Abstract
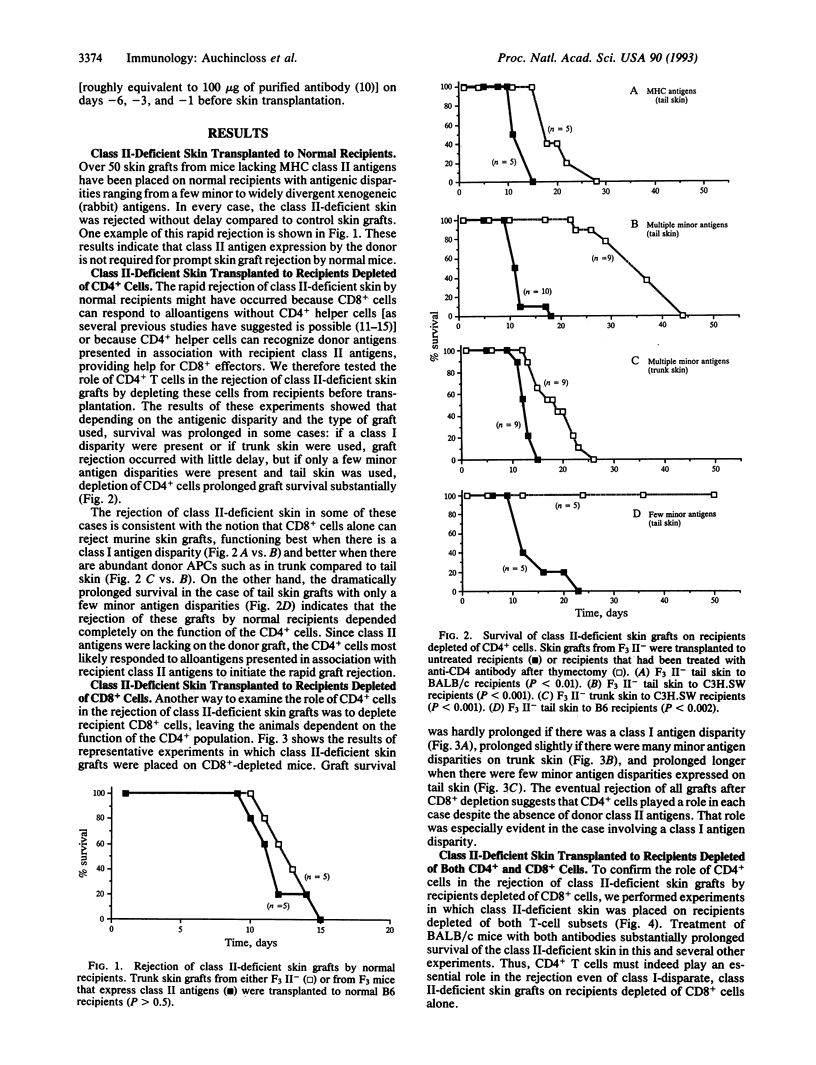
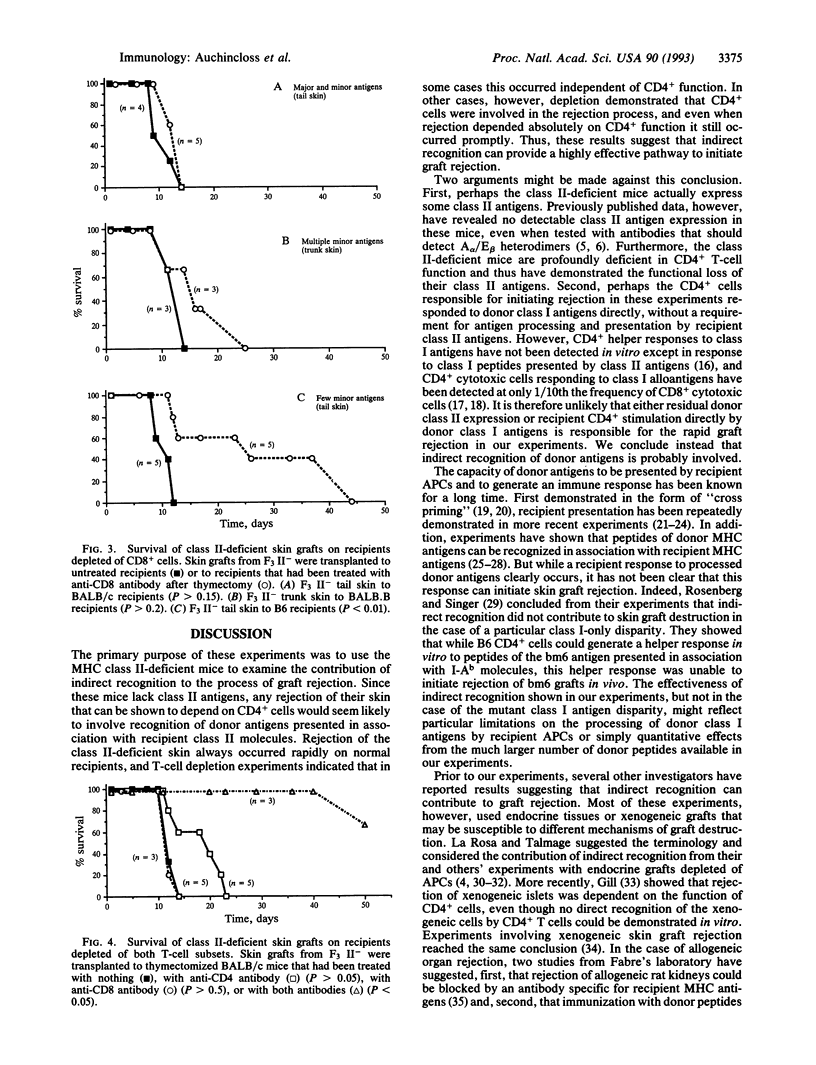
In vitro studies have revealed several pathways by which T cells can respond to alloantigens, including CD4+ direct responses to allogeneic class II antigens, CD8+ direct responses to allogeneic class I antigens, and CD4+ "indirect" responses to peptides of alloantigens presented in association with responder class II molecules. In vivo studies of skin graft rejection, however, have so far provided clear evidence for the contribution of only the two direct pathways and not for indirect recognition. We have used major histocompatibility complex class II-deficient mice as donors to test the role of indirect recognition in rejection of skin grafts. Class II-deficient skin was always rejected without delay by normal recipients. Removal of recipient CD8+ cells (to leave the animals dependent on CD4+ function) or depletion of recipient CD4+ cells revealed that CD4+ cells were usually involved and sometimes absolutely required in this rapid rejection. Since the donor grafts lacked class II antigens, the CD4+ cells must have recognized donor antigens presented in association with recipient class II molecules. These results therefore indicate that indirect recognition can initiate rapid skin graft rejection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auchincloss H., Jr, Ghobrial R. R., Russell P. S., Winn H. J. Prevention of alloantibody formation after skin grafting without prolongation of graft survival by anti-L3T4 in vivo. Transplantation. 1988 Jun;45(6):1118–1123. doi: 10.1097/00007890-198806000-00024. [DOI] [PubMed] [Google Scholar]
- Auchincloss H., Jr, Mayer T., Ghobrial R., Winn H. J. T-cell subsets, bm mutants, and the mechanisms of allogeneic skin graft rejection. Immunol Res. 1989;8(2):149–164. doi: 10.1007/BF02919076. [DOI] [PubMed] [Google Scholar]
- Bartlett S. T., Jennings A. S., Yu C., Naji A., Barker C. F., Silvers W. K. Influence of culturing on the survival of major histocompatibility complex-compatible and -incompatible thyroid grafts in rats. J Exp Med. 1983 Jan 1;157(1):348–352. doi: 10.1084/jem.157.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976 May 1;143(5):1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. J. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J Immunol. 1976 Dec;117(6):2233–2238. [PubMed] [Google Scholar]
- Bowen K. M., Andrus L., Lafferty K. J. Successful allotransplantation of mouse pancreatic islets to nonimmunosuppressed recipients. Diabetes. 1980;29 (Suppl 1):98–104. doi: 10.2337/diab.29.1.s98. [DOI] [PubMed] [Google Scholar]
