Abstract
Mast cells play crucial roles in both innate and adaptive arms of the immune system. Along with basophils, mast cells are essential effector cells for allergic inflammation that causes asthma, allergic rhinitis, food allergy and atopic dermatitis. Mast cells are usually increased in inflammatory sites of allergy and, upon activation, release various chemical, lipid, peptide and protein mediators of allergic reactions. Since antigen/immunoglobulin E (IgE)-mediated activation of these cells is a central event to trigger allergic reactions, innumerable studies have been conducted on how these cells are activated through cross-linking of the high-affinity IgE receptor (FcεRI). Development of mature mast cells from their progenitor cells is under the influence of several growth factors, of which the stem cell factor (SCF) seems to be the most important. Therefore, how SCF induces mast cell development and activation via its receptor, KIT, has been studied extensively, including a cross-talk between KIT and FcεRI signaling pathways. Although our understanding of the signaling mechanisms of the FcεRI and KIT pathways is far from complete, pharmaceutical applications of the knowledge about these pathways are underway. This review will focus on recent progresses in FcεRI and KIT signaling and chemotaxis.
Keywords: Mast cell, IgE receptor, KIT receptor, Signal transduction, Chemotaxis, Plasma membrane
1. Introduction
Mast cells are terminally differentiated cells of the hematopoietic origin that are involved in both innate and adaptive immunity (Bischoff, 2007; Kalesnikoff and Galli, 2008; Abraham and St John, 2010). Mast cells originate from myeloid precursors that are released from bone marrow into blood circulation. Once they acquire proper signals through their chemoattractant receptors, they migrate to the target tissues that are strategically located at the host-environment interface (Okayama and Kawakami, 2006; Halova et al., 2012). Numbers of tissue mast cells are tightly regulated not only by migration, but also by proliferation and survival, as mast cells are long-lived cells capable of surviving for months. Under pathological conditions, tissue mast cell homeostasis could be disturbed and the number and distribution of mast cells quickly changed (Okayama and Kawakami, 2006). Mast cell chemoattractants include antigens recognized by immunoglubulins E (IgE), stem cell factor (SCF), different chemokines, cytokines, and leukotrienes. Many of them are also produced by mast cells to attract various cell types of the immune system as well as other mast cells and their precursors to modulate their amount by autocrine and/or paracrine mechanisms (Halova et al., 2012).
Mature mast cells express on their plasma membrane numerous receptors which, after binding of the corresponding ligands, can induce cell activation leading to the release of various inflammatory mediators. The most prominent is the high-affinity receptor for IgE (FcεRI), which has been implicated in an array of acute as well as chronic reactions including allergic rhinitis, asthma, anaphylaxis and atopic dermatitis. Antigen/IgE-mediated activation of mast cells is a multistep process, eventually leading to degranulation of preformed granules containing histamine, heparin, various proteases, tumor necrosis factor (TNF)-α, and other inflammatory mediators and de novo synthesis of cytokines, chemokines, eicosanoids, and other immune mediators. FcεRI-mediated activation events are modulated by engagement of other surface receptors such as KIT, adenosine receptors, prostaglandin (PG) receptors and many others. These receptors play multiple roles in differentiation, proliferation, chemotaxis and in setting a threshold for mast cell triggering (Gilfillan and Tkaczyk, 2006).
In industrial countries, mast cell-associated diseases are a serious problem, solution of which requires new strategies for development of new therapeutics. Detailed understanding of mast cell signaling events at the molecular levels could contribute to such developments. In this review we summarize recent findings on the early stages of antigen- and SCF-induced mast cell activation as well as mast cell chemotaxis.
2. Signal transduction
Mast cells express on their plasma membrane numerous receptors that are involved in cell migration and activation. The most extensively studied are FcεRI and KIT.
2.1. FcεRI signaling
2.1.1. FcεRI
FcεRI belongs to the multichain immune receptor family that includes the T and B cell receptors and other Fc receptors. In mast cells and basophils the receptor is expressed as a tetrameric structure composed of one IgE-binding α subunit, one membrane-tetraspanning β subunit and a dimer of disulphide-linked γ subunits (Blank et al., 1989). In other cells such as monocytes, Langerhans cells and dendritic cells, FcεRI is also found in a trimeric form lacking the β subunit (Kinet, 1999). The chain is responsible for binding the Fc part of IgE. The β chain stabilizes the receptor complex (Donnadieu et al., 2000) and amplifies spleen tyrosine kinase (SYK) phosphorylation resulting in higher magnitude of calcium mobilization while the γ chain dimer functions as an autonomous activation module (Lin et al., 1996). Each β and γ chain possesses one immunoreceptor tyrosine-based activation motif (ITAM) located in their cytoplasmic tails which are responsible for signal transduction and after phosphorylation serve as docking sites for molecules containing one or two Src homology (SH)2 domains (Cambier, 1995; Kinet, 1999). The β and γ chains are shared with other Fc receptors.
2.1.2. Protein kinases and phosphatases
Transduction of the signal from FcεRI is mediated and regulated via several kinases and phosphatases (Figure 1). The Src family protein tyrosine kinases (SFKs) have a well-defined structure containing five functional domains: a variable N-terminal domain, an SH2 domain, an SH3 domain, a kinase domain and a C-terminal regulatory tail (Okada, 2012). LYN, FYN, HCK and FGR are the SFKs that have been shown to be involved in early stages of the FcεRI signaling. LYN is the most abundant SFK expressed in mast cells and its activity is essential for initial tyrosine phosphorylation of the ITAMs of the FcεRI β and γ chains. LYN plays both positive and negative regulatory roles in mast cell signaling but exact molecular mechanisms of its action still remain controversial. Discordant results were obtained from studies using LYN knockout mice (Table 1). All experiments concluded that in the absence of LYN Ca2+ mobilization is decreased (Nishizumi and Yamamoto, 1997; Kawakami et al., 2000; Parravicini et al., 2002; Hernandez-Hansen et al., 2004). However, some studies showed increased degranulation in bone marrow-derived cultured mast cells (BMMCs) from LYN knockout mice (Parravicini et al., 2002; Hernandez-Hansen et al., 2004; Odom et al., 2004), whereas in others absence of LYN had no effect on degranulation (Nishizumi and Yamamoto, 1997; Kawakami et al., 2000). An early study described opposite roles of LYN after activation of mast cells with high or low intensity; low-intensity stimulation suppressed LYN kinase activity and its association with FcεRI receptor, whereas high-intensity stimulation had an opposite effect (Xiao et al., 2005). Also studies on passive cutaneous anaphylaxis (PCA) and/or passive systemic anaphylaxis (PSA) gave different results. A first study showed an absence of PCA in LYN knockout mice (Hibbs et al., 1995). Later it was described that PSA in LYN knockout mice depends on age of the mice; in young mice (4 weeks old) it was increased, but in older mice (more than 7 weeks old) it was decreased (Odom et al., 2004). A follow-up study showed that the genetic background of mice affects the results. When BMMCs from Lyn knockout mice were compared to those from wild-type mice, antigen-induced degranulation was either decreased when derived from C57BL/6 mice, or increased when derived from 129/Sv mice (Yamashita et al., 2007). The authors suggested that different expression of FYN kinase in different mouse strains could be responsible for the observed differences.
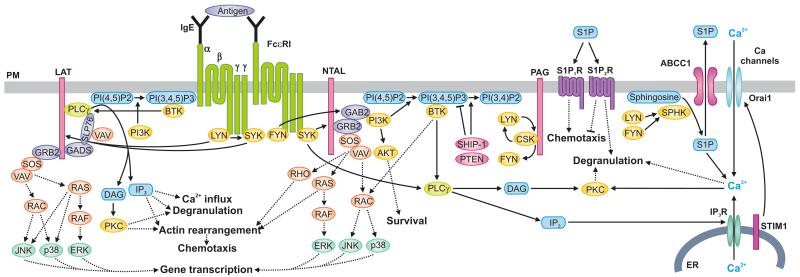
Fig. 1.
FcεRI-mediated signaling events. The first biochemically defined step in antigen-mediated aggregation of the IgE-FcεRI complexes is tyrosine phosphorylation of the FcεRI β and γ subunits by LYN kinase. This is followed by binding of SYK to FcεRI γ subunit leading to phosphorylation and activation of SYK. These kinases then phosphorylate downstream signaling targets. SYK phosphorylates transmembrane adaptor proteins NTAL and LAT and thus creates binding sites for various SH2-containing proteins like GRB2. In this way PI3K and GAB2 are brought to the plasma membrane (PM). PM-bound PI3K phosphorylates PI(4,5)P2 and generates PI(3,4,5)P3. Production of PI(3,4,5)P3 can be negatively regulated by SHIP-1 and PTEN by conversion of PI(3,4,5)P3 to PI(3,4)P2 and PI(4,5)P2, respectively. Several PH domain-containing proteins, including BTK and PLCγ, are recruited to the membrane-bound PI(3,4,5)P3. PLCγ hydrolyzes PI(4,5)P2 to generate the second messengers, diacylglycerol (DAG) and IP3. IP3 binds to the ER-bound IP3 receptor (IP3R) and triggers the release of Ca2+ from the ER. Depletion of Ca2+ from the ER leads to interaction of STIM1 with the Orai1 PM-associated protein, opening the PM-bound calcium channels and influx of extracellular Ca2+ into the cytoplasm. LYN and FYN kinases also activate SPHKs which induce conversion of sphingosine into S1P, which is secreted from the cell through ABCC1 (a member of the ATP-binding cassette transporter family). The extracellular S1P binds to S1P1R and S1P2R, which are involved in cell migration and degranulation. Some other signaling proteins (SLP-76, VAV, GADS, SOS, RAC, RAS, RAF, JNK, p38, ERK, PKC, RHO, AKT, PAG, and CSK), which are involved in Ca2+ influx, degranulation, actin rearrangement, chemotaxis, and/or gene transcription, are also indicated.
Table 1.
The effect of gene knockout of key signaling molecules on SCF- or antigen-mediated chemotaxis, degranulation, Ca2+ response, PCA, or PSA
↓, decreased response; ↑, increased response; No, no change in response
PSA was increased in younger mice (4 weeks old) and decreased in older mice (more than 7 weeks old).
STIM1 knockout mice die in utero and therefore STIM1 heterozygotic mice were used.
It has been found that LYN-deficient mast cells exhibit enhanced FYN-dependent signals and degranulation, but reduced calcium responses (Parravicini et al., 2002). In contrast, FYN deficiency resulted in impaired degranulation, whereas calcium response was normal. Both FYN and LYN were found to be associated with the β chain of FcεRI, but unlike LYN, FYN did not participate in the phosphorylation of FcεRI (Gomez et al., 2005a). Instead, FYN was found to play a role in regulating the activity of phosphatidylinositol 3-kinases (PI3K) (Parravicini et al., 2002).
Less is known about the role of FGR and HCK in mast cell function and activation. HCK deficiency resulted in enhanced LYN activity and FcεRI phosphorylation but decreased phosphorylation of SYK and degranulation without effect on calcium response (Hong et al., 2007). FGR was found to associate with FcεRI and positively regulate mast cell signaling via promoting phosphorylation of SYK and its substrates (Lee et al., 2011). Studies on FGR and HCK suggest that these two kinases also contribute to signaling and SFKs exhibit a hierarchical relationship, i.e., HCK inhibits LYN and LYN inhibits FYN.
SYK is a member of another non-receptor tyrosine kinase family, composed of N-terminal two tandem SH2 domains, a kinase domain, a short C-terminal tail, and two interdomains. SYK-deficient mice are lethal and mast cells derived from fetal liver of SYK knockout mice are deficient in degranulation and cytokine release (Costello et al., 1996). In line with this, SYK-deficient RBL-2H3 rat mast cell lines lack the ability to degranulate and produce cytokines (Zhang et al., 1996). Furthermore, in vivo inducible SYK knockout mice exhibited impaired PCA, as expected (Wex et al., 2011). SYK is associated with the γ chain of the FcεRI. Binding of SYK to phosphorylated γ chain ITAM through the SYK SH2 domains induces a conformational change in the kinase, leading to its increased enzymatic activity (Zhang et al., 2000; Siraganian et al., 2010).
Tec family kinases represent another class of non-receptor protein tyrosine kinases that are implicated in FcεRI-mediated activation. The Tec kinases are located in cytosol and contain an N-terminal pleckstrin homology (PH) domain, a Tec homology domain, an SH2 domain, an SH3 domain, and a C-terminal catalytic domain (Gilfillan and Rivera, 2009). Three members of this family, Bruton’s tyrosine kinase (BTK), interleukin (IL)-2-inducible T-cell kinase (ITK) and tyrosine kinase expressed in hepatocellular carcinoma (TEC) are expressed in mast cells and are activated upon antigen-induced activation (Kawakami et al., 1994; Kawakami et al., 1995). Regulatory steps in Tec kinases activation include recruitment to the membrane through their PH domain, phosphorylation by SFKs and subsequent autophosphorylation. A single point mutation in the PH domain of Btk gene leading to X-linked immunodeficiency (xid) confirms the importance of BTK membrane localization for its proper function (Rawlings et al., 1993). Studies with xid and BTK knockout mice showed the importance of BTK for PCA. Furthermore, BMMCs obtained from these mice exhibited mild impairment in degranulation, and more severe defects in the production of various cytokines (Hata et al., 1998). Absence of ITK led to impaired PCA as well as decreased degranulation of airway mast cells in vivo (Forssell et al., 2005). Another study showed that degranulation of ITK knockout BMMCs is unaffected, but their cytokine production is increased (Iyer and August, 2008). Further analyses of ITK/BTK double knockout mast cells suggested that although they may share substrates, ITK has both positive and negative regulatory roles, while BTK is primarily a positive regulator of FcεRI signaling (Iyer et al., 2011). In contrast to ITK and BTK, TEC knockout mice displayed normal histamine levels in an anaphylactic model and also degranulation was not affected, but TEC was found to play an important role in generation of leukotrienes and cytokines (Schmidt et al., 2009).
