Abstract
Purpose
To investigate the efficacy of topical applied aloe vera (AV) and to facilitate the repair of the standardized alkaline corneal ulcer in normal and diabetic rats.
Materials and methods
The corneal alkali-burn injury model was established unilaterally in Wistar rats by filter paper saturated with 0.01 M NaOH contacting the eyes for 45 seconds. Rats were divided into four groups: normal control (NC), normal AV (NAV), diabetic control (DC), and diabetic AV (DAV). NAV and DAV groups were treated with AV gel eye drops four times daily, and NC and DC groups were treated with normal saline for 3 days. Corneal epithelial wound closure and degree of edema were recorded using slit lamp and optical coherence tomography at 0, 24, 48, and 72 hours postwounding. Histological examination was conducted to evaluate the degree of inflammation and the healing effect.
Results
Corneal epithelial wound healing was better in the NAV group than in the NC group, and it was significantly higher in the DAV group than in the DC group (P<0.05). In comparison to the DC group, DAV treated with AV demonstrated a marked reduction in edema at 48 and 72 hours. Histologically, corneal re-epithelialization was complete and higher in DAV group than that in DC group; moreover, the inflammatory cells were increased in DC group than DAV group (P<0.05).
Conclusion
This study demonstrated the efficacy of AV for enhanced corneal re-epithelialization, as well as reduced inflammatory response after alkali burn in rats; therefore, it could be useful as a therapy for diabetic keratopathy.
Keywords: aloe vera, cornea, epithelium, diabetes, wound healing
Introduction
Corneal epithelial defects usually undergo rapid resurfacing by the surrounding epithelial cells unless profound concurrent inflammation or ocular surface disease occurs. Corneal epithelial defects have been associated with diabetes.1–3
Patients with diabetes are at increased risk for developing corneal disorders, termed diabetic keratopathy.4–7 Diabetic keratopathy has been estimated to occur in 47%–64% of diabetic patients during the course of their disease.8 Some corneal disorders associated with diabetic keratopathy, include in the form of nonhealing epithelial defects, which may result in infections, corneal ulcers, secondary scarring, and permanent visual loss.4–7 These epithelial abnormalities are resistant to conventional treatment regimens (eg, lubricants, antibiotics, bandage contact lens, and tarsorrhaphy).9 Such treatment is passive in nature in that it does not stimulate corneal epithelial wound healing, but rather protects the corneal surface from external stimuli or injuries until complete wound healing.10 Active treatment to stimulate corneal epithelial migration in eyes with nonhealing epithelial defects is a preferable therapeutic approach. However, no active treatment regimen for nonhealing epithelial defects secondary to diabetes has yet become widely established.11 Therefore, it is vital that novel methods for the treatment of this complication be devised and explored, and brought to clinical trial.
Aloe vera (AV) is a perennial succulent belong to the Lily (Liliaceae) family. This plant has been known as “the healing plant”.12 The therapeutic use of this herbal remedy with its wide range of applications has been well documented.13,14 Reported pharmacological actions of AV include wound healing, anti-inflammatory, antibacterial, antioxidant, antiviral, and antifungal actions, as well as antidiabetic activities.15–17 AV extract contains a wide range of principle bioactive compounds responsible for promoting wound healing.13,14,16 The effectiveness of AV in promoting skin wound healing was studied extensively.14,16 Application of AV extract shown to promote wound and burn healing as represented by reducing inflammation, greater collagen content, and increasing re-epithelialization rate.13,18–21 Considering the potential effect of AV extracts on skin re-epithelialization, this extract could be a possible candidate for treating a corneal epithelial wound. A previous study reported that lower concentrations of AV solution may be beneficial in the healing of superficial corneal wounds to help decrease fibrosis and speed epithelialization.22 A literature search indicates that almost no scientific information is available for the effect AV extract on corneal epithelial wound healing in an in vivo diabetic model. Therefore, this study was designed to evaluate the effect of AV on corneal epithelial wound closure in both diabetic and nondiabetic rats.
