol 75: 171–183, 1996. [DOI] [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci USA 108: 14974–14979, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