- Bumgardner G. L., Chen S., Almond P. S., Bach F. H., Ascher N. L., Matas A. J. Cell subsets responding to purified hepatocytes and evidence of indirect recognition of hepatocyte major histocompatibility complex class I antigen. I. The role of L3T4+ T cells in the development of allospecific cytotoxicity in hepatocyte-sponge matrix allografts. Transplantation. 1992 Apr;53(4):857–862. doi: 10.1097/00007890-199204000-00028. [DOI] [PubMed] [Google Scholar]
- Carbone F. R., Moore M. W., Sheil J. M., Bevan M. J. Induction of cytotoxic T lymphocytes by primary in vitro stimulation with peptides. J Exp Med. 1988 Jun 1;167(6):1767–1779. doi: 10.1084/jem.167.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D., Gray D., Dierich A., Kaufman J., Lemeur M., Benoist C., Mathis D. Mice lacking MHC class II molecules. Cell. 1991 Sep 6;66(5):1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- Cosimi A. B., Burton R. C., Kung P. C., Colvin R., Goldstein G., Lifter J., Rhodes W., Russell P. S. Evaluation in primate renal allograft recipients of monoclonal antibody to human T-cell subclasses. Transplant Proc. 1981 Mar;13(1 Pt 1):499–503. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Fangmann J., Dalchau R., Fabre J. W. Rejection of skin allografts by indirect allorecognition of donor class I major histocompatibility complex peptides. J Exp Med. 1992 Jun 1;175(6):1521–1529. doi: 10.1084/jem.175.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobrial R. R., Boublik M., Winn H. J., Auchincloss H., Jr In vivo use of monoclonal antibodies against murine T cell antigens. Clin Immunol Immunopathol. 1989 Sep;52(3):486–506. doi: 10.1016/0090-1229(89)90162-1. [DOI] [PubMed] [Google Scholar]
- Gill R. G. The role of direct and indirect antigen presentation in the response to islet xenografts. Transplant Proc. 1992 Apr;24(2):642–643. [PubMed] [Google Scholar]
- Grusby M. J., Johnson R. S., Papaioannou V. E., Glimcher L. H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991 Sep 20;253(5026):1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. The somatic generation of immune recognition. Eur J Immunol. 1971 Jan;1(1):1–9. doi: 10.1002/eji.1830010102. [DOI] [PubMed] [Google Scholar]
- Kievits F., Ivanyi P. A subpopulation of mouse cytotoxic T lymphocytes recognizes allogeneic H-2 class I antigens in the context of other H-2 class I molecules. J Exp Med. 1991 Jul 1;174(1):15–19. doi: 10.1084/jem.174.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa F. G., Talmage D. W. Synergism between minor and major histocompatibility antigens in the rejection of cultured allografts. Transplantation. 1985 May;39(5):480–485. doi: 10.1097/00007890-198505000-00004. [DOI] [PubMed] [Google Scholar]