Materials and methods
Animals
All experimental procedures were approved by the Ethics and Animal Experiments Committee at the University of Kafrelsheikh. Animals were treated in accordance with guidelines provided in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Six-week-old male Wistar rats weighing 130–150 g were used in this study. All the rats were given a period of 2 weeks to acclimatize to the surroundings before starting of experiment and housed under standard laboratory conditions; water and food were continuously available.
Induction of diabetes
Type 1 diabetes was induced as reported previously.23 An intraperitoneal (i.p.) injection of 50 mg/kg Streptozotocin (CAs Number 0018883664; Sigma-Aldrich, Cairo, Egypt) in ice-cold 0.1 M citrate buffer (pH 4.5) was administered. Another group of animals received only citrate buffer, and were considered normal. Blood glucose levels were monitored from the tail vein using glucose-oxidase-impregnated strips and a Blood Glucometer (Accu-Check III, Boehringer Mannheim Diagnostics, Indianapolis, IN), immediately prior to receiving STZ and at 1 week after injection of STZ. Glucose levels of ≥300 mg/dL were considered to be the minimum blood glucose level compatible with a stable, nontoxic diabetic state2 and were found to be fourfold higher than controls within 1 week; glucose levels in STZ rats remained elevated throughout the experimental period.
Model of corneal epithelial wound healing
The procedures for wounding and monitoring repair followed those reported earlier.24 In brief, 2 weeks after injection of STZ, all the rats were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (7 mg/kg) i.p. In each case, the central cornea of the right eye was injured by placing a filter paper (circular 3.0 mm diameter) saturated with 0.01 M NaOH on it for 45 seconds. The wound surface was then washed with 0.9% physiological saline. Rats were divided into four groups (seven rats each) as follows: normal control (NC), normal AV (NAV), diabetic control (DC), and diabetic AV (DAV). Any rat that experienced infection was not included in the study.
Photography
The animals were examined under a slit lamp microscope (Topcon slit lamp SL-D7, Topcon Medical Systems, Inc., Santa Clara, CA, USA) after the alkali burns. Wound sizes were determined by staining the ocular surfaces with 1% fluorescein sodium and then observing under cobalt blue light and photographing immediately after injury (time 0), 24, 48, and 72 hours postwounding. Areas of wounds were quantified from photographs using NIH Image J analyzer software (downloaded from http://imagej.nih.gov/ij/). The percentage of wound healing was calculated using the following formula: (Initial wound area – Wound area)/Initial wound area ×100.
Topical administration
AV was prepared by dissolving AV lyophilized powder (Air Green Co., Lid., Fukui, Japan; (60 mg/mL) in saline. AV-treated groups were treated with AV eye drops as a single drop (0.05 mL) to the central cornea of the injured eye four times daily for 3 days, and the control groups were treated with vehicle (saline) eye drops in the same manner. Eye drops were delivered to unanesthetized rats.
Noninvasive measurements of the cornea
Two measures of ocular health were assessed in all rats for both eyes prior to injury and drug administration and for the injured right eye at 0, 24, 48, and 72 hours postwounding. These measures included general overall morphology and pathology, which include corneal edema examination with slit lamp and corneal topography from a spectral optical coherence tomography (OCT) system (3D OCT 2000 FA plus, Topcon, Tokyo, Japan). The slit lamp and pachymeter were tested on rats anesthetized with a mixture of ketamine (70 mg/kg) and xylazine (7 mg/kg); measures with the slit lamp were conducted before and after dilation with phenylephrine hydrochloride ophthalmic solution 2.5% (Bausch & Lomb Inc., Tampa, FL, USA) and tropicamide ophthalmic solution (Falcon Pharmaceuticals Ltd., Forth Worth, TX, USA). All noninvasive measurements were conducted by observers masked as to treatment group.
Histology and morphometric analysis
Animals were euthanized at 72 hours postwounding with an injection of sodium pentobarbital i.p. (>100 mg/kg), decapitated, and the eyes proptosed and enucleated. Eyes were fixed in 10% neutral buffered formalin for 24 hours, embedded in paraffin, and corneal sections of 5 μm thickness were stained with hematoxylin and eosin and evaluated by light microscopy to observe the structure of the basement membrane, epithelium, and stroma. Using at least two sections per rat cornea, and three animals per experimental group, assessment of the epithelization of the cornea, stroma in the region of the peripheral cornea, and inflammatory cell infiltration were assessed in 4–6 independent fields per section.