The tyrosine kinase network is regulated by dephosphorylation events that are mediated by protein tyrosine phosphatases, which selectively catalyze the removal of a phosphate group from the tyrosine residue. In mast cells, the cytoplasmic SH2-domain-containing protein tyrosine phosphatase (SHP)-1 and SHP-2 are implicated in the control of cellular proliferation, survival and signaling (Heneberg and Dráber, 2002). They contain two adjacent N-terminal SH2 domains, and SHP-2 also possesses a proline-rich sequence at the C-terminus that can interact with SH3 domains of other proteins. After FcεRI engagement, these phosphatases negatively regulate mast cell activation via the immunoreceptor tyrosine-based inhibitory motif (ITIM) as documented by experiments with genetically modified mice.
2.1.3. Lipid kinases and phosphatases
PI3Ks are a family of lipid kinases that are involved in diverse biological functions. PI3Ks phosphorylate phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) to phosphatidylinositol-3,4,5-trisphosphate (PI(3,4,5)P3). Mast cells possess class IA PI3Ks, consisting of a catalytic subunit (p110α, β or δ) and a regulatory subunit (p85α, p85β or p50α). The p85 subunits carry two SH2 domains, which allow the p85/p110 complex to be recruited to phosphotyrosine residues. Class IA PI3Ks are utilized after activation of FcεRI and KIT receptors, whereas class IB PI3K, consisting of a catalytic subunit p110γ and a regulatory subunit p84 or p101, is activated through G protein-coupled receptors (GPCRs) (Rommel et al., 2007; Kim et al., 2008). Whereas mice lacking p110α or p110β are embryonic lethal, p110δ-null mice are viable, with specific defects in B and T cells (Okkenhaug and Vanhaesebroeck, 2003). BMMCs isolated from mice deficient in p85 subunit exhibited no defects in antigen-induced signaling (Fukao et al., 2002; Tkaczyk et al., 2003), suggesting that class IA PI3Ks are not important for FcεRI signaling. However, subsequent studies with mice expressing either wild-type p110δ or p110δD910A, a loss-of-function allele of p110δ, as well as a chemical inhibition of p110δ, showed indispensability of these PI3Ks for mast cell activation (Ali et al., 2004). Thus, in the absence of p85, p110 may be capable of cooperating with the remaining regulatory subunits. However, a recent study called into question the role of p110δ in mast cell activation (see below).
Two phosphatases oppose activity of PI3Ks. SH2-containing inositol phosphatase (SHIP)1 removes a phosphate group from PI(3,4,5)P3 at the 5′ position (Rauh et al., 2003). Absence of SHIP1 leads to hyperresponsiveness of mast cells to antigen stimulation under in vivo or in vitro conditions (Huber et al., 1998; Haddon et al., 2009). Phosphatase and tensin homologue deleted on chromosome ten (PTEN) dephosphorylates PI(3,4,5)P3 at the 3′ position. Deficiency in PTEN had a very similar effect as the absence of SHIP1 (Furumoto et al., 2011).
2.1.4. Adaptor proteins
Adaptor proteins play important roles as scaffolds enabling the assembly of large spacio-temporarily controlled signaling complexes that contribute to degranulation, cytokine production, chemotaxis and other physiological events. Adaptors are proteins without intrinsic enzymatic function composed of multiple protein-protein or protein-lipid interacting domains. Membrane-bound adaptors are anchored scaffolds that provide docking sites mainly through phosphorylation of tyrosine residues. Cytosolic adaptors usually possess more motifs that allow them to bind other molecules (Alvarez-Errico et al., 2009; Draber et al., 2012).
The transmembrane adaptor protein linker for activation of T-cells (LAT), also known as LAT1, is the first adaptor described in T cells as playing an important role after T-cell receptor engagement (June et al., 1990). In mast cells, LAT is phosphorylated by SYK and associates with numerous cytoplasmic proteins, including growth factor receptor-bound protein 2 (GRB2), phospholipase (PLC)γ1, guanine nucleotide exchange factor VAV, SH2-domain-containing leukocyte protein of 76 kDa (SLP-76), Casitas B-lineage lymphoma (CBL) and GRB2-related adaptor downstream of SHC (GADS). LAT deficiency causes reduced phosphorylation of PLCγ1 and SLP-76, decreased mitogen-activated protein kinase (MAPK) activity, Ca2+ signaling and degranulation (Saitoh et al., 2000). Non-T-cell activation linker (NTAL), also termed as LAB or LAT2, is an adaptor structurally related to LAT, but it lacks a motif for PLCγ1 binding (Brdička et al., 2002). It is phosphorylated by SYK and LYN (Iwaki et al., 2008). There are conflicting results about NTAL function in mast cells. NTAL-deficient BMMCs exhibited enhanced antigen-induced degranulation, calcium mobilization, and phosphorylation of LAT, PLCγ1, and extracellular signal-regulated protein kinase (ERK) (Volná et al., 2004; Zhu et al., 2004). This was in contrast with the results obtained with human mast cells and RBL cells in which LAT knockdown (KD) by RNA silencing techniques resulted in reduced degranulation (Tkaczyk et al., 2004; Draberova et al., 2007). A recent study showed that mouse BMMCs with NTAL KD have similar properties as BMMCs from NTAL knockout mice (Polakovicova et al., 2014). These data indicate that NTAL in mouse BMMCs is a negative regulator of FcεRI signaling, independently of possible compensatory developmental alterations in NTAL-deficient mice.
Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), also called C-terminal SRC kinase (CSK)-binding protein (CBP), is phosphorylated by LYN and FYN but not SYK. It can bind the negative regulatory kinase CSK (Brdicka et al., 2000; Kawabuchi et al., 2000). Experiments with RBL cells showed that PAG is a negative regulator of FcεRI signaling (Ohtake et al., 2002). Interestingly, BMMCs from PAG-deficient mice exhibited impaired degranulation, calcium mobilization and tyrosine phosphorylation of FcεRI subunits and PLCγ, suggesting that PAG could also have positive regulatory roles (Draberova et al., 2014).
Cytosolic adaptor proteins lack the hydrophobic transmembrane domain that would anchor them to the plasma membrane; instead they possess various motifs which allow them to associate with plasma membrane molecules, cytoskeleton and/or other organelles (Alvarez-Errico et al., 2009). One of the cytosolic adaptors being recruited to tyrosine phosphorylated LAT is SLP-76 (Saitoh et al., 2000). Mast cells lacking SLP-76 show impaired FcεRI signaling, including decreased degranulation, PLCγ and PI3K activity (Pivniouk et al., 1999). Upon FcεRI triggering, SLP-76 becomes tyrosine-phosphorylated by SYK and provide binding sites for VAV, non-catalytic region of tyrosine kinase (NCK), and BTK (Kettner et al., 2003). SLP-76 is recruited to LAT by another cytosolic adaptor, GADS, which constitutively associates with proline-rich regions of SLP-76. This interaction is important for proper FcεRI-mediated degranulation (Silverman et al., 2006). The absence of GADS causes disruption of association of SLP-76 to LAT, and impaired degranulation and calcium mobilization; however this effect is not as strong as SLP-76 deletion (Yamasaki et al., 2008).
GRB2-associated-binding protein 2 (GAB2) is a cytosolic adaptor important in PI3K signaling (Gu et al., 1998). An initial study suggested that GAB2 is phosphorylated by FYN and is involved in downstream signaling events from FcεRI and KIT, but a later study showed that the kinase activity of SYK is reduced in FYN-deficient mast cells and SYK phosphorylates GAB2 (Yu et al., 2006). The FYN signaling is complementary to the LYN-LAT pathway (Gu et al., 2001; Parravicini et al., 2002). GAB2-deficient mast cells show impaired degranulation, cytokine production, and PI3K activation (Gu et al., 2001).
Related to GAB2 is GRB2, which interacts with the guanine exchange factor SOS and thus activates the RAS-ERK cascade (Lowenstein et al., 1992). Besides SOS, GRB2 was reported to interact with LAT, NTAL, SLP-76 and GAB2 (Alvarez-Errico et al., 2009).
Adaptors are involved not only in promoting signaling but also act in a negative regulatory manner. For instance, downstream of tyrosine kinase 1 (DOK1) is constitutively associated with RAS GTPase-activating protein (RAS-GAP). After SHIP recruitment to the ITIM in a LYN-dependent manner, DOK1 associates with SHIP and the negative regulatory complex SHIP/RAS-GAP/DOK1 down-regulates PI(3,4,5)P3 levels and inhibits RAS activation via Ras-GAP (Kepley et al., 2004; Tamir et al., 2000). Nevertheless, DOK1-deficient mast cells show no defect in activation (Ott et al., 2002).
2.1.5. FcεRI signaling pathways
The binding of multivalent antigen to IgE-FcεRI complexes on the plasma membrane causes aggregation of the complexes and triggers a series of activation events starting with enhanced phosphorylation of the FcεRI β and γ chains by LYN kinase. The exact molecular mechanism behind this event is not completely understood, but it is expected that there are changes in access of LYN to tyrosine residues in the ITAM motifs of β and γ chains and/or decrease dephosphorylation of the target phosphotyrosines (Bugajev et al., 2010). Phosphorylation of FcεRI β and γ chains facilitates recruitment of LYN and SYK, respectively, and their activation. Activated SYK in FcεRI signalosome is instrumental in downstream propagation of the signal through phosphorylation of many substrates, including adaptor proteins LAT, NTAL, and SLP-76. Phosphorylated LAT serves as an anchor for PLCγ, which after phosphorylation promotes calcium signaling.
Another pathway of FcεRI signaling is initiated by FYN. After FcεRI aggregation, FYN phosphorylates the adaptor GAB2 and AKT. Phosphorylated GAB2 serves as a docking site for additional FYN and promotes degranulation by its subsequent association with PI3K. PI3K then phosphorylates PI(4,5)P2 to PI(3,4,5)P3. FYN is required for normal mast cell degranulation and maintenance and/or amplification of Ca2+ signal (Parravicini et al., 2002).
The transmembrane adaptor LAT, phosphorylated by SYK recruits cytosolic adaptors, such as GADS, GRB2 and SLP-76, guanine nucleotide exchangers VAV and SOS, and signaling enzymes PLCγ1 and PLCγ2. Assembly of this complex and recruitment of PI3K enables PLCγ phosphorylation by SYK and BTK. BTK recruitment to the plasma membrane is regulated via its PH domain-mediated binding to PI(3,4,5)P3, generated by activity of PI3K. Activated PLCγ catalyzes the hydrolysis of membrane bound lipid, PI(4,5)P2, to generate two important second messengers, 1,2-diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (IP3). DAG activates conventional protein kinase C (PKC) isoforms that together with the free Ca2+ released via the action of IP3 initiates the degranulation event and its maximum is reached by sustained high levels of PI(3,4,5)P3 and Ca2 (Kraft and Kinet, 2007).
2.1.6. Calcium mobilization
IP3 generated by activity of PLCγ diffuses through the cytosol and induces cytosolic calcium mobilization via binding to IP3 receptors located in the membrane of the endoplasmic reticulum (ER). IP3 receptors are Ca2+ channels allowing the release of Ca2+ from the ER stores to the cytoplasm. The depletion of Ca2+ from intracellular stores leads to opening of plasma membrane store-operated Ca2+ release-activated Ca2+ (CRAC) channels that allow a strong Ca2+ influx into the cytoplasm. The stromal interaction molecule 1 (STIM1) located in the membrane of ER is a Ca2+ sensor detecting the ER Ca2+ concentration (Roos et al., 2005; Liou et al., 2005; Draber and Draberova, 2005). STIM1-deficient mast cells had impaired FcεRI-mediated Ca2+ influx, degranulation, cytokine production, and activation of the transcription factors NF-κB and nuclear factor for T cell activation (NFAT) (Baba et al., 2008). Depletion of Ca2+ from ER induces clustering of STIM1 in the ER membrane which then moves to the close proximity of the plasma membrane, and STIM1 directly interacts with the CRAC channel subunit ORAI1. This enables entering of Ca2+ into the cells, leading to numerous changes, including reorganization of microtubules in activated mast cells (Hajkova et al., 2011). Mast cell degranulation is critically dependent on increased intracellular calcium and activation of PKC (Ozawa et al., 1993). The Ca2+ influx triggers the fusion of mast cell granules to the membrane and is essential for actin cytoskeleton reorganization.
Calcium mobilization has an impact on activation of many signal-transducing proteins including VAV, GRB2, SOS, Rho GTPases and MAPKs. A key role in Ca2+ mobilization is played by LAT which after phosphorylation allows anchoring of PLCγ1, GRB2, and GADS via their SH2 domains. Guanine nucleotide exchange factors (GEFs) VAV and SOS associate with these complexes and activate the RAS and Rho family GTPases and thus modulating cytoskeletal organization and vesicle movement as well as initiating MAPKs activation. Activated RAS positively regulates the RAF-dependent pathway and activates the ERK, JNK, and p38 MAPK pathways, leading to activation of FOS and JUN, NFAT, NF-κB, ATF2, and ELK1 (Gilfillan and Rivera, 2009; Gilfillan and Beaven, 2011).