- La Rosa F. G., Talmage D. W. The failure of a major histocompatibility antigen to stimulate a thyroid allograft reaction after culture in oxygen. J Exp Med. 1983 Mar 1;157(3):898–906. doi: 10.1084/jem.157.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty K. J., Bootes A., Dart G., Talmage D. W. Effect of organ culture on the survival of thyroid allografts in mice. Transplantation. 1976 Aug;22(2):138–149. doi: 10.1097/00007890-197608000-00009. [DOI] [PubMed] [Google Scholar]
- Liu Z., Braunstein N. S., Suciu-Foca N. T cell recognition of allopeptides in context of syngeneic MHC. J Immunol. 1992 Jan 1;148(1):35–40. [PubMed] [Google Scholar]
- Macphail S., Stutman O. Anti-L3T4 antibody inhibits the lysis of H-2 class II antigen-negative target cells by L3T4+ cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5205–5209. doi: 10.1073/pnas.85.14.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macphail S., Stutman O. L3T4+ cytotoxic T lymphocytes specific for class I H-2 antigens are activated in primary mixed lymphocyte reactions. J Immunol. 1987 Dec 15;139(12):4007–4015. [PubMed] [Google Scholar]
- Madsen J. C., Peugh W. N., Wood K. J., Morris P. J. The effect of anti-L3T4 monoclonal antibody treatment on first-set rejection of murine cardiac allografts. Transplantation. 1987 Dec;44(6):849–852. [PubMed] [Google Scholar]
- Markowitz J. S., Rogers P. R., Grusby M. J., Parker D. C., Glimcher L. H. B lymphocyte development and activation independent of MHC class II expression. J Immunol. 1993 Feb 15;150(4):1223–1233. [PubMed] [Google Scholar]
- Matzinger P., Bevan M. J. Hypothesis: why do so many lymphocytes respond to major histocompatibility antigens? Cell Immunol. 1977 Mar 1;29(1):1–5. doi: 10.1016/0008-8749(77)90269-6. [DOI] [PubMed] [Google Scholar]
- McCarthy S. A., Kaldjian E., Singer A. Induction of anti-CD8 resistant cytotoxic T lymphocytes by anti-CD8 antibodies. Functional evidence for T cell signaling induced by multi-valent cross-linking of CD8 on precursor cells. J Immunol. 1988 Dec 1;141(11):3737–3746. [PubMed] [Google Scholar]
- Mitchison N. A., O'Malley C. Three-cell-type clusters of T cells with antigen-presenting cells best explain the epitope linkage and noncognate requirements of the in vivo cytolytic response. Eur J Immunol. 1987 Nov;17(11):1579–1583. doi: 10.1002/eji.1830171109. [DOI] [PubMed] [Google Scholar]
- Parker K. E., Dalchau R., Fowler V. J., Priestley C. A., Carter C. A., Fabre J. W. Stimulation of CD4+ T lymphocytes by allogeneic MHC peptides presented on autologous antigen-presenting cells. Evidence of the indirect pathway of allorecognition in some strain combinations. Transplantation. 1992 Apr;53(4):918–924. doi: 10.1097/00007890-199204000-00038. [DOI] [PubMed] [Google Scholar]