Statistical analysis
Data were expressed as mean ± SD. The area of defect was analyzed at each time point using analysis of variance and Newman–Keuls tests, where P<0.05 was considered statistically significant. The statistical analysis was conducted with software (GraphPad Prism for Windows, version 5.0; GraphPad Software Inc., San Diego, CA, USA).
Results
Corneal epithelial wound healing
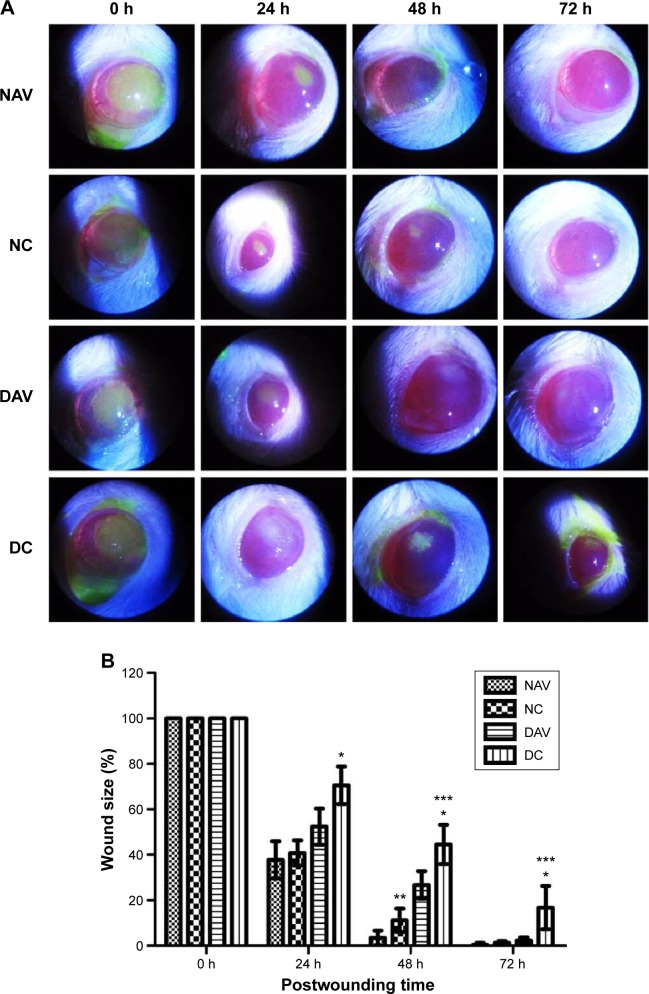
Measurement of the areas of corneal injury stained with fluorescein was used to calculate the size of the wound defect area at 0, 24, 48, and 72 hours postwounding (Figure 1A). No significant difference in the size of the wounds in all rats was observed immediately after induction of burn wound injury. The percentage of wound defect areas of DC group was significantly larger than that of NC at 24, 48, and 72 hours postwounding (P<0.05), while the wound defect areas were consistently smaller in the NAV groups than in the NC group but significant only at 48 hour postwounding (P<0.05). The percentage of wound area in DC group was significantly greater than in the DAV group at 48 and 72 hours postwounding (P<0.05). At 72 hours postwounding, the corneal epithelial defects of NAV, NC, and DAV groups had undergone complete re-epithelialization (Figure 1B).
Figure 1.
Corneal wound healing in normal and diabetic rats treated with AV.
Notes: (A) Slit lamp photographs of fluorescein staining of corneal epithelial defects in NAV, NC, DAV, and DC groups at 0, 24, 48, and 72 hours postwounding. Photographs of fluorescein staining showing better wound healing in the NAV group than in NC group at 24 and 48 hours postwounding, while DAV showing smaller corneal epithelial defect than DC at 24, 48, and 72 hours postwounding. (B) The percentage of wound area in NAV, NC, DAV, and DC groups at 0, 24, 48, and 72 hours postwounding. Data are expressed as mean ± SD (n=7 rats, each group). *Significant difference in the wound area between DC group and NC group (P<0.05). **Significant difference in the wound area between NAV group and NC group (P<0.05). ***Significant difference in the wound area between DAV group and DC group (P<0.05).