An increase in free intracellular calcium concentration activates a protein phosphatase calcineurin by binding a regulatory subunit and activating calmodulin binding. Calcineurin dephosphorylates NFAT and the activated NFAT then translocates to the nucleus, where it regulates the transcription of several cytokine genes (Rivera and Gilfillan, 2006).
Another second messenger for mast cell activation and calcium mobilization is sphingosine 1-phosphate (S1P). LYN and FYN kinases activate sphingosine kinases SPHK1 and SPHK2, which induce conversion of sphingosine into S1P. S1P works as a ligand for a subset of G protein-coupled S1P receptors, which are known to regulate a variety of cellular responses including cytoskeletal reorganization, formation of adherent junctions, proliferation, angiogenesis, and cell movement (Spiegel and Milstien, 2003). Mast cells activated via the FcεRI secrete S1P through transporter ABCC1 (a member of the ATP-binding cassette transporter family) and the extracellular S1P binds to S1P1 and S1P2 receptors (Prieschl et al., 1999; Jolly et al., 2004). Mast cells isolated from mice lacking SPHK1 didńt show any marked alteration of degranulation and calcium response while SPHK2-deficient cells were found to have impaired degranulation, cytokine secretion, and defective calcium influx (Olivera et al., 2007). In contrast to murine mast cells, S1P induced degranulation of human mast cells and SPHK1, but not SPHK2, has been shown to play a critical role in this process (Oskeritzian et al., 2008). Direct interaction of SPHK1 with LYN, but not with SYK, causes SPHK1 recruitment to membrane rafts and to FcεRI (Oskeritzian et al., 2008; Urtz et al., 2004). The sphingosine kinase activity induced by FcεRI crosslinking plays a role in maintaining the balance between sphingosine and S1P. While high levels of sphingosine are associated with apoptosis, high levels of S1P with cell proliferation (Hait et al., 2006).
2.1.7. Regulation of the FcεRI signaling
A recent study showed that FcεRI is capable of reacting differently to high- and low-affinity stimuli (Suzuki et al., 2014). The low-affinity stimulation led to reduced degranulation and leukotriene B4 and cytokine production, but enhanced chemokine production. The observed differences were not due to variable receptor phosphorylation but to differences of the size, mobility, and distribution of the receptor clusters. In low-affinity stimulation bigger and less mobile receptor clusters were formed. While in high-affinity stimulation LAT, PLCγ1 and PLCγ2 were strongly phosphorylated, in low-affinity stimulation the signal was shifted to NTAL that exhibited higher phosphorylation and more extensive colocalization with the FcεRI. Also the association of FGR with the receptor was increased.
Mast cells also express inhibitory cell-surface receptors to control the effector functions. These receptors contain ITIMs, which are used to suppress the activation by promoting the dephosphorylation carried out by the phosphatases. Lipid phosphatase SHIP binds to the low-affinity receptor for IgG, FcγRIIB, and to β and γ subunits of the FcεRI and inhibits the degranulation. Through interactions of its SH2 domain with ITIMs, SHIP is recruited to the plasma membrane where it degrades PI(3,4,5)P3 to PI(4,5)P2, subsequently leading to reduced BTK activation and PLCγ-mediated Ca2+ mobilization. PTEN opposes PI3K activity. PTEN catalyzes the hydrolysis of PI(3,4,5)P3 to PI(4,5)P2, thus functioning as a negative regulator of FcεRI-induced calcium flux, degranulation and cytokine production (Furumoto et al., 2006).
2.2. KIT signaling
2.2.1 KIT receptor
An important receptor localized on the plasma membrane of mast cells is KIT (CD117), the receptor for SCF. KIT activation is crucial for growth, survival, differentiation and homing of mast cells into target tissues. SCF, existing in two isoforms, soluble and membrane bound, that arise by alternative splicing of one RNA, is produced by different cell types including fibroblasts and endothelial cells. SCF, similarly to IgE-antigen complexes, can be an activator/co-activator of mast cells (Gilfillan and Tkaczyk, 2006). When activated by antigen, SCF markedly increases mast cell degranulation and potentiates and prolongs calcium signals. It also influences mast cell chemotaxis and adhesion (Vosseller et al., 1997). KIT is a single chain receptor with protein-tyrosine kinase activity. Its extracellular part comprises of five immunoglobulin-like domains, the first three of which bind SCF and the fourth is important for the receptor dimerization. The intracellular part has a split catalytic domain. SCF binding to KIT leads to dimerization and auto/transphosphorylation at tyrosine residues.
2.2.2. KIT signaling pathways
Signaling pathways elicited by KIT share several similar features with FcεRI-mediated events such as involvement of SFKs, PLCγ1, PI3K, calcium mobilization and MAPK-cascade activation (Figure 2). However, activated KIT does not recruit or activate SYK or phosphorylate LAT. The ability of KIT to potentiate FcεRI-dependent degranulation seems to be due to the involvement of NTAL and BTK and their ability to regulate PLCγ1-dependent calcium mobilization and PKC activation (Gilfillan and Beaven, 2011). KIT can phosphorylate tyrosine residues in NTAL, which are different from those phosphorylated by SYK in FcεRI-activated cells (Iwaki et al., 2008).
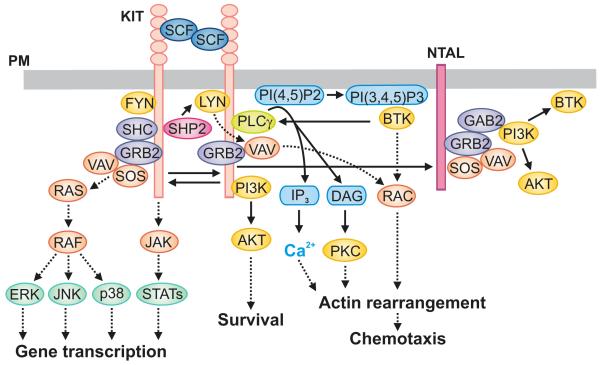
Fig. 2.
KIT-mediated signaling events. Dimerization of the plasma membrane (PM)-anchored KIT by SCF leads to the receptor auto-transphosphorylation. This is followed by recruitment of SFKs LYN and FYN, SHP2, PI3K, GRB2, SHC and other SH2 domain-containing proteins to phosphorylated KIT. Activated PI3K generates PI(3,4,5)P3 through which PH domain-containing proteins such as BTK are recruited to the PM and further propagate the signal. An increased activity of PLCγ leads to production of DAG and IP3, responsible for enhanced levels of free cytoplasmic Ca2+. This is followed by actin rearrangements and chemotactic response. KIT also phosphorylates NTAL that can bind other adaptor proteins, including GRB2. Activated GRB2 orchestrates activation of RAS-RAF pathway by recruiting GEFs, SOS and VAV, which subsequently leads to activation of MAP kinases ERK, JNK and p38. Some other signaling proteins (JAK, STATs, AKT, PKC, and RAC), which are involved in gene transcription, survival, actin rearrangement, and/or chemotaxis, are also indicated.
After SCF-induced KIT dimerization and activation of its intrinsic kinase activity, phosphorylated tyrosine residues recruit SH2-domain containing proteins, including cytosolic adaptors SHC and GRB2, SFKs (LYN and FYN), PLCγ, and PI3K (Linnekin et al., 1997; Roskoski, Jr., 2005). This creates a signaling complex that further propagates the downstream signaling leading to activation of other signaling molecules. Among them are components of the JAK-STAT and RAS-RAF-MAPK pathways, which lead to mast cell growth, differentiation, survival, adhesion and chemotaxis. KIT receptor can directly bind the p85α subunit of PI3K and in this way contributes to subsequent generation of membrane-associated PI(3,4,5)P3 (Gilfillan and Rivera, 2009).
2.3. Other surface receptors
Besides FcεRI and KIT, mast cells express other surface receptors that enable them to perform various functions including recognition of microbes, signaling to other immune cells, interactions with neurons and positive or negative modulation of other surface receptors. Several recent reviews have dealt with such receptors in mast cells (Pundir and Kulka, 2010; Sandig and Bulfone-Paus, 2012; Gilfillan and Beaven, 2011).
Several surface receptors are important for recognition and/or defense against pathogens. Toll-like receptors (TLRs) enable mast cells to respond to products of Gram-negative and Gram-positive bacteria. The tissue origin of mast cells and/or culture conditions can influence the expression of different TLRs (Marshall and Jawdat, 2004). TLR2 and TLR4, the most well-studied TLRs in mast cells, act synergistically with antigen to enhance cytokine production (Gilfillan and Beaven, 2011). Closely related is ST2 (also known as IL1RL1), which binds IL-33 and forms a complex with IL-1 receptor accessory protein (IL1RAP). IL-33 can activate NF-κB and MAP kinase signaling pathways, drives maturation of human mast cells and directly stimulates them to produce several proinflammatory cytokines and chemokines (Allakhverdi et al., 2007). IL-33 has a pro-inflammatory role in rheumatoid arthritis and promotes anaphylaxis by mast cell activation. Furthermore, increased levels of IL-33 were reported in asthmatic patients, and in skin cells of patients with atopic dermatitis (Liew et al., 2010). Nucleotide-binding oligomerization domain-like receptors (NLRs) such as NOD1-5 and NLRP1-14 are involved in intracellular recognition of bacterial infection. NLRs are expressed in the cytosol of epithelial cells and antigen presenting cells, and some of them are also in mast cells (Haidl et al., 2011). Antimicrobial peptides are ancient and essential elements expressed by different immune cells to execute innate immune response against pathogens. Antimicrobial peptides can induce mast cell degranulation independently of antigen stimulation. Many of these peptides work through GPCRs but their identity and mechanisms of action are poorly understood (Pundir and Kulka, 2010).
Mast cells not only produce a variety of cytokines but also express various receptors for cytokines such as IL-3R, IL-4R, IL-5R, IL-10R, and TGFβR1. Binding of IL-3 and IL-4 to their receptors causes increased FcεRI-dependent histamine release (Gebhardt et al., 2002; Bischoff et al., 1999), while IL-5 binding to IL-5R has no effect on degranulation. IL-10 has an anti-inflammatory effect on mast cells by inhibiting their release of TNF-α, IL-8 and histamine (Royer et al., 2001). It also causes suppression of FcεRI expression (Kennedy et al., 2008). Mast cell-derived TGF-β also acts as a negative regulator, working in an autocrine manner on mast cells. TGF-β inhibits the release of histamine and TNF-α from mast cells (Bissonnette et al., 1997) and the surface expression of FcεRI (Gomez et al., 2005b).
FcεRI-mediated mast cell activation can be modified by GPCRs, which are the most common targets of anti-allergy therapy. GPCRs with 7 membrane-spanning regions associate with heterotrimeric G proteins, composed of α,β, and γ subunits. In the resting state, the α subunit of a G protein is bound to GDP. After ligand binding to the receptor, the bound GDP is exchanged for GTP resulting in temporary dissociation of this subunit from the β and γ subunits and allowing the free α subunits to mediate downstream signaling (Kuehn and Gilfillan, 2007). Mast cells express many classes of GPCRs including the receptors for the complement components and chemokines, S1P, PGE2, adenosine, neuropeptide and antimicrobial peptides.
Mast cells express complement receptors for C3a and C5a, which can act as mast cell chemoattractants (Nilsson et al., 1996). C3a (anaphylatoxin peptide) can modulate mast cell degranulation and the release of chemokines (Woolhiser et al., 2004). The chemokine receptor CCR3 seems to play an important role in allergic diseases. This receptor binds a variety of chemokines including CCL2/MCP-1, CCL5/RANTES and CCL11/eotaxin-1, which either induce mast cell chemotaxis or degranulation. Also expressed are CCR1, CCR5, and CXCR4 (Kuehn and Gilfillan, 2007; Marshall and Jawdat, 2004).
As described above, S1P can act as a second messenger for intracellular calcium mobilization and regulate mast cell activation and chemotaxis by binding to one of the two S1P receptors, S1P1R and S1P2R, expressed on mast cells (Choi et al., 1996; Jolly et al., 2004). These two receptors have distinct roles; S1P1R induces mast cell chemotaxis while S1P2R modifies degranulation and chemokine and cytokine release (Jolly et al., 2004).
PGE2 is an important modulator of inflammatory responses that is involved in several inflammatory diseases including rheumatoid arthritis. It is produced by various cell types and multiple GPCRs for PGE2 have been described. Mast cells express two of them, EP2 and EP3 receptors. The EP2 receptor is linked with down-regulation of antigen-mediated degranulation and cytokine production in mast cells. In contrast, the EP3 receptor enhances FcεRI-mediated degranulation and production of cytokines (Feng et al., 2006).
Adenosine has been shown to mediate pathogenic mechanisms of asthma and rheumatoid arthritis. The adenosine A2A receptor is expressed on most inflammatory cells implicated in asthma including mast cells. This receptor down-regulates mast cell activation, while the A2B receptor up-regulates degranulation and cytokine secretion. Finally, the A3 receptor seems to be uninvolved in the regulation of degranulation and is mainly expressed by lung mast cells in mice (Brown et al., 2008).