- Pierson R. N., 3rd, Winn H. J., Russell P. S., Auchincloss H., Jr Xenogeneic skin graft rejection is especially dependent on CD4+ T cells. J Exp Med. 1989 Sep 1;170(3):991–996. doi: 10.1084/jem.170.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley C. A., Spencer S. C., Sawyer G. J., Fabre J. W. Suppression of kidney allograft rejection across full MHC barriers by recipient-specific antibodies to class II MHC antigens. Transplantation. 1992 May;53(5):1024–1032. doi: 10.1097/00007890-199205000-00011. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. S., Mizuochi T., Sharrow S. O., Singer A. Phenotype, specificity, and function of T cell subsets and T cell interactions involved in skin allograft rejection. J Exp Med. 1987 May 1;165(5):1296–1315. doi: 10.1084/jem.165.5.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. S., Mizuochi T., Singer A. Analysis of T-cell subsets in rejection of Kb mutant skin allografts differing at class I MHC. 1986 Aug 28-Sep 3Nature. 322(6082):829–831. doi: 10.1038/322829a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. S., Mizuochi T., Singer A. Evidence for involvement of dual-function T cells in rejection of MHC class I disparate skin grafts. Assessment of MHC class I alloantigens as in vivo helper determinants. J Exp Med. 1988 Jul 1;168(1):33–45. doi: 10.1084/jem.168.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. S., Munitz T. I., Maniero T. G., Singer A. Cellular basis of skin allograft rejection across a class I major histocompatibility barrier in mice depleted of CD8+ T cells in vivo. J Exp Med. 1991 Jun 1;173(6):1463–1471. doi: 10.1084/jem.173.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. S., Singer A. Cellular basis of skin allograft rejection: an in vivo model of immune-mediated tissue destruction. Annu Rev Immunol. 1992;10:333–358. doi: 10.1146/annurev.iy.10.040192.002001. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Silvers W. K., Fleming H. L., Naji A., Barker C. F. Evidence for major histocompatibility complex restriction in transplantation immunity. Proc Natl Acad Sci U S A. 1982 Jan;79(1):171–174. doi: 10.1073/pnas.79.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A., Munitz T. I., Golding H., Rosenberg A. S., Mizuochi T. Recognition requirements for the activation, differentiation and function of T-helper cells specific for class I MHC alloantigens. Immunol Rev. 1987 Aug;98:143–170. doi: 10.1111/j.1600-065x.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Stock P. G., Ascher N. L., Chen S., Field J., Bach F. H., Sutherland D. E. Evidence for direct and indirect pathways in the generation of the alloimmune response against pancreatic islets. Transplantation. 1991 Oct;52(4):704–709. doi: 10.1097/00007890-199110000-00023. [DOI] [PubMed] [Google Scholar]