Abbreviations: NC, normal control; NAV, normal aloe vera; DC, diabetic control; DAV, diabetic aloe vera; h, hours; SD, standard deviation; AV, aloe vera.
Corneal morphology with slit lamb and OCT
There was a slight decrease in corneal edema in the normal group and DAV group, while there was a significant reduction in the NAV group, and there was significant difference between the DAV group and DC group at 72 hours postwounding.
OCT examination at 0 hour postwounding in all groups revealed a corneal ulcer with irregularity of the epithelium and surrounding edema (Figure 2). At 24, 48, and 72 hours postwounding, corneal ulcer with severe corneal edema is evident and larger in the DC group than other groups. At 72 hours postwounding, epithelium healing is complete in both the NAV and NC groups, with only residual edema in NC group. Corneal edema and incomplete epithelium healing were still evident till 72 hours postwounding in the DC group, while edema was decreased with complete epithelium healing in DAV.
Figure 2.
OCT images.
Notes: (A) Normal cornea, (B) corneal ulcer immediately after alkali burn (0 hour) (long arrow), (C) cornea of DC group at 72 hours postwounding showed corneal edema (short arrow) and incomplete epithelium, (D) cornea of DAV group at 72 hours postwounding showed complete epithelium with some corneal edema, (E) cornea of NC group at 72 hours postwounding showed partial complete epithelium with residual edema, and (F) cornea of NAV at 72 hours postwounding showed complete epithelium.
Abbreviations: OCT, optical coherence tomography; NC, normal control; NAV, normal aloe vera; DC, diabetic control; DAV, diabetic aloe vera.
Histological examinations
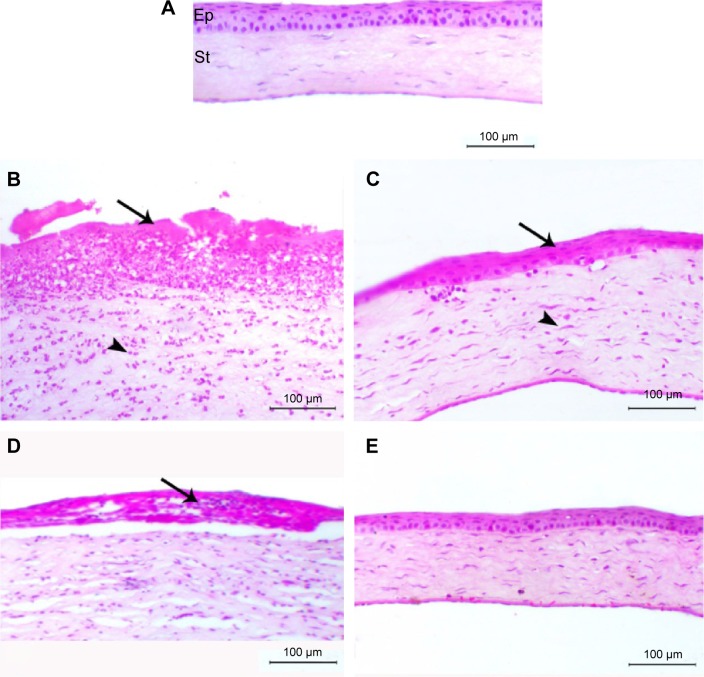
The normal rat eyes showed normal corneal stroma covered with normal epithelium. DC group showed marked corneal epithelial degeneration associated with massive inflammatory cells infiltration, predominately neutrophils, and a lesser number of macrophages. Severe edema within the corneal stroma was also noticed. In NC group, the animals showed corneal epithelial vacuoles, stromal edema, and cellular infiltration. NAV and DAV groups showed complete re-epithelialization represented by multilayered of squamous-like corneal epithelium and some fibroblastic cell proliferation within the stroma associated with minimal inflammatory cells infiltration (Figure 3).