During an inflammatory response mast cells may respond to stimuli such as neuropeptides in an FcεRI-independent manner. Since mast cells are often located in close proximity to neurons and blood vessels, they may respond to neuropeptides produced by nearby neurons. Acute psychological stress can trigger skin mast cell degranulation (Singh et al., 1999). Different types of mast cells were reported to respond to various neuropeptides such as substance P, vasoactive intestinal polypeptide (VIP), neuropeptide Y, and calcitonin gene-related peptide (CGRP). Substance P and VIP induce degranulation and cytokine and chemokine release in mast cells. These cells constitutively express the receptor for substance P, neurokinin 1 receptor (NK1R), the receptor for VIP, VIP receptor type 2 (VPAC2), and neuropeptide receptors NK2R and NK3R. FcεRI-mediated activation of human mast cells up-regulated expression of VPAC2, NK2R, and NK3R, suggesting that exposure to allergen may enhance neuronal inflammation (Kulka et al., 2008). Mouse mast cells express the neuropeptide Y receptor, Y1, and to a lesser extent Y2 and Y5. Recently, the overexpression of neuropeptide Y was shown to contribute to the development of atherosclerosis. Neuropeptide Y causes a significant increase in perivascular mast cell activation and induced pro-inflammatory mediator release from isolated mast cells (Lagraauw et al., 2014).
Antigen-induced responses can be modulated by various inhibitory receptors. They contain in their cytoplasmic domains up to four ITIMs that recruit phosphatases and other inhibitory signaling molecules. Most of the inhibitory receptors recruit the phosphatases SHP-1 and SHP-2, while FcγRIIB primarily recruits SHIP-1. FcγRIIB binds allergen-specific IgG and prevents activation of mast cells caused by allergen-specific IgE bound to FcεRI (Kraft and Kinet, 2007). Other inhibitory receptors include sialic acid-binding immunoglobulin-like lectin (Siglec) family, among them the most studied Siglec 8, mast cell function-associated antigen (MAFA), platelet endothelial cell adhesion molecule (PECAM1), Gp49B1, and many others whose properties have been reviewed elsewhere (Li and Yao, 2004; Shik and Munitz, 2010). CD200, also known as OX2, is a ligand for surface receptor CD200R that lacks ITIMs but can also suppress FcεRI signaling (Kraft and Kinet, 2007).
Apart from the inhibitory FcγRIIB, mast cells express other activating high- or low-affinity receptors for IgG. FcγRI (CD64) and FcγRIII (CD16) share the same γ subunit dimer with FcεRI, but their α chains bind IgG. Their expression may vary according to the local environment, presence of cytokines, and species origin of the cells. The types of Fcγ receptors and differences in expression on human and mouse have been extensively reviewed (Malbec and Daeron, 2007; Jonsson and Daeron, 2012).
3. Chemotaxis
3.1. FcεRI-mediated chemotaxis
As mentioned above, binding of IgE to the α chain of FcεRI leads to sensitization of mast cells which is a prerequisite for their activation by multivalent antigen recognized by the IgE. The chain of activation events is initiated by phosphorylation of the ITAM motifs of the FcεRI β and γ subunits by LYN (and other SFKs). Inhibition of enzymatic activity of the SFKs with PP2 and SYK with piceatannol or ER-27319 diminished FcεRI-mediated migration, whereas inhibition of TEC kinase with terreic acid had no effect. Experiments with mast cells from mice deficient in LYN or FYN kinase showed that for FcεRI-mediated migration the presence of LYN is more important than FYN. Furthermore, cells deficient in SYK were unable to migrate towards (Kitaura et al., 2005). On the other hand, BTK-deficient mast cells did not migrate towards antigen, but exhibited normal migration towards SCF and PGE2 (Kuehn et al., 2010). However, the role of SYK in mast cell physiology is more complex; recent studies with inducible SYK knockout mice showed that SYK is dispensable for mast cell chemotaxis towards SCF (Wex et al., 2011). SYK propagates the signal, among others, by phosphorylating LAT and NTAL. Different roles of these two adaptors in chemotaxis and degranulation have been demonstrated: NTAL was found to be a predominantly negative regulator of chemotaxis (Tumova et al., 2010), whereas LAT was found to be expendable in chemotaxis (Halova et al., 2013). Interestingly, NTAL/LAT doubly deficient mast cells had diminished phosphorylation of numerous substrates, calcium release and degranulation, but exhibited increased migration towards antigen when compared to wild-type cells. However, this increase was lower in magnitude when compared to NTAL knockout cells, suggesting that in the absence of NTAL, LAT could negatively regulate chemotaxis (Halova et al., 2013). These results are consistent with the results obtained with SYK-deficient cells and suggest that the differences in activation leading to the release of secretory granules and chemotaxis are larger than previously thought. The negative regulatory role of NTAL in antigen-induced chemotaxis seems to be mediated trough small GTPase RhoA and its kinase ROCK; on the other hand NTAL does not seem to be involved in chemotaxis towards SCF (Tumova et al., 2010). RhoA is known to regulate cortical filamentous (F)-actin disassembly depending on levels of free cytoplasmic calcium (Sullivan et al., 1999). The importance of SOCE influx on mast cell chemotaxis was shown in cells with decreased levels of STIM1. These cells exhibited decreased calcium influx, formation of microtubule protrusions and impaired chemotaxis (Hajkova et al., 2011). In accordance with these data are findings that knockdown of the Ca2+ entry channel protein ORAI1 led to inhibition of spontaneous motility as well as directional chemotaxis (Lee et al., 2012). Recent study also indicated that chemotaxis towards antigen is positively regulated by adaptor protein PAG, which, however, had no effect on KIT-mediated chemotaxis in BMMCs (Draberova et al., 2014).
The role of PI3K isoforms in mast cell migration is controversial. In initial studies with p110δ knockout mice, p110δD910A transgenic mice with inactive catalytic subunit, and p110δ-selective inhibitor IC87114 showed importance of this subunit for SCF-mediated proliferation, adhesion and chemotaxis, as well as antigen-induced degranulation and cytokine production (Ali et al., 2004). Further in vivo comparative analyses showed that p110δ, but not p110γ, is important for antigen-dependent hypersensitivity responses in mice (Ali et al., 2008). Studies with IC 87114 and AS 252424, which selectively block PI3Kδ and PI3Kγ, respectively, showed PI3Kδ as the major isoform regulating the responses to antigen alone, or in combination with other stimulants and PI3Kγ contributing only in GPCR activation (Kuehn et al., 2010). The absence of PI3Kγ inhibited G protein-coupled signaling and mast cell degranulation in vitro, and protected mice from antigen-induced PSA by disrupting an adenosine-dependent autocrine/paracrine signaling loop (Laffargue et al., 2002). PI3Kγ is also critically involved in an autocrine/paracrine mast cell migration towards antigen (Kitaura et al., 2005; Endo et al., 2009). In contrast to the results obtained previously (Kuehn et al., 2010; Ali et al., 2008), the most recent work (Collmann et al., 2013) ruled out the role of PI3Kδ in antigen-mediated mast cell chemotaxis. Using p110γ knockout mice, p110γKR mice (expressing catalytically inactive subunit of p110γ), p110δ knockout mice, the p110δ-specific inhibitor IC87114 and a novel p110γ-specific inhibitor NVS-PI3-4, the authors showed that PI3Kγ alone is responsible for accumulation of mast cells in IgE-challenged skin, cytokine release, and mast cell/endothelial interaction and chemotaxis. As expected, NVS-PI3-4 also blocked direct migration through GPCRs; PI3Kδ was necessary only for migration and adhesion caused by SCF. The blocking of mast cell recruitment protected mice against anaphylaxis. These in vivo results demonstrated the advantage of blocking chemotaxis in treatment because they required 10 times lower drug doses than those used for inhibition of degranulation. Interestingly, transient inhibition of PI3Kγ enzymatic activity was sufficient to block the recruitment of mast cell progenitors into target tissues. In addition to PI3Kγ, another GPCR signal transducer PLC-β3 plays an important role in FcεRI signaling, as PLC-β3-deficient mast cells exhibit reduced antigen-induced chemotaxis and cytokine production but normal degranulation (Xiao et al., 2011). Interestingly, PLC-β3 constitutively interacts with FcεRI, LYN and SHP-1. These observations might simply indicate that multiple signaling molecules are commonly used by GPCRs and FcεRI pathways. Alternatively, FcεRI might interact with some GPCR(s) at a receptor or G protein level as well.
As already mentioned, an amount of PI(3,4,5)P3 produced by PI3K is negatively regulated by activity of two lipid phosphates, PTEN and SHIP1. It has been shown that PTEN-deficient human mast cells exhibited increased calcium levels, cytokine production, constitutive phosphorylation of AKT, p38 MAPK, and JNK as well as enhanced cell survival (Furumoto et al., 2006). Further studies showed that PTEN knockout mice exhibited mast cell hyperplasia in various organs. Selective depletion of PTEN in mast cells revealed that this phenomenon is intrinsic to the mast cell (Furumoto et al., 2011). In this study no changes in mast cell chemotaxis towards antigen and SCF in PTEN-deficient mast cell were found. However, PTEN was important for migration of macrophages (Papakonstanti et al., 2007), and one cannot exclude the possibility that the increased number of mast cells in PTEN knockout mice could be caused not only by hyper-proliferation but also by increased migration of their progenitors. Similarly, SHIP1 knockout mice also exhibited mast cell hyperplasia, increased cytokine production and anaphylactic response (Haddon et al., 2009). A new small-molecule SHIP1 activator, AQX-1125, reduced cytokine production in splenocytes, inhibited the activation of mast cells and human leukocyte chemotaxis (Stenton et al., 2013b; Stenton et al., 2013a). However, the role of these phosphatases in mast cell chemotaxis remains to be determined.
3.2. KIT-mediated chemotaxis
SCF is a major chemotactic attractant for mast cells and their precursors (Chabot et al., 1988; Meininger et al., 1992; Nilsson et al., 1994; Nilsson et al., 1998). Once bound to KIT, SCF causes KIT tyrosine phosphorylation and formation of docking sites for SH2 domain-containing molecules, such as LYN and FYN, SFKs that are recruited to phosphorylated Y567 of the KIT. This results in activation of the kinases and further propagation of the signal. Activation of FYN leads to phosphorylation of GAB2 and subsequent activation of the small GTPase RAC that is responsible for the cytoskeletal reorganization and mast cell migration (Linnekin et al., 1997; Timokhina et al., 1998; Ueda et al., 2002; Samayawardhena et al., 2007). Proper functioning of the GAB2/SHP2/VAV/PAK/RAC/JNK signaling axis requires protein tyrosine phosphatase PTPα as determined by reduced migration towards SCF in PTPα knockout mice (Samayawardhena and Pallen, 2008). Chemotaxis towards SCF also requires another phosphatase, SHP2. BMMCs from conditional SHP2 knockout mice exhibit decreased chemotaxis. SHP2 directly activated LYN kinase by dephosphorylation of its inhibitory tyrosine. This resulted in enhanced phosphorylation of VAV1, RAC activation and F-actin polymerization. In accordance with these data, chemotaxis was also reduced in cells exposed to the SHP2 inhibitor, II-B08 (Sharma et al., 2014). An important activation step is phosphorylation of KIT Y719 that is crucial for recruitment of PI3K (Ueda et al., 2002). As already mentioned PI3K plays an important role in mast chemotaxis towards different chemoattractants.
3.3. FcεRI and KIT cross-talk in chemotaxis
SCF- and antigen/IgE-activation pathways converge into a final common pathway leading to cell migration towards a chemoattractant. Therefore, it is of importance to comprehend cooperative actions of these pathways during cell migration. This topic is controversial. An early study showed that pretreatment of mast cells with SCF causes inhibition of migration towards antigen (Sawada et al., 2005). The authors proposed that SCF at sites of inflammation could lead to inhibition of migration towards antigen. This would result in preferential accumulation and degranulation of mast cells at sites of high levels of antigen. However, further experiments showed that when the IgE-sensitized cells are exposed to antigen and SCF simultaneously the chemotactic response is higher than when the cells are exposed to the individual activators (Kuehn et al., 2010). Another study showed that the response of cells exposed simultaneously to antigen and SCF was higher than that toward antigen alone, but lower than that induced by SCF alone (Tumova et al., 2010). These discrepancies could be due to differences in experimental procedures. Clarification of this issue requires further study.
4. Pharmacological targeting of signal transduction and chemotaxis pathways
Current pharmacological approaches to inhibit activity of mast cells and basophils have been summarized in a recent review (Harvima et al., 2014). Three inhibitory levels have been identified: (1) targeting soluble mediators released from the cells, (2) interfering with the intracellular signaling pathways, and (3) modulating the action of surface activating or inhibitory receptors. In the category of intracellular signal transduction targets the promising drugs are those which inhibit key enzymes involved in signal transduction from the plasma membrane receptors to the cytoplasmic effectors. To fulfill a set of the criteria as a therapeutic, they should be specific to mast cells and their action should not cause severe effects in other cell types. For this reason, SFKs may not be suitable targets, because they are expressed in numerous other cells types. SYK has a more limited distribution and therefore is more suitable as a drug target. In fact, several SYK inhibitors have been developed and some of them have entered clinical trials. These include fostamatinib, also called R-788 (Braselmann et al., 2006; Weinblatt et al., 2013) and PRT062607 (Simmons, 2013). Fostamanitib, an oral SYK inhibitor, has been successfully used in a phase II clinical trial for treatment of patients with rheumatoid arthritis (Weinblatt et al., 2010; Weinblatt et al., 2013). When administrated to mice it caused a significant reduction of neutrophils and macrophages, whereas systemic leukocyte counts were not affected (Hilgendorf et al., 2011). BTK can be selectively inhibited by ibrutinib that, via its binding to the active site, disables its phosphorylation and thus causes inactivation. It forms a specific covalent bond with cysteine 481 in BTK and abrogates its full activation by inhibiting autophosphorylation at Tyr-223 (Pan et al., 2007; Honigberg et al., 2010). Another drug AVL-292/CC-292 was designed on the same principle and is under investigation in clinical trials to treat rheumatoid arthritis (Evans et al., 2013).
Promising as targets are also PI3Ks which are involved in various signaling pathways, although these kinases are expressed in many cell types. IC87114 and its chemical derivatives CAL-101 and CAL-263 are highly selective inhibitors of PI3Kδ and have clinical potential for treatment of allergic rhinitis (Blunt and Ward, 2012), asthma (Lee et al., 2006) and rheumatoid arthritis (Randis et al., 2008). IPI-145, a small-molecule inhibitor of PI3K-δ and PI3K-γ, showed potent activity in collagen-induced arthritis, ovalbumin-induced asthma, and systemic lupus erythematosus in rodent models. This drug blocked neutrophil migration, lymphocyte proliferation, and reduce basophil and mast cell activation (Winkler et al., 2013). IPI-145 is in clinical trials for various hematologic malignancies and inflammatory diseases, such as asthma and rheumatoid arthritis. A wide range of other PI3K inhibitors have entered clinical trials (Marone et al., 2008).
Enhancement of the activity of negative regulators of degranulation and termination of the activation events can be achieved by stimulating the phosphatase SHIP1. A recent double blind (placebo/controlled) study with AQX-1125, a novel oral SHIP1 activator, reports significantly reduced late responses to allergen challenge, with a trend to reduce airway inflammation and claims AQX-1125 as a safe and well tolerated drug (Leaker et al., 2014).
Several diseases including mastocytosis are associated with a gain of function mutation (D816V) in KIT (Ustun et al., 2011). Also an aberrant bone marrow mast-cell population carrying the clonal markers found in mastocytosis was reported in patients with recurrent anaphylaxis (Akin et al., 2007). The KIT receptor is related to platelet-derived growth factor receptor (PDGFR), and the known KIT inhibitors also inhibit PDGFR and some other molecules with tyrosine kinase activity. Inhibition of KIT activity is mainly desired in myeloid leukemia but could also be used for treatment of systemic mastocytosis. Several KIT inhibitors varying in their inhibitory specificity and sensitivity to the activating mutation are available, including dasatinib (Kneidinger et al., 2008), nilotinib (Verstovsek et al., 2006), imatinib (Vega-Ruiz et al., 2009) and masitinib (Humbert et al., 2009; Paul et al., 2010). Two KIT inhibitors, masitinib and imatinib, that are promising for treatment of mastocytosis, arthritis and allergen-induced asthma, were also found to be efficient inhibitors of mast cell migration, where masitinib was more efficient then imatinib (Dubreuil et al., 2009).
5. Future directions and concluding remarks
First of all, one should realize that our understanding of FcεRI signaling is far from complete. Although everyone would agree on the fundamental importance of tyrosine and lipid phosphorylation in FcεRI signaling pathways, multiple studies fail to agree on detailed mechanisms by which SFKs and PI3K isoforms regulate mast cell activation, as discussed above. Furthermore, more than one hundred tyrosine-phosphorylated proteins (Cao et al., 2007) await to be investigated. There are also many poorly explored areas in FcεRI signaling, including potential roles of other post-translational modifications, miRNAs and other noncoding RNAs, epigenetic regulation of signaling molecules, etc. However, as we are in the post-human/mouse genome era with an armamentarium of proteomics, next-generation sequencing, epigenetics, siRNA and CRISPR/Cas techniques, super-resolution microscopy, multi-photon microscopy, etc., our pace of discoveries in FcεRI signaling per se will be accelerated in the next few years. Second, one needs to understand more how FcεRI signaling in mast cells contributes to in vivo allergic inflammation. This issue involves not only the influence of soluble factors on FcεRI signaling, but also that of direct mast cell-other cell interactions. Studies on interactions of mast cells with T cells, eosinophils, and other immune cells have already begun to provide novel insights into the latter area (Mekori and Hershko, 2012; Gangwar and Levi-Schaffer, 2014). This type of studies will deepen our understanding of the cellular and molecular mechanisms of allergic diseases. Third, one need to pay more attention to the effect of the developmental stages of mast cells and different types of mast cells on FcεRI signaling. In this regard, Mcpt5-Cre mice, which allows to generate conditional null alleles in connective tissue-type mast cells, are very useful (Scholten et al., 2008; Reber et al., 2013; Oh et al., 2014). As described above, all these aspects of new knowledge will be subsequently followed by pharmaceutical development of anti-allergic drugs.
Acknowledgements
Work in the P. D. laboratory is supported by projects P302-14-09807S, P302/12/G101, and P305-14-00703S from the Czech Science Foundation; Action BM1007 from European Cooperation in Science and Technology; project LD12073 COST-CZ-MAST from the Ministry of Education Youth and Sports of the Czech Republic, and by the Institute of Molecular Genetics of the Acad. Sci. Czech Republic (RVO 68378050). T.K. is supported in part by the US National Institutes of Health grants R01 AR064418-01A1 and 1R01 HL124283-01 and a grant from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
References
- Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin C, Scott LM, Kocabas CN, Kushnir-Sukhov N, Brittain E, Noel P, Metcalfe DD. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with “idiopathic” anaphylaxis. Blood. 2007;110:2331–2333. doi: 10.1182/blood-2006-06-028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110 phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- Ali K, Camps M, Pearce WP, Ji H, Ruckle T, Kuehn N, Pasquali C, Chabert C, Rommel C, Vanhaesebroeck B. Isoform-specific functions of phosphoinositide 3-kinases: p110δ but not p110γ promotes optimal allergic responses in vivo. J. Immunol. 2008;180:2538–2544. doi: 10.4049/jimmunol.180.4.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J. Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- Alvarez-Errico D, Lessmann E, Rivera J. Adapters in the organization of mast cell signaling. Immunol. Rev. 2009;232:195–217. doi: 10.1111/j.1600-065X.2009.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat. Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat. Rev. Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- Bischoff SC, Sellge G, Lorentz A, Sebald W, Raab R, Manns MP. IL-4 enhances proliferation and mediator release in mature human mast cells. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8080–8085. doi: 10.1073/pnas.96.14.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette EY, Enciso JA, Befus AD. TGF-β1 inhibits the release of histamine and tumor necrosis factor-α from mast cells through an autocrine pathway. Am. J. Respir. Cell Mol. Biol. 1997;16:275–282. doi: 10.1165/ajrcmb.16.3.9070612. [DOI] [PubMed] [Google Scholar]
- Blank U, Ra C, Miller L, White K, Metzger H, Kinet J-P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989;337:187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- Blunt MD, Ward SG. Pharmacological targeting of phosphoinositide lipid kinases and phosphatases in the immune system: success, disappointment, and new opportunities. Front Immunol. 2012;3:226. doi: 10.3389/fimmu.2012.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braselmann S, Taylor V, Zhao H, Wang S, Sylvain C, Baluom M, Qu K, Herlaar E, Lau A, Young C, Wong BR, Lovell S, Sun T, Park G, Argade A, Jurcevic S, Pine P, Singh R, Grossbard EB, Payan DG, Masuda ES. R406, an orally available spleen tyrosine kinase inhibitor blocks Fc receptor signaling and reduces immune complex-mediated inflammation. J. Pharmacol. Exp. Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- Brdička T, Pavlištová D, Leo A, Bruyns E, Kořínek V, Angelisová P, Scherer J, Shevchenko A, Hilgert I, Černý J, Drbal K, Kuramitsu Y, Kornacker B, Hořejší V, Schraven B. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase Csk and is involved in regulation of T cell activation. J. Exp. Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brdička T, Imrich M, Angelisová P, Brdičková N, Horváth O, Špička J, Hilgert I, Lusková P, Dráber P, Novák P, Engels N, Wienands J, Simeoni L, Osterreicher J, Aguado E, Malissen M, Schraven B, Hořejší V. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med. 2002;196:1617–1626. doi: 10.1084/jem.20021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Wilson TM, Metcalfe DD. The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin. Exp. Allergy. 2008;38:4–18. doi: 10.1111/j.1365-2222.2007.02886.x. [DOI] [PubMed] [Google Scholar]
- Bugajev V, Bambousková M, Dráberova L, Dráber P. What precedes the initial tyrosine phosphorylation of the high affinity IgE receptor in antigen-activated mast cell? FEBS Lett. 2010;584:4949–4955. doi: 10.1016/j.febslet.2010.08.045. [DOI] [PubMed] [Google Scholar]
- Cambier JC. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J. Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- Cao L, Yu K, Banh C, Nguyen V, Ritz A, Raphael BJ, Kawakami Y, Kawakami T, Salomon AR. Quantitative time-resolved phosphoproteomic analysis of mast cell signaling. J. Immunol. 2007;179:5864–5876. doi: 10.4049/jimmunol.179.9.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signalling by the FcεRI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- Collmann E, Bohnacker T, Marone R, Dawson J, Rehberg M, Stringer R, Krombach F, Burkhart C, Hirsch E, Hollingworth GJ, Thomas M, Wymann MP. Transient targeting of phosphoinositide 3-kinase acts as a roadblock in mast cells’ route to allergy. J. Allergy Clin. Immunol. 2013;132:959–968. doi: 10.1016/j.jaci.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Costello PS, Turner M, Walters AE, Cunningham CN, Bauer PH, Downward J, Tybulewicz VL. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 1996;13:2595–2605. [PubMed] [Google Scholar]
- Dillahunt SE, Sargent JL, Suzuki R, Proia RL, Gilfillan A, Rivera J, Olivera A. Usage of sphingosine kinase isoforms in mast cells is species and/or cell type determined. J. Immunol. 2013;190:2058–2067. doi: 10.4049/jimmunol.1201503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnadieu E, Jouvin MH, Kinet JP. A second amplifier function for the allergy-associated FcεRI-β subunit. Immunity. 2000;12:515–523. doi: 10.1016/s1074-7613(00)80203-4. [DOI] [PubMed] [Google Scholar]
- Draber P, Draberova L. Lifting the fog in store-operated Ca2+ entry. Trends Immunol. 2005;26:621–624. doi: 10.1016/j.it.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Draber P, Halova I, Levi-Schaffer F, Draberova L. Transmembrane adaptor proteins in the high-affinity IgE receptor signaling. Frontiers Immunol. 2012;2:1–11. doi: 10.3389/fimmu.2011.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draberova L, Bugajev V, Potuckova L, Halova I, Bambouskova M, Polakovicova I, Xavier RJ, Seed B, Draber P. Transmembrane adaptor protein PAG/CBP is involved in both positive and negative regulation of mast cell signaling. Mol. Cell Biol. 2014 doi: 10.1128/MCB.00983-14. doi: 10.1016/j.celrep.2014.08.066. Epub 20114 Oct. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dráberová L, Shaik GM, Volná P, Heneberg P, Tůmova M, Lebduška P, Korb J, Dráber P. Regulation of Ca2+ signaling in mast cells by tyrosine-phosphorylated and unphosphorylated non-T cell activation linker. J. Immunol. 2007;179:5169–5180. doi: 10.4049/jimmunol.179.8.5169. [DOI] [PubMed] [Google Scholar]
- Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Casteran N, Borge L, Hajem B, Lermet A, Sippl W, Voisset E, Arock M, Auclair C, Leventhal PS, Mansfield CD, Moussy A, Hermine O. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS. One. 2009;4:e7258. doi: 10.1371/journal.pone.0007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo D, Gon Y, Nunomura S, Yamashita K, Hashimoto S, Ra C. PI3Kγ differentially regulates FcεRI-mediated degranulation and migration of mast cells by and toward antigen. Int. Arch. Allergy Immunol. 2009;149(Suppl 1):66–72. doi: 10.1159/000211375. [DOI] [PubMed] [Google Scholar]
- Evans EK, Tester R, Aslanian S, Karp R, Sheets M, Labenski MT, Witowski SR, Lounsbury H, Chaturvedi P, Mazdiyasni H, Zhu Z, Nacht M, Freed MI, Petter RC, Dubrovskiy A, Singh J, Westlin WF. Inhibition of Btk with CC-292 Provides Early Pharmacodynamic Assessment of Activity in Mice and Humans. J. Pharmacol. Exp. Ther. 2013 doi: 10.1124/jpet.113.203489. [DOI] [PubMed] [Google Scholar]