Figure 3.
Photomicrograph of corneal sections of normal and diabetic rats treated with AV.
Notes: (A) The figure showed normal corneal Ep and St; (B) DC group showed marked corneal epithelial degeneration (arrow) with severe neutrophilic infiltration (arrowhead); (C) DAV group showed epithelization of corneal epithelium (arrow) with fibroblastic cell in the stroma (arrowhead); (D) NC group showed corneal epithelial vacuolation with cellular infiltration (arrow) and stromal edema; (E) NAV group showed marked epithelization of cornea. (H&E, bar =100 μm.)
Abbreviations: AV, aloe vera; Ep, epithelium; St, stroma; NC, normal control; NAV, normal aloe vera; DC, diabetic control; DAV, diabetic aloe vera; H&E, hematoxylin and eosin.
Discussion
This study documented for the first time that topical application of AV accelerated corneal wound healing in an in vivo corneal alkali burn model in diabetic rats. The accelerated corneal wound healing was associated with rapid re-epithelialization and reduced inflammation.
Delayed wound healing, erosions, and keratitis, the most serious complications of diabetic keratopathy, are treated only symptomatically.4–7 Numerous treatments have been used in the management of delayed corneal wound healing, such as stem-cell-based therapeutics, growth factors, and gene therapy;25–28 however, despite their beneficial effects, they cannot be used for human therapy on a large scale because of the significant cost and limited commercial quantities.
AV contains multiple pharmacologically active substances including polysaccharides, anthraquinone, lectin, superoxide dismutase, glycoprotein, vitamins A, C, and E, and minerals that reportedly provide anti-inflammatory, immunomodulatory, and wound-healing effects.29 AV is well known for its wound-healing properties.13 It has been reported to stimulate skin wound healing in different wound models.14 Moreover, our previous studies demonstrated that AV accelerated skin wound healing in diabetic, radiation delayed, and burn wound models by increasing fibroblast proliferation, collagen production, and re-epithelialization.19–21 More recently, an in vitro study suggested that an AV solution may be beneficial in the healing of superficial wounds to help decrease fibrosis and speed re-epithelialization.22 In this study, the rates of corneal wound healing were faster following the installation of AV than in the case of saline instillation.
Corneal wound healing is a complex process requiring integrated function of multiple tissues, cell lineages, growth factors, and cytokines.30 Re-epithelialization is a critical contributing process for successful corneal healing. Delayed corneal re-epithelialization is a complication of diabetes, increases the risk of infection, and is associated with heightened inflammation and insufficient stromal remodeling, resulting in loss of transparency of corneal tissues.2,8,31 In our study, corneal epithelial wound healing in diabetic rats was delayed as compared with healing in nondiabetic rats, and topically applied AV facilitated the corneal epithelial wound closure, regardless of whether or not the rats were diabetic.
The previous studies stated that the progressive delay expressed in the healing process of the ocular surface epithelium was time dependent with respect to the diabetic state. Thus, by comparison of the diabetic and control subjects at 4 and 8 weeks, results showed marked delays in the repair of the corneal epithelium.32 However, this study used 2 weeks diabetic rats, but the results in this article stand in agreement with those of other studies, showing a decrease in healing of the corneal epithelium in diabetic rats after 2 weeks of induction of diabetes.2,33 Therefore, further studies of the effects of AV in corneal re-epithelialization after alkali burn in advanced state of diabetes are needed before final recommendations can be made.
Corneal injury induced by an alkali burn usually requires more time for wound closure than those by mechanical deepithelization.34 Such a difference is due to the fact that an alkali burn elicits stromal dysregulated inflammation and scar formation by inducing immune cell infiltration and myofibroblast formation from keratocytes. This type of inflammatory response is not self-limiting and is associated with epithelial and stromal degradation.35 These pathological findings were the basis for our choice of the NaOH alkali burn model to evaluate the effects of AV on corneal wound healing.
The basic mechanisms of corneal re-epithelialization are similar to those seen in other mucous membranes and consist of epithelial migration and proliferation.1 Several biochemical factors, including epidermal growth factor, insulinlike growth factor 1 (IGF-1), platelet-derived growth factor, transforming growth factor (TGF-β), and basic fibroblast growth factor (bFGF), are known to be involved in re-epithelialization.36–38 These factors have been identified as key materials in epithelialization, particularly in cell migration and mitosis.38 Nakamura et al33 have revealed that topical application of substance P and IGF-1 accelerated the corneal epithelial wound-healing process in diabetic animals. Our previous in vivo study reported that AV accelerated skin wound healing and increased epithelialization through increasing TGF and bFGF expression.20 Therefore, the present results suggested that rapid corneal re-epitheization induced by topical AV was also mediated through increased growth factor production. In the future, further studies are required to examine the expression of growth factors in corneal wound healing after AV administration.
Literature data indicate that anti-inflammatory effect might also be connected with AV extract.18,39–42 Mechanistic studies have clearly shown that AV can inhibit inflammatory process, not only by decreasing the level of proinflammatory cytokines,42 but also by reduction of leukocyte adhesion and infiltration in the place of a wound or injury, and decrease in edema.14,43 Our results have also shown a decrease in leukocyte infiltration and edema. Taken together, the instillation of AV decreases inflammation, thus preventing the delay in corneal wound healing in diabetic rats.
Conclusion
Our preliminary data suggest that the instillation of AV has a potent effect in promoting corneal wound healing through facilitating re-epithilaization and reduction in inflammation in diabetic rats. Therefore, AV may be an effective and safe drug to promote corneal wound healing in diabetic keratopathy.
Acknowledgments
This work was financially supported by a grant (number KFURF-12) from Kafrelsheikh University (Kafrelsheikh, Egypt).
Footnotes
Disclosure
The authors report no conflicts of interests in this work.
References
- 1.Foulks GN, Thoft RA, Perry HD, et al. Factors related to corneal epithelial complications after closed vitrectomy in diabetics. Arch Ophthalmol. 1979;97:1076–1078. doi: 10.1001/archopht.1979.01020010530002. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura M, Sato N, Chikama T-I, Hasegawa Y, Nishida T. Fibronectin facilitates corneal epithelial wound healing in diabetic rats. Exp Eye Res. 1997;64(3):355–359. doi: 10.1006/exer.1996.0216. [DOI] [PubMed] [Google Scholar]
- 3.Nagai N, Ito Y. Therapeutic effects of sericin on diabetic keratopathy in Otsuka Long-Evans Tokushima fatty rats. World J Diabetes. 2013;4(6):282–289. doi: 10.4239/wjd.v4.i6.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Diabetic keratopathy. Trans Am Ophthalmol Soc. 1981;79:180–199. [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez-Thorin JC. The cornea in diabetes mellitus. Int Ophthalmol Clin. 1998;38(2):19–36. [PubMed] [Google Scholar]
- 6.Cisarik-Fredenburg P. Discoveries in research on diabetic keratopathy. Optometry. 2001;72(11):691–704. [PubMed] [Google Scholar]