- Feng C, Beller EM, Bagga S, Boyce JA. Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood. 2006;107:3243–3250. doi: 10.1182/blood-2005-07-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssell J, Sideras P, Eriksson C, Malm-Erjefalt M, Rydell-Tormanen K, Ericsson PO, Erjefalt JS. Interleukin-2-inducible T cell kinase regulates mast cell degranulation and acute allergic responses. Am. J. Respir. Cell Mol. Biol. 2005;32:511–520. doi: 10.1165/rcmb.2004-0348OC. [DOI] [PubMed] [Google Scholar]
- Fukao T, Yamada T, Tanabe M, Terauchi Y, Ota T, Takayama T, Asano T, Takeuchi T, Kadowaki T, Hata JJ, Koyasu S. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat. Immunol. 2002;3:295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- Fukuoka S-I, Freedman SD, Yu H, Sukhatme VP, Scheele GA. GP-2/THP gene family encodes self-binding glycosylphosphstidylinositol-anchored proteins in apical secretory compartments of pancreas and kidney. Proc. Natl. Acad. Sci. USA. 1992;89:1189–1193. doi: 10.1073/pnas.89.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumoto Y, Brooks S, Olivera A, Takagi Y, Miyagishi M, Taira K, Casellas R, Beaven MA, Gilfillan AM, Rivera J. Cutting Edge: Lentiviral short hairpin RNA silencing of PTEN in human mast cells reveals constitutive signals that promote cytokine secretion and cell survival. J. Immunol. 2006;176:5167–5171. doi: 10.4049/jimmunol.176.9.5167. [DOI] [PubMed] [Google Scholar]
- Furumoto Y, Charles N, Olivera A, Leung WH, Dillahunt S, Sargent JL, Tinsley K, Odom S, Scott E, Wilson TM, Ghoreschi K, Kneilling M, Chen M, Lee DM, Bolland S, Rivera J. PTEN deficiency in mast cells causes a mastocytosis-like proliferative disease that heightens allergic responses and vascular permeability. Blood. 2011;118:5466–5475. doi: 10.1182/blood-2010-09-309955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwar RS, Levi-Schaffer F. Eosinophils interaction with mast cells: the allergic effector unit. Methods Mol. Biol. 2014;1178:231–249. doi: 10.1007/978-1-4939-1016-8_20. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Sellge G, Lorentz A, Raab R, Manns MP, Bischoff SC. Cultured human intestinal mast cells express functional IL-3 receptors and respond to IL-3 by enhancing growth and IgE receptor-dependent mediator release. Eur. J. Immunol. 2002;32:2308–2316. doi: 10.1002/1521-4141(200208)32:8<2308::AID-IMMU2308>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Crit Rev. Immunol. 2011;31:475–529. doi: 10.1615/critrevimmunol.v31.i6.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol. Rev. 2009;228:149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- Gomez G, Gonzalez-Espinosa C, Odom S, Baez G, Cid ME, Ryan JJ, Rivera J. Impaired FcεRI-dependent gene expression and defective eicosanoid and cytokine production as a consequence of Fyn deficiency in mast cells. J. Immunol. 2005a;175:7602–7610. doi: 10.4049/jimmunol.175.11.7602. [DOI] [PubMed] [Google Scholar]
- Gomez G, Ramirez CD, Rivera J, Patel M, Norozian F, Wright HV, Kashyap MV, Barnstein BO, Fischer-Stenger K, Schwartz LB, Kepley CL, Ryan JJ. TGF-β1 inhibits mast cell FcεRI expression. J. Immunol. 2005b;174:5987–5993. doi: 10.4049/jimmunol.174.10.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Pratt JC, Burakoff SJ, Neel BG. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol. Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- Gu H, Saito K, Klaman LD, Shen J, Fleming T, Wang Y, Pratt JC, Lin G, Lim B, Kinet J-P, Neel BG. Essential role for Gab2 in the allergic response. Nature. 2001;412:186–190. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- Haddon DJ, Antignano F, Hughes MR, Blanchet MR, Zbytnuik L, Krystal G, McNagny KM. SHIP1 is a repressor of mast cell hyperplasia, cytokine production, and allergic inflammation in vivo. J. Immunol. 2009;183:228–236. doi: 10.4049/jimmunol.0900427. [DOI] [PubMed] [Google Scholar]
- Haidl ID, McAlpine SM, Marshall JS. Enhancement of mast cell IL-6 production by combined toll-like and nucleotide-binding oligomerization domain-like receptor activation. Int. Arch. Allergy Immunol. 2011;154:227–235. doi: 10.1159/000321109. [DOI] [PubMed] [Google Scholar]
- Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Hájková Z, Bugajev V, Dráberová E, Vinopal S, Dráberová L, Janáček J, Dráber P, Dráber P. STIM1-directed reorganization of microtubules in activated mast cells. J. Immunol. 2011;186:913–923. doi: 10.4049/jimmunol.1002074. [DOI] [PubMed] [Google Scholar]
- Hálová I, Dráberová L, Bambousková M, Machyna M, Stegurová L, Smrž D, Dráber P. Crosstalk between tetraspanin CD9 and transmembrane adaptor protein non-T cell activation linker (NTAL) in mast cell activation and chemotaxis. J. Biol. Chem. 2013;288:9801–9814. doi: 10.1074/jbc.M112.449231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halova I, Draberova L, Draber P. Mast cell chemotaxis - chemoattractants and signaling pathways. Front Immunol. 2012;3:119. doi: 10.3389/fimmu.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvima IT, Levi-Schaffer F, Draber P, Friedman S, Polakovicova I, Gibbs BF, Blank U, Nilsson G, Maurer M. Molecular targets on mast cells and basophils for novel therapies. J. Allergy Clin. Immunol. 2014;134:530–544. doi: 10.1016/j.jaci.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Hata D, Kawakami Y, Inagaki N, Lantz CS, Kitamura T, Khan WN, Maeda-Yamamoto M, Miura T, Han W, Hartman SE, Yao L, Nagai H, Goldfeld AE, Alt FW, Galli SJ, Witte ON, Kawakami T. Involvement of Bruton’s tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J. Exp. Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneberg P, Dráber P. Nonreceptor protein tyrosine and lipid phosphatases in type I Fcε receptor-mediated activation of mast cells and basophils. Int. Arch. Allergy Immunol. 2002;128:253–263. doi: 10.1159/000063864. [DOI] [PubMed] [Google Scholar]
- Hernandez-Hansen V, Smith AJ, Surviladze Z, Chigaev A, Mazel T, Kalesnikoff J, Lowell CA, Krystal G, Sklar LA, Wilson BS, Oliver JM. Dysregulated FcεRI signaling and altered Fyn and SHIP activities in Lyn-deficient mast cells. J. Immunol. 2004;173:100–112. doi: 10.4049/jimmunol.173.1.100. [DOI] [PubMed] [Google Scholar]
- Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Hilgendorf I, Eisele S, Remer I, Schmitz J, Zeschky K, Colberg C, Stachon P, Wolf D, Willecke F, Buchner M, Zirlik K, Ortiz-Rodriguez A, Lozhkin A, Hoppe N, von Zur MC, zur HA, Bode C, Zirlik A. The oral spleen tyrosine kinase inhibitor fostamatinib attenuates inflammation and atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2011;31:1991–1999. doi: 10.1161/ATVBAHA.111.230847. [DOI] [PubMed] [Google Scholar]
- Hong H, Kitaura J, Xiao W, Horejsi V, Ra C, Lowell CA, Kawakami Y, Kawakami T. The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn. Blood. 2007;110:2511–2519. doi: 10.1182/blood-2007-01-066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Helgason CD, Scheid MP, Duronio V, Humphries RK, Krystal G. Targeted disruption of SHIP leads to Steel factor-induced degranulation of mast cells. EMBO J. 1998;17:7311–7319. doi: 10.1093/emboj/17.24.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert M, de BF, Garcia G, Prud’homme A, Leroyer C, Magnan A, Tunon-de-Lara JM, Pison C, Aubier M, Charpin D, Vachier I, Purohit A, Gineste P, Bader T, Moussy A, Hermine O, Chanez P. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64:1194–1201. doi: 10.1111/j.1398-9995.2009.02122.x. [DOI] [PubMed] [Google Scholar]
- Iwaki S, Spicka J, Tkaczyk C, Jensen BM, Furumoto Y, Charles N, Kovarova M, Rivera J, Horejsi V, Metcalfe DD, Gilfillan AM. Kit- and FcεRI-induced differential phosphorylation of the transmembrane adaptor molecule NTAL/LAB/LAT2 allows flexibility in its scaffolding function in mast cells. Cell Signal. 2008;20:195–205. doi: 10.1016/j.cellsig.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki S, Tkaczyk C, Satterthwaite AB, Halcomb K, Beaven MA, Metcalfe DD, Gilfillan AM. Btk plays a crucial role in the amplification of FcεRI-mediated mast cell activation by kit. J. Biol. Chem. 2005;280:40261–40270. doi: 10.1074/jbc.M506063200. [DOI] [PubMed] [Google Scholar]
- Iyer AS, August A. The Tec family kinase, IL-2-inducible T cell kinase, differentially controls mast cell responses. J. Immunol. 2008;180:7869–7877. doi: 10.4049/jimmunol.180.12.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer AS, Morales JL, Huang W, Ojo F, Ning G, Wills E, Baines JD, August A. Absence of Tec family kinases interleukin-2 inducible T cell kinase (Itk) and Bruton’s tyrosine kinase (Btk) severely impairs FcεRI-dependent mast cell responses. J. Biol. Chem. 2011;286:9503–9513. doi: 10.1074/jbc.M110.165613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by FcεRI triggering is required for normal mast cell degranulation and chemotaxis. J. Exp. Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson F, Daëron M. Mast cells and company. Front Immunol. 2012;3:16. doi: 10.3389/fimmu.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, Fletcher MC, Ledbetter JA, Schieven GL, Siegel JN, Phillips AF, Samelson LE. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc. Natl. Acad. Sci. U. S. A. 1990;87:7722–7726. doi: 10.1073/pnas.87.19.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat. Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Kitaura J, Satterthwaite AB, Kato RM, Asai K, Hartman SE, Maeda-Yamamoto M, Lowell CA, Rawlings DJ, Witte ON, Kawakami T. Redundant and opposing functions of two tyrosine kinases, Btk and Lyn, in mast cell activation. J. Immunol. 2000;165:1210–1219. doi: 10.4049/jimmunol.165.3.1210. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Yao L, Miura T, Tsukada S, Witte ON, Kawakami T. Tyrosine phosphorylation and activation of Bruton tyrosine kinase upon FcεRI cross-linking. Mol. Cell Biol. 1994;14:5108–5113. doi: 10.1128/mcb.14.8.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Yao L, Tashiro M, Gibson S, Mills GB, Kawakami T. Activation and interaction with protein kinase C of a cytoplasmic tyrosine kinase, Itk/Tsk/Emt, on FcεRI cross-linking on mast cells. J. Immunol. 1995;155:3556–3562. [PubMed] [Google Scholar]
- Kennedy NS, Barnstein B, Brenzovich J, Bailey DP, Kashyap M, Speiran K, Ford J, Conrad D, Watowich S, Moralle MR, Kepley CL, Murray PJ, Ryan JJ. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J. Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
- Kepley CL, Taghavi S, Mackay G, Zhu D, Morel PA, Zhang K, Ryan JJ, Satin LS, Zhang M, Pandolfi PP, Saxon A. Co-aggregation of FcγRII with FcεRI on human mast cells inhibits antigen-induced secretion and involves SHIP-Grb2-Dok complexes. J. Biol. Chem. 2004;279:35139–35149. doi: 10.1074/jbc.M404318200. [DOI] [PubMed] [Google Scholar]
- Kettner A, Pivniouk V, Kumar L, Falet H, Lee JS, Mulligan R, Geha RS. Structural requirements of SLP-76 in signaling via the high-affinity immunoglobulin E receptor (FcεRI) in mast cells. Mol. Cell Biol. 2003;23:2395–2406. doi: 10.1128/MCB.23.7.2395-2406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Radinger M, Gilfillan AM. The multiple roles of phosphoinositide 3-kinase in mast cell biology. Trends Immunol. 2008;29:493–501. doi: 10.1016/j.it.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinet JP. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu. Rev. Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- Kitaura J, Kinoshita T, Matsumoto M, Chung S, Kawakami Y, Leitges M, Wu D, Lowell CA, Kawakami T. IgE- and IgE+Ag-mediated mast cell migration in an autocrine/paracrine fashion. Blood. 2005;105:3222–3229. doi: 10.1182/blood-2004-11-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneidinger M, Schmidt U, Rix U, Gleixner KV, Vales A, Baumgartner C, Lupinek C, Weghofer M, Bennett KL, Herrmann H, Schebesta A, Thomas WR, Vrtala S, Valenta R, Lee FY, Ellmeier W, Superti-Furga G, Valent P. The effects of dasatinib on IgE receptor-dependent activation and histamine release in human basophils. Blood. 2008;111:3097–3107. doi: 10.1182/blood-2007-08-104372. [DOI] [PubMed] [Google Scholar]
- Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat. Rev. Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcεRI-mediated mast cell activation. Immunol. Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn HS, Radinger M, Brown JM, Ali K, Vanhaesebroeck B, Beaven MA, Metcalfe DD, Gilfillan AM. Btk-dependent Rac activation and actin rearrangement following FcεRI aggregation promotes enhanced chemotactic responses of mast cells. J. Cell Sci. 2010;123:2576–2585. doi: 10.1242/jcs.071043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, Hirsch E, Wymann MP. Phosphoinositide 3-kinase γ is an essential amplifier of mast cell function. Immunity. 2002;16:441–451. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- Lagraauw HM, Westra MM, Bot M, Wezel A, van Santbrink PJ, Pasterkamp G, Biessen EA, Kuiper J, Bot I. Vascular neuropeptide Y contributes to atherosclerotic plaque progression and perivascular mast cell activation. Atherosclerosis. 2014;235:196–203. doi: 10.1016/j.atherosclerosis.2014.04.025. [DOI] [PubMed] [Google Scholar]