- 7.Kaji Y. Prevention of diabetic keratopathy. Br J Ophthalmol. 2005;89(3):254–255. doi: 10.1136/bjo.2004.055541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zagon IS, Sassani JW, Carroll MA, McLaughlin PJ. Topical application of naltrexone facilitates reepithelialization of the cornea in diabetic rabbits. Brain Res Bull. 2010;81(2–3):248–255. doi: 10.1016/j.brainresbull.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulze SD, Sekundo W, Kroll P. Autologous serum for the treatment of corneal epithelial abrasions in diabetic patients undergoing vitrectomy. Am J Ophthalmol. 2006;142(2):207–211. doi: 10.1016/j.ajo.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Kabosova A, Kramerov AA, Aoki AM, Murphy G, Zierske JD, Ljubimov AV. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp Eye Res. 2003;77:211–217. doi: 10.1016/s0014-4835(03)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada N, Morishige N, Yanai R, et al. Open clinical study of eye drops containing the fibronectin-derived peptide PHSRN for treatment of persistent corneal epithelial defects. Cornea. 2012;31(12):1408–1413. doi: 10.1097/ICO.0b013e31824afd6c. [DOI] [PubMed] [Google Scholar]
- 12.Choi SW, Son BW, Son YS, Park YI, Lee SK, Chung MH. The wound-healing effect of a glycoprotein fraction isolated from aloe vera. Br J Dermatol. 2001;145:535–545. doi: 10.1046/j.1365-2133.2001.04410.x. [DOI] [PubMed] [Google Scholar]
- 13.Maenthaisong R, Chaiyakunapruk N, Niruntraporn S, Kongkaew C. The efficacy of aloe vera used for burn wound healing: a systematic review. Burns. 2007;33:713–718. doi: 10.1016/j.burns.2006.10.384. [DOI] [PubMed] [Google Scholar]
- 14.Haniadka R, Kamble P, Azmidha A, et al. Review on the use of aloe vera (Aloe) in dermatology. In: Watson RR, Zibadi S, editors. Bioactive Dietary Factors and Plant Extracts in Dermatology. New York, NY: Humana Press; 2013. pp. 125–133. [Google Scholar]
- 15.Choi S, Chung M-H. A review on the relationship between aloe vera components and their biologic effects. Sem Integrative Med. 2003;1:53–62. [Google Scholar]
- 16.Hamman JH. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13:1599–1616. doi: 10.3390/molecules13081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph B, Raj SJ. Pharmacognostic and phytochemical properties of Aloe Vera Linn – an overview. Int J Pharm Sci Rev Res. 2010;4:106–110. [Google Scholar]
- 18.Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on collagen characteristics in healing dermal wounds in rats. Mol Cell Biochem. 1998;181:71–76. doi: 10.1023/a:1006813510959. [DOI] [PubMed] [Google Scholar]
- 19.Atiba A, Ueno H, Uzuka Y. The effect of aloe vera oral administration on cutaneous wound healing in type 2 diabetic rats. J Vet Med Sci. 2011;73:583–589. doi: 10.1292/jvms.10-0438. [DOI] [PubMed] [Google Scholar]
- 20.Atiba A, Nishimura M, Kakinuma S, et al. Aloe vera oral administration accelerates acute radiation-delayed wound healing by stimulating transforming growth factor-beta and fibroblast growth factor production. Am J Surg. 2011;201:809–818. doi: 10.1016/j.amjsurg.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Atiba A, Marzok M, Ghazy A. Comparison of aloe vera and silver sulfadiazine in the treatment of deep second-degree burn in dogs. Global Veterinaria. 2014;13(5):733–737. [Google Scholar]
- 22.Curto EM, Labelle A, Chandler HL. Aloe vera: an in vitro study of effects on corneal wound closure and collagenase activity. Vet Ophthalmol. 2014;17:403–410. doi: 10.1111/vop.12163. [DOI] [PubMed] [Google Scholar]
- 23.Prasad SK, Kulshreshtha A, Qureshi TN. Antidiabetic activity of some herbal plants in streptozotocin induced diabetic albino rats. Pak J Nutr. 2009;8:551–557. [Google Scholar]
- 24.Kim EC, Kim TK, Park SH, Kim MS. The wound healing effects of vitamin A eye drops after a corneal alkali burn in rats. Acta Ophthalmol. 2012;90:540–546. doi: 10.1111/j.1755-3768.2012.02496.x. [DOI] [PubMed] [Google Scholar]