- Leaker BR, Barnes PJ, O’Connor BJ, Ali FY, Tam P, Neville J, Mackenzie LF, Macrury T. The effects of the novel SHIP1 activator AQX-1125 on allergen-induced responses in mild-to-moderate asthma. Clin. Exp. Allergy. 2014;44:1146–1153. doi: 10.1111/cea.12370. [DOI] [PubMed] [Google Scholar]
- Lee J, Veatch SL, Baird B, Holowka D. Molecular mechanisms of spontaneous and directed mast cell motility. J. Leukoc. Biol. 2012;92:1029–1041. doi: 10.1189/jlb.0212091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim JW, Kim d.K., Kim HS, Park HJ, Park DK, Kim AR, Kim B, Beaven MA, Park KL, Kim YM, Choi WS. The Src family kinase Fgr is critical for activation of mast cells and IgE-mediated anaphylaxis in mice. J. Immunol. 2011;187:1807–1815. doi: 10.4049/jimmunol.1100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Lee HK, Hayflick JS, Lee YC, Puri KD. Inhibition of phosphoinositide 3-kinase δ attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. FASEB J. 2006;20:455–465. doi: 10.1096/fj.05-5045com. [DOI] [PubMed] [Google Scholar]
- Li L, Yao Z. Mast cell and immune inhibitory receptors. Cell Mol. Immunol. 2004;1:408–415. [PubMed] [Google Scholar]
- Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat. Rev. Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- Lin S, Cicala C, Scharenberg AM, Kinet JP. The FcεRIβ subunit functions as an amplifier of FcεRIγ-mediated cell activation signals. Cell. 1996;85:985–995. doi: 10.1016/s0092-8674(00)81300-8. [DOI] [PubMed] [Google Scholar]
- Linnekin D, DeBerry CS, Mou S. Lyn associates with the juxtamembrane region of c-Kit and is activated by stem cell factor in hematopoietic cell lines and normal progenitor cells. J. Biol. Chem. 1997;272:27450–27455. doi: 10.1074/jbc.272.43.27450. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Do HW, Jones JT, Myers JW, Ferrell JE, Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ Influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein EJ, Daly RJ, Batzer AG, Li W, Margolis B, Lammers R, Ullrich A, Skolnik EY, Bar-Sagi D, Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- Malbec O, Daëron M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol. Rev. 2007;217:206–221. doi: 10.1111/j.1600-065X.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Manetz TS, Gonzalez-Espinosa C, Arudchandran R, Xirasagar S, Tybulewicz V, Rivera J. Vav1 regulates phospholipase Cγ activation and calcium responses in mast cells. Mol. Cell Biol. 2001;21:3763–3774. doi: 10.1128/MCB.21.11.3763-3774.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim. Biophys. Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Marshall JS, Jawdat DM. Mast cells in innate immunity. J. Allergy Clin. Immunol. 2004;114:21–27. doi: 10.1016/j.jaci.2004.04.045. [DOI] [PubMed] [Google Scholar]
- McPherson VA, Sharma N, Everingham S, Smith J, Zhu HH, Feng GS, Craig AW. SH2 domain-containing phosphatase-2 protein-tyrosine phosphatase promotes FcεRI-induced activation of Fyn and Erk pathways leading to TNFα release from bone marrow-derived mast cells. J. Immunol. 2009;183:4940–4947. doi: 10.4049/jimmunol.0900702. [DOI] [PubMed] [Google Scholar]
- Meininger CJ, Yano H, Rottapel R, Bernstein A, Zsebo KM, Zetter BR. The c-kit receptor ligand functions as a mast cell chemoattractant. Blood. 1992;79:958–963. [PubMed] [Google Scholar]
- Mekori YA, Hershko AY. T cell-mediated modulation of mast cell function: heterotypic adhesion-induced stimulatory or inhibitory effects. Front Immunol. 2012;3:6. doi: 10.3389/fimmu.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Yoshimaru T, Suzuki Y, Inoue T, Ra C, Yakura H, Mizuno K. Positive and negative regulation of high affinity IgE receptor signaling by Src homology region 2 domain-containing phosphatase 1. J. Immunol. 2008;181:5414–5424. doi: 10.4049/jimmunol.181.8.5414. [DOI] [PubMed] [Google Scholar]
- Nilsson G, Butterfield JH, Nilsson K, Siegbahn A. Stem cell factor is a chemotactic factor for human mast cells. J. Immunol. 1994;153:3717–3723. [PubMed] [Google Scholar]
- Nilsson G, Hjertson M, Andersson M, Greiff L, Svensson C, Nilsson K, Siegbahn A. Demonstration of mast-cell chemotactic activity in nasal lavage fluid: characterization of one chemotaxin as c-kit ligand, stem cell factor. Allergy. 1998;53:874–879. doi: 10.1111/j.1398-9995.1998.tb03994.x. [DOI] [PubMed] [Google Scholar]
- Nilsson G, Johnell M, Hammer CH, Tiffany HL, Nilsson K, Metcalfe DD, Siegbahn A, Murphy PM. C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J. Immunol. 1996;157:1693–1698. [PubMed] [Google Scholar]
- Nishizumi H, Yamamoto T. Impaired tyrosine phosphorylation and Ca2+ mobilization, but not degranulation, in lyn-deficient bone marrow-derived mast cells. J. Immunol. 1997;158:2350–2355. [PubMed] [Google Scholar]
- O’Laughlin-Bunner B, Radosevic N, Taylor ML, Shivakrupa, DeBerry C, Metcalfe DD, Zhou M, Lowell C, Linnekin D. Lyn is required for normal stem cell factor-induced proliferation and chemotaxis of primary hematopoietic cells. Blood. 2001;98:343–350. doi: 10.1182/blood.v98.2.343. [DOI] [PubMed] [Google Scholar]
- Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, Gonzalez-Espinosa C, Hibbs ML, Harder KW, Rivera J. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J. Exp. Med. 2004;199:1491–1502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SY, Brandal S, Kapur R, Zhu Z, Takemoto CM. Global microRNA expression is essential for murine mast cell development in vivo. Exp Hematol. 2014 doi: 10.1016/j.exphem.2014.07.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake H, Ichikawa N, Okada M, Yamashita T. Cutting Edge: Transmembrane phosphoprotein Csk-binding protein/phosphoprotein associated with glycosphingolipid-enriched microdomains as a negative feedback regulator of mast cell signaling through the FcεRI. J. Immunol. 2002;168:2087–2090. doi: 10.4049/jimmunol.168.5.2087. [DOI] [PubMed] [Google Scholar]
- Okada M. Regulation of the SRC family kinases by Csk. Int. J. Biol. Sci. 2012;8:1385–1397. doi: 10.7150/ijbs.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol. Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat. Rev. Immunol. 2003;3:317–330. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Olivera A, Urtz N, Mizugishi K, Yamashita Y, Gilfillan AM, Furumoto Y, Gu H, Proia RL, Baumruker T, Rivera J. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J. Biol. Chem. 2006;281:2515–2525. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- Oskeritzian CA, Alvarez SE, Hait NC, Price MM, Milstien S, Spiegel S. Distinct roles of sphingosine kinases 1 and 2 in human mast-cell functions. Blood. 2008;111:4193–4200. doi: 10.1182/blood-2007-09-115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott VL, Tamir I, Niki M, Pandolfi PP, Cambier JC. Downstream of kinase, p62dok, is a mediator of FcγIIB inhibition of FcεRI signaling. J. Immunol. 2002;168:4430–4439. doi: 10.4049/jimmunol.168.9.4430. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Szallasi Z, Kazanietz MG, Blumberg PM, Mischak H, Mushinski JF, Beaven MA. Ca2+-dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J. Biol. Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- Pan Z, Scheerens H, Li SJ, Schultz BE, Sprengeler PA, Burrill LC, Mendonca RV, Sweeney MD, Scott KC, Grothaus PG, Jeffery DA, Spoerke JM, Honigberg LA, Young PR, Dalrymple SA, Palmer JT. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007;2:58–61. doi: 10.1002/cmdc.200600221. [DOI] [PubMed] [Google Scholar]
- Papakonstanti EA, Ridley AJ, Vanhaesebroeck B. The p110δ isoform of PI 3-kinase negatively controls RhoA and PTEN. EMBO J. 2007;26:3050–3061. doi: 10.1038/sj.emboj.7601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O’Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- Paul C, Sans B, Suarez F, Casassus P, Barete S, Lanternier F, Grandpeix-Guyodo C, Dubreuil P, Palmerini F, Mansfield CD, Gineste P, Moussy A, Hermine O, Lortholary O. Masitinib for the treatment of systemic and cutaneous mastocytosis with handicap: a phase 2a study. Am. J. Hematol. 2010;85:921–925. doi: 10.1002/ajh.21894. [DOI] [PubMed] [Google Scholar]
- Pivniouk VI, Martin TR, Lu-Kuo JM, Katz HR, Oettgen HC, Geha RS. SLP-76 deficiency impairs signaling via the high-affinity IgE receptor in mast cells. J. Clin. Invest. 1999;103:1737–1743. [PMC free article] [PubMed] [Google Scholar]
- Polakovicova I, Draberova L, Simicek M, Draber P. Multiple Regulatory Roles of the Mouse Transmembrane Adaptor Protein NTAL in Gene Transcription and Mast Cell Physiology. PLoS. One. 2014;9:e105539. doi: 10.1371/journal.pone.0105539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieschl EE, Csonga R, Novotny V, Kikuchi GE, Baumruker T. The balance between sphingosine and sphingosine-1-phosphate is decisive for mast cell activation after Fc receptor I triggering. J. Exp. Med. 1999;190:1–8. doi: 10.1084/jem.190.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pundir P, Kulka M. The role of G protein-coupled receptors in mast cell activation by antimicrobial peptides: is there a connection? Immunol. Cell Biol. 2010;88:632–640. doi: 10.1038/icb.2010.27. [DOI] [PubMed] [Google Scholar]
- Randis TM, Puri KD, Zhou H, Diacovo TG. Role of PI3Kδ and PI3Kγ in inflammatory arthritis and tissue localization of neutrophils. Eur. J. Immunol. 2008;38:1215–1224. doi: 10.1002/eji.200838266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh MJ, Kalesnikoff J, Hughes M, Sly L, Lam V, Krystal G. Role of Src homology 2-containing-inositol 5′-phosphatase (SHIP) in mast cells and macrophages. Biochem. Soc. Trans. 2003;31:286–291. doi: 10.1042/bst0310286. [DOI] [PubMed] [Google Scholar]
- Rawlings DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, Mohr RN, Bazan JF, Howard M, Copeland NG. Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- Reber LL, Marichal T, Mukai K, Kita Y, Tokuoka SM, Roers A, Hartmann K, Karasuyama H, Nadeau KC, Tsai M, Galli SJ. Selective ablation of mast cells or basophils reduces peanut-induced anaphylaxis in mice. J Allergy Clin. Immunol. 2013;132:881–888. doi: 10.1016/j.jaci.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Rommel C, Camps M, Ji H. PI3Kδ and PI3Kγ: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat. Rev. Immunol. 2007;7:191–201. doi: 10.1038/nri2036. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr. Signaling by Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem. Biophys. Res. Commun. 2005;337:1–13. doi: 10.1016/j.bbrc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Royer B, Varadaradjalou S, Saas P, Guillosson JJ, Kantelip JP, Arock M. Inhibition of IgE-induced activation of human mast cells by IL-10. Clin. Exp. Allergy. 2001;31:694–704. doi: 10.1046/j.1365-2222.2001.01069.x. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Arudchandran R, Manetz TS, Zhang W, Sommers CL, Love PE, Rivera J, Samelson LE. LAT is essential for FcεRI-mediated mast cell activation. Immunity. 2000;12:525–535. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- Samayawardhena LA, Hu J, Stein PL, Craig AW. Fyn kinase acts upstream of Shp2 and p38 mitogen-activated protein kinase to promote chemotaxis of mast cells towards stem cell factor. Cell Signal. 2006;18:1447–1454. doi: 10.1016/j.cellsig.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Samayawardhena LA, Kapur R, Craig AW. Involvement of Fyn kinase in Kit and integrin-mediated Rac activation, cytoskeletal reorganization, and chemotaxis of mast cells. Blood. 2007;109:3679–3686. doi: 10.1182/blood-2006-11-057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samayawardhena LA, Pallen CJ. Protein-tyrosine phosphatase α regulates stem cell factor-dependent c-Kit activation and migration of mast cells. J. Biol. Chem. 2008;283:29175–29185. doi: 10.1074/jbc.M804077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samayawardhena LA, Pallen CJ. PTPα activates Lyn and Fyn and suppresses Hck to negatively regulate FcεRI-dependent mast cell activation and allergic responses. J Immunol. 2010;185:5993–6002. doi: 10.4049/jimmunol.1001261. [DOI] [PubMed] [Google Scholar]
- Sanchez-Miranda E, Ibarra-Sanchez A, Gonzalez-Espinosa C. Fyn kinase controls FcεRI receptor-operated calcium entry necessary for full degranulation in mast cells. Biochem. Biophys. Res. Commun. 2010;391:1714–1720. doi: 10.1016/j.bbrc.2009.12.139. [DOI] [PubMed] [Google Scholar]