- 25.Ye J, Yao K, Kim JC. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: engraftment and involvement in wound healing. Eye. 2005;20(4):482–490. doi: 10.1038/sj.eye.6701913. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin PJ, Sassani JW, Klocek MS, Zagon IS. Diabetic keratopathy and treatment by modulation of the opioid growth factor (OGF) – OGF receptor (OGFr) axis with naltrexone: a review. Brain Res Bull. 2010;81(2–3):236–247. doi: 10.1016/j.brainresbull.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdelkader H, Patel DV, McGhee CNJ, Alany RG. New therapeutic approaches in the treatment of diabetic keratopathy: a review. Clin Experiment Ophthalmol. 2011;39(3):259–270. doi: 10.1111/j.1442-9071.2010.02435.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Q, Chen P, Di G, et al. Ciliary neurotrophic factor promotes the activation of corneal epithelial stem/progenitor cells and accelerates corneal epithelial wound healing. Stem Cells. 2015;33(5):1566–1576. doi: 10.1002/stem.1942. [DOI] [PubMed] [Google Scholar]
- 29.Surjushe A, Vasani R, Saple DG. Aloe vera: a short review. Indian J Dermatol. 2008;53(4):163–166. doi: 10.4103/0019-5154.44785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueno M, Lyons BL, Burzenski LM, et al. Accelerated wound healing of alkali-burned corneas in MRL mice is associated with a reduced inflammatory signature. Invest Ophthalmol Vis Sci. 2005;46(11):4097–4106. doi: 10.1167/iovs.05-0548. [DOI] [PubMed] [Google Scholar]
- 31.Zagon IS, Sassani JW, Myers RL, McLaughlin PJ. Naltrexone accelerates healing without compromise of adhesion complexes in normal and diabetic corneal epithelium. Brain Res Bull. 2007;72(1):18–24. doi: 10.1016/j.brainresbull.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Zagon IS, Jenkins JB, Sassani JW, et al. Naltrexone, an opioid antagonist, facilitates reepithelialization of the cornea in diabetic rat. Diabetes. 2002;51(10):3055–3062. doi: 10.2337/diabetes.51.10.3055. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura M, Kawahara M, Morishige N, Chikama T, Nakata K, Nishida T. Promotion of corneal epithelial wound healing in diabetic rats by the combination of a substance P-derived peptide (FGLM-NH2) and insulin-like growth factor-1. Diabetologia. 2003;46:839–842. doi: 10.1007/s00125-003-1105-9. [DOI] [PubMed] [Google Scholar]
- 34.Čejková J, Lojda Z, Dropčová S, Kadlecová D. The histochemical pattern of mechanically or chemically injured rabbit cornea after aprotinin treatment: relationships with the plasmin concentration of the tear fluid. Histochem J. 1993;25(6):438–445. doi: 10.1007/BF00157808. [DOI] [PubMed] [Google Scholar]
- 35.Martin LFT, Rocha EM, Garcia SB, Paula JS. Topical Brazilian propolis improves corneal wound healing and inflammation in rats following alkali burns. BMC Complement Altern Med. 2013;13:337–337. doi: 10.1186/1472-6882-13-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19(1):113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 37.Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med. 2001;226(7):653–664. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- 38.Yu FS, Yin J, Xu K, Huang J. Growth factors and corneal epithelial wound healing. Brain Res Bull. 2010;81(2–3):229–235. doi: 10.1016/j.brainresbull.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis RH, Leitner MG, Russo JM. Biological activity of aloe vera. Med Sci Res. 1987;15:530–535. [Google Scholar]
- 40.Davis RH, Didonato JJ, Hartman GM, Haas RC. Anti-inflammatory and wound healing activity of a growth substance in aloe vera. J Am Podiatr Med Assoc. 1994;84:77–81. doi: 10.7547/87507315-84-2-77. [DOI] [PubMed] [Google Scholar]
- 41.Visuthikosol V, Chowchuen B, Sukwanarat Y, Sriurairatana S, Boonpucknavig V. Effect of aloe vera gel to healing of burn wound a clinical and histologic study. J Med Assoc Thai. 1995;78:403–409. [PubMed] [Google Scholar]
- 42.Vázquez B, Avila G, Segura D, Escalante B. Antiinflammatory activity of extracts from A. vera gel. J Ethnopharmacol. 1996;55:69–75. doi: 10.1016/s0378-8741(96)01476-6. [DOI] [PubMed] [Google Scholar]
- 43.Duansak D, Somboonwong J, Patumraj S. Effects of Aloe vera on leukocyte adhesion and TNF-alpha and IL-6 levels in burn wounded rats. Clin Hemorheol Microcirc. 2003;29:239–246. [PubMed] [Google Scholar]