- Sandig H, Bulfone-Paus S. TLR signaling in mast cells: common and unique features. Front Immunol. 2012;3:185. doi: 10.3389/fimmu.2012.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada J, Shimizu S, Tamatani T, Kanegasaki S, Saito H, Tanaka A, Kambe N, Nakahata T, Matsuda H. Stem Cell Factor Has a Suppressive Activity to IgE-Mediated Chemotaxis of Mast Cells. J. Immunol. 2005;174:3626–3632. doi: 10.4049/jimmunol.174.6.3626. [DOI] [PubMed] [Google Scholar]
- Schmidt U, Abramova A, Boucheron N, Eckelhart E, Schebesta A, Bilic I, Kneidinger M, Unger B, Hammer M, Sibilia M, Valent P, Ellmeier W. The protein tyrosine kinase Tec regulates mast cell function. Eur. J. Immunol. 2009;39:3228–3238. doi: 10.1002/eji.200838839. [DOI] [PubMed] [Google Scholar]
- Scholten J, Hartmann K, Gerbaulet A, Krieg T, Muller W, Testa G, Roers A. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res. 2008;17:307–315. doi: 10.1007/s11248-007-9153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi R, Kinashi T, Sagara H, Hirosawa K, Takatsu K. Defective degranulation and calcium mobilization of bone-marrow derived mast cells from Xid and Btk-deficient mice. Immunol. Lett. 1998;64:109–118. doi: 10.1016/s0165-2478(98)00086-8. [DOI] [PubMed] [Google Scholar]
- Sharma N, Everingham S, Ramdas B, Kapur R, Craig AW. SHP2 phosphatase promotes mast cell chemotaxis toward stem cell factor via enhancing activation of the Lyn/Vav/Rac signaling axis. J. Immunol. 2014;192:4859–4866. doi: 10.4049/jimmunol.1301155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Kumar V, Everingham S, Mali RS, Kapur R, Zeng LF, Zhang ZY, Feng GS, Hartmann K, Roers A, Craig AW. SH2 domain-containing phosphatase 2 is a critical regulator of connective tissue mast cell survival and homeostasis in mice. Mol. Cell Biol. 2012;32:2653–2663. doi: 10.1128/MCB.00308-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shik D, Munitz A. Regulation of allergic inflammatory responses by inhibitory receptors. Clin. Exp. Allergy. 2010;40:700–709. doi: 10.1111/j.1365-2222.2010.03501.x. [DOI] [PubMed] [Google Scholar]
- Silverman MA, Shoag J, Wu J, Koretzky GA. Disruption of SLP-76 interaction with Gads inhibits dynamic clustering of SLP-76 and FcεRI signaling in mast cells. Mol. Cell Biol. 2006;26:1826–1838. doi: 10.1128/MCB.26.5.1826-1838.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DL. Targeting kinases: a new approach to treating inflammatory rheumatic diseases. Curr. Opin. Pharmacol. 2013;13:426–434. doi: 10.1016/j.coph.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: A link to neurogenic skin disorders. Brain Behav. Immun. 1999;13:225–239. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- Siraganian RP, de Castro RO, Barbu EA, Zhang J. Mast cell signaling: the role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010;584:4933–4940. doi: 10.1016/j.febslet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Stenton GR, Mackenzie LF, Tam P, Cross JL, Harwig C, Raymond J, Toews J, Chernoff D, Macrury T, Szabo C. Characterization of AQX-1125, a small-molecule SHIP1 activator: Part 2. Efficacy studies in allergic and pulmonary inflammation models in vivo. Br. J. Pharmacol. 2013a;168:1519–1529. doi: 10.1111/bph.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenton GR, Mackenzie LF, Tam P, Cross JL, Harwig C, Raymond J, Toews J, Wu J, Ogden N, Macrury T, Szabo C. Characterization of AQX-1125, a small-molecule SHIP1 activator: Part 1. Effects on inflammatory cell activation and chemotaxis in vitro and pharmacokinetic characterization in vivo. Br. J. Pharmacol. 2013b;168:1506–1518. doi: 10.1111/bph.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Price LS, Koffer A. Rho controls cortical F-actin disassembly in addition to, but independently of, secretion in mast cells. J. Biol. Chem. 1999;274:38140–38146. doi: 10.1074/jbc.274.53.38140. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Leach S, Liu W, Ralston E, Scheffel J, Zhang W, Lowell CA, Rivera J. Molecular editing of cellular responses by the high-affinity receptor for IgE. Science. 2014;343:1021–1025. doi: 10.1126/science.1246976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir I, Stolpa JC, Helgason CD, Nakamura K, Bruhns P, Daeron M, Cambier JC. The RasGAP-binding protein p62dok is a mediator of inhibitory FcγRIIB signals in B cells. Immunity. 2000;12:347–358. doi: 10.1016/s1074-7613(00)80187-9. [DOI] [PubMed] [Google Scholar]
- Tan BL, Yazicioglu MN, Ingram D, McCarthy J, Borneo J, Williams DA, Kapur R. Genetic evidence for convergence of c-Kit- and α4 integrin-mediated signals on class IA PI-3kinase and the Rac pathway in regulating integrin-directed migration in mast cells. Blood. 2003;101:4725–4732. doi: 10.1182/blood-2002-08-2521. [DOI] [PubMed] [Google Scholar]
- Timokhina I, Kissel H, Stella G, Besmer P. Kit signaling through PI 3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J. 1998;17:6250–6262. doi: 10.1093/emboj/17.21.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkaczyk C, Beaven MA, Brachman SM, Metcalfe DD, Gilfillan AM. The phospholipase Cγ1-dependent pathway of FcεRI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase. J. Biol. Chem. 2003;278:48474–48484. doi: 10.1074/jbc.M301350200. [DOI] [PubMed] [Google Scholar]
- Tkaczyk C, Horejsi V, Shoko I, Draber P, Samelson LE, Satterthwaite AB, Nahm DH, Metcalfe DD, Gilfillan AM. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following kit activation and FcεRI aggregation. Blood. 2004;104:207–214. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- Tůmová M, Koffer A, Šimíček M, Dráberova L, Dráber P. The transmembrane adaptor protein NTAL signals to mast cell cytoskeleton via the small GTPase Rho. Eur. J. Immunol. 2010;40:3235–3245. doi: 10.1002/eji.201040403. [DOI] [PubMed] [Google Scholar]
- Ueda S, Mizuki M, Ikeda H, Tsujimura T, Matsumura I, Nakano K, Daino H, Honda ZZ, Sonoyama J, Shibayama H, Sugahara H, Machii T, Kanakura Y. Critical roles of c-Kit tyrosine residues 567 and 719 in stem cell factor-induced chemotaxis: contribution of src family kinase and PI3-kinase on calcium mobilization and cell migration. Blood. 2002;99:3342–3349. doi: 10.1182/blood.v99.9.3342. [DOI] [PubMed] [Google Scholar]
- Urtz N, Olivera A, Bofill-Cardona E, Csonga R, Billich A, Mechtcheriakova D, Bornancin F, Woisetschlager M, Rivera J, Baumruker T. Early activation of sphingosine kinase in mast cells and recruitment to FcεRI are mediated by its interaction with Lyn kinase. Mol. Cell Biol. 2004;24:8765–8777. doi: 10.1128/MCB.24.19.8765-8777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustun C, DeRemer DL, Akin C. Tyrosine kinase inhibitors in the treatment of systemic mastocytosis. Leuk. Res. 2011;35:1143–1152. doi: 10.1016/j.leukres.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Vega-Ruiz A, Cortes JE, Sever M, Manshouri T, Quintas-Cardama A, Luthra R, Kantarjian HM, Verstovsek S. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk. Res. 2009;33:1481–1484. doi: 10.1016/j.leukres.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S, Akin C, Manshouri T, Quintas-Cardama A, Huynh L, Manley P, Tefferi A, Cortes J, Giles FJ, Kantarjian H. Effects of AMN107, a novel aminopyrimidine tyrosine kinase inhibitor, on human mast cells bearing wild-type or mutated codon 816 c-kit. Leuk. Res. 2006;30:1365–1370. doi: 10.1016/j.leukres.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Volná P, Lebduška P, Dráberová L, Šímová S, Heneberg P, Boubelík M, Bugajev V, Malissen B, Wilson BS, Hořejši V, Malissen M, Dráber P. Negative regulation of mast cell signaling and function by the adaptor LAB/NTAL. J. Exp. Med. 2004;200:1001–1013. doi: 10.1084/jem.20041213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosseller K, Stella G, Yee NS, Besmer P. c-kit receptor signaling through its phosphatidylinositide-3′-kinase-binding site and protein kinase C: role in mast cell enhancement of degranulation, adhesion, and membrane ruffling. Mol. Biol. Cell. 1997;8:909–922. doi: 10.1091/mbc.8.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinblatt ME, Kavanaugh A, Genovese MC, Jones DA, Musser TK, Grossbard EB, Magilavy DB. Effects of Fostamatinib (R788), an Oral Spleen Tyrosine Kinase Inhibitor, on Health-related Quality of Life in Patients with Active Rheumatoid Arthritis: Analyses of Patient-reported Outcomes from a Randomized, Double-blind, Placebo-controlled Trial. J. Rheumatol. 2013 doi: 10.3899/jrheum.120923. [DOI] [PubMed] [Google Scholar]
- Weinblatt ME, Kavanaugh A, Genovese MC, Musser TK, Grossbard EB, Magilavy DB. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N. Engl. J. Med. 2010;363:1303–1312. doi: 10.1056/NEJMoa1000500. [DOI] [PubMed] [Google Scholar]
- Wex E, Bouyssou T, Duechs MJ, Erb KJ, Gantner F, Sanderson MP, Schnapp A, Stierstorfer BE, Wollin L. Induced Syk deletion leads to suppressed allergic responses but has no effect on neutrophil or monocyte migration in vivo. Eur. J. Immunol. 2011;41:3208–3218. doi: 10.1002/eji.201141502. [DOI] [PubMed] [Google Scholar]
- Winkler DG, Faia KL, DiNitto JP, Ali JA, White KF, Brophy EE, Pink MM, Proctor JL, Lussier J, Martin CM, Hoyt JG, Tillotson B, Murphy EL, Lim AR, Thomas BD, Macdougall JR, Ren P, Liu Y, Li LS, Jessen KA, Fritz CC, Dunbar JL, Porter JR, Rommel C, Palombella VJ, Changelian PS, Kutok JL. PI3K-δ and PI3K-γ inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem. Biol. 2013;20:1364–1374. doi: 10.1016/j.chembiol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Woolhiser MR, Brockow K, Metcalfe DD. Activation of human mast cells by aggregated IgG through FcεRI: additive effects of C3a. Clin. Immunol. 2004;110:172–180. doi: 10.1016/j.clim.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Xiao W, Kashiwakura J, Hong H, Yasudo H, Ando T, Maeda-Yamamoto M, Wu D, Kawakami Y, Kawakami T. Phospholipase C-β3 regulates FcεRI-mediated mast cell activation by recruiting the protein phosphatase SHP-1. Immunity. 2011;34:893–904. doi: 10.1016/j.immuni.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Nishimoto H, Hong H, Kitaura J, Nunomura S, Maeda-Yamamoto M, Kawakami Y, Lowell CA, Ra C, Kawakami T. Positive and negative regulation of mast cell activation by Lyn via the FcεRI. J. Immunol. 2005;175:6885–6892. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Saito T. Progress in allergy signal research on mast cells: signal regulation of multiple mast cell responses through FcεRI. J. Pharmacol. Sci. 2008;106:336–340. doi: 10.1254/jphs.fm0070251. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Takase-Utsugi M, Ishikawa E, Sakuma M, Nishida K, Saito T, Kanagawa O. Selective impairment of FcεRI-mediated allergic reaction in Gads-deficient mice. Int. Immunol. 2008;20:1289–1297. doi: 10.1093/intimm/dxn085. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Charles N, Furumoto Y, Odom S, Yamashita T, Gilfillan AM, Constant S, Bower MA, Ryan JJ, Rivera J. Cutting edge: genetic variation influences FcεRI-induced mast cell activation and allergic responses. J. Immunol. 2007;179:740–743. doi: 10.4049/jimmunol.179.2.740. [DOI] [PubMed] [Google Scholar]
- Yu M, Lowell CA, Neel BG, Gu H. Scaffolding adapter Grb2-associated binder 2 requires Syk to transmit signals from FcRI. J Immunol. 2006;176:2421–2429. doi: 10.4049/jimmunol.176.4.2421. [DOI] [PubMed] [Google Scholar]
- Zhang J, Berenstein EH, Evans RL, Siraganian RP. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor-mediated degranulation in a Syk-negative variant of rat basophilic leukemia RBL-2H3 cells. J. Exp. Med. 1996;184:71–79. doi: 10.1084/jem.184.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Billingsley ML, Kincaid RL, Siraganian RP. Phosphorylation of Syk activation loop tyrosines is essential for Syk function. An in vivo study using a specific anti-Syk activation loop phosphotyrosine antibody. J. Biol. Chem. 2000;275:35442–35447. doi: 10.1074/jbc.M004549200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Oh SY, Wu X, Oh MH, Wu F, Schroeder JT, Takemoto CM, Zheng T, Zhu Z. SHP-1 deficient mast cells are hyperresponsive to stimulation and critical in initiating allergic inflammation in the lung. J. Immunol. 2010;184:1180–1190. doi: 10.4049/jimmunol.0901972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Oh SY, Zhou Y, Yuan B, Wu F, Oh MH, Wang Y, Takemoto C, Van RN, Zheng T, Zhu Z. SHP-1 regulation of mast cell function in allergic inflammation and anaphylaxis. PLoS. One. 2013;8:e55763. doi: 10.1371/journal.pone.0055763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Liu Y, Koonpaew S, Granillo O, Zhang W. Positive and negative regulation of FcεRI-mediated signaling by adaptor protein LAB/NTAL. J. Exp. Med. 2004;200:991–1000. doi: 10.1084/jem.20041223. [DOI] [PMC free article] [PubMed] [Google Scholar]